Abstract
Climate change is expected to amplify drought frequency and intensity, with a significant alteration of the ecosystems. It affects the livelihoods, communities, and productivity of many important crops globally, including cereals. These crops are vulnerable to drought stress due to disturbed growth, nutrient acquisition, and cell functioning and biochemistry, which decrease yields and grain quality. Thus, understanding plants’ drought stress tolerance mechanisms could be an effective strategy for ensuring continuous productivity.
Drought stresses plants and associated factors (e.g. soil microorganisms), inducing adjusted responses involving plant-soil signalling via phytohormones. The coordination of plant responses to drought by phytohormones is of great interest owing to the plants’ sessility, and their subsistence relies mainly on their aptitude to quickly regulate their growth and physiology processes and to alleviate drought stress effects. Phytohormones are typically involved in these processes. Research interest has recently been focused on understanding phytohormones’ multiple functions that are critical regulators of plant functioning, including cell expansion and division, endogenous level of metabolites, and gene expression. Phytohormones, especially abscisic acid (ABA), salicylic acid (SA), jasmonate (JA), ethylene (ET), auxin (IAA), cytokinins (CKs), brassinosteroids (BRs), gibberellins (GAs), and strigolactones (SLs) are known to be involved in plants’ tolerance to drought. Some reduce water loss through regulating stomata opening, while others induce root development, accumulation of osmolytes, and antioxidant enzymes to protect plant cells from stress-related damages. Some of these signalling molecules could be either produced by plants suffering from water deficiency or induced by the presence of microorganisms. Besides, they could also be exogenously applied to vegetative tissues and soil. Despite several studies on phytohormone effects, their mechanisms and possible crosstalk are still a subject of debate. Thus, this chapter presents an overview of the different roles of phytohormones in regulating cereals’ adaptive responses to drought stress severity and the potential factors to alter their effectiveness in mitigating this constraint in cereals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Phytohormones: Key Mediators of Cereal Responses to Drought Stress
Alarming environmental challenges have mounted and become more evident due to exacerbating water scarcity, industrialization, and extreme weather events. According to FAO (2018), up to 60% of the global population will suffer from water deficiency by 2025. Drought has recently received great importance since it is among the critical constraints that control and reduce the agricultural production of global crops (Iqbal et al. 2020; Meza et al. 2020). This constraint results in wide morphological, biochemical, and genetic disturbances and changes in plants and subsequently affects development, productivity, and yield (Ullah et al. 2018a; Boutasknit et al. 2021; Lahbouki et al. 2022). Thus, the impact of droughts threatens complex global food security and may reduce agricultural yields, including cereals (up to 10% decrease) (Lesk et al. 2016; Fu et al. 2021). To address this global issue, research in agriculture has targeted improving genetic resources and agricultural practices to enhance water use efficiency (WUE) (Haile et al. 2020; Ahluwalia et al. 2021). Additionally, this worrying scenario requires the urgent implementation of sustainable measures to improve crop yield and quality. Increasing attention has been focused on applying beneficial biostimulants to minimize the effect of drought.
Cereal crops, mainly rice, wheat, maize, sorghum, barley, and pearl millet provide more than 50% of the population’s caloric requirements, particularly in developing countries in South Asia, sub-Saharan Africa, and Latin America (Olugbire et al. 2021). Cereal crops are known as an excellent source of total calories (48%) and total protein (42%) that contribute to more than two-thirds of dietary energy intake worldwide (Curiel et al. 2020). Cereals have been considered staple foods for most human populations for about 10,000 years due to specific characteristics such as ease of growth, development, storage, and transport (Wendin et al. 2020). In addition, cereals are classified as essential materials for producing animal feed and biofuels (Olugbire et al. 2021). However, cereal crops have experienced yield losses of up to 40% globally in wheat and maize caused by drought stress effects (Daryanto et al. 2016; Fu et al. 2021). Cereals are mostly grown in (semi-) arid areas and are often exposed to intense and prolonged drought episodes. Hence, understanding drought-responsive mechanisms in cereals and molecular signalling pathways is a time-demanding task.
Phytohormones are considered regulators of plant growth and development since they act through several pathways such as additive, synergistic, or antagonistic pathways to enhance growth under normal and stressful conditions (Sadiq et al. 2020; Li et al. 2021). These molecules also develop potential phenological and biochemical processes to keep the cells’ relative water content (RWC) and water potential constant (Yadav et al. 2021). In response to drought stress conditions, several processes are elaborated by various phytohormones, such as abscisic acid (ABA), which plays a significant role in stomatal behaviour by responding to water deficiency (Li et al. 2021). Other phytohormones intervene in coordinating plant responses under drought conditions to different degrees, namely, salicylic acid (SA), jasmonic acid (JA), ethylene (ET), auxins (IAA), cytokinins (CKs), brassinosteroids (BRs), gibberellins (GAs), and strigolactones (SLs) (Tardieu et al. 2018; Ilyas et al. 2021; Yadav et al. 2021). These molecules act as chemical regulators of plant responses to multiple environmental stresses. Once the perception of the stress signal is established, a series of chemical reactions are released and activated in a network of interactions to enhance specific protective mechanisms, including stomatal closure, osmolyte accumulation, and antioxidant defense, in an attempt to escape water deficiency (Gupta et al. 2020; Yadav et al. 2021).
2 Correlations Between Phytohormones and Drought Stress Tolerance in Cereals
Phytohormones have essential roles in controlling multiple plants’ acclimatization processes in response to water deficiency. ABA is the primary hormone that strengthens plant water stress tolerance via many processes such as stomatal movements, root expansion, and stimulation of ABA-dependent pathways (Cardoso et al. 2020; Nawaz and Wang 2020; Wang et al. 2021a). Additionally, JA, SA, ET, IAA, GAs, CKs, SLs, and BRs are also necessary to meet the challenges of water deficiency. These molecules commonly are in cross-talk to ensure plant survivability under drought stress (Ullah et al. 2018a). Various investigations have highlighted the impact of water stress on phytohormones and vice versa, usually concluding that there is a high correlation between drought stress and phytohormone production. For instance, Bano et al. (2012) compared the effects of ABA and drought stress application at pre-sowing and 55 days after sowing in two drought tolerance contrasting wheat varieties. The drought-resistant variety developed a great defense mechanism to mitigate reactive oxygen species (ROS) effects through stimulation of the antioxidant enzyme activity. Under water deficiency, ABA induced a significant increase in superoxide dismutase (SOD) and peroxidase (POD) activity, with a significant decrease in this activity in re-watering. For both wheat cultivars, ABA treatment significantly enhanced RWC under drought conditions. Furthermore, the sensitive variety was more responsive to ABA treatment and showed low concentrations of endogenic ABA.
On the other hand, the tolerant cultivar presented a great recovery from water stress at re-watering. In addition, the grain weight was significantly improved by ABA treatment for tolerant cultivars under water deficiency (Nayyar and Walia 2003; Bano et al. 2012). Drought stress decreased GA and IAA and increased ABA and proline in two wheat cultivars (Bano and Yasmeen 2010). ABA and benzyladenine (BA) application at the anthesis stage induced osmoregulation by proline production. BA was more effective at the early stages of grain filling, while ABA was more effective at the later stages (Bano and Yasmeen 2010).
Similarly, leaf GA and IAA content significantly decreased under drought stress (Xie et al. 2003). The correlation analyses between yields, starch, and protein content in grains and levels and ratios of four hormones indicated that the changes were associated with IAA and GA reduction and ABA increase, especially in grains. The overall results of these studies suggested that the varying concentrations of endogenic hormones under post-anthesis drought conditions could alter grain starch and protein content by regulating the activity and processes of the enzyme, which might be attributed to synthesis decrease (Xie et al. 2004) or a degradation decrease (Davenport et al. 1980) of IAA and GA. Foliar application of glycine betaine (GB) improved drought tolerance and yield of maize and sorghum, but not wheat (Agboma et al. 1997). In contrast, drought significantly decreased maize and sorghum’s grain numbers and yields. Foliar application of GB minimally enhanced biomass production in the three crops and significantly increased maize (P = 0.001) and sorghum (P = 0.003) grain yield.
3 Hormones’ Signalling for Drought Stress Response and Tolerance
The evolution has enabled plants to develop multiple strategies to manage water scarcity. They have established mechanisms, including altered molecular, biochemical, and physiological processes (Bhargava and Sawant 2013; Seleiman et al. 2021). Such response mechanisms occur via plant hormone signalling pathways (Ilyas et al. 2021). Phytohormones are indispensable molecules that participate in various biological processes. Besides, they are vital to stress signalling pathways. The abiotic stress signalling in plants depends on the nature, intensity, and length of exposure.
In the signalling cascade, phytohormones are instrumental in orchestrating plant development, growth, and tolerance mechanisms (Tiwari et al. 2017; Tardieu et al. 2018; Jogawat et al. 2021).
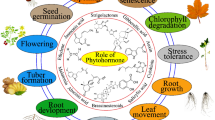
Here, we will focus on the signalling pathways related to each of the nine hormones involved in drought tolerance in cereals.
3.1 Abscisic Acid (ABA)
ABA is a sesquiterpenoid phytohormone often labelled as “stress hormone” due to its discrete association with plant abiotic stress mitigation (Zhao et al. 2021). Therefore, this molecule is the hormone of abscission and is probably the most studied of plant hormones (Chen et al. 2021b). ABA is formed via the carotenoid pathway (Fig. 13.1). Zeaxanthin is transformed to all-trans-violaxanthin by antheraxanthin. This reaction is activated by the zeaxanthin epoxidase enzyme (ZEP; EC 1.14.13.90) (Agrawal et al. 2001), followed by the conversion of trans-violaxanthin to 9-cis-violaxanthin and 9-cis-neoxanthin. Neoxanthin synthase (NXS; EC 5.3.99.9) is involved in these reactions (North et al. 2007). The final reaction to ABA production takes place in chloroplasts and is catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED; EC 1.13.11.51) (Iuchi et al. 2001). The first-ever NCED gene was studied in the maize vp14 mutant and is activated under water deficiency in the course of seed maturation (Tan et al. 1997). ABA orchesters diverse physiological functions and developmental phases, such as seed development and dormancy (Sano and Marion-Poll 2021), stomatal opening (Hasan et al. 2021), embryo morphogenesis (Kruglova et al. 2021), and biosynthesis of storage lipids and proteins (Ali et al. 2022). Furthermore, ABA is also implicated in controlling the expression of genes implicated in the ABA signalling pathways (Some et al. 2021). The role of ABA as a crucial messenger in mitigating stress and improving plant tolerance has received much attention (Wilkinson and Davies 2002). To cope with drought stress, cellular ABA content tends to rise, leading to the plant hormone binding to the receptors of pyrabactin resistance (PYR/PYL/RCARs), which inactivates type 2C protein phosphatases (PP2Cs) (Siodmak and Hirt 2021). The protein kinases, SnRK2s, are auto-stimulated after dissociation from PP2Cs, which triggers ABA responses. In addition, calcium-dependent protein kinases (CDPKs) play a role in ABA signalling. CDPKs were described in rice (29 CDPKs), wheat (20), and maize (35) (Schulz et al. 2013). Under drought stress, implicated signalling pathways and gene expression levels (more than 10% of protein-encoding genes) are de-regulated in response to increased endogenous ABA levels (Nemhauser et al. 2006; Canales et al. 2021), which results in limiting water loss through the reduction of leaf expansion and stomatal opening (Wilkinson et al. 2012). Another attribute to ABA resides in its implication in robustness and development of root architecture (Wilkinson et al. 2012; Benderradji et al. 2021). In addition, ABA interferes in synthesizing dehydrins and Late Embryogenesis Abundant (LEA) proteins (Sun et al. 2021). Tolerance is indeed conferred on plants owing to the upregulation by ABA of production of osmoprotectants, maintenance of cell turgor, and stimulation of antioxidant defense (Chaves et al. 2003). In a study conducted by Dominguez and Carrari (2015) on drought-tolerant Zhengdan 958 and drought-sensitive Xundan 20 maize hybrids, exogenous S-ABA helped mitigate drought stress by enhancing Asr1 (ABA, stress, ripening) gene expression levels.
