Abstract
The development of effective drug delivery systems for poorly water-soluble drugs remains a significant challenge for the pharmaceutical industry because of their limited solubility, bioavailability, permeability and stability and their polymorphic conversion. A well-established approach to address these limitations is to convert the active compounds to salts; however, the challenges related to bioavailability, permeability and polymorphic transformation of crystalline drugs remain. The incorporation of active pharmaceutical ingredients (APIs) into ionic liquids (ILs) has been shown to be an attractive method for resolving these challenges and/or significantly increasing the pharmacokinetic and pharmacodynamic properties of drugs. To date, API-ILs have been designed to enhance the solubility of poorly water-soluble drugs in both water and simulated fluids, and to disrupt physiological barriers to deliver drugs to target sites. This chapter highlights the progress of ILs in API-related research. The discussion is focussed on the importance and advantages of the API-IL approach for the development of novel drugs, considering not only the physicochemical properties but also the pharmacological profiles of the API-ILs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
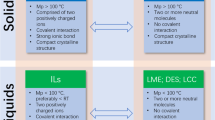
The pharmaceutical industry is facing unprecedented challenges related to the delivery of many solid active pharmaceutical ingredients (APIs) because of their limited solubility, insufficient bioavailability, polymorphism and poor stability, as well as formulation difficulties (Egorova et al. 2017; Ali et al. 2020). Approximately 40% of marketed drugs and up to 70% of drugs under development are poorly water-soluble, which leads to poor bioavailability and delivery difficulties, and thus causes them to fail in the later stages of development (Rodriguez-Aller et al. 2015; Moshikur et al. 2020b). Generally, the therapeutic efficacy of APIs is strongly influenced by their chemical structures, which contain various functional groups capable of forming strong inter/intramolecular interactions via hydrogen and/or halogen bonding, resulting in the formation of highly crystalline solids (Shamshina et al. 2015; Ali et al. 2021). These crystalline forms of APIs are accompanied by a suite of issues, such as lower aqueous solubility, irregular gastrointestinal absorption, pre-systemic metabolism, possible toxicity and side-effects of polymorphs and challenging particle size modification (Shamshina et al. 2015; Egorova et al. 2017). Large differences in bioavailability have been observed for different polymorphs of APIs, leading to toxic or potentially lethal ramifications if the wrong polymorph is administered (Ghielmetti et al. 1976). Difficulty controlling the particle size of solid APIs is another common problem that is often critical to the development of formulations with therapeutic efficacy. Based on the structural diversity of drug molecules (ionisable or non-ionisable), several approaches such as salt formation, prodrug conversion, nanoemulsion formation, micellisation and nanoparticle formation, are now widely used to improve their aqueous solubility and bioavailability (Rodriguez-Aller et al. 2015; Egorova et al. 2017). However, these techniques often use large quantities of organic solvents, leading to concerns for human health and ecosystems (Clarke et al. 2018). Green techniques that do not compromise the therapeutic efficacy of drugs are therefore required for effective delivery.
The use of API-ionic liquids (API-ILs, organic salts prepared by pairing an ionisable API with an appropriate IL-forming counterion that melt below body temperature) is a promising tool for addressing the polymorphism and aqueous solubility of solid drugs. ILs comprising APIs are expected to provide many advantageous physicochemical and biopharmaceutical properties over solid or crystalline APIs (Egorova et al. 2017; Moshikur et al. 2020b). The strategic design and appropriate choice of IL-forming counterions have the potential to not only stimulate the synergetic actions of API-ILs, but to allow tuning of physico-thermal properties such as solubility, polymorphism, hygroscopicity, permeability and thermal stability. In addition, the scientific and technical advantages of APIs in liquid form could allow facile formulation and delivery via various routes of administration compared with the solid or crystalline drug. Therefore, the API-IL strategy is a potential opportunity for pharmaceutical companies as one of their options in a competitive market.
The research activity related to the use of ILs in drug formulation and delivery has significantly expanded over the last two decades (Egorova et al. 2017; Moshikur et al. 2020b). Figure 2.1a shows the number of research publications that have reported the application of ILs/IL technologies in the pharmaceutical field since 2000. Notably, the API-ILs approach is the most studied area among the IL-based drug delivery strategies (Fig. 2.1b). Consequently, many companies that produce generic pharmaceuticals are increasingly motivated to design effective API-IL bearing drug delivery systems. However, the objective of this chapter is to summarise and further motivate biomedical application-driven exploration of API-ILs.
a Publications related to ionic liquids (ILs)/IL technologies over the past two decades. b A comparison of the prevalence of API-IL technology and other commonly used IL-related technologies in the pharmaceutics literature, reproduced with permission from (Moshikur et al. 2020b)
2.2 Design of API-ILs
The design of novel liquid forms of APIs is an appealing strategy for addressing the innate difficulties of many crystalline drugs. ILs derived from APIs may also provide new perspectives in terms of lowering the production costs or repurposing of classical drugs, and address the risk of toxicity arising from different undetected polymorphic phases that could cause harmful effects in patients. Converting solid drugs into API-ILs by pairing with tailor-made IL-forming counterions can lead to desirable physicochemical and biopharmaceutical properties. Their charged liquid state allows fine-tuning of their melting enthalpy barrier as well as the solubility/bioavailability. With effectively infinite options for design and flexibility, the use of the API-IL platform in drug delivery systems has already made advances through several approaches reported in the literature (Fig. 2.2). In this section, several implementation strategies for the API-IL platform developed over the last decade will be discussed.
2.2.1 Single-Active API-ILs
The combination of crystalline APIs with an appropriate IL-forming counterion is a promising technique for converting conventional pharmaceuticals into IL salts (Fig. 2.2a). These salts usually melt below body temperature and comprise one pharmaceutically active API and an IL-forming counterion. The appropriate selection of an IL-forming counterion allows control of the physicochemical and biological properties of the corresponding parent API, including the solubility, dissolution, permeability and bioavailability. API-ILs can reduce the issues of polymorphism and crystallinity that are related to the low aqueous solubility, poor therapeutic efficiency and thermal instability of drugs. A series of API-ILs with different pharmacological activities have been reported where solid APIs such as lidocaine, ibuprofen, sulfacetamide, sulfasalazine, indomethacin, procaine, aspirin, salicylic acid, methotrexate, piperacillin and penicillin are converted to the IL form by combining with IL-forming cations such as cholinium, amino acid ester, ammonium or phosphonium (Egorova et al. 2017; Moshikur et al. 2020b).
