Abstract
Many promising solid drug candidates suffer late-stage failure and process difficulty owing to their insolubility leading to poor absorption. Preparation of biocompatible ionic liquid salts can bypass these delivery and absorption issues and offer exciting prospects. There is an ample scope for configuring/designing cations and anions to add new function and/or deliver more than one active ingredient simultaneously. With drug discovery pipeline clogged, there is a need to look at novel alternatives to generate new entities and salt forms. Regulatory agencies should be more focused on clinical properties rather than the physical state or form. Whereas, academia can identify and develop a pool of biocompatible and degradable ions. Looking beyond ionic interaction and developing design principles to combine these ions to include wide range of non-covalent interactions seems to be a viable approach. However, the route is not free of any challenges. There is also need to work on methods toward purification of liquid salts and any contaminants or account for differences in biological activity that may emerge. It is imperative to telescope into ionic liquid-API complex that can be tightly held together even in solution through hydrogen-bonding and other non-covalent interactions, and remaining unaffected by dilution. Finally, modulating the counterions to introduce a second biological function like, easing permeability, enhancing stability and selectivity, alleviating side effects of the primary drug, to name a few seems promising. Overall, ionic liquid-based API organic salts are destined to play a significant role in coming years to produce drugs with superior function.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 The Pharmaceutical Landscape is Dominated by Solid Active Forms
One unique feature that defines a solid is the ability of its constituents to arrange themselves in different ways. It is common for the component ions, atoms, or molecules to adopt more than one arrangement in three dimensions. Thus, it is possible to construct crystalline solids displaying different physical properties from a single-component organic crystal given the name polymorphism arising mainly due to differences in free energy of their respective crystalline and solvated state [1, 2]. One such property that gets affected due to different crystalline arrangement is the aqueous solubility, thus directly affecting the bioavailability and hence absorption of the solid drugs [2]. However, crystals containing more than one type of atom, ionic compound, or molecule will have different properties than do crystals made from singular constituent and are called cocrystals [1].
Cocrystals wherein active pharmaceutical ingredient (API) may be embedded in a pharmaceutically acceptable guest molecules have gained prominence as it offers a possibility to improve the physical characteristics of the API. However, cocrystals also suffer from the same old problem of polymorphism as do API [3].
Pharmaceutical industry hinges heavily on solid active forms like powders and tablets owing to ease of taking a pill orally and handling convenience. Liquid forms, on the other hand, not only offers different modes of delivery options but can also enhance the bioavailability translating to enhanced absorption [4]. The insolubility of the solid forms results in 40–70% failure of the total compounds entering the development stage because of the inability to convert them into more soluble forms for release into the bloodstream [4]. A pressing example is the temporary removal of antiretroviral medication Norvir in 1998 due to the appearance of an unknown polymorph Form II with substantially less water solubility, resulting in precipitation and late-stage failure even after product launch [5].
2 Discussion
2.1 Why Ionic Liquids (IL’s) Are Emerging as a Rich Toolbox for Developing New Generation Liquid Drugs
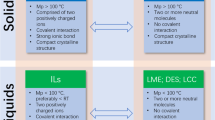
While ionic liquids have not received enough attention largely due some toxicity and regulatory concerns [4]. It is important to realize though that they offer exciting prospect due to their tuneability and multitude of interaction between the drug and counter ions in contrast ionic interaction which is the mainstay for more than half of the solid drugs. In addition, their low symmetry and diffused charge can lead to poor packing of the constituent ions leading to the appearance of liquid state and may be an effective means to bypass the delivery problems encountered by solid dosage forms (Fig. 1).