Schematic diagrams of the major plant hormone generation and signalling pathways. (a) Abscisic acid, (b) jasmonates, (c) salicylic acid, (d) ethylene, (e) indole-3-acetic acid, (f) cytokinins, (g) gibberellins, (h) brassinosteroids, and (i) strigolactones. 13-LOX 13-lipoxygenase, 9’-c-n 9’-cis-neoaxanthin, AAO ABA aldehyde oxidase, ACO 1-aminocyclopropane-1-carboxylic acid oxidase, ACS 1-aminocyclopropane-1-carboxylic acid synthase, AOC allene oxide cyclase, AOS allene oxide synthase, a-t-n all-trans-neoxanthin, a-t-v all-trans-violaxanthin, CCD carotenoid cleavage dioxygenase, CP450 cytochrome P450, CPS copalyl diphosphate synthase, D27 dwarf27, DET2 DEETIOLATED2 gene, DMAPP dimethylallyl pyrophosphate, DWF4 dwarf4, EDS5 enhanced disease susceptibility 5, EPS1 enhanced pseudomonas susceptibility 1, ICS isochorismate synthase, IPA indole-3-pyruvate, IPT isopentenyl transferase, JA-Ile jasmonoyl isoleucine, JIH1 jasmonoyl-l-isoleucine hydrolase 1, JMT jasmonic acid carboxyl methyltransferase, KAO ent-kaurenoic acid oxidase, KS ent-kaurene synthase, MeJA methyl jasmonate, MJE methyl jasmonate esterase, NCED 9-cis-epoxycarotenoid dioxygenase, NCEI neoxanthin synthase, NSY neoxanthin synthase, OPDA cis-oxo-phytodienoic acid, OPR3 12-oxophytodienoate reductase 3, PBS5 phosphate-buffered saline 5, SDR short-chain dehydrogenase/reductase-like, TAA tryptophan aminotransferase of Arabidopsis, Trp tryptophan, tz trans-zeatin
3.2 Jasmonates (JAs)
JAs are a group of hormones formed by cyclopentanone that include free jasmonic acid (JA) (the most common form) and methyl jasmonate (MeJA) (Fig. 13.1). These plant hormones are very abundant across different plant species. JAs are crucial for plant development and survival. They are required for senescence, flowering, fruiting, direct and indirect defense responses, and secondary metabolism (Wu et al. 2008; Fahad et al. 2015). JAs are synthesised from α-linolenic acid (α-LeA) through the galactolipase (Li et al. 2021a). JA formation is initiated by oxygenation of 13-lipoxygenase, the 13(S)-hydroperoxy-octadecatrienoic acid is transformed to an epoxide by a 13-allene oxide synthase and cyclized to the cyclopentenone (cis)-12-oxophytodienoic acid (OPDA) by an allene oxide cyclase. In this step, the enantiomeric structure of the naturally occurring (þ)-7-iso-JA ((3R,7S)-JA) is established (Wasternack et al. 2013). JAs act as a plant defense activator when subjected to abiotic stresses, including drought (Pauwels et al. 2009; Seo et al. 2011). JA ZIM-domain (JAZ) proteins play a significant role in the JA signalling pathway. JAI3/JAZ proteins bind to transcription factors (TFs) under normal conditions. Under stress conditions, however, JA and its derivatives accumulate, and JAZ proteins degrade, leading to the activation of TFs, which upregulate the genes implicated in stress responses (Liu et al. 2017; Han and Luthe 2021). Furthermore, JA induces the development of roots and ROS scavenging and the closure of stomata via the JA precursor, OPDA (Wang et al. 2021b). Allagulova et al. (2020) indicated that dehydrins might participate in the methyl jasmonate (MeJA)-induced protecting effect in wheat plants subjected to drought stress. Treated plants with 0.1 μM of exogenous MeJA decreased membrane structure lesions. Furthermore, TADHN dehydrin transcripts and dehydrin protein expression increased during dehydration.
3.3 Salicylic Acid (SA)
SA is a naturally occurring phenolic compound implicated in pathogenesis-associated protein expressions (Chen et al. 2012). It plays a crucial role in regulating plant growth (Bernal-Vicente et al. 2018), ripening and development (Changwal et al. 2021), and in triggering plants’ responses to abiotic stresses; including drought (Miura et al. 2013), salt (Khodary 2004), and heat (Fayez and Bazaid 2014). Two routes are involved in the biosynthesis of SA: the isochorismate (IC) and the phenylalanine ammonia-lyase (PAL) pathways, with the IC pathway being the major one (Fig. 13.1) (Uppalapati et al. 2007). While low concentrations of SA can increase the antioxidant machinery, high concentrations can lead to cell death and vulnerability under abiotic constraints (Prakash et al. 2021). SA triggers the expression of genes encoding chaperones, heat shock proteins (HSP), antioxidants, and genes involved in the biosynthesis of cytochrome P450 and secondary metabolites, sinapyl alcohol dehydrogenase, and cinnamyl alcohol dehydrogenase (Jumali et al. 2011).
SA responses to the environment occur through the nonexpressor of pathogenesis-related genes, NPR1, expressing PR genes (Maier et al. 2011). Activated genes like WRKYs and TGAs improve plants’ tolerance against biotic and abiotic stresses (Chen et al. 2012; Ullah et al. 2018b). SA content in barley roots was enhanced ca. 2x under water stress (Bandurska 2005). SA-inducible pathogenesis-related genes PR1 and PR2 were incited in response to water deficits (Miura et al. 2013). NPR1 protein acts as a regulator of the SA-controlled signalling pathway, especially under pathogen infection. SA accumulation implicates antimicrobial NPR1 proteins in the defense process, leading to the import of monomeric NPR1 to the nucleus, where it binds with SA and generates a conformational change that conducts to the C-terminal activation domain that triggers transcription. Another work on two wheat varieties, drought-tolerant Kundan and drought-sensitive Lok1, subjected to 75 and 50% reduction of RWC and recovery, revealed that SA-induced thioredoxins activated defense responses against ROS, leading to drought tolerance (Sharma et al. 2017).
3.4 Ethylene (ET)
ET constitutes a simple hydrocarbon gaseous molecule that acts as a plant hormone. ET is implicated in specific stages of plant growth and development, particularly fruit ripening (Liu et al. 2021), flower senescence (Naing et al. 2021) and the abscission of petals and leaves (Yang et al. 2021). The primary precursor of ET is 1-aminocyclopropane-1-carboxylic acid (ACC) (Fig. 13.1). ACC synthase is the enzyme that catalyzes the conversion of S-adenosyl-l-methionine to ACC, which is later converted to ET by the action of ACC oxidase (Bleecker and Kende 2000). One of the prominent roles of ET is the regulation of drought tolerance in plants. APETELA2/Ethylene-Responsive Element-Binding Protein (AP2/EREBP) genes have been found to respond to drought (Dong et al. 2021). The expression of GmERF3, an ethylene response factor (ERF), was shown to be induced under drought and salt stresses, conferring tolerance to plants (Zhang et al. 2009; Ilyas et al. 2021). Another ERF gene, AtERF019, acted positively in delaying senescence (Scarpeci et al. 2017), whereas transgenic plants have shown improved tolerance to drought by lowering transpiration rate and regulating stomatal regulation aperture and cuticle-thinning.
Environmental stress leads to ET accumulation, which improves the survival of plants under adverse environmental conditions (Gamalero and Glick 2012; Salazar et al. 2015). SUB1A, an ethylene-response-factor-like gene and an ETS2 Receptor Factor present in limited rice accessions, attributed submergence tolerance to rice (Xu et al. 2006; Fukao et al. 2011). Genotypes with the SUB1A gene could recover after desubmergence via the establishment of new leaves (Fukao et al. 2006). These findings suggest that SUB1A also may help the process of enduring several abiotic stresses. Under drought stress, EIN2 downstream signalling components are kept inactive by the action of a Raf-like kinase and critical negative regulator of ET, CTR1 kinase-dependent phosphorylation (Eppel and Rachmilevitch 2016). Besides, Dubois et al. (2013) highlighted SIERF5, AtERF5, and AtER6 as central regulators of salt and drought stresses. It is worth mentioning that AtERF6 stimulates the expression of different osmotic stress-responsive genes such as STZ, MYB51, and WRKY33.
3.5 Auxins
Indole-3-acetic acid (IAA) is the most common phytohormone of the auxin family involved in signalling and several aspects of plant growth and responses to environmental conditions (Jin et al. 2021). Auxins are deemed as the first phytohormones to be characterized (Masuda and Kamisaka 2000), being IAA the most abundant and versatile (Zhao 2010), yet the mechanisms governing IAA biosynthesis, transport, and signalling pathways are still to be elucidated (Wang et al. 2015). This is due to the complexity of the process itself. Available data suggest that the amino acid tryptophan (Trp) acts as a precursor or the major IAA precursor (Fig. 13.1). The conversion of tryptophan to IAA has also been reported (Bartel 1997). Coleoptile tips excised tissues with l4C-Trp-seeds (kernels) of maize (Zea mays L. cv. Golden Cross Bantam 70) have shown label incorporation, demonstrating the conversion of tryptophan into IAA (Koshiba et al. 1995). Moreover, IAA can release from IAA conjugates through the hydrolysis of IAA-amino acids, IAA-sugar, and IAA-methyl ester (Bartel 1997; Qin et al. 2005).
IAA acts as a plant growth regulator. It induces cell elongation (Rayle and Cleland 1992), differentiation of cell and vascular tissues (Ding and Friml 2010; Casanova-Sáez et al. 2021) and axial elongation (Campanoni and Nick 2005). It acts as a mediator of apical dominance (Booker et al. 2003). The role of IAA extends to influencing gametogenesis (Zhao 2010), embryogenesis (Cheng et al. 2007), seedling growth (Hu et al. 2017), vascular patterning (Berleth et al. 2000), and flower development (Cheng and Zhao 2007).
IAA plays an integral part in plant adaptation to drought, heavy metal, salinity, and fungal stresses by increasing root and shoot growth at the transcriptional level (Yuan et al. 2013; Tiwari et al. 2020). In tobacco, it has been shown that auxin-inducible glutathione S-transferase (GST), PjGSTU1 from Prosopis juliflora confers drought tolerance (George et al. 2010; Cicero et al. 2015). In Arabidopsis, increased endogenous levels of IAA-inducing drought tolerance were attained by the activation of flavin monooxygenase encoding genes involved in the tryptophan-dependent IAA biosynthesis pathway (Niyogi and Fink 1992). Similar results were obtained by overexpressing YUCCA7 in Arabidopsis (Lee et al. 2012). Induction of the OsGH3–2 gene encoding an enzyme for IAA activation in rice subjected to water deficit exhibited drought resistance (Ahammed et al. 2013). Furthermore, the OsPIN gene family, particularly OsPIN2 and OsPIN5b, was upregulated under drought stress (Lu et al. 2015) and TLD1/OsGH3.13—an IAA amino synthetase encoding gene—overexpression stimulated LEA, leading ultimately to drought tolerance (Rao et al. 2014).
3.6 Cytokinins (CKs)
Natural forms of CKs occur as N6 substituted adenine derivatives, with distinct substitutions attached to the N6 position of the adenine ring (Fig. 13.1). N6-(Δ2-isopentenyl)-adenine (iP), trans-zeatin (tZ), cis-zeatin, and dihydrozeatin are the most common forms. The significant derivatives are tZ, iP, and their sugar conjugates (Sakakibara 2021). CKs are biosynthesized in roots. The addition of the prenyl group derived from the prenol phosphate, named dimethylallyl diphosphate, to the N6-terminus of ADP/ATP constitutes the first step catalyzed by isopentenyl transferase (IPT), a multigene family that is encoded in most plants (Kakimoto 2001; Sakakibara 2021). Cytochrome P450 enzymes, CYP735A1 and CYP735A2, participate in the hydroxylation of the isoprenoid side chain, which converts the resulting iP-ribotides into tZ-type CKs (Wheeldon and Bennett 2021). CKs then move the xylem upward to other plant parts, functioning as long-distance messengers to regulate plant growth and development (Aloni et al. 2006; Dun et al. 2006). Prominent roles include cell division (Riou-khamlichi et al. 1999), sink/source relations and nutrient uptake (Roitsch and Ehneß 2000), phyllotaxis (Reinhardt 2004), and gametophyte and embryonic development (Wybouw and De Rybel 2019).
CKs regulate protective responses in plants under abiotic stresses from roots to shoots (Wu et al. 2021). The local stress sensed by roots might implicate changes in the levels of CKs in different plant organs, leading to the plant’s drought adaptation, thanks to an enhanced apical dominance stimulated by reduced CK levels (O’Brien and Benková 2013). It has been reported that deregulation of CKs (up- or downregulation) leads to drought tolerance. Enhanced endogenous CK levels were registered in transgenic plants expressing an IPT gene, resulting in delayed senescence by suppressing drought-induced leaf senescence. Overexpression of CK oxidase/dehydrogenase (CKX; EC. 1.5. 99.12), which catalyzes CK breakdown, improved drought tolerance possibly due to endogenous plant hormone concentration reduction (Prerostova et al. 2018).
A suggested model, under environmental stresses, revealed that IPT gene expression decreases, leading to a decrease in CKs accumulation. Consequently, the triggered expression of stress-responsive genes, following the alleviation of the inhibitory effect of CK signalling, leads to an enhanced plant tolerance. Results displaying altered activity of CK metabolic enzymes in mutant and transgenic cells and tissues corroborate it (Nishiyama et al. 2011). CKs are believed to negatively regulate the branching and growth of roots (Tessi et al. 2021). CKs are degraded under drought stress, thereby enhancing primary root growth and branching (Werner et al. 2010). Batool et al. (2019) reported that two wheat (Triticum aestivum L.) genotypes, Heshangtou and Longchun 8275, were subjected to a cascade of water deficit treatments ranging from 80 to 45% reduced CK concentration and closed stomata, resulting in less gas exchange. These changes improved antioxidant machinery and osmotic regulation, leading to enhanced WUE. Altogether, these findings pointed out the CK implication in the root-to-shoot signaling process under environmental stress.