2.2.2 Dual-Active API-ILs
The dual-active API-IL strategy appears very attractive for the design of effective drug delivery systems owing to the dual-functional performances and possible synergistic effects beyond those of the parent APIs. Generally, any combination of two or more APIs is possible if both drugs form stable ions. Dual-active API-ILs are composed of an active cation and an active anion with different pharmaceutical activities (Fig. 2.2b). The role of the counterions is not only to influence the crystallinity of the drug molecules, but also to retain their own biomedical activity, resulting in dual-functional properties or providing new therapeutic properties not attainable with the two isolated APIs or known salt forms. Several notable examples of dual-active API-ILs have been reported to enhance their physicochemical and biomedical properties. A dual-functional API-IL was formed by the combination of acetylsalicylate with its main metabolite salicylic acid, resulting in the enhanced solubility of acetylsalicylate with minimal gastrointestinal distress (Endres 2010). Similar dual-active API-ILs were also formed from both salicylate and acetylsalicylate when paired with an analgesic tramadolium cation, antibacterial benzethonium cation, local anaesthetic lidocainium and procainium cations and an antiarrhythmic procainiumamide cation. Most of these API-ILs were liquids at room temperature and showed improved stability in air and moisture (Endres 2010). Antibacterial cations such as benzalkonium and didecyldimethylammonium were paired with the ‘sweet’ anions saccharinate (Sac) and acesulfame (Acesuf), which led to improved antimicrobial activity as well as good insect deterrent activity compared with the individual drugs. However, some of these API-ILs ([(C10)2(C1)2 N][Sac] and [(C10)2(C1)2 N][Acesulf]) caused oral toxicity and skin irritation (Hough-Troutman et al. 2009). Ampicillin is a popular antibiotic that formed a dual-active API-IL when paired with the antiseptic cetylpyridinium (C16Pyr), demonstrating significantly higher activity against several gram-positive and gram-negative bacterial strains compared with parent [Na][Amp] or [C16Pyr][Cl] (Ferraz et al. 2014, 2015). Notably, [C16Pyr][Amp] showed significantly higher growth inhibition in some tumour cell lines than [Na][Amp] (Ferraz et al. 2015). To improve the transdermal penetration, the nonsteroidal anti-inflammatory drugs, etodolac and ibuprofen, were combined with the local anaesthetic lidocaine, resulting in significant aqueous solubility as well as more efficient skin permeability. Although, the permeation of etodolac from lidocainium etodolac was significantly higher than that of parent etodolac, the opposite was observed for lidocaine (Miwa et al. 2016).
2.2.3 Oligomeric API-ILs
A nonstoichiometric approach for converting crystalline drugs into liquid forms is the formation of oligomeric API-ILs. Generally, oligomeric API-ILs are composed of hydrogen-bonded cations/anions and neutral non-ionised drug molecules, which share the delocalised protons between the deprotonated anion and protonated cation causing them to resist crystal formation (Fig. 2.2c). These API-ILs can be tuned by simply altering the stoichiometric ratio and/or complexity of the ions by introducing the free acids or bases of the conjugate bases or acids within the salt formulations. Use of the term ‘ionic liquid’ for this type of liquid salt formulation is debatable unless non-covalently bonded species behave as a single ion, because all species in API-ILs are partially to fully ionised liquids. Rogers’ group first introduced this concept in 2010 by preparing ‘oligomeric’ tetrabutylphosphonium salicylates ([P4,4,4,4][Hn(Saln+1)]) (Bica and Rogers 2010). The formation of these oligomeric API-ILs can be explained by the formation of hydrogen-bonded dimer complexes between salicylic acid and the salicylate anion. All compositions in the range [P4,4,4,4][Sal]1.3–3H0.7–2 were liquids. Lidocainium salicylate ([HLid][Saln+1Hn] or [HLidn+1][Sal]), a viscous liquid, is another example of an oligomeric API-IL, which was prepared by adding an excess of salicylic acid or lidocaine to lidocainium salicylate (Bica and Rogers 2010).
2.2.4 Prodrug API-ILs
The prodrug strategy is a promising approach for improving the therapeutic efficacy of APIs and extending their delivery to different routes of administration (Fig. 2.2d). A prodrug is a pharmacologically inactive molecule that is converted into an active substance through chemical and/or enzymatic transformation within the body (Pedro et al. 2020). Prodrugs are generally used to increase the solubility and site specificity, reduce rapid drug metabolism and cellular toxicity and achieve controlled drug release (Shamshina et al. 2013). However, prodrugs can exist as several polymorphs that suffer the same polymorphic problems as any solid API. For example, chloramphenicol palmitate—a prodrug of chloromycetin—exists in three polymorphic forms, A, B and C, but only the B polymorph is able to transfer into the blood plasma at the effective level (Aguiar et al. 1967). Combining the advantages of API-IL with a prodrug approach can be a potential mean of not only improving the drug efficacy, but also eliminating the unwanted toxicity of the solid drug. Prodrug API-ILs are prepared by adding hydrolysable functional groups, which can be easily biochemically cleaved, to neutral APIs, and then pairing them with IL-forming counterions to give new ILs (Cojocaru et al. 2013). The appropriate choice of IL-forming counterion would lead to a prodrug API-IL with the desired physicochemical and biopharmaceutical properties. A series of API-IL prodrugs prepared by pairing the acetaminophen bearing imidazolium, phosphonium, pyridinium and pyrrolidinium prodrugs with docusate, resulted in no melting point, lower aqueous solubilities and controlled release profiles in physiological fluids (Cojocaru et al. 2013).
