Abstract
In the wake of increasing antimicrobial resistance due to the indiscriminate use of antibiotics, the need of the hour has been to look for novel antimicrobial agents that have least potential for eliciting resistance from microbes. Current antibiotics that target bacterial cell wall or biochemical processes in the cell have thus largely failed as microbes evolve faster to counteract these mechanisms. Carbon nanomaterials (CNMTs) have been studied as a novel class of antibiotics in the last decade, and functionalized carbon nanoparticles and nanocomposites have shown to possess antimicrobial activity. Carbon nanocomposites like nanotubes, fullerenes, and graphene and their derivatives have also shown great potential to act as antimicrobial compounds. One of the main contributors to antimicrobial activity is the large surface to volume ratio of CNMTs that allows easy and efficient binding to microbes to elicit the antimicrobial activity by various mechanisms including physical cell membrane damage, oxidative stress, affecting bacterial enzymes, and respiration. This chapter focusses on the antimicrobial properties of various types of CNMTs, their mechanism of action, their advantages for combating antimicrobial resistance, and challenges in use as next-generation antimicrobial agents.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Antimicrobial resistance
- Antimicrobial agents
- Bacterial cell wall
- Antibiotics
- Carbon nanocomposites
- Carbon nanoparticles
- Carbon nanotubes
- Membrane disruption
- Nanoliposomes
- Oxidative stress
- Antioxidant
- Reactive oxygen species
- Photosensitizers
- Multidrug resistance
- Fullerenes
- Photodynamic therapy
- Gram-positive bacteria
- Gram-negative bacteria
- Liposomes
- Graphene
1 Introduction
Antimicrobial resistance is a global crisis, affecting lives of millions of people suffering from various infections. Developing countries are the worst affected. Indiscriminate use of antibiotics and the lack of new effective drugs have made multidrug-resistant infections common, impacting the clinical outcomes of many especially in hospitals and intensive care units, surgery patients, and immunocompromised patients including cancer patients and transplant patients (Ventola 2015). Typically treating drug-resistant bacteria is not only cost-intensive but extremely long, impacting not only the affected individual but also hospitals and governments. This is especially causing endemics in the case of communicable infectious diseases, especially in developing countries like Africa, India, South America, and Southeast Asia, making it a worldwide public threat (Mogasale et al. 2021).
With the increasing global population, and pollution in the environment and lack of sanitation, there is a wide spread of infectious diseases, and therefore, antibiotics are being indiscriminately used. However, over the years, bacteria both individual and those in biofilms have developed resistance through adaptations, selection of mutants. There are several mechanisms by which bacteria protect themselves—some secrete enzymes that destroy antibiotics, and others produce efflux pumps that pump out antibiotics from cellular interior. Others excrete extracellular polymeric substances that preserve them from host responses. Due to existence of bacteria in biofilms in respiratory infections, chronic wounds, infective endocarditis, and osteomyelitis, bacteria are protected due to advantages of spatial and chemical heterogeneities allowing for persistence of infections for reasons other than resistance mutations.
Currently treatment of MDR requires multiple or high dosages of antibiotic agents that result in long and expensive treatments, with the possibility of adverse effects and uncertain outcomes. Nanomaterials are not naturally present in the environment and hence are new materials for bacteria, and any mechanisms against them have not yet been developed. This presents an opportunity for carbon nanomaterials to be used as nanocarriers to address multidrug-resistant microbes—both planktonic and in biofilms in therapeutics (Mulani et al. 2019).
Carbon allotropy is one of the most interesting phenomena in material science giving rise to a variety of nanostructures that differ in their properties while all being composed of the same carbon atoms. In the last 20 years, apart from the two well-known natural allotypes, graphite and diamond, there have been other allotypes synthesized in labs, exploiting the versatile sp2 and sp3 bonding ability of carbon nanotubes (CNTs), graphite, graphene/graphene oxide (G/GO), and fullerenes (Rajeswari et al. 2021; Wang et al. 2014a). This chapter focuses on each of the different types of carbon nanotubes, their antimicrobial properties, and their mechanism of action allowing them to be effective as next generation of antibiotics.
2 Carbon Nanotubes and Their Antimicrobial Properties
Carbon nanotubes are hollow carbon-walled structures that are broadly classified into single-walled carbon nanotubes (SWNTs), double-walled carbon nanotubes (DWCNTs), and multiwalled nanotubes (MWNTs) (Iijima 1991). The differences are in the wall diameter and thickness and properties that are conferred due to addition of cylinders. Their methods of synthesis have been detailed in Azizi-Lalabadi et al. (2020). They are known for their high electrical conductivity (~3000 W/m/K) high adsorption (17.44 mg g−1), and surface/volume ratios. These structures not only possess high electrical and thermal conductivity but also mechanical resistance and photoluminescence, making them useful for many biosensing applications at the interface of electronics and have thus been used in a variety of biosensing applications in agriculture and medicine (Rdest and Janas 2021; Chik et al. 2019; Wang et al. 2017; Cataldo and Da Ros 2008).
The antimicrobial properties of carbon nanotubes have come to light while investigating the possible interactions of carbon nanotubes with human cells for assessing toxicity. These studies were important as carbon nanotubes have wide applications in electronics and could be used as biosensors, hence could contaminate our environment. Their constant presence and interactions would help us evaluate their applications which are in vivo sensing capabilities. Carbon nanotubes are hydrophobic in nature. Therefore, in order to use carbon nanotubes for biological applications, they need to be functionalized. Functionalization is carried out using solubilizing agents like surfactants, harsh chemical treatments that could be toxic to human cells and elicit immune responses if used in medical applications (Vardharajula et al. 2012; Sun et al. 2002). Additionally, physicochemical properties of SWCNTs, such as properties such as structure, diameter, and cleanliness (e.g., % metal), also contribute to toxicity (Mohanta et al. 2019; Allegri et al. 2016; Jafar et al. 2016). These studies propelled groups to look into carbon nanotubes’ effect on microbes (Kang et al. 2007).
2.1 Carbon Nanotubes Damage Bacterial Cell Membranes by Direct Contact
In 2007, for the first time, the antimicrobial properties of commercial SWCNTs (diameter: 0.75 nm to 1.2 nm; length: ~13 μm) were described on E. coli using fluorescence-based cytotoxicity assays utilizing dyes like propidium iodide (PI) and 4′6diamidino2phenylindole (DAPI) whose emission spectra did not overlap with SWCNTs. E. coli treated with SWCNTs were found to exhibit severe cell membrane damage (Kang et al. 2007). They had concluded that direct contact of SWCNTs with cells was essential and that the membrane damage increased with time. Damage observed in the first 30 min was associated with loss of viability at 73.1% that increased to 87.6% viability loss in 120 min. Direct contact is dependent on many factors: size and concentration of nanotubes and time of treatment (Kang et al. 2007). This was confirmed from many subsequent studies showing direct contact by microscopical observations under different treatment conditions and from different types of nanotubes and bacteria studied. The main factors contributing to direct contact required to elicit antimicrobial activity against bacteria are discussed below.