3.7 Gibberellins (GA)
GAs form a large group of naturally tetracyclic diterpenoid carboxylic acids based on their ent-gibberellin carbon skeletal structure. GAs range from GA1 to GA136 and are present in 128 vascular plant species (Sponsel and Hedden 2010). The biosynthesis of GAs occurs in the plastid from trans-geranylgeranyl diphosphate, via the methylerythritol phosphate pathway (Kasahara et al. 2002), through the plastid-localized sequential action of two terpene cyclases (Fig. 13.1). The following step is the oxidation by cytochrome P450 monooxygenases, which occurs in the endoplasmic reticulum, and then by soluble 2-oxoglutarate-dependent dioxygenases (Yamaguchi 2008). The dioxygenases involve the GA 20-oxidase and GA 3-oxidase families of isozymes, whereas the GA 2-oxidases (GA2ox), another class of dioxygenases, lead to the formation of inactive products in order to enable GA turnover. Drought tolerance is promoted by reducing the endogenous GA level in plants (Colebrook et al. 2014; Zhou et al. 2020). Arabidopsis gibberellin methyl transferase1 (AtGAMT1) gene was shown to encode an enzyme that catalyzes the methylation of active GA—generating GA methyl esters—resulting in drought tolerance improvement. The overexpression of the SiDREB gene was shown to suppress GA biosynthesis genes and increase drought tolerance (Nir et al. 2014; Ullah et al. 2018b).
Besides, GA2ox genes are considered to be the most receptive genes to abiotic stress (Ben Saad et al. 2020; Li et al. 2021b). A previous study suggested that restrained plant growth due to exposure to abiotic stress can be partially mediated by DELLA (Asp-Glu-Leu-Leu-Ala) protein—a negative regulator of GA signaling—acting downstream of the GA receptor (Yoshida et al. 2014). Upregulation of specific GA2ox genes by dehydration-responsive element binding proteins/C-repeat binding factor (DREB/CBF) family belonging to APETALA2 (AP2) family TFs regulates the expression of stress-responsive genes (Martin et al. 2021). The most significant role of GAs under abiotic stress appears to be associated with cell elongation, as they stimulate DELLA proteins to regulate gene expressions under water scarcity (Krugman et al. 2011). The genotypes of wheat drought-resistant, Y12-3, and drought susceptible, A24-39, subjected to 7-day drought stress, showed shifts in the expression of gibberellin-related genes.
3.8 Brassinosteroids (BRs)
BRs are a group of polyhydroxy steroidal plant hormones. Over 70 BRs have been characterized in plants. The most bioactive BRs are brassinolide, 28-homobrassinolide, and 24-epibrassinolide, and they are ubiquitous in the whole plant (Bajguz and Hayat 2009). The first steps in the biosynthesis of BRs are arguably the conversion of campesterol into campestanol, which is later converted to castasterone (Fig. 13.1). These reactions can occur via early or late C-6 oxidation. Finally, castasterone is transformed into brassinolide, which is the first isolated brassinosteroid. Further investigation into the BRs biosynthesis pathways revealed transformations between teasterone and typhasterol via 3-dehydroteasterone. Available data indicates that early and late C-6 oxidation pathways occur in many plants (Hu et al. 2021). BRs act in stem and root growth (Wei and Li 2016), floral initiation (Clouse 2008), and the development of flowers and fruits (Ali et al. 2021). BRs can mitigate abiotic stresses such as high-temperatures (Chen et al. 2021a), salinity (Vázquez et al. 2019), drought (Farooq et al. 2009; Chen et al. 2021b), flooding (Wani et al. 2016), metals/metalloids (Kour et al. 2021), and organic pollutants (Ahammed et al. 2013) by modulating antioxidant machinery components. BRs bind to BR-Insensitive 1 (BRI1) Leucine-Rich Repeat (LRR)-RLK family members on the plasma membrane in response to environmental stress. A ligand elicits BRI1 to act together with the co-receptor BRI1 Associated Receptor Kinase 1, primordial for early BR signalling events (He et al. 2000; Anwar et al. 2018). This is followed by the initiation of a signalling cascade of phosphorylation governing multiple BR-regulated gene expression through BRI1-EMS-SUPPRESSOR1 (BES1) and Brassinazole Resistant1 (BZR1) TFs (Fàbregas et al. 2018). WRKY TFs were implicated in plant growth and response to water deficit stress. AtDWF4 gene confers better growth, yield, and tolerance against drought in Brassica napus (Sahni et al. 2016). Farooq et al. (2009) corroborate the role of BRs in rice subjected to 50% field capacity (FC) water limitation after exogenous application of BRs, 28-homobrassinolide and 24-epibrassinolide, which the cv. Basmati showed drought tolerance by modulating leaf water economy and growth promotion.
3.9 Strigolactones (SLs)
SLs are a group of terpenoid lactones that are derived from carotenoids (Fig. 13.1). They play a crucial role in developing the overall root architecture (Xu et al. 2021). SLs play a crucial role in the germination of seeds and plant-microorganism interactions (Mitra et al. 2021). Treatment of strigolactone-response mutant (MAX2) Arabidopsis thaliana seedlings with GR24 (a synthetic and biologically active strigolactone) did not repress the lateral root formation, suggesting that the negative effect of strigolactones on lateral root formation is MAX2-dependent (Kapulnik et al. 2011). While SLs are synthesized and exuded essentially in roots, they can be produced in other plant parts (Bradley and Lumba 2021). Moreover, cytochrome P450 and MAX (more axillary growth) genes were displayed to operate SL biosynthesis (Yoneyama and Brewer 2021). A new class of Fe-containing protein, D27, was also identified as an SL biosynthetic element (Lin et al. 2009).
SLs are generally influenced by environmental stimuli and act on shoot and root architecture depending on nutritional conditions (Raza et al. 2021). SLs act in drought acclimatization in plant shoots, while their biosynthesis is suppressed in roots. Since SLs are transported acropetally, their downregulation can indicate affected shoots (Visentin et al. 2016). SLs act as signalling molecules in nodulation during the legume-rhizobium interactions (Soto et al. 2010; Foo and Davies 2011). In barley, the HvD14 gene has been shown to encode α/β hydrolase implicated in SL signalling and is an orthologue to D14 described in rice. All these results highlight the potential of SLs to mitigate drought stress (Marzec et al. 2020).
4 Hormone Signalling Crosstalk in Cereal Under Drought Stress Conditions
ABA, JA, ET, and SA are primal actors in drought stress mitigation, where ABA plays a leading role in controlling osmotic stress (Lata and Prasad 2011) (Fig. 13.2). GA, BRs, IAA, SLs, and CKs also act with stress-related gene factors and other phytohormones to sustain plant growth under drought stress. Increasing ABA level under water deficiency was closely linked with ABA-gene activation (Du et al. 2010), thereby inducing drought resistance through stomatal closure and osmolyte accumulation (Tiwari et al. 2017). SA and JA were also shown to be critical factors in water stress signalling based on their increase during this stress and their overall beneficial controlling role in drought resistance mechanisms such as stomatal closing (Savchenko et al. 2014; Tiwari et al. 2016). JA or MeJA (methyl-JA) is converted into an active form JA-Ile ((+)-7-iso-Jasmonoyl-L-isoleucine) after their exogenous supplementation to plants. JA-Ile gets bounded by the SCF-COI complex receptor, which has the COI1 (coronatine insensitive1) F-box protein (Fonseca et al. 2009). This action induces the degradation of JAZ (Jasmonate ZIM-domain) repressors, leading to the JA response gene activation by MYC2 (helix-loop-helix transcription factor 2) (Thines et al. 2007). In the absence of JA, JAZ blocks MYC2, which is then incapable of activating JA-inducible gene transcription. During the stress response, GA in interaction with SA and its exogenous supplementation induced the NPR1 expression and SA-related genes implicated in SA effect.
Schematic presentation of potential crosstalk between phytohormone signalling in cereals under drought stress conditions. ABA is the key operating phytohormone against drought stress due to its ability to adapt to stress signals and address downstream stress responses. Stress adaptation regulation is directly controlled by synergistic/antagonistic crosstalk between ABA and other phytohormones. CDK cyclin-dependent kinase, CKX cytokinin oxidase/dehydrogenase, COP1 E3 ubiquitin ligase constitutive photomorphogenic 1, CTR1 constitutive triple response, EIL ethylene insensitive-like protein, EIN2 membrane protein ethylene insensitive 2, ERF ethylene response factors, ETR ethylene receptor, HOOKLESS ethylene response gene, MAPKKK/MPK mitogen-activated protein kinase, MFT mother of FT and TFL1, MYC2 helix-loop-helix (bHLH) transcription factor, NPR1 non-expressor of pathogenesis-related gene 1, PIN1/3/7 efflux proteins PIN FORMED 1/3/7, RHA2A-E3 ubiquitin-protein ligase, Type A RR response regulator, WUS homeodomain transcription factor Wuschel
ABA also regulates the BR signalling pathway through BIN2 or its upstream components via the protein phosphatase 2C (PP2C) gene family (Zhang et al. 2009). Under drought stress, ABA also restrains BR-induced responses in plants (Divi et al. 2010). CKs regulate the drought stress responses that reduce the biosynthesis and transport of CKs (Tran et al. 2010). Both auxins and BRs, with the presence of MeJA, activate ACO (1-aminocyclopropane-1-carboxylic acid oxidase) enzymes that increase ethylene production (Arraes et al. 2015).
5 Factors Modifying the Phytohormonal Activity in Conferring Drought Tolerance in Cereal
Plants are deeply influenced by environmental factors such as light, temperature, infection by microorganisms (beneficial or pathogenic), low nutrients, precipitation, and contamination with metals. These external stimuli affect phytohormone levels in all plant tissues. Therefore, phytohormones are considered a challenge that limits an accurate understanding of plant physiology changes and reaches the mechanistic insight of the implication of phytohormones in regulating drought stress tolerance. Nevertheless, applying natural and chemical substances, minerals, organic amendments, and beneficial microorganisms is the most effective approach for inducing the resistance of plants to drought stress.
5.1 Substances, Minerals, and Organic Amendments
Applying priming agents such as injuries or exogenous substances could prepare the plant to respond faster and more effectively against future stresses. A recent study reported that primed wheat plants with polyethylene glycol (PEG) solution maintained higher water potential, increased net photosynthetic rate and proline and GB levels for better dehydration resistance, and activated ABA receptor PYL4 gene that regulates stomatal closure (Wang et al. 2021a) (Table 13.1). The application of other polymers, such as chitooligosaccharides, chitosan (Ch) and Ch nanoparticles, stimulated plant growth and alleviated water stress in cereals (Zou et al. 2015; Behboudi et al. 2018; Hafez et al. 2020; El Amerany et al. 2020; Almeida et al. 2020). Polymers’ effects are due either to their structures containing free amino groups-NH2 that could interact with ROS or to their ability to decrease the concentration of malondialdehyde (MDA) and electrolyte leakage (EL) and to improve the photosynthetic efficiency, diameter of vascular bundle, RWC, and antioxidant enzymes (i.e., POD, SOD, and CAT) activity (Hidangmayum et al. 2019; El Amerany et al. 2020). However, the effects of these polymers on hormone levels in cereals have not yet been investigated. Zhang et al. (2018) reported that the exogenous supplementation of Ch improved ABA, CKs, and GA levels under drought stress conditions but reduced IAA in white clover (Trifolium repens L.).
The application of mineral nutrients (i.e., zinc and ZnO nanoparticles) either to leaves or roots and organic amendments (i.e., formulated fertilizer synergist, biochar, vermicompost, humic acid, and compost) has been commonly applied in agriculture to increase seedling growth and to alleviate drought stresses in cereals (Wang et al. 2007; Moghadam et al. 2013; García et al. 2014; Sun et al. 2020; Ding et al. 2021). Their effects are related to the modulation of phytohormone levels implicated in stress tolerance (Table 13.1). The mineral imbalance in the soil (starvation or accumulation) negatively affected the hormonal interaction between the above-ground and underground parts of plants (Battal 2004). It has been shown that phosphate starvation increased ABA production in roots and inhibited the translocation of CKs from roots to shoots of barley plants, which therefore inhibited roots extension and vacuolar invertase activity and reduced ATP content (Werner et al. 2008; Vysotskaya et al. 2016; Vysotskaya et al. 2020). The excess supply of phosphate increased the ethylene response, which reduced the acquisition of other nutrients (Fe, Zn, Mn, Ca) and the number of meristematic cortical cells and inhibited the growth of the primary root (Shukla et al. 2017).
Organic amendments are considered essential components of soil organic carbon and are well known as biostimulants that may achieve phytohormone-like effects to stimulate nutrient absorption (Canellas et al. 2020). For instance, the application of humic acid to rice seedlings activated plasma membrane H+-ATPase and increased the activity of calcium-dependent protein kinases (CPKs/CDPKs) implicated in phytohormone production and signalling (Ramos et al. 2015; Xu and Huang 2017). Additionally, the application of seaweed extract (SWE) that contains osmoprotectants (i.e., GB) and phytohormones mixtures (IAA, GA, and CKs) decreased the oxidative damage of wheat grown under limited water supply by inducing the biosynthesis of ascorbate and CAT (Kasim et al. 2015). Shemi et al. (2021) reported that the individual application of GB, Zn, or SA increased the growth of maize seedlings under drought conditions due to their ability to stabilize chlorophyll pigments, maintain water equilibrium, decrease ROS and MDA content, and increase gas exchanges, RWC, and antioxidant enzymes (SOD, CAT, POD, glutathione reductase (GR), and ascorbate peroxidase (APX)). Although there is scarce information about SWE functioning to alleviate drought, they could positively affect the expression of genes responsible for host hormonal biosynthesis, like auxin (Ali et al. 2021).