2.3 Synthesis and Characterisation of API-ILs
2.3.1 Synthesis of API-ILs
The conversion of APIs to liquid forms is a promising strategy for boosting the efficacy and delivery of APIs. This can be effectively tuned by selecting biologically active ions and appropriate IL-forming counterions with a high degree of asymmetry and diffuse charge as well as a minimal number of potential H-bonds among the molecules (Balk et al. 2015a). The biologically active ions should also be on the list of substances ‘generally regarded as safe’ under sections 201(s) and 409 of the Federal Food, Drug, and Cosmetic Act (the Act), which is appraised and approved by the United States FDA. The initial development of API-ILs demanded an instinctual knowledge not only of the pharmacological activity of the ions, but also of their ability to form ILs. In general, API-ILs are synthesised by neutralisation of an acid with a base and/or metathesis reactions between the biologically active anion and the cation bearing salt (Moshikur et al. 2020b). In most cases, available salt forms of the cations and anions are dissolved in a solvent (e.g. water, methanol, ethanol, acetone, chloroform, tetrahydrofuran) and stirred at room temperature or a given temperature to afford the desired API-ILs by eliminating inorganic salts. For example, the first reported API-IL, lidocainium docusate, was synthesised by metathesis of lidocainium hydrochloride and sodium docusate in methanol. The efficiency of this method was also proven by synthesising a series of API-ILs including benzalkonium based penicillinate G, trans-cinnamate and thiomersalate. Alternative metathesis methods have also been used to synthesise API-ILs, for example, cationic salts were converted to the hydroxide forms in methanol using ion exchange or Amberlite resin, and then the basic solution was neutralised by adding the acidic API solutions. However, the use of large amounts of organic solvents during the preparation of API-ILs led to the formation of undesired impurities, which may be harmful to human health as well as to the environment. To address these issues, mechanochemical processing—a green alternative method for synthesising API-ILs—has attracted significant attention because it is based on a grinding technique and uses only small amounts of organic solvents, or no solvents at all (Fig. 2.3). Martins et al (2017) introduced this strategy to synthesise gabapentin and L-glutamic acid bearing mechnoAPI-ILs by simply grinding the API precursors with IL-forming counterions, suggesting a faster, solvent-free, reproducible, higher yield and greener synthetic approach for API-IL preparation. A series of ketoprofen-containing mechnoAPI-ILs were prepared with benzocainium, procainium or tetracainium using a mortar agate pestle. However, additional studies are required to clearly determine the mechanisms as well as to generalise these techniques for wider API-IL preparation.
Schematic diagrams of a the traditional method and b the mechanochemical method of API-IL preparation, reproduced with permission from Egorova et al. (2018)
2.3.2 Characterisation of API-ILs
Designing API-ILs for pharmaceutical applications requires careful assessment to ensure both appropriate physico-thermal properties and high therapeutic efficacy, with the minimal possible side-effects. Generally, the formation of API-ILs is confirmed by NMR spectroscopy through the qualitative characterisation of proton transfer—where the chemical shifts of 1H, 13C and 15N are closely monitored to determine the degree of proton transfer from an acidic API to a basic IL-forming counterion, which indicates ionisation or H-bonded complex formation (Moreira et al. 2015). 1H and 13C NMR are used to monitor the reactions and to confirm the stoichiometry of the cation and anion of an API-IL that contains hydrogen and carbon atoms, from the change in the chemical shifts upon the proton transfer related to IL formation (Balk et al. 2015b). They are also used to evaluate the presence of by-products and degradation by simple integration of signals in the compound. Recently, nuclear overhauser enhancement experiments such as NOESY, ROESY and HOESY have been used to confirm the chemical structures of API-ILs. To eliminate the influence of the NMR solvents, several deuterated solvents such as methanol-d4, DMSO-d6 and chloroform-d have been used to measure the 1H and 13C NMR spectra of API-ILs by simply dissolving the ILs with the deuterated solvents in an NMR tube. In particular, 1H NMR spectra of ILs can reflect the differences in intra- and intermolecular interaction between ions that vary in size and structure (Cremer et al. 2010). Infrared spectroscopy is also used to qualitatively assess the proton transfer, complete ionisation and impurities of API-IL moieties. X-ray diffraction of single crystals or powders is used to assess the crystallinity of API-ILs or the homogeneous distribution of APIs in the IL-forming counterionic matrix. Halide analyses are conducted to detect the presence of inorganic impurities using the metathesis reactions. Thermoanalytical methods such as differential scanning calorimetry, thermal gravimetric analysis or derivative thermal gravimetric analysis are carried out to assess the decomposition temperatures, melting point and phase transitions (glass transition temperature or solid–solid transitions) (Ali et al. 2019; Moshikur et al. 2020c). In addition, Karl Fischer titration is used to determine the water content, and hygroscopicity is assessed by dynamic vapour sorption during storage.
2.4 Physico-Thermal and Solubility Behaviour of API-ILs
The conversion of neutral APIs into IL forms is a promising approach for overcoming the inadequate physico-thermal properties and solubility challenges of APIs. The physicochemical and biomedical properties of API-ILs mainly depend on the appropriate choice of IL-forming cations. It has been suggested that using APIs in an IL form allows fine-tuning of their physicochemical and biological properties. Several notable studies have been conducted to emphasise the effect of counterions in controlling the various pharmaceutical cocktail properties of API-ILs, i.e. solubility, physico-thermal properties, stability, biological activity and bioavailability.
2.4.1 Physico-Thermal Properties
When crystalline solid APIs are converted into API-ILs, the thermal properties such as the glass transition temperature (Tg) and melting point (Tm) are significantly lower than those of the corresponding parent drugs. Cholinium-containing ibuprofen, ketoprofen and naproxen are liquid at room temperature (Tg in the range −90 to −70 °C), whereas the Tg values of cholinium-based acyclovir and methothexate were −50.2 and 27 °C, respectively (Shamshina et al. 2017; Chantereau et al. 2019; Moshikur et al. 2019). The Tg values of lidocaine ibuprofenate, ranitidinium ibuprofenate and ranitidinium sulfacetamide were −30, −12 and 25 °C, respectively; the melting points of these API-ILs were not detected in the range −80 to 300 °C. Similarly, docusate-containing diphenhydraminium, glycinium and ethylglycinium ILs showed no melting point, and their Tg values were observed below 0 °C (Araújo et al. 2014; Panić et al. 2020). Ampicillin- and penicillin-based ILs with cholinium, ammonium, pyridinium, phosphonium and imidazolium as cations, as well as cholinium-bearing nalidixic acid, pyrazinoic acid, niflumic acid, 4-amino-salicylic acid and other APIs as anions; showed significantly lower Tm than their corresponding starting APIs or conventional API salts (Balk et al. 2015c; Chantereau et al. 2019; Ferraz et al. 2020). A wide panel of ibuprofen-containing dual-active API-ILs with lidocaine, procainium, ranitidine, diphenhydramine and other counterions exhibited relatively low Tg and Tm, indicating acceptable thermal stability (Abednejad et al. 2019; Wu et al. 2019; Panić et al. 2020). The same was observed for dual-active API-ILs combining lidocaine with salicylate, diclofenate, naproxenate, etodolac and docusate (Miwa et al. 2016; Berton et al. 2017; Abednejad et al. 2019; Maneewattanapinyo et al. 2019; Panić et al. 2020). Methotrexate and acyclovir containing API-IL prodrugs with cholinium, ammonium, amino acid esters, phosphonium and/or imidazolium and docusate also demonstrated lower Tg and Tm values than the parent APIs. The obtained prodrug-ILs showed fast hydrolysis in both water and simulated body fluids (phosphate-buffered saline, simulated gastric and simulated intestinal fluids) (Shamshina et al. 2017; Moshikur et al. 2019). Lidocainium ibuprofenate was suggested for use as a stabiliser for the preparation of IL-mediated silver nanoparticles (20–30 nm), and was readily released from the nanoparticles (Jiang et al. 2018). An IL with the surface-active API 1-alkyl-3-methylimidazolium ibuprofenate, formed aggregate structures where the number of imidazolium cations in the micelles increased with the increasing alkyl chain length of the cations. API-ILs of mefenamic acid containing ammonium were suggested for use as an initiator for ring opening polymerisation of L-lactide, and the shape and size of API-IL-grafted–poly(L-lactide) was controlled by altering the number of hydroxyl groups in the cation (Halayqa et al. 2017). However, lidocaine salicylate showed higher density and viscosity with lower conductivity compared with lidocaine ibuprofenate, suggesting the smaller size of the salicylate anions facilitates dense packing of the lidocainium cations (Panić et al. 2020). A similar feature was found for diphenhydramine bearing ibuprofen and naproxen, where the diphenhydramine naproxenate showed higher viscosity with lower iconicity, diffusivity and ionic conductivity compared with diphenhydramine ibuprofenate (Wang et al. 2018).