2.2 Antimicrobial Activity Is Dependent on CNT Concentration, Buffer, and Treatment Time
Antimicrobial activity of SWCNTs and MWCNTs was studied against various groups of bacteria—gram-positive and gram-negative cocci/rods (Arias and Yang 2009). There was no significant difference in activities of nanotubes against various groups of bacteria studied. All of them showed approximately a 7-log reduction in cell count in viability testing.
This study also shed a light on the following properties of carbon nanotubes and their effect on antimicrobial activity: surface charge due to functionalization (presence of –OH and –COOH groups), concentration of nanotubes, and the buffer composition. They concluded that surface charge due to –OH and –COOH groups does not seem to be a factor for determining toxicity as SWNTs and MWNTs with the same surface charges behaved differently. While charged SWCNTs exhibited strong antimicrobial activity, MWCNTs did not exhibit significant antimicrobial property. They found that increasing concentration of nanotubes increased antimicrobial activities on both types of charged SWCNTs (with surface groups of −OH and –COOH), starting at 50–250 μg/ml (at the highest concentration, these could inactivate 107 cfu/ml Salmonella cells in 15 min). Interestingly, buffer composition was also found to be a critical factor in eliciting antimicrobial properties. SWCNTs in deionized water or 0.9% NaCl solution exhibited extremely strong antimicrobial activity but lost activity in PBS buffer and brain heart infusion broth.
Another study investigating antimicrobial property of SWCNTs against Salmonella enteric, E. coli, and Enterococcus faecium at concentrations 0.3–1 mg/ml showed that antibacterial activity was positively dependent on nanotube concentration (Dong et al. 2012). SWCNTs are generally treated with surfactants for dispersing bundles into individual tubes for applications. Many of these surfactants themselves show antimicrobial activities, and hence the use of the right one is essential for applicability in biomedical applications. A study of various surfactants—sodium dodecyl benzenesulfonate (SDB), sodium cholate, and sodium dodecyl sulfate (SDS) in combination with SWCNTs—showed that sodium cholate exhibited least inhibitory effect on bacterial growth at concentrations of 1% while allowing for SWCNT dispersion effectively; SDS and SDB showed total effectiveness against antimicrobial growth at 1% (Dong et al. 2009). Moreover, sodium cholate shows lower toxicity to 1321Ni human astrocytoma cells and hence is optimal for use in biomedical applications. This allows for use of sodium cholate SWCNTs in drug delivery applications especially those that require long treatment times like those against multidrug-resistant microbes. In contrast to previous study, the time of treatment from 5 min to 2 h did not seem to affect antimicrobial activity (Dong et al. 2012). The antimicrobial mechanisms of CNTs are depicted in Fig. 14.1.
Antimicrobial mechanisms of CNTs. When introduced to suspensions containing bacteria, CNTs aggregate with them and cause membrane damage, resulting in the release of DNA (red) and microbial cell death (left). Bacterial viability decreases with increasing CNT concentration and decreasing size (right)
2.3 The Size of Nanotubes Determines Antimicrobial Activity
Nanotube size depends upon its diameter, length, and volume/surface area. A detailed study on the types of NTs and their effect showed that SWCNTs displayed better antibacterial activity compared to MWCNTs owing to their smaller nanotube diameter and large surface area allowing for better interaction with bacterial membranes (Maleki Dizaj et al. 2015; Yang et al. 2010).
SWCNTs of similar weights but varying lengths were studied, and 5 μm long were found to exhibit higher antibacterial activity compared to smaller-length tubes of <1 μm and <5 μm. The longer nanotubes aggregated better with bacterial cells, while shorter ones were more prone to self-aggregation excluding the bacterial cells. Interestingly, longer nanotubes also showed better antibacterial activity that increased with nanotube concentration and treatment time (Vecitis et al. 2010; Kang et al. 2008).
2.4 Carbon Nanotube Composites and Their Antimicrobial Properties
Carbon nanotube composites with biopolymers and nanoparticles like CuO, Ag, TiO2, ZnO, etc. are known for their high antimicrobial activity (Kim et al. 2008; Cha et al. 2005). However, the utilization of these NPs requires much care considering their toxicity. To resolve this problem, carriers or supporters such as polymers, magnetic NPs, or CNMs like CNTs, GO, and fullerenes can be used, which can potentially boost the antimicrobial activity of the NPs too (Ballatore et al. 2015; Duri et al. 2017). CNMs have good functionalization potentials considering their chemical groups and excellent dispersion capability. The synergistic effect in antimicrobial properties is very important. CNT chitosan, CNT Ag, GO–Ag, and C60 ZnO and C60 CuO show increased antibacterial properties against microbes, including E. coli (Hussain et al. 2019; Sakib et al. 2019; Dinh et al. 2015; Das et al. 2011). This synergist effect is related to their mechanisms in disrupting the cell wall or membrane which permits easier availability for other small antimicrobial materials to penetrate into cells. AgNP deposition on MWCNTs with polyamidoamine has been shown to induce bacteriostatic effects against S. aureus, E. coli, and P. aeruginosa (Yun 2013; Yuan et al. 2008).
2.5 Carbon Nanotubes for Delivery of Antibiotics
Due to the high adsorption properties of carbon nanotubes, imparted on it by its high surface area, electronic properties, and stability, CNTs have been used as drug delivery systems in therapeutic use.
CNTs have been used for delivery of vectors and biomolecules (like DNA, RNA, and proteins) to cells, tissues, and organs (He et al. 2013). And they have been used as biosensors for diagnostic applications as well (Zhang et al. 2011).
Due to the hollow nature of carbon nanotubes, drugs can be loaded into two ways (Martincic and Tobias 2015). It can either be packed into the hollow CNT structure or covalently conjugated to the CNT surface (Heister et al. 2012). CNT has been shown to act as an antimicrobial both as a drug deliverer without internalization into cells and being internalized into the cells along with the drugs. This was found to occur via either by penetration followed by diffusion mechanism or the endocytosis pathway. The internalization of drug into CNT hollow tube has been shown to be more effective than surface attachment. CNT works by releasing conjugated drug inside the cells, once internalized. If the drug were to be attached to the surface, they are in danger of degradation by the physiological fluids prior to be internalized by the cells (Debnath and Srivastava 2021; Roldo 2016; Bianco et al. 2005). CNTs have therefore promising agents for antibiotic drug delivery.