5.2 Beneficial Microorganisms
Other strategies plants adopt to deal with (a)biotic stresses, especially drought, are interaction with beneficial soil microbes, including plant growth-promoting fungi (e.g. arbuscular mycorrhizal fungi (AMF)) and rhizobacteria. Significantly, the ‘cry-for-help’ strategy suggests that plants recruit beneficial microorganisms to resist environmental stresses by changing their root exudation chemistry (Rizaludin et al. 2021). These microorganisms have been described as effective biofertilizers for host plant growth-boosting, encompassing all the dynamic processes of growth, metabolism, and defense (Ait-El-Mokhtar et al. 2020; Anli et al. 2020; Ben-Laouane et al. 2021; Boutasknit et al. 2020; El Amerany et al. 2020; Hossain and Sultana 2020).
5.2.1 Plant Growth-Promoting Fungi (PGPF)
PGPF are nonpathogenic saprotroph fungi that have shown increasing interest in recent years due to their benefits, especially in promoting crop production and potentiating tolerance against stresses (Hossain and Sultana 2020; Cornejo-Ríos et al. 2021). These fungi could alter the physiology and biochemical processes of the host plants through various strategies, including the conversion of the insoluble phosphate to a soluble form, mineralization of organic substrate, production of enzymes, volatile compounds (i.e., sesquiterpenes and diterpenes), and phytohormones (Hossain and Sultana 2020). Several studies reported that Trichoderma atroviride ID20G application to maize seedlings reduced the injurious effects of water stress by stimulating photosystem II efficiency (Fv/Fm), pigment contents, and antioxidant enzyme activity (Guler et al. 2016). While no report related to phytohormone changes in cereals under water deficiency, either Phoma glomerata LWL2 or Penicillium sp. LWL3 application to cucumber seedlings mitigated water deficit by impairing polyphenol oxidase activity and reducing glutathione and endogenous SA, JA, and ABA levels that could impact GAs and IAA secretion (Waqas et al. 2012).
Arbuscular mycorrhizal fungi (AMF) could form symbiotic interactions with more than 70% of terrestrial plants (Cosme et al. 2018). Many reports showed that AMF-inoculated plants increased height, number and length of lateral roots, leaf area, number of flowers and fruits, and dry matter production (Elhindi et al. 2017; Boutasknit et al. 2021; Vosnjak et al. 2021). For instance, the plant weight of rice was ten times higher in Glomus intraradices inoculated plants than in non-inoculated plants (Ruíz-Sánchez et al. 2011). Moreover, the mutualistic relationship between plants and AMF is regulated by phytohormones. Some SLs are involved even before physical contact between the fungi and the host plants, whereas others are accumulated only at a late stage of mycorrhization (i.e., CKs) (Mitra et al. 2021). In addition, some phytohormones are accumulated to regulate root system architecture and fungal development (i.e., IAA, ABA, and JA) (Akiyama and Hayashi 2006; Hause et al. 2007). In rice, the overexpression of OsNAC14, a TF that is implicated in drought stress tolerance, induced SL biosynthesis (Shim et al. 2018). Nevertheless, the effect of CKs on the establishment of mycorrhization and water stress tolerance is still not well understood; specific authors pointed out that the accumulation of these hormones was positively correlated with the photosynthesis rate increase and fungal growth (Werner et al. 2001). Inoculation of Catalpa bungei with Rhizophagus intraradices reduced the content of zeatin in well-watered plants and those exposed to water deficiency (Chen et al. 2020). These findings showed that mycorrhization rate and the level of other hormones, IAA and ABA, were not adversely affected. Also, the level of phytohormones might vary between plant cultivars. For instance, in maize, while cv. B73 inoculated with R. irregularis reduced ABA levels, however, PR34B39 cv increased them (Table 13.1). Furthermore, a metabolomic study revealed that the inoculation of durum and bread wheat roots with Funneliformis mosseae under drought stress increased the levels of brassinosteroids (6 alpha-hydroxy-castasterone and brassinolide-23-O-glucoside), hydrolyzable abscisic acid (D-glucopyranosyl abscisate), a hormone similar to IAA (indole-3-acetyl-methionine), gibberellins (gibberellin A29-catabolite and gibberellin A34-catabolite), and jasmonate derivative (tuberonic acid glucoside) in roots (Bernardo et al. 2019).
5.2.2 Plant Growth-Promoting Rhizobacteria (PGPR)
The PGPR are beneficial bacteria that positively influence the growth and yield of different crops (Oleńska et al. 2020). They could fix and convert atmospheric N to ammonia, transform soil macromolecules into easily available compounds for plants, solubilize soil phosphate, release organic acids, and produce exopolysaccharides (EPS) and phytohormones (i.e., CKs, IAA, GA3, and zeatin) to alleviate drought stresses (Martínez-Viveros et al. 2010; Vardharajula et al. 2011; Zaheer et al. 2019). EPS are organic polymers made by bacteria to contact the host plants (Naseem et al. 2018). Despite the vital role of EPS in drought stress tolerance, their role in modulating phytohormone levels is unknown. A previous study showed that applying one or multiple rhizobacterial strains, individually or combined with either L-tryptophan or super absorbent polymer, significantly increased plant performance (Table 13.1). This positive effect is due to increased osmotic potential, proline and sugar content, antioxidant enzyme activity, ABA, GAs, CKs, and IAA levels (Table 13.1).
6 Metabolic Engineering of Phytohormones: New Strategies in Cereal to Mitigate Drought Stress
Using the most tolerant plant species to water stress and increasing cereal resistance through making crosses between inbred lines or applying a transgenic approach are considered the best-applied strategies for improving cereal productivity and minimizing land consumption of freshwater resources (Parmar et al. 2017). These advanced lines are characterized by deregulating genes of interest and stimulating regulatory factors (proteins) involved in the biosynthesis of metabolites, especially phytohormones, to ensure drought tolerance.
6.1 Breeding
Developing new drought-tolerant cereal lines using natural breeding becomes an important strategy. This technique provides changes in plant traits and creates plants with desirable characteristics based on crossing lines selected based on their high WUE or lines with contrasting phytohormone levels (Bruce et al. 2002). Despite the applicability of this approach to a broad type of plant species, however, it did not meet the general need yet, owing to the long time it takes to minimize linkage drag through phenotypic screening. Successful findings were reported in maize. For example, Pekić et al. (1995) reported that crossing maize lines “Polj17” (high ABA level) x F-2 (low ABA level) increased yield and ABA level in both leaves and kernels and reduced transpiration in the offspring compared to the parents. Moreover, the maize inbred line “RIL70”, from crossing Ph4CV × F9721, was more tolerant to stress than parental lines due to its ability to detoxify stress signals through increasing photosynthetic rate, cell wall biosynthesis, and the early expression of aquaporin-related genes. Min et al. (2016) reported that ABA level and bZIP gene expression involved in ABA synthesis and ABRE TFs were induced in the “RIL93” line (drought-sensitive) compared to the “RIL70” line.
6.2 Genetic Engineering
Genetic engineering is of the utmost importance for cereal growers since it offers a fast and exact way to attain the same objective as breeding crops for certain desirable traits in one generation rather than multiple. To achieve this goal, new cultivars are created by employing wild genes involved in drought tolerance, selecting marker-assisted breeding, and isolating trait locus genes (Khan et al. 2019). Plants could alleviate the deleterious effects of drought stress by stimulating the expression of TFs, i.e., NAC, MYB, bZIP, HDG, and WRKY, that activate phytohormone biosynthesis genes at the transcriptional level and deregulate the expression of stress-related genes (He et al. 2016; Shim et al. 2018). In rice, Shim et al. (2018) reported that overexpression of OsNAC14 induced SL biosynthesis and diminished DNA damage and the expression of drought-responsive marker genes (OsDIP1, Dehydration Stress-Inducible Protein 1, and OsRbcS, Small Subunit of Rubisco). Overexpression of OsMYB6 in rice could play a pivotal role in minimizing drought stress injuries because it elevated the expression of the NAC gene (SNAC1) (Tang et al. 2019). Overexpression of OsHBP1b in rice plants boosted plant growth and development and increased callose and antioxidant enzyme levels (Das et al. 2019).
Transgenic wheat plants over-expressing AtHDG11 and TaWRKY2 showed better growth than wild type plants. These transgenic plants are characterized by lower stomatal density, higher WUE, and accumulation of osmotically active molecules (i.e., proline, chlorophyll, and sugars, CAT, and SOD) to detoxify ROS molecules (Li et al. 2016; Gao et al. 2018). Exogenous application of phytohormones, such as BRs, induced OsDof12-type TFs in rice plants; however, the overexpression of OsDof12 in plants negatively regulated BRs signalling genes and affected plant cell architecture (Wu et al. 2015). Overexpression of JERF1, involved in ET biosynthesis, in rice exhibited better shoot and root development and a higher level of ABA (Table 13.2).
7 Conclusion and Future Perspectives
Drought stress is a constant factor that alters cereals’ growth, physiology, and metabolism. Substantial progress has been achieved recently to understand the mechanisms and the role of signalling molecules, such as phytohormones, on plant growth and yield and plant tolerance to environmental stress. As phytohormones upregulate various plant functioning processes, they can control the same traits to manage the adaptation of plants to stressful conditions. Under drought stress, the growth and development of plants are controlled by phytohormones including CKs, IAA, ET, GAs, ABA, JAs, BRs, SA, and SL through signalling cross-talk pathways orchestrated by ABA. These compounds are implicated in the drought adaptation process through stimulating stomatal closure, increasing WUE, metabolite adjustment, and expression of TFs and stress-responsive genes. The phytohormones’ cross-talk under water deficiency is carried out at different levels such as hormone activation, transcriptional activation, gene expression, and developmental variations. Despite the significant role of phytohormones in alleviating drought stress, their production and signalling pathways may be hindered owing to the vast difference in plant physiological characteristics and the duration and severity of stress. Therefore, many methods and technologies have been used to retrieve cereals from the negative impact of drought and deregulate the phytohormone concentration involved in tolerance to improve the yield. Selection of drought-tolerant species, exogenous application of minerals and organic amendments, inoculation with beneficial microorganisms (AMF, PGPF, and PGPR), and use of natural breeding/genetic engineering are factors and techniques that could help to boost cereals under future environmental stresses. With the development of omics-based research in recent decades, many gaps still need to be investigated, especially in the molecular mechanisms related to the cross-talk among phytohormones at cellular and transcriptional levels and their transport systems, receptors, mediators, and targets in plants. These aspects are highly complicated, particularly under drought stress. It is worth mentioning that genome-wide studies targeting phytohormone signalling responses under adverse environmental situations are needed and will significantly contribute to a better understanding of hormone interaction with the crosstalk network and developing ultimately effective strategies for improving cereal stress tolerance.