2.4.2 Enhanced Solubility
The main anticipated advantage of the API-IL technique is the enhanced aqueous solubility and bioavailability compared with neutral APIs, which depend on the nature of the IL-forming counterions (Table 2.1). For example, the poorly water-soluble drugs acyclovir (ACV) and methotrexate (MTX) have been converted into IL forms using a series of IL-forming counterions. The solubility of MTX bearing cholinium and ammonium was at least 5000 times higher in both water and simulated body fluids than that of the neutral MTX, whereas for amino acid ester-based MTX-ILs, it was 4000 times higher (Moshikur et al. 2019). Similar variable solubility was also found in both water and simulated body fluids (up to 400 times higher than parent drug) when acyclovir was combined with IL-forming counterions such as ammonium, phosphonium, cholinium and docusate (Shamshina et al. 2017). The solubility of cholinium sulfasalazine in saline was improved almost 4000-fold, whereas that of cholinium niflumate in water was 56 000 times higher than that of the neutral API (Araújo et al. 2014; Shadid et al. 2015). API-ILs with cholinium containing nalidixic acid, 4-amino-salicylic acid, picolinic acid, pyrazinoic acid, naprox, ketoprofen, ibuprofen and betulinic acid, exhibited significantly improved aqueous solubility compared with the neutral APIs and sodium salts (Araújo et al. 2014; Azevedo et al. 2017; Chantereau et al. 2019). Amino acid esters—potent green IL-forming cations—were found to enhance the solubility of API-ILs. L-valine alkyl ester bearing ibuprofen ILs showed approximately 42 times better solubility than the parent API, whereas amino acid ester (alanine, proline and aspartic acid) bearing salicylate ILs were miscible (soluble) at any ratio with water (Moshikur et al. 2018; Janus et al. 2020). The API-ILs of indomethacin with tetramethylguanidine, 2-dimethylaminoethanol, 1,8-diazabicyclo[5.4.0]undec-7-ene and 1,4-diazabicyclo[2.2.2]octane showed very high aqueous solubility (5 000 000 times higher than that of the parent indomethacin) (Tojo 2018). Other IL-forming cations such as ammonium, phosphonium and docusate bearing API-ILs also improved the solubility in water compared with indomethacin. API-ILs of tetrabutylphosphonium bearing ibuprofen, ketoprofen, naproxen, diclofenac, sulfamethoxazole, sulfadiazine and tolbutamide showed enhanced solubility (1000 times higher than the parent drugs) in water (Balk et al. 2015c). Moreover, API-ILs bearing the hydrophobic IL-forming counterion docusate with glycinium, ethylglycinium, diphenhydraminium, ranitidinium sulfacetamide and lidocaine, exhibited similar aqueous solubility, whereas docusate-containing lumefantrine, erlotinib, gefitinib, ceritinib, cabozantinib, amlodipine and metformin ILs showed improved solubility in lipidic formulations (Frizzo et al. 2016; Williams et al. 2018a, b; Tay et al. 2020). Several dual-active API-ILs of lidocaine bearing ibuprofen, naproxen, diclofenac and etodolac showed approximately 470-fold higher aqueous solubility, whereas diphenhydramine containing ibuprofen and naproxen were considerably more water-soluble than the neutral APIs (Wang et al. 2018; Abednejad et al. 2019).
Although API-ILs are considered to have significant potential for application in the pharmaceutical industry, some drawbacks of the concept should be noted. In some cases, the polymorphism problem of solid APIs persists in their IL forms (Egorova et al. 2017). Ethambutol dibenzoate formed three polymorphs with different thermal stability. Procainium acetate was found to form a dihydrate salt that underwent irreversible crystallisation. Ibuprofen with 1-(2-hydroxyethyl)-3-methyl imidazolium showed high sensitivity to the water content. These findings suggest the importance of further investigation into the formation of polymorphs and hydrates in API-ILs.
2.5 Biomedical Activity
Notwithstanding numerous reports on the preparation and physicochemical properties of API-ILs, their biomedical activity has still only been evaluated using in vitro models. Many of the API-ILs showed improved aqueous solubility compared with the parent drugs, indicating the possibility of higher in vivo bioavailability. In addition, API-ILs addressed the polymorphism problems of many conventional solid APIs, and the amorphous structure of API-ILs offered higher solubility with reduced polymorphism. However, the impact of converting the APIs into ILs on the biomedical activity is not always clear. Therefore, an ideal API-IL should be liquid with considerable physicochemical properties so polymorphs are not formed and should retain the level of biomedical activity exhibited by the parent API.