3 Fullerenes and Their Antimicrobial Properties
Fullerenes are a group of cage-like carbon nanostructures of various shapes from hollow spheres to ellipsoids. C60 or buckminsterfullerene is a well-known and the most abundant form of fullerene, resembling a soccer ball. Due to their unique structure and electronic properties, fullerenes like C60 have been shown to possess high antioxidant activity among other functions—organic photovoltaics and biopharmaceuticals and treatment of water.
They show bacteriostatic properties against gram-positive (B. subtilis) and gram-negative (E. coli) bacteria in saline or buffer systems; the antimicrobial properties of fullerene decrease with certain types of buffers that result in aggregation of fullerenes in solution (Fortner et al. 2005).
3.1 Functionalized Fullerenes Affect Viral Proteases and Possess Bacteriostatic Properties
Functionalization is essential for fullerenes to be used in biomedical applications due to their insolubility (Li et al. 2012a). Thus, functionalization with different agents imparts different properties leading to different mechanisms of action against microbes.
Functionalization of C60 has shown to impart antimicrobial activity due to better interaction with microbes. Studies of C60 with viral proteases showed that similar to carbon nanotubes, direct contact was shown to be essential for antimicrobial activity of fullerenes (Zhu et al. 2003; Shoji et al. 2013).
Heightened antimicrobial and antiviral activity was seen due to incorporation of hydroxyl, carboxylic acid, and glycolic oxide groups in C60, due to enhanced interaction of C60 with virus protease active site cavity due to the formation of various stable hydrogen bonds with supporting interactions between C60 and aromatic Phe53/Arg8 in protease active site (Barzegar et al. 2017; Thota et al. 2012). Amino derivatives of fullerenes have demonstrated potent antimicrobial activity in the water treatment. Fulleropyrolidine-containing amino groups have been shown to deactivate HIV-1 and HIV-2 proteases (Szunerits et al. 2015).
Hexakis carboxylic acid fullerene derivatives have shown to protect mice from Streptococcus infection (Tsao et al. 2001). Fulleropeptides, prepared by solid-phase peptide synthesis, have been shown to possess bacteriostatic activities and have been effective against Streptococcus and E. coli. Interestingly, fulleropeptides have shown better antimicrobial activity to gram-positive bacteria than parent peptide. But these showed decreased potency against gram-negative bacteria and yeasts (D’Souza and Kadish 2012). In silico studies have shown that fullerenes can be effective against SARS-CoV-2 virus, causative agent of COVID-19 pandemic (Hurmach et al. 2021; Serrano-Aroca et al. 2021).
3.2 Fullerenes Affect Microbial Energy Metabolism
Antimicrobial properties of fullerenes are due to multiple properties relating to structure, solubility, and electronic chemistry. Like carbon nanotubes, membrane contact is important for the mechanism of action of fullerenes, but they do not necessarily cause membrane damage. Below are detailed accounts of mechanism of action of fullerenes in context of antimicrobial activity.
3.2.1 Lipophilic Nature of Fullerenes Allow for Membrane Permeability
Due to their lipophilic nature, amino and other biofunctionalized fullerenes show membranotropic properties, i.e., easy absorption by cellular membranes (Kotelnikova et al. 1996). Studies show that even though direct contact is important, unlike carbon nanotubes, physical disruption of cell wall/membrane was not responsible for antimicrobial activity of fullerenes. Rather, fullerenes were shown to pass through cell membranes and interact with components of the cellular metabolism to affect their growth and survival (Zhang et al. 2021).
Fullerenes being capable of generating reactive oxygen species are thought to harm cell membrane metabolism. Studies in gram-positive bacteria Bacillus subtilis and gram-negative bacteria on the effect of C60 on cell membrane lipid composition showed that the presence of fullerenes changed the proportion of unsaturated fatty acids and saturated fatty acids and impacted membrane fluidity. P. putida responded by decreasing its unsaturated fatty acids and increasing cyclopropane fatty acids, while B. subtilis levels of iso- and anteiso-branched fatty acids or monosaturated fatty acids are dependent of the concentration of C60 (Fang et al. 2007).
3.2.2 Cationic Fullerenes Use ROS-Mediated Antimicrobial Mechanism
Functionalization of fullerene compounds has been carried out to generate positively charged, neutral, and negatively charged derivatives. Positively charged or cationic derivatives show increased antimicrobial activity on Shewanella oneidensis and E. coli, while the anionic derivatives are not that effective (Nakamura and Mashino 2009). Cationic fullerenes are thought to inhibit the bacterial respiratory chain by ROS production or direct reduction (Cataldo and Da Ros 2008).
Another study comparing a protonated amine and deprotonated carboxylic conjugated to fullerene cage via organic linkers showed that positively charged fullerenes bound effectively to E. coli and showed antibacterial activity, whereas those negatively charged did not bind nor show antimicrobial activity (Deryabin et al. 2014).
3.3 Fullerenes Used for Photodynamic Therapy Against Infections
Oxidative damage by ROS has been known to be toxic to human cells and microbial cells. Molecules that make ROS in response to light, called photosensitizers, have been used for generation of ROS for targeting cancers and microbes in vivo (Abrahamse and Hamblin 2016). This is called photodynamic therapy (PDT). Using localized light delivery, PDT can be performed to target specifically diseased tissue, cancer cells, or microbial cells while keeping the normal cells unharmed. Since functionalized fullerenes possess the ability to form excited singlet state, followed by transition to the long-lived excited triplet state, and can react with oxygen to make ROS, they are excellent candidates for photodynamic therapy (Li et al. 2012a). Figure 14.2 represented the PDT of microbe-infected tissue using fullerenes.
Photodynamic therapy (PDT) of microbe-infected tissue using fullerenes. Microbe-infected tissues can be targeted using fullerenes that can specifically enter infected cells. In the presence of light, fullerenes, effective photosensitizers by nature, are excited to higher electronic states that react with oxygen to form reactive oxygen species that induce oxidative stress-induced bacterial cell death (top). The excitation states are shown in the boxed figure (bottom). Gray fullerenes represent unexcited or ground state fullerenes, and yellow-green fullerenes represent excited states. PS1 and PS2 are the two excited states that give rise to fluorescence and phosphorescence as they give off energy to return to the ground state (PS0). * indicates ROS
Functionalization of fullerenes with the addition of hydrophilic groups enhances production of superoxides which selectively increases cytotoxicity toward microbial cells compared to mammalian cells in PDT. Photoradiation of fulleropyrrolidinium salts resulted in toxicity of 99.99% of bacterial and fungal cells (Tegos et al. 2005). Sulfobutyl fullerenes were found to be effective against environmental bacteria (Lu et al. 2010). Cationic fullerenes are found to be effective against a broad range of microbes in PDT (Sharma et al. 2011). Cationic fullerenes with quaternary amino groups are found to be most effective against S. aureus and E. coli with most resistance from C. albicans (Cataldo and Da Ros 2008).