Liquid salts at body temperature have the potential to enhance their solubility and absorptions. Also, ionic liquids can be customized to deliver more than one ingredient simultaneously. Judicious combination of drugs with “functional counterions” can expand the scope of treatment and/or reduce the side effects of the primary drug providing better therapeutic options. Effective use of “functional counterion” to produce liquid salts, rendering the “combined entity” to address more than one aspect of a disease is a promising area where quick strides can be made. A classic example involves combining pain-relieving properties of procaine with anti-inflammatory property of salicylic acid to generate procainium salicylate in a liquid state, opening up new, viable treatment regimen, and delivery options [6]. This is important as the current drug discovery processes are circuitous and clogged and in part responsible for their high price and accessibility. In addition, pharmaceutical research should look beyond ionic interactions and engineer functional designer ions with wider spectrum of non-covalent interactions for better control of physical and biological properties. Regulation should also be revamped to focus more on clinical properties rather than physical forms [4, 7].
The scientific understanding behind the emerging paradigm is still evolving. For example, is there a way to predict a priori how an ion can be made liquid? purification of non-crystallizing active ingredients is a challenge. Is there a way to design nontoxic ions that may retain its multiple interactions with the active agent simultaneously interacting with the solvent system like water? central to this is aiming for ions capable multiple non-covalent interaction, and ionic liquids fills up the role to a great extent provided toxicity issues are addressed. Recently, Banerjee et al. [8] reported a highly effective and scalable oral insulin formulation stabilized by ionic liquid choline geranate (CAGE) that may be delivered in enterically coated capsules. The biocompatible formulation demonstrated profound efficacy at very low insulin doses in adult nondiabetic male Wister rats and had good stability. Promising results may be awaited following pre-clinical and clinical studies. Additionally, studies involving Insulin—CAGE—intestinal fluid interactions may uncover greater insights into the process of insulin absorption. Recently, novel electrospinning methods to disrupt bacterial film formation have been reported [9]. The choline geranate-based ionic liquids displayed enhanced skin penetration and were used to treat difficult skin lesions. They have demonstrated that protein scaffolds doped with different amount of choline geranate had multiple clinical application in the area of wound healing. Ranitidine docusate ([Ran][Doc]), the first liquid room temperature API-ILs introduced by Rogers et al. in 2007 [10], displayed improved API absorption, thus opening the door for new liquid form APIs with specific physicochemical and biological properties and/or more than one pharmacological action. In an interesting report, Hough et al. [11] demonstrated modified solubility, higher thermal stability, and significant enhancement (relative to lidocaine hydrochloride) of topical analgesia for Lidocaine docusate, a hydrophobic IL obtained by combination of topical pain reliever (lidocaine, Lid) and an emollient (docusate, doc) in two different models of mouse antinociception. The data is also suggestive of different mechanisms of action for [Lid][Cl] and [Lid][doc]. A case where counter ion modification confers novel bioactivity and delivery options due to the slow release of the API. In a relatively recent review [12], Rahman et al. discussed the need for discovering low-toxicity ILs and how the intrinsic properties of the ILs can be modified to get access to the structural diversity tailored to meet the current biopharmaceutical challenges. The review also sheds light on how biocompatible Ionic Liquids (ILs) are emerging as a major player in the oral and transdermal application of small molecules as well as macromolecules like peptides and proteins. New generation API-ILs can also discovered by a prodrug strategy wherein one of the ions undergoes enzymatic transformation to its active form in vivo. Generation of oligomeric ions by simple manipulation of the stoichiometry or introducing free acid/base of the conjugate base/acid with in the interaction domain is also being pursued [10].