2.5.1 Enhanced Biological Activity
To date, only a few attempts have been made to investigate the biological activities of the numerous API-ILs that have been synthesised (Table 2.1). Imidazolium-containing API-ILs of amoxicillin and penicillin showed improved antibacterial activity compared with the conventional salts of the APIs. Ammonium, phosphonium and cetylpyridinium bearing API-ILs of ampicillin and penicillin exhibited improved antibacterial activity (Ferraz et al. 2020). Cholinium-bearing API-ILs of nalidixic, niflumic and pyrazinoic acids, showed similar cytotoxicity to that of the parent APIs, whereas the cholinium naproxenate and ketoprofenate API-ILs showed higher cytotoxicity (Araújo et al. 2014; Azevedo et al. 2017). N-methyl-2-pyrrolidone bearing ibprofenate exhibited lower toxicity than cholinium ibprofenate (Moshikur et al. 2020d). The introduction of amino acid esters (AAEs)—low toxicity cations—into the anticancer drug methotrexate (MTX) enhanced the antitumour activity, whereas the cholinium-bearing MTX showed similar antitumour activity compared with the parent drug. MTX-IL with IL-forming cations of different toxicity, showed different modes of antitumour activity (Moshikur et al. 2019). In addition, the low toxicity AAE-containing salicylate API-ILs showed reduced cytotoxicity compared with the parent salicylic acid drug, whereas the imidazolium-containing salicylate exhibited similar cytotoxicity to the parent drug (Egorova et al. 2015; Furukawa et al. 2016; Moshikur et al. 2018). The biological activity of API-ILs also depends on the structure of the ions. When antibacterial agents (benzalkonium, [N1,1,10,10], [C16Pyr] or 3-hydroxy-1-octyloxymethylpyridinium) were combined with an artificial sweetener (acesulfame or saccharinate), the antibacterial activity of the API-ILs was greatly influenced by the IL-forming cations (Hough-Troutman et al. 2009). Dual-active API-ILs of ibuprofenate-based ranitidinium and diphenhydraminium showed similar solubility and improved antibacterial activities compared with their precursors when tested against Candida. The same was observed for dual-active API-ILs combining docusate with glycinium, ethylglycinium and diphenhydraminium (Frizzo et al. 2016). Lidocainium docusate offered a longer and more pronounced analgesic effect than lidocainium hydrochloride, indicating a synergistic impact on the activity.
2.5.2 Enhanced Permeability
Many topical drugs have been found to be unable to penetrate the skin barrier and to crystallise prior to use (Moshikur et al. 2020a). For topical and transdermal delivery, the bioavailability and therapeutic efficacy are strongly related to the penetration of a drug through the skin. Therefore, a great deal of research is currently focussed on improving drug transportation through the skin barrier by employing a penetration enhancer (Table 2.1). As the salt forms of drugs have difficulty penetrating through the skin, API-ILs are a promising platform for skin delivery because of their skin enhancer properties. Several studies have been conducted to evaluate the role of ILs during permeation. In most cases, the in vitro permeability of API-ILs was evaluated using animal skins or artificial model membranes. For example, the Rogers group designed a series of API-ILs to investigate the permeability of a model membrane to various combinations of protic, acidic and basic APIs, demonstrating their more rapid penetration compared with that of the free APIs. They conducted a topical in vivo pharmacokinetic study on rats by dissolving the lidocaine-containing liquid co-crystal (Lid.Ibu) and salts ([Lid]Cl or [Lid][Doc]) in a carrier cream and observed faster transport/absorption of actives from the liquid co-crystals than from the salts. PEGylated salicylate IL exhibited faster transdermal transport (~2.5-fold) than PEG-free cations, and AAE-containing salicylate ILs enhanced the skin permeation compared with the parent drug (Zavgorodnya et al. 2017; Moshikur et al. 2018). Proline ethyl ester ibuprofenate exhibited tenfold better permeation than the parent API. In a study of ibuprofen ILs with L-valine alkyl esters, L-valine propyl and isopropyl ester bearing ibuprofenate exhibited higher rate of transport through the skin (Janus et al. 2020). Fatty acid-containing donepezil AIP-ILs improved the permeation through an artificial membrane by 1.9- and 1.55-fold for α-linolenic and docosahexaenoic acid based API-IL patches, respectively, compared with the corresponding free base patch (Wu et al. 2020). Compared with cholinium ibuprofenate, N-methyl-2-pyrrolidone-bearing ibuprofenate enhanced the skin retention 2.6 times, however, the skin penetration was 2.3-fold lower (Moshikur et al. 2020d).
2.6 Clinical Applications
Notwithstanding the progress in the preparation and characterisation of API-ILs, only one API-IL—lidocaine etodolac—has reached clinical trials to date (Egorova et al. 2017). In 2013, the Japanese company MEDRx published the phase I trial data for the MRX-7EAT lidocaine etodolac topical patch, which resulted in faster absorption of the active (etodolac) through the skin, with mild to moderate adverse effects. The Phase II and III trials have been conducted to evaluate the efficacy and safety in several types of pain treatment including ankle sprains (NCT01198834), acute tendonitis and bursitis (NCT01161615, NCT01506154), low back pain (NCT01968005) and shoulder pain (NCT01506154). At the end of November 2016, MEDRx decided to terminate the development of the MRX-7EAT topical patch owing to unsatisfactory trial results.
2.7 Conclusion and Future Directions
This chapter emphasised the importance and advantages of API-ILs as promising alternatives to solid crystalline APIs in pharmaceuticals. API-ILs have effectively addressed the solubility and polymorphism challenges observed with solid APIs, which were the primary barriers to the development of effective drug delivery systems. The possibility of rationally designing the API-ILs also allows the tuning of new drug forms with the desired physicochemical and biomedical properties. Advances in this area have revealed the ability of API-ILs to improve drug solubility in both water and simulated body fluids, as well as to enhance drug permeation through physiological skin barriers. The results reported have been very encouraging, with specific or dual pharmacological action and enhanced bioavailability and efficacy being the hallmarks of the IL-strategy. However, few studies have assessed the in vivo bioavailability of API-ILs, suggesting the necessity for pharmacokinetic and pharmacodynamic investigations to understand the pathways involved in their absorption, distribution, metabolism and elimination. In addition, experimental and fundamental methodologies are needed to synthesise new API-ILs and to better understand the physicochemical and biological properties of these systems. We believe that the current understanding of ILs provides an important starting point for the further exploration and development of API-ILs for pharmaceutical applications.