3.4 Fullerene Nanocomposites as Antimicrobial Agents
Alekseeva et al. found that the fullerene/polystyrene film had bacteriostatic properties versus S. aureus, E. coli, and C. albicans (Alekseeva et al. 2013). Duri et al. (2017) investigated the effect of combining fullerenes (C60 or hydroxyC60) with polysaccharides such as cellulose, chitosan, and γ-cyclodextrin. The results indicated that the γ-cyclodextrin/chitosan/fullerene film had considerable antimicrobial activity against vancomycin-resistant enterococci. Thus, γ-cyclodextrin and chitosan with fullerenes in composite films can be used in food packaging. Ballatore et al. (2015) assessed the inactivation of microorganisms in electrogenerated porphyrin-fullerene C60 polymeric films. These films exhibited photocytotoxic features versus S. aureus and E. coli; in the irradiated films, the microbial population decreased by as much as 4 logs after 30 and 60 min for S. aureus and E. coli, respectively. Therefore, porphyrin-fullerene films feature a fascinating, flexible, photodynamic, active surface that can annihilate microorganisms.
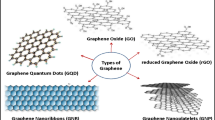
4 Antimicrobial Properties Graphene, Graphene Oxide (GO), and Their Derivatives
Graphene is the simplest form of carbon, made of carbon atoms arranged tightly in a monolayer, essentially thin sheets of graphite. It has unique electrochemical properties like high thermal conductivity, inertness to chemicals, very hydrophobic nature, and optical transmittance. The other graphite family members include graphene oxide, reduced graphene oxide, and graphite oxide.
A comparative study of antibacterial activity among graphite (Gt), graphite oxide (GtO), graphene oxide (GO), and reduced graphene oxide (rGO) toward E. coli showed that GO showed maximum antibacterial activity. After GO, rGO, Gt, and GtO showed decreasing antibacterial activity (Liu et al. 2011). Graphene oxide nanostructures are formed by functionalization of graphene with hydroxyl, epoxyl, or carboxyl groups (Sanchez et al. 2012). These hydrophilic functional groups along with hydrophobic graphenic regions allow for hydrophobic interactions in polar and hydrophilic solvents. Its large surface area also enables its use as an adsorbent or catalyst. The amphiphilic nature of GO makes them suitable for in vivo drug delivery of water-insoluble drugs and other applications in bioimaging and biosensing applications. Both graphene and graphene oxide are known for their antibacterial properties owing to two main properties: membrane damage and oxidative damage to bacterial cells. But recent studies have shown controversial results, indicating that other mechanisms may be responsible. In the coming sections, studies indicating the different mechanisms of action will be touched upon.
4.1 Graphene and Derivatives Cause Physical Damage to Microbial Membrane
Similar to carbon nanotubes, graphene and its derivatives show antimicrobial activity through direct contact and damage of cell membranes due to the sheets being sharp to penetrate membranes. This is why they are called nanoknives. Studies on both gram-positive and gram-negative bacteria showed that sheets caused membrane damage and consequent RNA efflux (Akhavan and Ghaderi 2010). Graphene nanowalls, vertically standing graphene nanostructures, have also been found effective against S. aureus (Gurunathan et al. 2012). Damage of cell membranes by GO and rGO has been reported against E. coli (Hu et al. 2010). Another report suggests that apart from physical piercing of cell membrane, hydrophobic regions of GOs have been found to strongly interact with lipids on the cell membrane resulting in their extraction and further destruction of cell membrane. In E. coli, large amounts of phospholipids were found to be extracted by graphene nanosheets (Tu et al. 2013).
Interestingly a study in understanding GO uptake by E. coli through atomic force microscopy studies found that hydrophilic GO sheets face high-energy barrier before entry into the cell due to repulsive interactions with the outer cell membrane. This was thought to result in lower incidence of adhesion events possibly bringing into question the physical piercing of cell membrane as the mechanism of action of GO sheets (Romero-Vargas Castrillón et al. 2015). Nano-wrapping of bacteria by carbon nanowalls has been another proposed antimicrobial mechanism (Akhavan et al. 2011).
4.2 GOs Cause Oxidative Damage to Microbial Cells
GO being an oxidated state of graphene has also been shown to induce cellular damage by oxidation of bacterial cell membrane components. But the type of oxidation event has not yet been clear due to contrasting reports. On the one hand, oxidative stress has been thought to be caused by generation of ROS and on the other hand, through nonsuperoxide-mediated oxidation of cellular components directly by GO (Fig. 14.3).
Antimicrobial properties of GO sheets. Sheets of graphene oxide and derivatives use three mechanisms against microbes: (1) cell piercing due to the sharp edges; (2) oxidative damage to cells by production of ROS or direct reduction of cellular respiration contents; and (3) cell wrapping preventing access of microbes to nutrients
Study on P. aeruginosa evaluating the antibacterial activities of GO and rGO showed that the presence of these nanostructures induces significant production of ROS, leading to cell death, in a time- and dose-dependent manner of GOs (Gurunathan et al. 2012).
Another report showed that ROS formation did not take place in the presence of GO and rGO, but oxidation of glutathione added for checking oxidation events in vitro indicated that GOs may be oxidating cellular substrates in microbial cells to induce oxidative stress (Gurunathan et al. 2012; Vecitis et al. 2010; Kang et al. 2008).
4.3 Size and Solubility of GO Determines Antimicrobial Activity
Solubility plays an important role in determining the antimicrobial activity in GO sheets. Depending on solubility antimicrobial activity of GO changes. In the case of GO suspensions, antimicrobial activity is through cell entrapment mechanism (Das et al. 2011). This antimicrobial effect of GO increases with increasing sheet area and size. In other cases, the antimicrobial activity of GO is attributed to oxidative mechanisms, where decreasing sheet area or smaller size was found to increase antimicrobial activity by fourfold. The higher antimicrobial effect of smaller GO sheets is attributed to higher defect density of smaller sheets. The oxidative damage is also more permanent in nature, while cell entrapment is reversible (Perreault et al. 2015).
4.4 GO Nanocomposites in Antimicrobial Therapy
Nanocomposites of GO with metal ions and other molecules have been shown to be effective antimicrobials. GO nanostructures, graphene oxide chlorophyllin and graphene oxide chlorophyllin Zn, were found to act against E. coli. Membrane damage was their proposed mechanism of action. Surface chemistry by way of hydrogen bonding of tetrapyrroles with cellular surface and metal toxicity due to Zn2+ leaching was thought to be additional antimicrobial mechanisms (Azimi et al. 2014).