Abbreviations
- 13-LOX:
-
13-Lipoxygenase
- 9’-c-n:
-
9’-Cis-neoaxanthin
- α-LeA:
-
α-Linolenic acid
- AAO:
-
ABA Aldehyde oxidase
- ABA:
-
Abscisic acid
- ACC:
-
1-aminocyclopropane-1-carboxylic acid
- ACO:
-
1-aminocyclopropane-1-carboxylic acid oxidase
- ACS:
-
1-aminocyclopropane-1-carboxylic acid synthase
- AMF:
-
Arbuscular mycorrhizal fungi
- AOC:
-
Allene oxide cyclase
- AOS:
-
Allene oxide synthase
- APX:
-
Ascorbate peroxidase
- BA:
-
Benzyladenine
- BR:
-
Brassinosteroid-Insensitive receptor
- BRs:
-
Brassinosteroids
- CAT:
-
Catalase
- CCD:
-
Carotenoid cleavage dioxygenase
- CDK:
-
Cyclin-dependent kinase
- CDPK:
-
Calcium-dependent protein kinase
- Ch:
-
Chitosan
- CKs:
-
Cytokinins
- CKX:
-
Cytokinin oxidase/dehydrogenase
- CP:
-
Cytochrome P450
- CPKs/CDPKs:
-
Calcium-dependent protein kinase
- CPS:
-
Copalyl diphosphate synthase
- DMAPP:
-
Dimethylallyl pyrophosphate
- DREB/CBF:
-
Dehydration-Responsive Element Binding proteins/C-repeat Binding Factor
- EDS:
-
Enhanced disease susceptibility
- EIL:
-
Ethylene insensitive-like protein
- EL:
-
Electrolyte leakage
- EPS:
-
Exopolysaccharides
- EPS1:
-
Enhanced Pseudomonas susceptibility 1
- ERF:
-
Ethylene response factor
- ET:
-
Ethylene
- ETR:
-
Ethylene receptor
- Fv/Fm:
-
Photosystem II efficiency
- GA2ox:
-
Gibberellin 2-oxidases
- GAs:
-
Gibberellins
- GB:
-
Glycine betaine
- GPX:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GST:
-
Glutathione S-transferase
- HSP:
-
Heat shock proteins
- IAA:
-
Indole-3-Acetic acid
- IC:
-
Isochorismate
- ICS:
-
Isochorismate synthase
- iP:
-
N6-(Δ2-isopentenyl)-adenine
- IPA:
-
Indole-3-pyruvate
- IPT:
-
Isopentenyl transferase
- JA:
-
Jasmonic acid
- JA-Ile:
-
Jasmonoyl isoleucine
- JAZ:
-
Jasmonate ZIM-domain
- JIH:
-
Jasmonoyl-l-isoleucine hydrolase
- JMT:
-
Jasmonic acid carboxyl methyltransferase
- LEA:
-
Late embryogenesis abundant
- MAPKKK/MPK:
-
Mitogen-activated protein kinase
- MDA:
-
Malondialdehyde
- MeJA:
-
Methyl jasmonate
- MJE:
-
Methyl jasmonate esterase
- NCED:
-
9-cis-epoxycarotenoid dioxygenase
- NCEI:
-
Neoxanthin synthase
- NPR:
-
Non-expressor of pathogenesis-related gene
- NSY:
-
Neoxanthin synthase
- NXS:
-
Neoxanthin synthase
- OPDA:
-
(cis)-12-oxophytodienoic acid
- OPR:
-
12-oxophytodienoate reductase
- PAL:
-
Phenylalanine ammonia-lyase
- PEG:
-
Polyethylene glycol
- PGPF:
-
Plant growth-promoting fungi
- PGPR:
-
Plant growth-promoting rhizobacteria
- POD:
-
Peroxidase
- PP2C:
-
2C protein phosphatase
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- SDR:
-
Short-chain dehydrogenase/reductase-like
- SLs:
-
Strigolactones
- SOD:
-
Superoxide dismutase
- SWE:
-
Seaweed extract
- TAA:
-
Tryptophan aminotransferase of Arabidopsis
- TF:
-
Transcription factor
- Trp:
-
Tryptophan
- tz:
-
Trans-zeatin
- WUE:
-
Water use efficiency
- ZEP:
-
Zeaxanthin epoxidase enzyme
References
Agboma PC, Jones MGK, Peltonen-Sainio P, Rita H, Pehu E (1997) Exogenous glycinebetaine enhances grain yield of maize, sorghum and wheat grown under two supplementary watering regimes. J Agron Crop Sci 178(1):29–37
Agrawal GK, Yamazaki M, Kobayashi M, Hirochika R, Miyao A, Hirochika H (2001) Screening of the rice viviparous mutants generated by endogenous retrotransposon Tos17 insertion. Tagging of a zeaxanthin epoxidase gene and a novel OsTATC gene. Plant Physiol 125(3):1248–1257
Ahammed GA, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Zhou Y, Yu J (2013) Role of brassinosteroids in alleviation of phenanthrene-cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:695–709
Ahluwalia O, Singh PC, Bhatia R (2021) A review on drought stress in plants: implications, mitigation and the role of plant growth promoting rhizobacteria. Resour Environ Sustain 5:100032
Ait-El-Mokhtar M, Baslam M, Ben-Laouane R, Anli M, Boutasknit A, Mitsui T, Wahbi S, Meddich A (2020) Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front Sustain Food Syst 4:13
Akiyama K, Hayashi H (2006) Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot 97(6):925–931
Ali F, Qanmber G, Li F, Wang Z (2022) Updated role of ABA in seed maturation, dormancy, and germination. J Adv Res 35(157):199–214. https://doi.org/10.1016/j.jare.2021.03.011
Ali O, Ramsubhag A, Jayaraman J (2021) Biostimulant properties of seaweed extracts in plants: implications towards sustainable crop production. Plan Theory 10(3):531
Allagulova C, Avalbaev A, Fedorova K, Shakirova F (2020) Methyl jasmonate alleviates water stress-induced damages by promoting dehydrins accumulation in wheat plants. Plant Physiol Biochem 155:676–682
Almeida LG, Magalhães PC, Karam D, Silva EMD, Alvarenga AA (2020) Chitosan application in the induction of water deficit tolerance in maize plants. Acta Sci Agron 42:e42463
Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot 97(5):883–893
Anli M, Symanczik S, El Abbassi A, Ait-El-Mokhtar M, Boutasknit A, Ben-Laouane R, Toubali S, Baslam M, Mäder P, Hafidi M, Meddich A (2020) Use of arbuscular mycorrhizal fungus Rhizoglomus irregulare and compost to improve growth and physiological responses of Phoenix dactylifera ‘Boufgouss’. Plant Biosyst 155(4):763–771
Ansary MH, Rahmani HA, Ardakani MR, Paknejad F, Habibi D, Mafakheri S (2012) Effect of Pseudomonas fluorescent on proline and phytohormonal status of maize (Zea mays L.) under water deficit stress. Ann Biol Res 3(2):1054–1062
Anwar A, Liu Y, Dong R, Bai L, Yu X, Li Y (2018) The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol Res 51(1):1–15. https://doi.org/10.1186/s40659-018-0195-2
Arraes FBM, Beneventi MA, de Sa MEL, Paixao JFR, Albuquerque EVS, Marin SRR, Purgatto E, Nepomuceno AL, Grossi-de-Sa MF (2015) Implications of ethylene biosynthesis and signaling in soybean drought stress tolerance. BMC Plant Biol 15(1):1–20
Arzanesh MH, Alikhani HA, Khavazi K, Rahimian HA, Miransari M (2011) Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J Microbiol Biotechnol 27(2):197–205
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47(1):1–8
Bandurska H (2005) The effect of salicylic acid on barley response to water deficit. Acta Physiol Plant 27(3):379–386
Bano A, Ullah F, Nosheen A (2012) Role of abscisic acid and drought stress on the activities of antioxidant enzymes in wheat. Plant Soil Environ 58(4):181–185
Bano A, Yasmeen S (2010) Role of phytohormones under induced drought stress in wheat. Pak J Bot 42(4):2579–2587
Bartel B (1997) Auxin biosynthesis. Annu Rev Plant Biol 48(1):51–66
Batool A, Akram NA, Cheng ZG, Lv GC, Ashraf M, Afzal M, Xiong J-L, Wang J-Y, Xiong YC (2019) Physiological and biochemical responses of two spring wheat genotypes to non-hydraulic root-to-shoot signalling of partial and full root-zone drought stress. Plant Physiol Biochem 139:11–20
Battal P (2004) Effects of some mineral nutrients on gibberellic acid levels in maize plants (Zea mays L.). Econ Bot 58(2):195–203
Behboudi F, Tahmasebi Sarvestani Z, Kassaee MZ, Modares Sanavi SAM, Sorooshzadeh A, Ahmadi SB (2018) Evaluation of chitosan nanoparticles effects on yield and yield components of barley (Hordeum vulgare L.) under late season drought stress. JWENT 3(1):22–39
Ben Saad R, Ben Romdhane W, Mihoubi W, Ben Hsouna A, Brini F (2020) A Lobularia maritima LmSAP protein modulates gibberellic acid homeostasis via its A20 domain under abiotic stress conditions. PLoS One 15(5):1–16. https://doi.org/10.1371/journal.pone.0233420
Benderradji L, Saibi W, Brini F (2021) Role of ABA in overcoming environmental stress: sensing, signaling and crosstalk. Ann Agric Crop Sci 6(1):1070. https://doi.org/10.26420/annagriccropsci.2021.1070
Ben-Laouane R, Ait-El-Mokhtar M, Anli M, Boutasknit A, Ait Rahou Y, Raklami A, Oufdou K, Wahbi S, Meddich A (2021) Green compost combined with mycorrhizae and rhizobia: a strategy for improving alfalfa growth and yield under field conditions. Gesunde Pflanzen 73(2):193–207
Berleth T, Mattsson J, Hardtke CS (2000) Vascular continuity and auxin signals. Trends Plant Sci 5(9):387–393
Bernal-Vicente A, Cantabella D, Hernández JA, Diaz-Vivancos P (2018) The effect of mandelonitrile, a recently described salicylic acid precursor, on peach plant response against abiotic and biotic stresses. Plant Biol 20(6):986–994
Bernardo L, Carletti P, Badeck FW, Rizza F, Morcia C, Ghizzoni R, Rouphael Y, Colla G, Terzi V, Lucini L (2019) Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol Biochem 137:203–212
Bhargava S, Sawant K (2013) Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breed 132(1):21–32
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16(1):1–18
Booker J, Chatfield S, Leyser O (2003) Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 15(2):495–507
Boutasknit A, Baslam M, Ait-El-Mokhtar M, Anli M, Ben-Laouane R, Douira A, El Modafar C, Mitsui T, Wahbi S, Meddich A (2020) Arbuscular mycorrhizal fungi mediate drought tolerance and recovery in two contrasting carob (Ceratonia siliqua L.) ecotypes by regulating stomatal, water relations, and (in) organic adjustments. Plan Theory 9(1):80
Boutasknit A, Baslam M, Ait-El-Mokhtar M, Anli M, Ben-Laouane R, Ait-Rahou Y, Mitsui T, Douira A, El Modafar C, Wahbi S, Meddich A (2021) Assemblage of indigenous arbuscular mycorrhizal fungi and green waste compost enhance drought stress tolerance in carob (Ceratonia siliqua L.) trees. Sci Rep 11(1):1–23
Bradley JM, Lumba S (2021) On the outside looking in : roles of endogenous and exogenous strigolactones. Plant J 105(2):322–334. https://doi.org/10.1111/tpj.15087
Bruce WB, Edmeades GO, Barker TC (2002) Molecular and physiological approaches to maize improvement for drought tolerance. J Exp Bot 53(366):13–25
Campanoni P, Nick P (2005) Auxin-dependent cell division and cell elongation. 1-Naphthaleneacetic acid and 2, 4-dichlorophenoxyacetic acid activate different pathways. Plant Physiol 137(3):939–948
Canales FJ, Rispail N, García-Tejera O, Arbona V, Pérez-de-Luque A, Prats E (2021) Drought resistance in oat involves ABA-mediated modulation of transpiration and root hydraulic conductivity. Environ Exp Bot 182:104333. https://doi.org/10.1016/j.envexpbot.2020.104333
Canellas LP, Canellas NO, Irineu LE, Olivares FL, Piccolo A (2020) Plant chemical priming by humic acids. Chem Biol Technol Agric 7(1):1–17
Cardoso AA, Gori A, Da-Silva CJ, Brunetti C (2020) Abscisic acid biosynthesis and signaling in plants: key targets to improve water use efficiency and drought tolerance. Appl Sci 10(18):6322
Casanova-Sáez R, Mateo-Bonmatí E, Ljung K (2021) Auxin metabolism in plants. Cold Spring Harb Perspect Med 11(3):1–23. https://doi.org/10.1101/cshperspect.a039867
Changwal C, Shukla T, Hussain Z, Singh N, Kar A, Singh VP, Abdin MZ, Arora A (2021) Regulation of postharvest tomato fruit ripening by endogenous salicylic acid. Front Plant Sci 12:768. https://doi.org/10.3389/fpls.2021.663943
Chareesri A, De Deyn GB, Sergeeva L, Polthanee A, Kuyper TW (2020) Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 30(2):315–328
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought-from genes to the whole plant. Funct Plant Biol 30(3):239–264
Chen J, Fei K, Zhang W, Wang Z, Zhang J, Yang J (2021a) Brassinosteroids mediate the effect of high temperature during anthesis on the pistil activity of photo-thermosensitive genetic male-sterile rice lines. Crop J 9(1):109–119. https://doi.org/10.1016/j.cj.2020.07.001
Chen L, Yang H, Fang Y, Guo W, Chen H, Zhang X, Dai W, Chen S, Hao Q, Yuan S, Zhang C, Huang Y, Shan Z, Yang Z, Qiu D, Liu X, Tran LSP, Zhou X, Cao D (2021b) Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnol J 19(4):702–716. https://doi.org/10.1111/pbi.13496
Chen L, Song Y, Li S, Zhang L, Zou C, Yu D (2012) The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta Gene Regul Mech 1819(2):120–128
Chen W, Meng P, Feng H, Wang C (2020) Effects of arbuscular mycorrhizal fungi on growth and physiological performance of Catalpa bungei CA Mey. Under drought stress. Forests 11(10):1117
Cheng Y, Dai X, Zhao Y (2007) Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19(8):2430–2439
Cheng Y, Zhao Y (2007) A role for auxin in flower development. J Integr Plant Biol 49(1):99–104
Cicero LL, Madesis P, Tsaftaris A, Piero ARL (2015) Tobacco plants over-expressing the sweet orange tau glutathione transferases (CsGSTUs) acquire tolerance to the diphenyl ether herbicide fluorodifen and to salt and drought stresses. Phytochemistry 116:69–77