Also, API designed to produce low melting liquid salt can be used to modify the solution properties of the API by enhancing the pairing interactions leading to superior transport through the cell membrane or skin barrier. Zakrewsky et al. [13] demonstrated a CAGE based deep eutectic solvent to treat biofilm penetration of recalcitrant Pseudomonas aeruginosa and Salmonella enterica and broad-spectrum antimicrobial activity against a number of drug-resistant bacteria, fungi, and viruses [13]. These include clinical isolates of Staphylococcus and Candida albicans as well as laboratory strains of Herpes Simplex Virus. Owing to the low toxicity of choline geranate ionic liquids, the molecular aggregation behavior was tracked using Small-angle X-ray scattering (SAXS) and 1NMR studies [14]. The SAXS pattern revealed structural transition from nanoscale aggregation (until 17 vol% water) to lamellar phase (in the 25–50 vol% water) to micellar phase with more than 67% water. 1H NMR studies indicated that water was located in close proximity to choline and the CO2H group in geranic acid to facilitate proton exchange up to 17 vol% of water. While the 13C NMR suggested that addition of water affected the hydrogen bonding between choline and geranic acid. Additionally, 13C cross polarization magic angle spinning NMR suggested that the rigid component of the lamellar phase was primarily geranic acid. The aggregation behavior of CAGE is an important study as it has received considerable attention as a biocompatible, relatively nontoxic IL for drug delivery systems [15]. Several studies reported CAGE as an excellent penetration enhancer in the transdermal administration of low molecular wt% flavonoid, peptides, and proteins like ovalbumin and bovine serum albumin [16].
2.2 Synthetic Routes
Most of the API-ILs syntheses reported in literature employ metathesis reaction for combining the cation and anion with the precipitation of stable salts like NaCl. The cations and anions are generally pre-dissolved separately in solvents and mixed together and stirred at room temperature or heated, if necessary. The simplicity of the method is encouraging, however, the purity of the final API-ILs can be challenging requiring the removal of inorganic salts like NaCl, especially if the API-ILs have considerable water solubility. The salt can be eliminated through adequate selection of solvent or by employing additional purification methods [17]. Dean et al. proposed an alternative anticrystal engineering strategy to synthesize API-ILs by deliberately choosing asymmetric ions that do not pack well [18]. Thus, in the temperature range of interest, emphasis must be on the preparation of amorphous phase as the thermodynamically most stable form, wherein the salt is in a liquid or a glassy state but not as supercooled liquid. Their study revealed that complementary functional groups leading to strong supramolecular attractive intermolecular or interionic hydrogen bonding between donor and acceptor must be avoided in the preparation of liquid API—ILs. However, more studies and larger sample pool size are required for better understanding.
3 Conclusion
3.1 Future Prospect and Challenges
Overall, the dual nature (discrete ions) of ILs can be conveniently modulated to generate liquid APIs with additional biological function or modify the properties of the existing drugs in a beneficial way. Some of the properties that have been looked at include controlling solubility, bioavailability, stability, elimination of polymorphism, and opening novel delivery options like transdermal penetration as API-IL complex and conferring slow release of the active form and development of pharmaceutical cocktails for peptide-based therapeutic agents [19, 20]. While there are possibilities in waiting for development for IL mediated vaccine stabilization/storage and enabling API with targeting ligands as counterions. Nevertheless, there are several challenges like limited in vivo studies, lack of pharmacokinetic, and pharmacodynamic data [21]. Study of metabolic pathway in their uptake and alteration of toxicity relative to the precursor API and identification of a larger pool of biocompatible ions from renewable sources and their toxicological profiles is also important. The slow progress might be partly attributed to the lack of guidelines from the pharmaceutical entities for API-ILs which renders testing of the formulations difficult. Nevertheless, ILs offer huge scope for finding new roles for old drugs and improving their delivery and efficacy.