4.4.1 Metal Nanocomposites Most Effective Antimicrobial Agents
GO-Ag nanocomposites have shown effective antibacterial activity against bacteria, gram- positive and gram-negative (Perreault et al. 2015; Yun 2013). GO-Ag composites significantly reduced E. coli and S. aureus populations by up to 99.99% and 99.96%, respectively. Membrane penetration, oxidative stress, ROS production, and disruption of bacterial DNA replication were found to be responsible. These were thought to be dependent on temperature, time, pH, and concentration.
A bacteriostatic activity was observed against E. coli by GO-ZnO nanocomposites (Wang et al. 2014b). Although gold NPs themselves have antimicrobial properties against E. coli, addition of GO or rGO resulted in enhanced action against S. aureus and B. subtilis as well. The antibacterial activity of GO-Ag and GO-ZnO against E. coli, Enterococcus faecium, S. aureus, and Klebsiella pneumonia was assessed. GO-Ag was found to be more effective than GO-ZnO in terms of antibacterial activity (Whitehead et al. 2017).
Nanocomposite of rGO, CuO, and poly-L-lysine (PLL) showed high antimicrobial activity against E. coli and S .aureus with a lethality rate of 99.9% (Ouyang et al. 2013). GO-Au or rGO-Au composites show enhanced activity against microorganisms such as E. coli, S. aureus, and B. subtilis (Hussain et al. 2014). Irradiated (758 nm) nanocomposites have also been found to be effective antimicrobial properties. GO-Au, GO-Fe, and rGO-TiO2Au were found to work against gram-positive, gram-negative, and fungal microorganisms. GO-TiO2 composites have been found to be cytotoxic to mammalian cells as well. They announced that this composite could damage the mitochondria, increase the number of lysosomes, and consequently disrupt and destroy the cell (Díez-Pascual 2020). rGO nanocomposites containing polyvinyl alcohol and Cu2O or TiO2 have shown bacteriostatic activity against Streptococcus oralis, S. aureus, E.coli, and P. aeruginosa (Dhanasekar et al. 2018). TiO2 particles and their nanocomposites have excellent potential to be used in food packaging applications. rGO-FeNP composites have shown bacteriostatic activity against S. aureus. These produce hydroxyl radicals inactivate vital cells and kill bacteria both in vivo and in vitro.
4.4.2 Other GO Nanocomposites and Their Antimicrobial Properties
Studies on polymer-GO composites like polyurethane-rGO-polyethyleneimine and GO- polyurethane composites showed that polyurethane-rGO-PEI composite had higher antibacterial property and hence has practical application in biomolecule encapsulation or immobilization (Tang et al. 2016). Agarose has also been used as an antibacterial hydrogel for its bacteriostatic properties. GO along with cationic surfactants such as benzalkonium bromide can be used to produce novel GO hydrogels for their antibacterial properties (Wang et al. 2015; Guo et al. 2020). The combination of polyvinyl-N-carbazole (PVC) and GO shows enhanced bacteriostatic activity against E. coli, Cupriavidus metallidurans, B. subtilis, and Rhodococcus opacus and few gram- positive bacteria (Carpio et al. 2012). For this reason, GO-PVC composites could be used to prevent biofilm formation.
GOs with surfactants like Tween are shown to be effective in inhibiting the bacterial growth (Szunerits and Boukherroub 2016). A composite with GO, LF, and chitosan enhanced antimicrobial activity of GO against bacteria (Nanda et al. 2015). GO-lysozyme composite showed excellent activity against E. coli. Nisin-G composites produced an active matrix that was used to identify, separate, and disinfect water contaminated with methicillin-resistant S. aureus. Due to the numerous properties of GO, they are under consideration for medical applications. One of the major challenges is its low biocompatibility. Functionalized GO are hence the solution for applicability.
5 Carbon Dots as Emerging Class of Photosensitizers for Antimicrobial Therapy
Carbon dots, also known as quantum carbon dots, are extremely tiny, quasi-spherical nanoparticles with diameter below 10 nm. They are a new class of carbon nanomaterials with enhanced photosensitizing properties. They have the ability of specific detection and inactivation of different bacterial species. Like other carbon nanoparticles, they possess chemical stability and outstanding photoelectric properties. Interestingly, they show high water solubility, low toxicity, and hence are known to be extremely biocompatible. They are easy to prepare and are affordable and hence ideal candidates for antimicrobial therapy. Discovered in 2004, CDs have been found to have applications ranging from use in semiconductors to biomedicine and agricultural applications (Li et al. 2012b).
Their photosensitizing properties have been exploited for testing their use in photodynamic therapy, like GOs. Bright carbon dots (Cipro@Cdots) synthesized using microwave assistance and gum arabic have been shown to be used to deliver broad-spectrum antibiotic nad ciprofloxacin hydrochloride into mammalian cells where release of antibiotic was regulated under physiological conditions. Release of ciprofloxacin was found to be extremely regulated under physiological conditions. Cipro@Cdots conjugate also exhibited antimicrobial activity against gram-positive and gram-negative microbes. CDs hence could be used as nanocarriers with abilities for controlled drug release, contributing to high antimicrobial activity. Table 14.1 summarizes the different types of nanoparticles described in this chapter and their mechanisms of action.
6 Future Perspectives for the Use of Carbon Nanomaterials as Antibiotics: Advantages and Challenges
Nanoparticles by themselves or in conjugation with other molecules are the next-generation antibiotics due to their varied mechanisms of action against bacterial cells. Current antibiotics fail due to the fast rate of evolution of microbes against the mechanisms of action of antibiotics. This is especially true for gram-negative ESKAPE pathogens that are more resistant than others (Santajit and Indrawattana 2016). This resistance is therefore due to the biological warfare and continuous arms race between biological molecules. Hence the only way to put an end to this never-ending fight is the use of physical forces that bacteria cannot evolve against. This is largely absent in the current in vivo antimicrobial therapies. Carbon nanomaterials hence have an advantage due to their properties of piercing cell membranes and carrying out physical damage to microbial cells. Carbon nanotubes and graphene and its derivatives are very well known for this property and hence can be used as carriers with antibiotics or standalone for antimicrobial in vivo therapy.
One of the main issues in the fight against antimicrobial resistance is the lack of biotherapeutic molecules for use as antibiotics in the case of multidrug-resistant infections. Thus, combinatorial therapy with existing antibiotics can be carried out to generate new drug combinations. Combination of nanoparticles along with existing antibiotics show a better therapeutic strategy to combat multidrug-resistant infections, targeting different mechanisms of action against bacteria. This would also mean more combinations can be created in therapy to prevent the rise of resistant strains.
Non-toxic inert carbon nanoparticles are used as carriers of antibiotics to allow for more permeability into bacterial cells due to the lipophilic nature of carbon nanoparticles like fullerenes. This makes better drug permeability and availability and also allows for targeted drug delivery mechanisms.