Clouse SD (2008) The molecular intersection of brassinosteroid-regulated growth and flowering in Arabidopsis. PNAS 105(21):7345–7346
Cohen AC, Travaglia CN, Bottini R, Piccoli PN (2009) Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 87(5):455–462
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217(1):67–75
Cornejo-Ríos K, Osorno-Suárez MDP, Hernández-León S, Reyes-Santamaría MI, Juárez-Díaz JA, Pérez-España VH, Peláez-Acero A, Madariaga-Navarrete A, Saucedo-García M (2021) Impact of trichoderma asperellum on chilling and drought stress in tomato (Solanum lycopersicum). Horticulturae 7(10):385
Cosme M, Fernández I, Van der Heijden MG, Pieterse CM (2018) Non-mycorrhizal plants: the exceptions that prove the rule. Trends Plant Sci 23(7):577–587
Curiel R, Domínguez L, Donovan M, Doody A, Johnson J, Listman GM, MacNeil M (2020) The cereals imperative of future food systems. Seeds of Change Annual Report 2019. CIMMYT (International Maize and Wheat Improvement Center), Mexico, pp 18–19
Daryanto S, Wang L, Jacinthe PA (2016) Global synthesis of drought effects on maize and wheat production. PLoS One 11(5):e0156362
Das P, Lakra N, Nutan KK, Singla-Pareek SL, Pareek A (2019) A unique bZIP transcription factor imparting multiple stress tolerance in Rice. Rice 12(1):1–16
Davenport TL, Morgan PW, Jordan WR (1980) Reduction of auxin transport capacity with age and internal water deficits in cotton petioles. Plant Physiol 65(5):1023–1025
Ding Z, Ali EF, Elmahdy AM, Ragab KE, Seleiman MF, Kheir AM (2021) Modeling the combined impacts of deficit irrigation, rising temperature and compost application on wheat yield and water productivity. Agric Water Manag 244:106626
Ding Z, Friml J (2010) Auxin regulates distal stem cell differentiation in Arabidopsis roots. PNAS 107(26):12046–12051
Divi UK, Rahman T, Krishna P (2010) Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol 10(1):1–14
Dominguez PG, Carrari F (2015) ASR1 transcription factor and its role in metabolism. Plant Signal Behav 10(4):e992751
Dong C, Xi Y, Chen X, Cheng ZM (2021) Genome-wide identification of AP2/EREBP in Fragaria vesca and expression pattern analysis of the FvDREB subfamily under drought stress. BMC Plant Biol 21(1):295. https://doi.org/10.1186/s12870-021-03095-2
Du H, Wang N, Cui F, Li X, Xiao J, Xiong L (2010) Characterization of the β-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiol 154(3):1304–1318
Dubois M, Skirycz A, Claeys H, Maleux K, Dhondt S, De Bodt S, Vanden Bossche R, De Milde L, Yoshizumi T, Matsui M, Inzé D (2013) Ethylene response factor6 acts as a central regulator of leaf growth under water-limiting conditions in Arabidopsis. Plant Physiol 162(1):319–332
Dun EA, Ferguson BJ, Beveridge CA (2006) Apical dominance and shoot branching. Divergent opinions or divergent mechanisms? Plant Physiol 142(3):812–819
El Amerany F, Rhazi M, Wahbi S, Taourirte M, Meddich A (2020) The effect of chitosan, arbuscular mycorrhizal fungi, and compost applied individually or in combination on growth, nutrient uptake, and stem anatomy of tomato. Sci Hortic 261:109015
Elhindi KM, El-Din AS, Elgorban AM (2017) The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi. J Biol Sci 24(1):170–179
Eppel A, Rachmilevitch S (2016) Photosynthesis and photoprotection under drought in the annual desert plant Anastatica hierochuntica. Photosynthetica 54(1):143–147
Fàbregas N, Lozano-Elena F, Blasco-Escámez D, Tohge T, Martínez-Andújar C, Albacete A, Osorio S, Bustamante M, Riechmann JL, Nomura T, Yokota T (2018) Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat Commun 9(1):1–13
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N, Faiq M (2015) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75(2):391–404
FAO (Food and Agriculture Organization) (2018) The future of food and agriculture-alternative pathways to 2050. FAO, Rome
Farooq M, Wahid A, Basra SMA (2009) Improving water relations and gas exchange with brassinosteroids in rice under drought stress. J Agron Crop Sci 195(4):262–269
Fayez KA, Bazaid SA (2014) Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. J Saudi Soc Agric Sci 13(1):45–55
Fonseca S, Chico JM, Solano R (2009) The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr Opin Plant Biol 12(5):539–547
Foo E, Davies NW (2011) Strigolactones promote nodulation in pea. Planta 234(5):1073–1081
Fu P, Jaiswal D, McGrath JM, Wang S, Long SP, Bernacchi CJ (2021) Drought imprints on crops can reduce yield loss: nature’s insights for food security. Food Energy Secur 11:e332
Fukao T, Xu K, Ronald PC, Bailey-Serres J (2006) A variable cluster of ethylene response factor–like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 18(8):2021–2034
Fukao T, Yeung E, Bailey-Serres J (2011) The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell 23(1):412–427
Gamalero E, Glick BR (2012) Ethylene and abiotic stress tolerance in plants. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, pp 395–412
Gao H, Wang Y, Xu P, Zhang Z (2018) Overexpression of a WRKY transcription factor TaWRKY2 enhances drought stress tolerance in transgenic wheat. Front Plant Sci 9:997
García AC, Santos LA, Izquierdo FG, Rumjanek VM, Castro RN, dos Santos FS, de Souza LGA, Berbara RLL (2014) Potentialities of vermicompost humic acids to alleviate water stress in rice plants (Oryza sativa L.). J Geochem Explor 136:48–54
George S, Venkataraman G, Parida A (2010) A chloroplast-localized and auxin-induced glutathione S-transferase from phreatophyte Prosopis juliflora confer drought tolerance on tobacco. J Plant Physiol 167(4):311–318
Guler NS, Pehlivan N, Karaoglu SA, Guzel S, Bozdeveci A (2016) Trichoderma atroviride ID20G inoculation ameliorates drought stress-induced damages by improving antioxidant defence in maize seedlings. Acta Physiol Plant 38(6):132
Gupta A, Rico-Medina A, Caño-Delgado AI (2020) The physiology of plant responses to drought. Science 368(6488):266–269
Habben JE, Bao X, Bate NJ, DeBruin JL, Dolan D, Hasegawa D, Helentjaris TG, Lafitte RH, Lovan N, Mo H, Reimann K (2014) Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnol J 12(6):685–693
Hafez Y, Attia K, Alamery S, Ghazy A, Al-Doss A, Ibrahim E, Rashwan E, El-Maghraby L, Awad A, Abdelaal K (2020) Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 10(5):630
Haile GG, Tang Q, Li W, Liu X, Zhang X (2020) Drought: progress in broadening its understanding. Wiley Interdiscip Rev Water 7(2):e1407
Han Y, Luthe D (2021) Identification and evolution analysis of the JAZ gene family in maize. BMC Genomics 22(1):1–21. https://doi.org/10.1186/s12864-021-07522-4
Hasan MM, Gong L, Nie ZF, Li FP, Ahammed GJ, Fang XW (2021) ABA-induced stomatal movements in vascular plants during dehydration and rehydration. Environ Exp Bot 186:104436. https://doi.org/10.1016/j.envexpbot.2021.104436
Hause B, Mrosk C, Isayenkov S, Strack D (2007) Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry 68(1):101–110
He GH, Xu JY, Wang YX, Liu JM, Li PS, Chen M, Ma YZ, Xu ZS (2016) Drought-responsive WRKY transcription factor genes TaWRKY1 and TaWRKY33 from wheat confer drought and/or heat resistance in Arabidopsis. BMC Plant Biol 16(1):1–16
He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288(5475):2360–2363
Hidangmayum A, Dwivedi P, Katiyar D, Hemantaranjan A (2019) Application of chitosan on plant responses with special reference to abiotic stress. Physiol Mol Biol Plant 25(2):313–326
Hossain MM, Sultana F (2020) Application and mechanisms of plant growth promoting fungi (PGPF) for phytostimulation. In: Das SK (ed) Organic agriculture. IntechOpen, pp 1–30. https://doi.org/10.1016/j.plaphy.2020.12.022
Hu D, Wei L, Liao W (2021) Brassinosteroids in plants: crosstalk with small-molecule compounds. Biomol Ther 11(12):1800. https://doi.org/10.3390/biom11121800
Hu Y, Vandenbussche F, Van Der Straeten D (2017) Regulation of seedling growth by ethylene and the ethylene–auxin crosstalk. Planta 245(3):467–489
Hussain MB, Zahir ZA, Asghar HN, Asgher M (2014) Can catalase and exopolysaccharides producing rhizobia ameliorate drought stress in wheat? Int J Agric Biol 16(1):3–13
Ilyas M, Nisar M, Khan N, Hazrat A, Khan AH, Hayat K, Fahad S, Khan A, Ullah A (2021) Drought tolerance strategies in plants: a mechanistic approach. J Plant Growth Regul 40(3):926–944
Iqbal MS, Singh AK, Ansari MI (2020) Effect of drought stress on crop production. In: Rakshit A, Singh H, Singh A, Singh U, Fraceto L (eds) New frontiers in stress management for durable agriculture. Springer, Singapore, pp 35–47. https://doi.org/10.1007/978-981-15-1322-0_3
Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27(4):325–333
Jin L, Yarra R, Zhou L, Cao H (2021) The auxin response factor (ARF) gene family in Oil palm (Elaeis guineensis Jacq.): genome-wide identification and their expression profiling under abiotic stresses. Protoplasma:1–14. https://doi.org/10.1007/s00709-021-01639-9
Jogawat A, Yadav B, Chhaya Lakra N, Singh AK, Narayan OP (2021) Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: a review. Physiol Plant 172(2):1106–1132. https://doi.org/10.1111/ppl.13328
Jumali SS, Said IM, Ismail I, Zainal Z (2011) Genes induced by high concentration of salicylic acid in ‘Mitragyna speciosa’. Aust J Crop Sci 5(3):296–303
Jung H, Lee DK, Do Choi Y, Kim JK (2015) OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci 236:304–312
Kakimoto T (2001) Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases. Plant Cell Physiol 42(7):677–685
Kapulnik Y, Delaux PM, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, Séjalon-Delmas N, Combier JP, Bécard G, Belausov E, Koltai H (2011) Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233(1):209–216
Kasahara H, Hanada A, Kuzuyama T, Takagi M, Kamiya Y, Yamaguchi S (2002) Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J Biol Chem 277(47):45188–45194
Kasim WA, Hamada EA, El-Din NS, Eskander S (2015) Influence of seaweed extracts on the growth, some metabolic activities and yield of wheat grown under drought stress. Int J Agr Agric Res 7(2):173–189
Khan S, Anwar S, Yu S, Sun M, Yang Z, Gao ZQ (2019) Development of drought-tolerant transgenic wheat: achievements and limitations. Int J Mol Sci 20(13):3350
Khodary SEA (2004) Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int J Agric Biol 6(1):5–8
Koshiba T, Kamiya Y, Iino M (1995) Biosynthesis of indole-3-acetic acid from L-tryptophan in coleoptile tips of maize (Zea mays L.). Plant Cell Physiol 36(8):1503–1510
Kour J, Kohli SK, Khanna K, Bakshi P, Sharma P, Singh AD, Ibrahim M, Devi K, Sharma N, Ohri P, Skalicky M (2021) Brassinosteroid signaling, crosstalk and, physiological functions in plants under heavy metal stress. Front Plant Sci 12:608061. https://doi.org/10.3389/fpls.2021.608061
Kruglova NN, Titova GE, Seldimirova OA, Zinatullina AE (2021) Cytophysiological features of the cereal-based experimental system “embryo in vivo–callus in vitro”. Russian J Dev Biol 52(4):199–214. https://doi.org/10.1134/s1062360421040044
Krugman T, Peleg Z, Quansah L, Chagué V, Korol AB, Nevo E, Saranga Y, Fait A, Chalhoub B, Fahima T (2011) Alteration in expression of hormone-related genes in wild emmer wheat roots associated with drought adaptation mechanisms. Funct Integr Genomics 11(4):565–583
Lahbouki S, Ben-Laouane R, Anli M, Boutasknit A, Ait-Rahou Y, Ait-El-Mokhtar M, El Gabardi S, Douira A, Wahbi S, Outzourhit A, Meddich A (2022) Arbuscular mycorrhizal fungi and/or organic amendment enhance the tolerance of prickly pear (Opuntia ficus-indica) under drought stress. J Arid Environ 199:104703
Lata C, Prasad M (2011) Role of DREBs in regulation of abiotic stress responses in plants. J Exp Bot 62(14):4731–4748
Lee M, Jung JH, Han DY, Seo PJ, Park WJ, Park CM (2012) Activation of a flavin monooxygenase gene YUCCA7 enhances drought resistance in Arabidopsis. Planta 235(5):923–938
Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529(7584):84–87
Li G, Hu S, Zhao X, Kumar S, Li Y, Yang J, Hou H (2021) Mechanisms of the morphological plasticity induced by phytohormones and the environment in plants. Int J Mol Sci 22(2):765