Abbreviations
- [C2MIM]:
-
1-Ethyl-3-methylimidazolium
- [C16Pyr]:
-
1-Hexadecylpyridium
- [N2,2,2,2]:
-
Tetraethylammonium
- [N4,4,4]:
-
Tributylammonium
- [N4,4,4,4]:
-
Tetrabutylammonium
- [N1,1,10,10]:
-
Didecyldimethylammonium
- [N6,6,6,6]:
-
Tetrahexylammonium
- [P4,4,4,4]:
-
Tetrabutylphosphonium
- [P6,6,6,14]:
-
Tributyl(tetradecyl)phosphonium
- [C16Pyr]:
-
Cetylpyridinium
- [HMPyr]:
-
1-Methylpyrrolidinium
- [AAE]:
-
Amino acid ester
- [AlaEt]:
-
Alanine ethyl ester
- [ProEt]:
-
Proline ethyl ester
- [AspEt]:
-
Aspartic diethyl ester
- [PheEt]:
-
Phenylalanine ethyl ester
- [C2OHMIM]:
-
1-(2-Hydroxyethyl)-3-methylimidazolium
- [mPEG3N444]:
-
Triethylene glycol monomethyl ether tributylammonium
- [(C10)2(C1)2 N]:
-
Didecyldimethylammonium
References
Abednejad A, Ghaee A, Morais ES, Sharma M, Neves BM, Freire MG, Nourmohammadi J, Mehrizi AA (2019) Polyvinylidene fluoride–hyaluronic acid wound dressing comprised of ionic liquids for controlled drug delivery and dual therapeutic behavior. Acta Biomater 100:142–157. https://doi.org/10.1016/j.actbio.2019.10.007
Aguiar AJ, Krc J, Kinkel AW, Samyn JC (1967) Effect of polymorphism on the absorption of chloramphenicol from chloramphenicol palmitate. J Pharm Sci 56:847–853. https://doi.org/10.1002/jps.2600560712
Ali MK, Moshikur RM, Wakabayashi R, Moniruzzaman M, Goto M (2021) Biocompatible ionic liquid-mediated micelles for enhanced transdermal delivery of paclitaxel. ACS Appl Mater Interface 13:19745–19755. https://doi.org/10.1021/acsami.1c03111
Ali MK, Moshikur RM, Wakabayashi R, Moniruzzaman M, Kamiya N, Goto M (2020) Biocompatible ionic liquid surfactant-based microemulsion as a potential carrier for sparingly soluble drugs. ACS Sustain Chem Eng. https://doi.org/10.1021/acssuschemeng.9b07773
Ali MK, Moshikur RM, Wakabayashi R, Moniruzzaman M, Kamiya N, Goto M (2019) Synthesis and characterization of choline–fatty-acid-based ionic liquids: a new biocompatible surfactant. J Colloid Interface Sci 551:72–80. https://doi.org/10.1016/j.jcis.2019.04.095
Araújo JMM, Florindo C, Pereiro AB, Vieira NSM, Matias AA, Duarte CMM, Rebelo LPN, Marrucho IM (2014) Cholinium-based ionic liquids with pharmaceutically active anions. RSC Adv 4:28126–28132. https://doi.org/10.1039/c3ra47615d
Azevedo AMO, Costa SPF, Dias AFV, Marques AHO, Pinto PCAG, Bica K, Ressmann AK, Passos MLC, Araújo ARTS, Reis S, Saraiva MLMFS (2017) Anti-inflammatory choline based ionic liquids: Insights into their lipophilicity, solubility and toxicity parameters. J Mol Liq 232:20–26. https://doi.org/10.1016/j.molliq.2017.02.027
Balk A, Holzgrabe U, Meinel L (2015a) Pro et contra’ ionic liquid drugs—challenges and opportunities for pharmaceutical translation. Eur J Pharm Biopharm 94:291–304. https://doi.org/10.1016/j.ejpb.2015.05.027
Balk A, Widmer T, Wiest J, Bruhn H, Rybak JC, Matthes P, Müller-Buschbaum K, Sakalis A, Lühmann T, Berghausen J, Holzgrabe U, Galli B, Meinel L (2015b) Ionic liquid versus prodrug strategy to address formulation challenges. Pharm Res 32:2154–2167. https://doi.org/10.1007/s11095-014-1607-9
Balk A, Wiest J, Widmer T, Galli B, Holzgrabe U, Meinel L (2015c) Transformation of acidic poorly water soluble drugs into ionic liquids. Eur J Pharm Biopharm 94:73–82. https://doi.org/10.1016/j.ejpb.2015.04.034
Berton P, Di Bona KR, Yancey D, Rizvi SAA, Gray M, Gurau G, Shamshina JL, Rasco JF, Rogers RD (2017) Transdermal bioavailability in rats of lidocaine in the forms of ionic liquids, salts, and deep eutectic. ACS Med Chem Lett 8:498–503. https://doi.org/10.1021/acsmedchemlett.6b00504
Bica K, Rogers RD (2010) Confused ionic liquid ions—a “liquification” and dosage strategy for pharmaceutically active salts. Chem Commun 46:1215–1217. https://doi.org/10.1039/b925147b
Butt FI, Muhammad N, Hamid A, Moniruzzaman M, Sharif F (2018) Recent progress in the utilization of biosynthesized polyhydroxyalkanoates for biomedical applications—review. Int J Biol Macromol 120:1294–1305. https://doi.org/10.1016/j.ijbiomac.2018.09.002
Celik S, Albayrak AT, Akyuz S, Ozel AE (2020) Synthesis, molecular docking and ADMET study of ionic liquid as anticancer inhibitors of DNA and COX-2, TOPII enzymes. J Biomol Struct Dyn 38:1354–1364. https://doi.org/10.1080/07391102.2019.1604263
Chantereau G, Sharma M, Abednejad A, Vilela C, Costa EM, Veiga M, Antunes F, Pintado MM, Sèbe G, Coma V, Freire MG, Freire CSR, Silvestre AJD (2020) Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J Mol Liq 302:112547. https://doi.org/10.1016/j.molliq.2020.112547
Chantereau G, Sharma M, Abednejad A, Neves BM, Sèbe G, Coma V, Freire MG, Freire CSR, Silvestre AJD (2019) Design of nonsteroidal anti-inflammatory drug-based ionic liquids with improved water solubility and drug delivery. ACS Sustain Chem Eng 7:14126–14134. https://doi.org/10.1021/acssuschemeng.9b02797
Clarke CJ, Tu WC, Levers O, Bröhl A, Hallett JP (2018) Green and sustainable solvents in chemical processes. Chem Rev 118:747–800. https://doi.org/10.1021/acs.chemrev.7b00571
Cojocaru OA, Bica K, Gurau G, Narita A, Shamshina MPD, JL, Barber PS, Rogers RD, (2013) Prodrug ionic liquids: functionalizing neutral active pharmaceutical ingredients to take advantage of the ionic liquid form. Medchemcomm 4:559–563. https://doi.org/10.1039/c3md20359j