References
Stahly GP (207) Diversity in single- and multiple-component crystals. The search for and prevalence of polymorphs and cocrystals. Cryst Growth Des 7:1007–1026
Cruz-Cabeza AJ, Feeder N, Davey RJ (2020) Open questions in organic crystal polymorphism. Commun Chem Nat Res 3:1–4
Guo M, Sun X, Chen J, Cai T (2021) Pharmaceutical cocrystals: a review of preparations, physicochemical properties and applications. Acta Pharmaceutica Sinica B. Chinese Acad Med Sci 11:2537–2564
Shamshina J, Kelley S, Gurau G (2015) Develop ionic liquid drugs. Nature 528:188–189
Chemburkar SR, Bauer J, Deming K, Spiwek H, Patel K, Morris J et al (2000) Dealing with the impact of ritonavir polymorphs on the late stages of bulk drug process development. Org Process Res Dev 4(5):413–417
Endres F (2010) Physical chemistry of ionic liquids. Phys Chem Chem Phys 12(8):1648–1648
Callréus T, Schneider CK (2013) The emergence of regulatory science in pharmaceutical medicine. Pharmaceut Med. 27(6):345–351
Banerjee A, Ibsen K, Brown T, Chen R, Agatemor C, Mitragotri S (2018) Ionic liquids for oral insulin delivery. Proc Natl Acad Sci USA 115(28):7296–7301
Akhmetova A, Heinz A (2020) pharmaceutics electrospinning proteins for wound healing purposes: opportunities and challenges. 2020; Available from: https://doi.org/10.3390/pharma
Marrucho IM, Branco LC, Rebelo LPN (2014) Ionic liquids in pharmaceutical applications. Ann Rev Chem Biomol Eng Ann Rev Inc 5:527–546
Hough WL, Smiglak M, Rodríguez H, Swatloski RP, Spear SK, Daly DT et al (2007) The third evolution of ionic liquids: active pharmaceutical ingredients. New J Chem 31(8):1429–1436
Md Moshikur R, Chowdhury MR, Moniruzzaman M, Goto M (2020) Biocompatible ionic liquids and their applications in pharmaceutics. Green Chem Roy Soc Chem 22:8116–8139
Zakrewsky M, Lovejoy KS, Kern TL, Miller TE, Le V, Nagy A et al (2014) Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc Natl Acad Sci USA 111(37):13313–13318
Takeda J, Iwao Y, Karashima M, Yamamoto K, Ikeda Y (2021) Structural evaluation of the choline and geranic acid/water complex by SAXS and NMR analyses. ACS Biomater Sci Eng 7(2):595–604
Zakrewsky M, Banerjee A, Apte S, Kern TL, Jones MR, Sesto RED et al (2016) Choline and geranate deep eutectic solvent as a broad-spectrum antiseptic agent for preventive and therapeutic applications. Adv Healthc Mater 5(11):1282–1289
Banerjee A, Ibsen K, Iwao Y, Zakrewsky M, Mitragotri S (2017) Transdermal protein delivery using choline and geranate (CAGE) deep eutectic solvent. Adv Healthc Mater 6(15)
Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD et al (2003) Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc 125(22):6632–6633
Dean PM, Turanjanin J, Yoshizawa-Fujita M, MacFarlane DR, Scott JL (2009) Exploring an anti-crystal engineering approach to the preparation of pharmaceutically active ionic liquids. Cryst Growth Des 9(2):1137–1145
Gomes A, Bessa LJ, Correia P, Fernandes I, Ferraz R, Gameiro P et al (2020) “Clicking” an Ionic liquid to a potent antimicrobial peptide: on the route towards improved stability. Int J Mol Sci 21(17):1–11
Saraswat J, Wani FA, Dar KI, Rizvi MMA, Patel R (2020) Noncovalent conjugates of ionic liquid with antibacterial peptide melittin: an efficient combination against bacterial cells. ACS Omega 5(12):6376–6388
Pedro SN, Freire CSR, Silvestre AJD, Freire MG (2020) The role of ionic liquids in the pharmaceutical field: an overview of relevant applications. Int J Molecular Sci MDPI AG; 21:1–50
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Bhawal, S. (2022). Exploring the Scope of Developing Ionic Liquid-Based Drugs. In: Mukherjee, K., Layek, R.K., De, D. (eds) Tailored Functional Materials. Springer Proceedings in Materials, vol 15. Springer, Singapore. https://doi.org/10.1007/978-981-19-2572-6_8
Download citation
DOI: https://doi.org/10.1007/978-981-19-2572-6_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2571-9
Online ISBN: 978-981-19-2572-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)