Graphene and metal nanocomposites of carbon nanoparticles have been shown to be very effective against multidrug-resistant bacteria due to their thermo-plasmonic properties. Therefore, antibiotic functionalized carbon nanoparticles could be used for photothermal lysis to increase the efficacy of antimicrobial therapy. Some composites of carbon nanodots have been shown to regulate the release of antibiotics as well depending on physiological conditions, allowing for controlled release of antibiotics in vivo.
One of the major factors in antibiotic resistance is efflux of antibiotics by the increased number of efflux pumps on membranes of resistant bacteria, affecting drug availability in the cellular interior. Carbon nanotubes and fullerenes have been shown to inhibit the activity of efflux pumps and components in bacterial membrane and metabolism, neutralizing the resistance mechanisms. This is thus a huge advantage of using nanoparticles in conjugation with antibiotics that will allow for more drug availability.
Although antibacterial effects of carbon nanoparticles have been studied for two decades, only few have come into mainstream treatment. The three main reasons for it are the lack of comprehensive understanding of cytotoxicity of nanoparticles on biological systems, selection of narrow size range of nanoparticles, and emerging news of resistance against nanoparticles that have mechanism of action other than physical damage. Cytotoxicity of carbon nanoparticles essentially is imparted by chemical treatments carried out during their preparation to arrive at the right size range as well as to functionalize them for use in biological applications. Although toxicity of various carbon nanoparticles and their functionalized derivatives have been studied in mammalian and microbial cells, and even mice, there needs to be studies carried out in higher mammalian models to understand accumulation of carbon nanoparticles (CNP) in tissues, organs, their half-life and clearance, and potential long-term effects. The antimicrobial mechanisms of CNPs effective against the evolving microbes are summarized in Fig. 14.4.
CNP mechanisms effective in combating the evolving microbial defense. Microbes develop antimicrobial resistance to antibiotics by either evolving enzymes that breakdown membrane-targeting antibiotics or evolving transmembrane efflux pumps that pump out antibiotics from cell interior reducing drug availability. Carbon nanoparticles in combination with traditional antibiotics are advantageous as CNPs by themselves can carry out physical damage and prevent efflux of antibiotics by blocking bacterial membrane pumps
Synthesis of carbon nanoparticles is fairly a complex process and extremely sensitive to various factors, including choice of precursors and method of synthesis to temperature and chemical environment. This makes their synthesis unpredictable as getting a narrow range of size of CNPs in nanoscale is a challenge, consistently, even with the same production method. Even if synthesis is achieved, slight variations may cause changes in their antimicrobial properties which need to be tested every time, unlike drugs that will behave similarly if a particular SOP is followed for its synthesis. Therefore, carbon nanoparticles used in therapy would require exhaustive structural and functional characterization studies.
Non-toxic carbon nanoparticles have been in use for nano-delivery and sustained release of antibiotics, to counter-attack toxicity from antibiotics themselves. Polymer-based and nonpolymeric nanoparticles as liposomes are used for nano-delivery for the treatment of infectious diseases (Zazo et al. 2016). There are now commercially available carbon nanoparticles used for treatment. Respiratory infections are generally difficult to treat and have longer treatment time that leads to systemic side effects. Broad-spectrum antibiotics like ciprofloxacin used for lung infections are now commercially available as a liposome formulation such as Lipoquin™, designed as an inhaled formula that releases drug in 24 h, eliminating the systemic effects of the high-dose antibiotic (Cipolla et al. 2016). Antifungal liposomal carrier AmBisome® (amphotericin B) reduces toxicity of amphotericin B and hence is used for highly immunocompromised patients with HIV infection and disseminated histoplasmosis (Meletiadis et al. 2008). Nano-drugs containing silver nanoparticles are also used in biosensors and medical devices like venous catheters to prevent biofilm formation (Wu et al. 2015; Wang et al. 2017). Other nanoparticles used in diagnosis or as medical devices include Endorem™ SPIONs, Verigene®, Silverline®, and Acticoat™ (Beal et al. 2013).
Recent reports indicating resistance against nanomaterials show an emerging threat against the use of nanomaterials as antibiotics. These concerns have arisen due to the widespread use of silver nanoparticles in consumer products as well as healthcare settings. Studies have shown the possibility of Acinetobacter baumannii, an opportunistic pathogen, causing nosocomial infections, to become resistant to silver nanoparticles. This raises concern over the use of other nanoparticles, especially those with heavy metal composites in antimicrobial therapy. Further studies are required to understand and combat these challenges before carbon nanoparticles could be deployed as antibiotics for the next generation therapeutics.
Abbreviations
- CNTs:
-
Carbon nanotubes
- DWCNTs:
-
Double-walled carbon nanotubes
- GO:
-
Graphene oxide
- Gt:
-
Graphite
- GtO:
-
Graphite oxide
- MDR:
-
Multidrug resistance
- MWNTs:
-
Multiwalled nanotubes
- PDT:
-
Photodynamic therapy
- rGO:
-
Reduced graphene oxide
- ROS:
-
Reactive oxygen species
- SWNTs:
-
Single-walled carbon nanotubes
References
Abrahamse H, Hamblin MR (2016) New photosensitizers for photodynamic therapy. Biochem J 473(4):347–364
Akhavan O, Ghaderi E (2010) Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4(10):5731–5736
Akhavan O, Ghaderi E, Esfandiar A (2011) Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J Phys Chem B 115(19):6279–6288
Alekseeva ОV, Bagrovskaya NA, Noskov AV et al (2013) Polystyrene film composites filled with fullerenes. 2(3): 3–24
Allegri M, Perivoliotis DK, Bianchi MG et al (2016) Toxicity determinants of multi-walled carbon nanotubes: the relationship between functionalization and agglomeration. Toxicol Rep 3:230–243
Arias LR, Yang L (2009) Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir 25(5):3003–3012. https://doi.org/10.1021/la802769m