Li L, Zheng M, Deng G, Liang J, Zhang H, Pan Z, Long H, Yu M (2016) Overexpression of AtHDG11 enhanced drought tolerance in wheat (Triticum aestivum L.). Mol Breed 36(3):23
Li Y, Shan X, Jiang Z, Zhao L, Jin F (2021b) Genome-wide identification and expression analysis of the GA2ox gene family in maize (Zea mays L.) under various abiotic stress conditions. Plant Physiol Biochem 166:621–633. https://doi.org/10.1016/j.plaphy.2021.06.043
Li M, Yu G, Cao C, Liu P (2021a) Metabolism, signaling, and transport of jasmonates. Plant Commun 2(5):100231. https://doi.org/10.1016/j.xplc.2021.100231
Lim C, Kang K, Shim Y, Yoo SC, Paek NC (2021) Inactivating transcription factor OsWRKY5 enhances drought tolerance through abscisic acid signaling pathways. Plant Physiol 00:1–17
Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, Wang Y (2009) DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21(5):1512–1525
Liu F, Sun T, Wang L, Su W, Gao S, Su Y, Xu L, Que Y (2017) Plant jasmonate ZIM domain genes: shedding light on structure and expression patterns of JAZ gene family in sugarcane. BMC Genomics 18(1):1–17
Liu Y, Shi Y, Su D, Lu W, Li Z (2021) SlGRAS4 accelerates fruit ripening by regulating ethylene biosynthesis genes and SlMADS1 in tomato. Hortic Res 8(1):1–11. https://doi.org/10.1038/s41438-020-00431-9
Lu G, Coneva V, Casaretto JA, Ying S, Mahmood K, Liu F, Nambara E, Bi YM, Rothstein SJ (2015) OsPIN5b modulates rice (Oryza sativa) plant architecture and yield by changing auxin homeostasis, transport and distribution. Plant J 83(5):913–925
Lu Y, Li Y, Zhang J, Xiao Y, Yue Y, Duan L, Zhang M, Li Z (2013) Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize (Zea mays L.). PLoS One 8(1):e52126
Maier F, Zwicker S, Hueckelhoven A, Meissner M, Funk J, Pfitzner AJ, Pfitzner UM (2011) Nonexpressor of pathogenesis-related proteins1 (NPR1) and some NPR1-related proteins are sensitive to salicylic acid. Mol Plant Pathol 12(1):73–91
Martin RC, Kronmiller BA, Dombrowski JE (2021) Transcriptome analysis of lolium temulentum exposed to a combination of drought and heat stress. Plan Theory 10(11):2247. https://doi.org/10.3390/plants10112247
Martínez-Viveros O, Jorquera MA, Crowley DE, Gajardo GMLM, Mora ML (2010) Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr 10(3):293–319
Marzec M, Daszkowska-Golec A, Collin A, Melzer M, Eggert K, Szarejko I (2020) Barley strigolactone signaling mutant hvd14.d reveals the role of strigolactones in ABA dependent response to drought. Plant Cell Enviro 43(9):2239–2253. https://doi.org/10.1111/pce.13815
Masuda Y, Kamisaka K (2000) Discovery of auxin. In: Kung SD, Yang SF (eds) Discoveries in plant biology, vol III. World Scientific, Singapore, pp 43–57
Meza I, Siebert S, Döll P, Kusche J, Herbert C, Eyshi Rezaei E, Nouri H, Gerdener H, Popat E, Frischen J, Hagenlocher M (2020) Global-scale drought risk assessment for agricultural systems. NHESS 20(2):695–712
Min H, Chen C, Wei S, Shang X, Sun M, Xia R, Liu X, Hao D, Chen H, Xie Q (2016) Identification of drought tolerant mechanisms in maize seedlings based on transcriptome analysis of recombination inbred lines. Front Plant Sci 7:1080
Mitra D, Guerra Sierra BE, Khoshru B, De Los Santos Villalobos S, Belz C, Chaudhary P, Shahri FN, Djebaili R, Adeyemi NO, El-Ballat EM, Mohapatra PKD (2021) Impacts of arbuscular mycorrhizal fungi on rice growth, development, and stress management with a particular emphasis on strigolactone effects on root development. Commun Soil Sci Plant Anal:1–31
Miura K, Okamoto H, Okuma E, Shiba H, Kamada H, Hasegawa PM, Murata Y (2013) SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J 73(1):91–104
Moghadam HRT, Zahedi H, Ashkiani A (2013) Effect of zinc foliar application on auxin and gibberellin hormones and catalase and superoxide dismutase enzyme activity of corn (Zea mays L) under water stress. Maydica 58(3–4):218–223
Moslemi Z, Habibi D, Asgharzadeh A, Ardakani MR, Mohammadi A, Mohammadi M (2011) Response of phytohormones and biochemical markers of maize to super absorbent polymer and plant growth promoting rhizobacteria under drought stress. AEJAES 10(5):787–796
Naing AH, Soe MT, Kyu SY, Kim CK (2021) Nano-silver controls transcriptional regulation of ethylene- and senescence-associated genes during senescence in cut carnations. Sci Hortic 287:110280. https://doi.org/10.1016/j.scienta.2021.110280
Naseem H, Ahsan M, Shahid MA, Khan N (2018) Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J Basic Microbiol 58(12):1009–1022
Nawaz M, Wang Z (2020) Abscisic acid and glycine betaine mediated tolerance mechanisms under drought stress and recovery in Axonopus compressus: a new insight. Sci Rep 10(1):1–10
Nayyar H, Walia DP (2003) Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol Plant 46(2):275–279
Naz R, Sarfraz A, Anwar Z, Yasmin H, Nosheen A, Keyani R, Roberts TH (2021) Combined ability of salicylic acid and spermidine to mitigate the individual and interactive effects of drought and chromium stress in maize (Zea mays L.). Plant Physiol Biochem 159:285–300
Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126(3):467–475
Nir IDO, Moshelion M, Weiss D (2014) The Arabidopsis Gibberellin Methyl Transferase 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Enviro 37(1):113–123
Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, Tran LSP (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23(6):2169–2183
Niyogi KK, Fink GR (1992) Two anthranilate synthase genes in Arabidopsis: defense-related regulation of the tryptophan pathway. Plant Cell 4(6):721–733
North HM, Almeida AD, Boutin JP, Frey A, To A, Botran L, Sotta B, Marion-Poll A (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50(5):810–824
O’Brien JA, Benková E (2013) Cytokinin cross-talking during biotic and abiotic stress responses. Front Plant Sci 4:451
Oleńska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J (2020) Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci Total Environ 743:140682
Olugbire OO, Olorunfemi S, Oke DO (2021) Global utilisation of cereals: sustainability and environmental issues. Agro-Science 20(1):9–14
Parmar N, Singh KH, Sharma D, Singh L, Kumar P, Nanjundan J, Khan YJ, Chauhan DK, Thakur AK (2017) Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops: a comprehensive review. 3. Biotech 7(4):1–35
Pauwels L, Inzé D, Goossens A (2009) Jasmonate-inducible gene: what does it mean? Trends Plant Sci 14(2):87–91
Pekić S, Stikić R, Tomljanović L, Andjelković V, Ivanović M, Quarrie SA (1995) Characterization of maize lines differing in leaf abscisic acid content in the field. 1 abscisic acid physiology. Ann Bot 75(1):67–73
Peleg Z, Blumwald E (2011) Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol 14(3):290–295
Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E (2011) Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol J 9(7):747–758
Pospíšilová H, Jiskrova E, Vojta P, Mrizova K, Kokáš F, Čudejková MM, Bergougnoux V, Plíhal O, Klimešová J, Novák O, Galuszka P (2016) Transgenic barley overexpressing a cytokinin dehydrogenase gene shows greater tolerance to drought stress. New Biotechnol 33(5):692–705
Prakash V, Singh VP, Tripathi DK, Sharma S, Corpas FJ (2021) Nitric oxide (NO) and salicylic acid (SA): a framework for their relationship in plant development under abiotic stress. Plant Biol 23(S1):39–49. https://doi.org/10.1111/plb.13246
Prerostova S, Dobrev PI, Gaudinova A, Knirsch V, Körber N, Pieruschka R, Fiorani F, Brzobohatý B, Černý M, Spichal L, Vankova R (2018) Cytokinins: their impact on molecular and growth responses to drought stress and recovery in Arabidopsis. Front Plant Sci 9:655
Qin G, Gu H, Zhao Y, Ma Z, Shi G, Yang Y, Pichersky E, Chen H, Liu M, Chen Z, Qu LJ (2005) An indole-3-acetic acid carboxyl methyltransferase regulates Arabidopsis leaf development. Plant Cell 17(10):2693–2704
Quiroga G, Erice G, Aroca R, Zamarreño ÁM, García-Mina JM, Ruiz-Lozano JM (2018) Arbuscular mycorrhizal symbiosis and salicylic acid regulate aquaporins and root hydraulic properties in maize plants subjected to drought. Agric Water Manag 202:271–284
Ramos AC, Dobbss LB, Santos LA, Fernandes MS, Olivares FL, Aguiar NO, Canellas LP (2015) Humic matter elicits proton and calcium fluxes and signaling dependent on Ca2+-dependent protein kinase (CDPK) at early stages of lateral plant root development. Chem Biol Technol Agric 2(1):1–12
Rao G, Sui J, Zeng Y, He C, Duan A, Zhang J (2014) De novo transcriptome and small RNA analysis of two Chinese willow cultivars reveals stress response genes in Salix matsudana. PLoS One 9(10):e109122
Rayle DL, Cleland RE (1992) The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol 99(4):1271–1274
Raza A, Javed R, Zahid Z, Sharif R, Nawaz MU, Siddiqui MH (2021) Strigolactones for sustainable plant growth and production under adverse environmental conditions. In: Husen A (ed) Plant performance under environmental stress. Springer, Cham, pp 129–166. https://doi.org/10.1007/978-3-030-78521-5
Reinhardt D (2004) Regulation of phyllotaxis. Int J Dev Biol 49(5–6):539–546
Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283(5407):1541–1544
Rizaludin MS, Stopnisek N, Raaijmakers JM, Garbeva P (2021) The chemistry of stress: understanding the ‘cry for help’ of plant roots. Meta 11(6):357
Roitsch T, Ehneß R (2000) Regulation of source/sink relations by cytokinins. Plant Growth Regul 32(2):359–367
Ruíz-Sánchez M, Armada E, Muñoz Y, de Salamone IEG, Aroca R, Ruíz-Lozano JM, Azcón R (2011) Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J Plant Physiol 168(10):1031–1037
Sadiq Y, Zaid A, Khan MMA (2020) Adaptive physiological responses of plants under abiotic stresses: role of phytohormones. In: Hasanuzzaman M (ed) Plant ecophysiology and adaptation under climate change: mechanisms and perspectives I. Springer, Singapore, pp 797–824. https://doi.org/10.1007/978-981-15-2156-0_28
Sahni S, Prasad BD, Liu Q, Grbic V, Sharpe A, Singh SP, Krishna P (2016) Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci Rep 6(1):1–14
Sakakibara H (2021) Cytokinin biosynthesis and transport for systemic nitrogen signaling. Plant J 105(2):421–430. https://doi.org/10.1111/tpj.15011
Salazar C, Hernández C, Pino MT (2015) Plant water stress: associations between ethylene and abscisic acid response. Chilean J Agric Res 75:71–79
Sano N, Marion-Poll A (2021) ABA metabolism and homeostasis in seed dormancy and germination. Int J Mol Sci 22(10):5069. https://doi.org/10.3390/ijms22105069
Savchenko T, Kolla VA, Wang CQ, Nasafi Z, Hicks DR, Phadungchob B, Chehab WE, Brandizzi F, Froehlich J, Dehesh K (2014) Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol 164(3):1151–1160
Scarpeci TE, Frea VS, Zanor MI, Valle EM (2017) Overexpression of AtERF019 delays plant growth and senescence, and improves drought tolerance in Arabidopsis. J Exp Bot 68(3):673–685
Schulz P, Herde M, Romeis T (2013) Calcium-dependent protein kinases: hubs in plant stress signaling and development. Plant Physiol 163(2):523–530
Seiler C, Harshavardhan VT, Reddy PS, Hensel G, Kumlehn J, Eschen-Lippold L, Rajesh K, Korzun V, Wobus U, Lee J, Sreenivasulu N (2014) Abscisic acid flux alterations result in differential abscisic acid signaling responses and impact assimilation efficiency in barley under terminal drought stress. Plant Physiol 164(4):1677–1696
Seleiman MF, Al-suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Dindaroglu T, Abdul-wajid HH, Battaglia ML (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plan Theory 10(2):1–25
Seo JS, Joo J, Kim MJ, Kim YK, Nahm BH, Song SI, Cheong JJ, Lee JS, Kim JK, Choi YD (2011) OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J 65(6):907–921
Sharma M, Gupta SK, Majumder B, Maurya VK, Deeba F, Alam A, Pandey V (2017) Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J Proteome 163:28–51
Shemi R, Wang R, Gheith ESM, Hussain HA, Hussain S, Irfan M, Cholidah L, Zhang K, Zhang S, Wang L (2021) Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci Rep 11(1):1–14
Shim JS, Oh N, Chung PJ, Kim YS, Choi YD, Kim JK (2018) Overexpression of OsNAC14 improves drought tolerance in rice. Front Plant Sci 9:310
Shohat H, Eliaz NI, Weiss D (2021) Gibberellin in tomato: metabolism, signaling and role in drought responses. Mol Hortic 1(1):1–12. https://doi.org/10.1186/s43897-021-00019-4