Cremer T, Kolbeck C, Lovelock KRJ, Paape N, Wölfel R, Schulz PS, Wasserscheid P, Weber H, Thar J, Kirchner B, Maier F, Steinrück HP (2010) Towards a molecular understanding of cation-anion interactions-Probing the electronic structure of imidazolium ionic liquids by NMR spectroscopy, X-ray photoelectron spectroscopy and theoretical calculations. Chem Eur J 16:9018–9033. https://doi.org/10.1002/chem.201001032
Egorova KS, Gordeev EG, Ananikov VP (2018) Fundamental importance of ionic interactions in the liquid phase: a review of recent studies of ionic liquids in biomedical and pharmaceutical applications. J Mol Liq 272:271–300. https://doi.org/10.1016/j.molliq.2018.09.025
Egorova KS, Gordeev EG, Ananikov VP (2017) Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev 117:7132–7189. https://doi.org/10.1021/acs.chemrev.6b00562
Egorova KS, Seitkalieva MM, Posvyatenko AV, Khrustalev VN, Ananikov VP (2015) Cytotoxic activity of salicylic acid-containing drug models with ionic and covalent binding. ACS Med Chem Lett 6:1099–1104. https://doi.org/10.1021/acsmedchemlett.5b00258
Endres F (2010) Physical chemistry of ionic liquids. Phys Chem Chem Phys 12:1648–1648. https://doi.org/10.1039/c001176m
Ferraz R, Costa-Rodrigues J, Fernandes MH, Santos MM, Marrucho IM, Rebelo LPN, Prudêncio C, Noronha JP, Petrovski Ž, Branco LC (2015) Antitumor activity of ionic liquids based on ampicillin. ChemMedChem 10:1480–1483. https://doi.org/10.1002/cmdc.201500142
Ferraz R, Silva D, Dias AR, Dias V, Santos MM, Pinheiro L, Prudêncio C, Noronha JP, Petrovski Ž, Branco LC (2020) Synthesis and antibacterial activity of ionic liquids and organic salts based on penicillin g and amoxicillin hydrolysate derivatives against resistant bacteria. Pharmaceutics 12:221. https://doi.org/10.3390/pharmaceutics12030221
Ferraz R, Teixeira V, Rodrigues D, Fernandes R, Prudêncio C, Noronha JP, Petrovski Ž, Branco LC (2014) Antibacterial activity of ionic liquids based on ampicillin against resistant bacteria. RSC Adv 4:4301–4307. https://doi.org/10.1039/c3ra44286a
Frizzo CP, Wust K, Tier AZ, Beck TS, Rodrigues LV, Vaucher RA, Bolzan LP, Terra S, Soares F, Martins MAP (2016) Novel ibuprofenate- and docusate-based ionic liquids: emergence of antimicrobial activity. RSC Adv 6:100476–100486. https://doi.org/10.1039/c6ra22237d
Furukawa S, Hattori G, Sakai S, Kamiya N (2016) Highly efficient and low toxic skin penetrants composed of amino acid ionic liquids. RSC Adv 6:87753–87755. https://doi.org/10.1039/C6RA16926K
Ghielmetti G, Bruzzese T, Bianchi C, Recusani F (1976) Relationship between acute toxicity in mice and polymorphic forms of polyene antibiotics. J Pharm Sci 65:905–907. https://doi.org/10.1002/jps.2600650625
Halayqa M, Zawadzki M, Domańska U, Plichta A (2017) API-ammonium ionic liquid—polymer compounds as a potential tool for delivery systems. J Mol Liq 248:972–980. https://doi.org/10.1016/j.molliq.2017.10.136
Hough-Troutman WL, Smiglak M, Griffin S, Matthew Reichert W, Mirska I, Jodynis-Liebert J, Adamska T, Nawrot J, Stasiewicz M, Rogers RD, Pernak J (2009) Ionic liquids with dual biological function: sweet and anti-microbial, hydrophobic quaternary ammonium-based salts. New J Chem 33:26–33. https://doi.org/10.1039/b813213p
Janus E, Ossowicz P, Klebeko J, Nowak A, Duchnik W, Kucharski Ł, Klimowicz A (2020) Enhancement of ibuprofen solubility and skin permeation by conjugation with l-valine alkyl esters. RSC Adv 10:7570–7584. https://doi.org/10.1039/d0ra00100g
Jiang Q, Yu S, Li X, Ma C, Li A (2018) Evaluation of local anesthetic effects of Lidocaine-Ibuprofen ionic liquid stabilized silver nanoparticles in Male Swiss mice. J Photochem Photobiol B Biol 178:367–370. https://doi.org/10.1016/j.jphotobiol.2017.11.028
Maneewattanapinyo P, Yeesamun A, Watthana F, Panrat K, Pichayakorn W, Suksaeree J (2019) Controlled release of lidocaine-diclofenac ionic liquid drug from freeze-thawed gelatin/poly(vinyl alcohol) transdermal patches. AAPS PharmSciTech 20:1–9. https://doi.org/10.1208/s12249-019-1545-2
Martins ICB, Oliveira MC, Diogo HP, Branco LC, Duarte MT (2017) MechanoAPI-ILs: pharmaceutical ionic liquids obtained through mechanochemical synthesis. Chemsuschem 10:1360–1363. https://doi.org/10.1002/cssc.201700153
Miwa Y, Hamamoto H, Ishida T (2016) Lidocaine self-sacrificially improves the skin permeation of the acidic and poorly water-soluble drug etodolac via its transformation into an ionic liquid. Eur J Pharm Biopharm 102:92–100. https://doi.org/10.1016/j.ejpb.2016.03.003
Moreira DN, Fresno N, Pérez-Fernández R, Frizzo CP, Goya P, Marco C, Martins MAP, Elguero J (2015) Brønsted acid-base pairs of drugs as dual ionic liquids: NMR ionicity studies. Tetrahedron 71:676–685. https://doi.org/10.1016/j.tet.2014.12.003
Moshikur RM, Chowdhury MR, Fujisawa H, Wakabayashi R, Moniruzzaman M, Goto M (2020a) Design and characterization of fatty acid-based amino acid ester as a new “green” hydrophobic ionic liquid for drug delivery. ACS Sustain Chem Eng 8:13660–13671. https://doi.org/10.1021/acssuschemeng.0c03419
Moshikur RM, Chowdhury MR, Moniruzzaman M, Goto M (2020b) Biocompatible ionic liquids and their applications in pharmaceutics. Green Chem 22:8116–8139. https://doi.org/10.1039/d0gc02387f
Moshikur RM, Ali MK, Wakabayashi R, Moniruzzaman M, Goto M (2020c) Formation and potential application of micelles composed of biocompatible N-lauroyl-amino acid ionic liquids surfactant. J Mol Liq 320:114424. https://doi.org/10.1016/j.molliq.2020.114424