Azimi S, Behin J, Abiri R, Rajabi L, Derakhshan AA, Karimnezhad H (2014) Synthesis, characterization and antibacterial activity of chlorophyllin functionalized graphene oxide nanostructures. Sci Adv Mater 6(4):771–781
Azizi-Lalabadi M, Hashemi H, Feng J et al (2020) Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv Colloid Interf Sci 284:102250
Ballatore MB, Durantini J, Gsponer NS et al (2015) Photodynamic inactivation of bacteria using novel electrogenerated porphyrin-fullerene C60 polymeric films. Environ Sci Technol 49(12):7456–7463
Barzegar A, Naghizadeh E, Zakariazadeh M, Azamat J (2017) Molecular dynamics simulation study of the HIV-1 protease inhibit ion using fullerene and new fullerene derivatives of carbon nanostructures. Mini Rev Med Chem 17(7):633–647
Beal SG, Ciurca J, Smith G, John J, Lee F, Doern CD et al (2013) Evaluation of the nanosphere verigene gram- positive blood culture assay with the VersaTREK blood culture system and assessment of possible impact on selected patients. J Clin Microbiol 51(12):3988–3992
Bianco A, Kostarelos K, Prato M (2005) Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol 9(6):674–679
Carpio IEM et al (2012) Toxicity of a polymer–graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 4(15):4746–4756. https://doi.org/10.1039/C2NR30774J
Cataldo F, Da Ros T (eds) (2008) Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes, vol 1. Springer, Dordrecht. ISBN: 978-1-4020-6844-7
Cha SI, Kim K, Arshad SN et al (2005) Extraordinary strengthening effect of carbon nanotubes in metal- matrix nanocomposites processed by molecular-level mixing. Adv Mater 17(11):1377–1381
Chik MW, Hussain Z, Zulkefeli M et al (2019) ePolymer-wrapped single-walled carbon nanotubes: a transformation toward better applications in healthcare. Drug Deliv Transl Res 9(2):578–594
Cipolla D, Blanchard J, Gonda I (2016) Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics 8(1):6. https://doi.org/10.3390/pharmaceutics8010006
D’Souza F, Kadish KM (2012) Handbook of carbon nano materials, vol 4. World Scientific, Singapore
Das MR, Rupak KS, Ratul S (2011) Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surf B Biointerfaces 83(1):16–22
Debnath SK, Srivastava R (2021) Drug delivery with carbon-based nanomaterials as versatile nanocarriers: progress and prospects. Front Nanotechnol 3:644564
Deryabin D, Davydova OK, Yanikna ZZH et al (2014) The activity of fullerene derivatives bearing amine and carboxylic solubilizing groups against Escherichia coli: a comparative study. J Nanomater 2014:907435
Dhanasekar M, Jenefer V, Nambair RB et al (2018) Ambient light antimicrobial activity of reduced graphene oxide supported metal doped TiO2 nanoparticles and their PVA based polymer nanocomposite films. Mater Res Bull 97:238–243
Díez-Pascual AM (2020) Antibacterial action of nanoparticle loaded nanocomposites based on graphene and its derivatives: a mini-review. Int J Mol Sci 21(10):3563
Dinh NX, Quy MV, Huy TQ et al (2015) Decoration of silver nanoparticles on multiwalled carbon nanotubes: antibacterial mechanism and ultrastructural analysis. J Nanomater 16:63
Dong L, Witkowski CM, Craig MM et al (2009) Cytotoxicity effects of different surfactant molecules conjugated to carbon nanotubes on human astrocytoma cells. Nanoscale Res Lett 4(12):1517–1523
Dong L, Henderson A, Field C (2012) Antimicrobial activity of single-walled carbon nanotubes suspended in different surfactants. J Nanotechnol 130:2626–2633
Duri S, Harkins AL, Frazier AJ, Tran CD (2017) Composites containing fullerenes and polysaccharides: green and facile synthesis, biocompatibility, and antimicrobial activity. ACS Sustain Chem Eng 5(6):5408–5417
Fang J, Lyon DY, Wiesner MR, Dong J, Alvarez PJ (2007) Effect of a fullerene water suspension on bacterial phospholipids and membrane phase behavior. Environ Sci Technol 41(7):2636–2642
Fortner JD, Lyon DY, Sayes CM et al (2005) C60 in water: nanocrystal formation and microbial response. Environ Sci Technol 39(11):4307–4316
Guo A et al (2020) Facile synthesis of polydopamine/reduced graphene oxide nanosheets with incorporated copper ions for high antibacterial performance. Micro Nano Lett 15(2):114–118
Gurunathan S, Han JW, Dayem AA, Eppakayala V et al (2012) Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomedicine 7:5901–5914
He H, Pham-Huy LA, Dramou P et al (2013) Carbon nanotubes: applications in pharmacy and medicine. Biomed Res Int 2013:578290
Heister E, Neves V, Lamprecht C et al (2012) Drug loading, dispersion stability, and therapeutic efficacy in targeted drug delivery with carbon nanotubes. Carbon 50(2):622–632
Hu W, Peng C, Luo W, Lv M, Li X, Li D, Huang Q, Fan C (2010) Graphene-based antibacterial paper. ACS Nano 4(7):4317–4323. https://doi.org/10.1021/nn101097v
Hurmach V, Platonov MO, Prylutska SV et al (2021) C60 fullerene against SARS-CoV-2 coronavirus: an in silico insight. Sci Report 11:1
Hussain N, Gogoi A, Sarma RK et al (2014) Reduced graphene oxide nanosheets decorated with Au nanoparticles as an effective bactericide: investigation of biocompatibility and leakage of sugars and proteins. ChemPlusChem 79(12):1774–1784
Hussain MZ et al (2019) Porous ZnO/Carbon nanocomposites derived from metal organic frameworks for highly efficient photocatalytic applications: a correlational study. Carbon 146:348–363
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354(6348):56–58
Jafar A, Alshatti Y, Ahmad A (2016) Carbon nanotube toxicity: the smallest biggest debate in medical care. Cogent Med 3(1):1217970
Kang S, Pinault M, Elimelech M et al (2007) Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 23(17):8670–8673
Kang S, Herzberg M, Rodrigues DF et al (2008) Antibacterial effects of carbon nanotubes: size does matter. Langmuir 24(13):6409–6413
Kim KT et al (2008) The role of interfacial oxygen atoms in the enhanced mechanical properties of carbon-nanotube- reinforced metal matrix nanocomposites. Small 4(11):1936–1940
Kotelnikova RA, Kotelnikov AI, Bogdanov GN et al (1996) Membranotropic properties of the water-soluble amino acid and peptide derivatives of fullerene C60. FEBS Lett 389(2):111–114
Li CZ, Yip HL, Jen AKY (2012a) Functional fullerenes for organic photovoltaics. J Mater Chem 22(10):4161–4177
Li H, Kang Z, Liu Y, Lee ST (2012b) Carbon nanodots: synthesis, properties and applications. J Mater Chem 22(46):24230–24253
Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R et al (2011) Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5(9):6971–6980
Lu Z, Dai T, Huang L et al (2010) Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine (Lond) 5(10):1525–1533
Maleki Dizaj S, Mennati A, Jafari S, Khezri K et al (2015) Antimicrobial activity of carbon-based nanoparticles. Adv Pharm Bull 5(1):19–23