Shukla D, Rinehart CA, Sahi SV (2017) Comprehensive study of excess phosphate response reveals ethylene mediated signaling that negatively regulates plant growth and development. Sci Rep 7(1):1–16
Siodmak A, Hirt H (2021) Stomatal regulation: role of H2S-induced persulfidation in ABA signaling. Mol Plant 14(6):858–860. https://doi.org/10.1016/j.molp.2021.04.004
Some F, Takahashi F, Yamaguchi-Shinozaki K, Shinozaki K (2021) Cellular phosphorylation signaling and gene expression in drought stress responses: ABA-dependent and ABA-independent regulatory systems. Plan Theory 10(4):756
Soto MJ, Fernández-Aparicio M, Castellanos-Morales V, García-Garrido JM, Ocampo JA, Delgado MJ, Vierheilig H (2010) First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol Biochem 42(2):383–385
Sponsel VM, Hedden P (2010) Gibberellin biosynthesis and inactivation. In: Davies PJ (ed) Plant hormones. Springer, Dordrecht, pp 63–94
Sun L, Song F, Guo J, Zhu X, Liu S, Liu F, Li X (2020) Nano-ZnO-induced drought tolerance is associated with melatonin synthesis and metabolism in maize. Int J Mol Sci 21(3):782
Sun Z, Li S, Chen W, Zhang J, Zhang L, Sun W, Wang Z (2021) Plant dehydrins: expression, regulatory networks, and protective roles in plants challenged by abiotic stress. Int J Mol Sci 22(23):12619. https://doi.org/10.3390/ijms222312619
Tan BC, Schwartz SH, Zeevaart JA, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. PNAS 94(22):12235–12240
Tang Y, Bao X, Zhi Y, Wu Q, Guo Y, Yin X, Zeng L, Li J, Zhang J, He W, Liu K (2019) Overexpression of a MYB family gene, OsMYB6, increases drought and salinity stress tolerance in transgenic rice. Front Plant Sci 10:168
Tardieu F, Simonneau T, Muller B (2018) The physiological basis of drought tolerance in crop plants: a scenario-dependent probabilistic approach. Annu Rev Plant Biol 69:733–759
Tessi TM, Brumm S, Winklbauer E, Schumacher B, Pettinari G, Lescano I, González CA, Wanke D, Maurino VG, Harter K, Desimone M (2021) Arabidopsis AZG2 transports cytokinins in vivo and regulates lateral root emergence. New Phytol 229(2):979–993. https://doi.org/10.1111/nph.16943
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF COI1 complex during jasmonate signalling. Nature 448(7154):661–665
Tiwari P, Indoliya Y, Chauhan AS, Singh P, Singh PK, Singh PC, Srivastava S, Pande V, Chakrabarty D (2020) Auxin-salicylic acid cross-talk ameliorates OsMYB–R1 mediated defense towards heavy metal, drought and fungal stress. J Hazard Mater 399:122811
Tiwari S, Lata C, Chauhan PS, Nautiyal CS (2016) Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem 99:108–117
Tiwari S, Lata C, Singh Chauhan P, Prasad V, Prasad M (2017) A functional genomic perspective on drought signalling and its crosstalk with phytohormone-mediated signalling pathways in plants. Curr Genomics 18(6):469–482
Tran LSP, Shinozaki K, Yamaguchi-Shinozaki K (2010) Role of cytokinin responsive two-component system in ABA and osmotic stress signalings. Plant Signal Behav 5(2):148–150
Ullah A, Manghwar H, Shaban M, Khan AH, Akbar A, Ali U, Ali E, Fahad S (2018a) Phytohormones enhanced drought tolerance in plants: a coping strategy. ESPR 25(33):33103–33118
Ullah A, Sun H, Yang X, Zhang X (2018b) A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol Plant 162(4):439–454
Uppalapati SR, Ishiga Y, Wangdi T, Kunkel BN, Anand A, Mysore KS, Bender CL (2007) The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with EPS1 syringae pv. Tomato DC3000. MPMI 20(8):955–965
Vardharajula S, Zulfikar Ali S, Grover M, Reddy G, Bandi V (2011) Drought-tolerant plant growth promoting bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Interact 6(1):1–14
Vázquez MN, Guerrero YR, de la Noval WT, Gonzalez LM, Zullo MAT (2019) Advances on exogenous applications of brassinosteroids and their analogs to enhance plant tolerance to salinity: a review. Aust J Crop Sci 13(1):115–121
Visentin I, Vitali M, Ferrero M, Zhang Y, Ruyter-Spira C, Novák O, Strnad M, Lovisolo C, Schubert A, Cardinale F (2016) Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol 212(4):954–963
Vosnjak M, Likar M, Osterc G (2021) The effect of mycorrhizal inoculum and phosphorus treatment on growth and flowering of Ajania (Ajania pacifica (Nakai) Bremer et Humphries) plant. Horticulturae 7(7):178
Vysotskaya L, Akhiyarova G, Feoktistova A, Akhtyamova Z, Korobova A, Ivanov I, Dodd I, Kuluev B, Kudoyarova G (2020) Effects of phosphate shortage on root growth and hormone content of barley depend on capacity of the roots to accumulate ABA. Plan Theory 9(12):1722
Vysotskaya LB, Trekozova AW, Kudoyarova GR (2016) Effect of phosphorus starvation on hormone content and growth of barley plants. Acta Physiol Plant 38(5):1–6
Wang SX, Xia ST, Peng KQ, Kuang FC, Yong CAO, Xiao LT (2007) Effects of formulated fertilizer synergist on abscisic acid accumulation, proline content and photosynthetic characteristics of rice under drought. Rice Sci 14(1):42–48
Wang X, Li Q, Xie J, Huang M, Cai J, Zhou Q, Dai T, Jiang D (2021a) Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J 9(1):120–132
Wang Y, Mostafa S, Zeng W, Jin B (2021b) Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int J Mol Sci 22(16):8568. https://doi.org/10.3390/ijms22168568
Wang Z, Ke Q, Kim MD, Kim SH, Ji CY, Jeong JC, Lee HS, Park WS, Ahn MJ, Li H, Xu B (2015) Transgenic alfalfa plants expressing the sweetpotato Orange gene exhibit enhanced abiotic stress tolerance. PLoS One 10(5):e0126050
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4(3):162–176
Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM, Kim YH, Lee IJ (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17(9):10754–10773
Wasternack C, Forner S, Strnad M, Hause B (2013) Jasmonates in flower and seed development. Biochimie 95(1):79–85
Wei Z, Li J (2016) Brassinosteroids regulate root growth, development, and symbiosis. Mol Plant 9(1):86–100
Wendin K, Mustafa A, Ortman T, Gerhardt K (2020) Consumer awareness, attitudes and preferences towards heritage cereals. Foods 9(6):742
Werner T, Holst K, Pörs Y, Guivarc’h A, Mustroph A, Chriqui D, Grimm B, Schmülling T (2008) Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J Exp Bot 59(10):2659–2672
Werner T, Motyka V, Strnad M, Schmülling T (2001) Regulation of plant growth by cytokinin. PNAS 98(18):10487–10492
Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schmülling T (2010) Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22(12):3905–3920
Wheeldon CD, Bennett T (2021) There and back again: an evolutionary perspective on long-distance coordination of plant growth and development. Semin Cell Dev Biol 109:55–67. https://doi.org/10.1016/j.semcdb.2020.06.011
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25(2):195–210
Wilkinson S, Kudoyarova GR, Veselov DS, Arkhipova TN, Davies WJ (2012) Plant hormone interactions: innovative targets for crop breeding and management. J Exp Bot 63(9):3499–3509
Wong WS, Tan SN, Ge L, Chen X, Yong JWH (2015) The importance of phytohormones and microbes in biofertilizers. In: Maheshwari DK (ed) Bacterial metabolites in sustainable agroecosystem. Springer, Cham, pp 105–158
Wu K, Zhang L, Zhou C, Yu CW, Chaikam V (2008) HDA6 is required for jasmonate response, senescence and flowering in Arabidopsis. J Exp Bot 59(2):225–234
Wu Q, Li D, Li D, Liu X, Zhao X, Li X, Li S, Zhu L (2015) Overexpression of OsDof12 affects plant architecture in rice (Oryza sativa L.). Front Plant Sci 6:833
Wu Y, Liu H, Wang Q, Zhang G (2021) Roles of cytokinins in root growth and abiotic stress response of Arabidopsis thaliana. Plant Growth Regul 94(2):151–160. https://doi.org/10.1007/s10725-021-00711-x
Wybouw B, De Rybel B (2019) Cytokinin–a developing story. Trends Plant Sci 24(2):177–185
Xie Z, Jiang D, Cao W, Dai T, Jing Q (2003) Relationships of endogenous plant hormones to accumulation of grain protein and starch in winter wheat under different post-anthesis soil water statuses. Plant Growth Regul 41(2):117–127
Xie Z, Jiang D, Dai T, Jing Q, Cao W (2004) Effects of exogenous ABA and cytokinin on leaf photosynthesis and grain protein accumulation in wheat ears cultured in vitro. Plant Growth Regul 44(1):25–32
Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442(7103):705–708
Xu W, Huang W (2017) Calcium-dependent protein kinases in phytohormone signaling pathways. Int J Mol Sci 18(11):2436
Xu Y, Wang J, Wang R, Wang L, Zhang C, Xu W, Wang S (2021) The role of strigolactones in the regulation of root system architecture in grapevine (Vitis vinifera L.) in response to root-restriction cultivation. Int J Mol Sci 22(16):8799
Yadav B, Jogawat A, Gnanasekaran P, Kumari P, Lakra N, Lal SK, Pawar J, Narayan OP (2021) An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene 25:100264
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59:225–251
Yang Y, Takenaga S, Ohno S, Doi M (2021) Ethylene-sensitive abscission layer formation and petal senescence in cut dahlia inflorescences. Hort J 90(4):460–468. https://doi.org/10.2503/hortj.UTD-281
Yasmin H, Nosheen A, Naz R, Bano A, Keyani R (2017) L-tryptophan-assisted PGPR-mediated induction of drought tolerance in maize (Zea mays L.). J Plant Intera 12(1):567–578
Yoneyama K, Brewer PB (2021) ScienceDirect plant biology Strigolactones, how are they synthesized to regulate plant growth and development ? Curr Opin Plant Biol 63:102072. https://doi.org/10.1016/j.pbi.2021.102072
Yoshida H, Hirano K, Sato T, Mitsuda N, Nomoto M, Maeo K, Koketsu E, Mitani R, Kawamura M, Ishiguro S, Ueguchi-Tanaka M (2014) DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. PNAS 111(21):7861–7866
Yuan HM, Liu WC, Jin Y, Lu YT (2013) Role of ROS and auxin in plant response to metal-mediated stress. Plant Signal Behav 8(7):e24671
Zaheer MS, Raza MAS, Saleem MF, Erinle KO, Iqbal R, Ahmad S (2019) Effect of rhizobacteria and cytokinins application on wheat growth and yield under normal vs drought conditions. Commun Soil Sci Plant Anal 50(20):2521–2533
Zhang Q, Li J, Zhang W, Yan S, Wang R, Zhao J, Li Y, Qi Z, Sun Z, Zhu Z (2012) The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J 72(5):805–816
Zhang SW, Li CH, Cao J, Zhang YC, Zhang SQ, Xia YF, Sun DY, Sun Y (2009) Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3. 13 activation. Plant Physiol 151(4):1889–1901
Zhang Y, Li Z, Li YP, Zhang XQ, Ma X, Huang LK, Yan YH, Peng Y (2018) Chitosan and spermine enhance drought resistance in white clover, associated with changes in endogenous phytohormones and polyamines, and antioxidant metabolism. Funct Plant Biol 45(12):1205–1222
Zhang Z, Li F, Li D, Zhang H, Huang R (2010) Expression of ethylene response factor JERF1 in rice improves tolerance to drought. Planta 232(3):765–774
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Zhao Y, Zhang W, Abou-Elwafa SF, Shabala S, Xu L (2021) Understanding a mechanistic basis of ABA involvement in plant adaptation to soil flooding: the current standing. Plan Theory 10(10):1–15. https://doi.org/10.3390/plants10101982
Zhou J, Li Z, Xiao G, Zhai M, Pan X, Huang R, Zhang H (2020) CYP71D8L is a key regulator involved in growth and stress responses by mediating gibberellin homeostasis in rice. J Exp Bot 71(3):1160–1170. https://doi.org/10.1093/jxb/erz491
Zou P, Li K, Liu S, Xing R, Qin Y, Yu H, Zhou M, Li P (2015) Effect of chitooligosaccharides with different degrees of acetylation on wheat seedlings under salt stress. Carbohydr Polym 126:62–69
Acknowledgement
This work was supported by the FOSC project (Sus-Agri-CC) from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 862555, Grant-in-Aid for Early-Career Scientists to M.B. (JSPS KAKENHI Grant Number 20 K15425), and by a Grant for Promotion of KAAB Projects (Niigata University) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ait-El-Mokhtar, M. et al. (2022). Cereals and Phytohormones Under Drought Stress. In: Abdel Latef, A.A.H. (eds) Sustainable Remedies for Abiotic Stress in Cereals. Springer, Singapore. https://doi.org/10.1007/978-981-19-5121-3_13
Download citation
DOI: https://doi.org/10.1007/978-981-19-5121-3_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5120-6
Online ISBN: 978-981-19-5121-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)