Moshikur RM, Chowdhury MR, Wakabayashi R, Moniruzzaman M, Kamiya N, Goto M (2020d) Ionic liquids with N-methyl-2-pyrrolidonium cation as an enhancer for topical drug delivery: Synthesis, characterization, and skin-penetration evaluation. J Mol Liq 299:112166. https://doi.org/10.1016/j.molliq.2019.112166
Moshikur RM, Chowdhury MR, Wakabayashi R, Moniruzzaman M, Goto M (2019) Ionic liquids with methotrexate moieties as a potential anticancer prodrug: synthesis, characterization and solubility evaluation. J Mol Liq 278:226–233. https://doi.org/10.1016/j.molliq.2019.01.063
Moshikur RM, Chowdhury MR, Wakabayashi R, Moniruzzaman M, Goto M (2018) Characterization and cytotoxicity evaluation of biocompatible amino acid esters used to convert salicylic acid into ionic liquids. Int J Pharm 546:31–38. https://doi.org/10.1016/j.ijpharm.2018.05.021
Panić J, Tot A, Janković N, Drid P, Gadžurić S, Vraneš M (2020) Physicochemical and structural properties of lidocaine-based ionic liquids with anti-inflammatory anions. RSC Adv 10:14089–14098. https://doi.org/10.1039/c9ra08815f
Pedro SN, Freire CSR, Silvestre AJD, Freire MG (2020) The role of ionic liquids in the pharmaceutical field: an overview of relevant applications. Int J Mol Sci 21:1–50. https://doi.org/10.3390/ijms21218298
Pore Y, Mangrule V, Mane M, Chopade A (2017) Preparation, characterization and physicochemical studies of diclofenac ionic liquids. Asian J Pharm Pharmacol 3:208–214
Rodriguez-Aller M, Guillarme D, Veuthey JL, Gurny R (2015) Strategies for formulating and delivering poorly water-soluble drugs. J Drug Deliv Sci Technol 30:342–351. https://doi.org/10.1016/j.jddst.2015.05.009
Shadid M, Gurau G, Shamshina JL, Chuang BC, Hailu S, Guan E, Chowdhury SK, Wu JT, Rizvi SAA, Griffin RJ, Rogers RD (2015) Sulfasalazine in ionic liquid form with improved solubility and exposure. Medchemcomm 6:1837–1841. https://doi.org/10.1039/c5md00290g
Shamshina JL, Kelley SP, G, Gurau RDR, (2015) Develop ionic liquid drugs. Nature 528:188–189
Shamshina JL, Barber PS, Rogers RD (2013) Ionic liquids in drug delivery. Expert Opin Drug Deliv 10:1367–1381. https://doi.org/10.1517/17425247.2013.808185
Shamshina JL, Cojocaru OA, Kelley SP, Bica K, Wallace SP, Gurau G, Rogers RD (2017) Acyclovir as an ionic liquid cation or anion can improve aqueous solubility. ACS Omega 2:3483–3493. https://doi.org/10.1021/acsomega.7b00554
Tay E, Nguyen TH, Ford L, Williams HD, Benameur H, Scammells PJ, Porter CJH (2020) Ionic liquid forms of the antimalarial lumefantrine in combination with LFCS type IIIB lipid-based formulations preferentially increase lipid solubility, in vitro solubilization behavior and in vivo exposure. Pharmaceutics 12:17–24. https://doi.org/10.3390/pharmaceutics12010017
Tojo E (2018) New active pharmaceutical ingredient-ionic liquids (api-ils) derived from indomethacin and mebendazole. Proceedings 9:48. https://doi.org/10.3390/ecsoc-22-05781
Wang C, Chopade SA, Guo Y, Early JT, Tang B, Wang E, Hillmyer MA, Lodge TP, Sun CC (2018) Preparation, characterization, and formulation development of drug-drug protic ionic liquids of diphenhydramine with ibuprofen and naproxen. Mol Pharm 15:4190–4201. https://doi.org/10.1021/acs.molpharmaceut.8b00569
Wang Z, Zhang J, Lu B, Li Y, Liang Y, Yuan J, Zhao M, Wang B, Mai C, Zhang J (2019) Novel bio-renewable matrinium-based ionic liquids derived from Chinese herb medicine: synthesis, physicochemical properties and biological activity. J Mol Liq 296:111822. https://doi.org/10.1016/j.molliq.2019.111822
Williams HD, Ford L, Han S, Tangso KJ, Lim S, Shackleford DM, Vodak DT, Benameur H, Pouton CW, Scammells PJ, Porter CJH (2018a) Enhancing the oral absorption of kinase inhibitors using lipophilic salts and lipid-based formulations. Mol Pharm 15:5678–5696. https://doi.org/10.1021/acs.molpharmaceut.8b00858
Williams HD, Ford L, Lim S, Han S, Baumann J, Sullivan H, Vodak D, Igonin A, Benameur H, Pouton CW, Scammells PJ, Porter CJH (2018b) Transformation of biopharmaceutical classification system class I and III drugs into ionic liquids and lipophilic salts for enhanced developability using lipid formulations. J Pharm Sci 107:203–216. https://doi.org/10.1016/j.xphs.2017.05.019
Wu H, Deng Z, Zhou B, Qi M, Hong M, Ren G (2019) Improved transdermal permeability of ibuprofen by ionic liquid technology: correlation between counterion structure and the physicochemical and biological properties. J Mol Liq 283:399–409. https://doi.org/10.1016/j.molliq.2019.03.046
Wu H, Fang F, Zheng L, Ji W, Qi M, Hong M, Ren G (2020) Ionic liquid form of donepezil: preparation, characterization and formulation development. J Mol Liq 300:112308. https://doi.org/10.1016/j.molliq.2019.112308
Zavgorodnya O, Shamshina JL, Mittenthal M, McCrary PD, Rachiero GP, Titi HM, Rogers RD (2017) Polyethylene glycol derivatization of the non-active ion in active pharmaceutical ingredient ionic liquids enhances transdermal delivery. New J Chem 41:1499–1508. https://doi.org/10.1039/C6NJ03709G
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Moshikur, R. ., Goto, M. (2021). Ionic Liquids as Active Pharmaceutical Ingredients (APIs). In: Goto, M., Moniruzzaman, M. (eds) Application of Ionic Liquids in Drug Delivery. Springer, Singapore. https://doi.org/10.1007/978-981-16-4365-1_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-4365-1_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4364-4
Online ISBN: 978-981-16-4365-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)