Martincic M, Tobias G (2015) Filled carbon nanotubes in biomedical imaging and drug delivery. Expert Opin Drug Deliv 12(4):563–581
Meletiadis J, Chanock S, Walsh TJ (2008) Defining targets for investigating the pharmacogenomics of adverse drug reactions to antifungal agents. Pharmacogenomics 9(5):561–584. https://doi.org/10.2217/14622416.9.5.561
Mogasale VV et al (2021) A descriptive analysis of antimicrobial resistance patterns of WHO priority pathogens isolated in children from a tertiary care hospital in India. Sci Rep 11:5116
Mohanta D, Patnaik S, Sood S et al (2019) Carbon nanotubes: evaluation of toxicity at biointerfaces. J Pharm Anal 9(5):293–300
Mulani MS, Kamble EE, Kumkar SN, Tawre MS et al (2019) Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol 1(10):539
Nakamura S, Mashino T (2009) Biological activities of water-soluble fullerene derivatives. J Phys Conf Ser 159(1):012003
Nanda SS, An SSA, Yi DK (2015) Oxidative stress and antibacterial properties of a graphene oxide-cystamine nanohybrid. Int J Nanomedicine 10:549–556
Ouyang W, Sun J, Memon J, Wang C, Geng J et al (2013) Scalable preparation of three-dimensional porous structures of reduced graphene oxide/cellulose composites and their application in supercapacitors. Carbon 62:501–509
Perreault F, de Faria AF, Nejati S, Elimelech M (2015) Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano 9(7):7226–7236
Rajeswari SR, Nandini V, Perumal A (2021) Influence of titania nanotubes diameter on its antibacterial efficacy against periodontal pathogens: an in vitro analysis. J Pharm Bioallied Sci 13:S284–S288
Rdest M, Janas D (2021) Carbon nanotube wearable sensors for health diagnostics. Sensors (Basel) 21(17):5847
Roldo M (2016) Carbon nanotubes in drug delivery: just a carrier? Ther Deliv 7(2):55–57
Romero-Vargas Castrillón S, Perreault F, De Faria AF et al (2015) Interaction of graphene oxide with bacterial cell membranes: insights from force spectroscopy. Environ Sci Technol Lett 2(4):112–117
Sakib AA, Masum SM, Hoinkis J, Islam R, Molla M et al (2019) Synthesis of CuO/ZnO nanocomposites and their application in photodegradation of toxic textile dye. J Compos Sci 3(3):91
Sanchez VC, Jachak A, Hurt RH et al (2012) Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol 25(1):15–34
Santajit S, Indrawattana N (2016) Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int 2016:2475067
Serrano-Aroca Á, Takayama K, Tuñón-Molina A et al (2021) Carbon-based nanomaterials: promising antiviral agents to combat COVID-19 in the microbial-resistant era. ACS Nano 15(5):8069–8086
Sharma SK, Chiang LY, Hamblin MR (2011) Photodynamic therapy with fullerenes in vivo: reality or a dream? Nanomedicine (Lond) 6(10):1813–1825
Shoji M, Takahashi E, Hatakeyama D et al (2013) Anti-influenza activity of c60 fullerene derivatives. PLoS One 8(11):e66337
Sun YP et al (2002) Functionalized Carbon nanotubes: properties and applications. Acc Chem Res 35(12):1096–1104
Szunerits S, Boukherroub R (2016) Antibacterial activity of graphene-based materials. J Mater Chem B 4(43):6892–6912
Szunerits S et al (2015) Nanostructures for the inhibition of viral infections. Molecules 20(8):14051–14081
Tang XZ et al (2016) Flexible polyurethane composites prepared by incorporation of polyethylenimine- modified slightly reduced graphene oxide. Carbon 98:432–440
Tegos GP, Demidova TN, Arcila-Lopez D et al (2005) Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol 12(10):1127–1135
Thota S et al (2012) Synthesis and characterization of positively charged pentacationic [60]fullerene monoadducts for antimicrobial photodynamic inactivation. Molecules 17:5225–5243
Tsao N, Luh TY, Chou CK et al (2001) Inhibition of group a streptococcus infection by Carboxyfullerene. Antimicrob Agents Chemother 45(6):1788
Tu Y, Lv M, Xiu P et al (2013) Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat Nanotechnol 8(8):594–601
Vardharajula S et al (2012) Functionalized carbon nanotubes: biomedical applications. Int J Nanomedicine 7:5361
Vecitis CD, Zodrow KR, Kang S, Elimelech M (2010) Electronic-structure-dependent bacterial cytotoxicity of single-walled carbon nanotubes. ACS Nano 4(9):5471–5479
Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. P T 40(4):277–283
Wang J-T, Chen C, Wang E et al (2014a) A new carbon allotrope with six-fold helical chains in all-sp2 bonding networks. Sci Report 4:4339
Wang YW, Cao A, Jiang Y et al (2014b) Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl Mater Interfaces 6(4):2791–2798
Wang X, Liu Z, Ye X et al (2015) A facile one-pot method to two kinds of graphene oxide-based hydrogels with broad-spectrum antimicrobial properties. Chem Eng J 260:331–337
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine 12:1227
Whitehead KA, Vaidya M, Liauw C et al (2017) Antimicrobial activity of graphene oxide-metal hybrids. Int Biodeterior Biodegradation 123:182–190
Wu K, Yang Y, Zhang Y, Deng J et al (2015) Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int J Nanomedicine 10:7241–7252
Yang C, Mamouni J, Tang Y, Yang L (2010) Antimicrobial activity of single-walled carbon nanotubes: length effect. Langmuir 26(20):16013–16019
Yuan W, Jiang G, Che J et al (2008) Deposition of silver nanoparticles on multiwalled Carbon nanotubes grafted with hyperbranched poly(amidoamine) and their antimicrobial effects. J Phys Chem C 112(48):18754–18759
Yun HJDHCCW (2013) Antibacterial activity of CNT-Ag and GO-Ag nanocomposites against gram- negative and gram-positive bacteria. Bull Kor Chem Soc 34(11):3261–3264
Zazo H, Colino CI, Lanao JM (2016) Current applications of nanoparticles in infectious diseases. J Control Release 224:86–102
Zhang W, Zhang Z, Zhang Y (2011) The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett 6(1):555
Zhang C et al (2021) Membrane perturbation of fullerene and graphene oxide distinguished by pore-forming peptide melittin. Carbon 180:67–76
Zhu Z, Schuster DI, Tuckerman ME (2003) Molecular dynamics study of the connection between flap closing and binding of fullerene-based inhibitors of the HIV-1 protease. Biochemistry 42(5):1326–1333
Acknowledgments
None to declare.
Conflict of Interest
None to declare.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Deb, S., Sriram, D. (2022). Carbon Nanoparticles as the Next-Generation Antimicrobial Agents. In: Saha, T., Deb Adhikari, M., Tiwary, B.K. (eds) Alternatives to Antibiotics. Springer, Singapore. https://doi.org/10.1007/978-981-19-1854-4_14
Download citation
DOI: https://doi.org/10.1007/978-981-19-1854-4_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1853-7
Online ISBN: 978-981-19-1854-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)