Abstract
Nutrition and temperature are the primary factors affecting development of fish larvae during the early feeding stage. This chapter discusses how nutrient enhancement and ambient temperature affect the rearing performance of golden pompano Trachinotus ovatus. Artemia nauplii enriched with Algamac 3080 enhanced fish growth and reduced malformation. Fish fed Artemia nauplii enriched by Nanochloropsis achieved high survival but high jaw malformation. The water temperature of 26–29 °C enhanced growth and survival, while 23 °C was too low for both parameters. Jaw, vertebral column, and caudal vertebra deformity significantly increased at 33 °C. Therefore, the temperature range of 26–29 °C is optimal, and temperature >33 °C and <23 °C may have adverse impacts on fish performance. Information presented in this chapter will improve hatchery management on the production efficiency of golden pompano fingerlings. These findings may also apply to other similar species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Malformation adversely impacts the market value of marine fish (Cobcroft and Battaglene 2009, 2013; Sandel et al. 2010; Ma et al. 2014c). Malformed fish are usually sold at a low price or manually removed during the production phase, thus significantly increasing the production cost (Koumoundourous et al. 1997). In addition, the deformation has adversely affected fish’s growth and survival, food conversation, swimming ability, and susceptibility to stress and pathogens (Koumoundourous et al. 1997; Andrades et al. 1996; Boglione et al. 2001). Such impact can greatly add production cost on marine fish aquaculture. Existing evidence indicates that malformation during larval development occurs on over 27% of fish, leading to severe mortality at the grow-out phase (Andrades et al. 1996). Although the rearing environment (such as temperature, water current, tank color, salinity, dissolved oxygen) (Koumoundouros et al. 1999, 2001; Sfakianakis et al. 2004; Hattori et al. 2004; Okamoto et al. 2009; Georgakopoulou et al. 2010; Owen et al. 2012), genetic factors (Ferguson and Danzmann 1998; Castro et al. 2007), pesticides, and parasites (Liang et al. 2012; Kusuda and Sugiyama 1981; Liu et al. 2012) have been identified to be associated with boney malformation, and more and more evidence has indicated the nutritional factors in the process of larval fish rearing as a direct result of the deformation of the fish (Afonso et al. 2000; Andrades et al. 1996; Sandel et al. 2010; Cahu et al. 2003a).
In the past decades, the requirements of essential fatty acids in fish larvae have been widely studied (Izquierdo et al. 1992; Morais et al. 2007; Kjørsvik et al. 2009). The long-chain polyunsaturated fatty acids (PUFA) such as eicosapentaenoic acid 20:5n-3 (EPA), docosahexaenoic acid 22:6n-3 (DHA), and arachidonic acid 20:4n-6 (ARA) play critical roles in growth, survival, and stress resistance in most marine fish larvae (Watanabe 1993; Bell and Sargent 2003; Faulk and Holt 2003). As the essential fatty acids in marine fish larvae, the dietary requirements of EPA, DHA, and ARA have been well quantified in fish nutrition (Hamre et al. 2002; Bell and Sargent 2003; Faulk et al. 2005). DHA plays a vital role in neutral membrane structure and functions (Sargent et al. 1999a; Copeman et al. 2002), and its requirement differs among fish species (McEvoy et al. 1998; Planas and Cunha 1999; Copeman et al. 2002). The abundances of DHA and EPA in cell membranes serve as a major source of energy to absorb fat-soluble vitamins (A, D, E, and K) and as precursors for prostaglandin hormones (Sargent et al. 1999a, b; Rezek et al. 2010). The DHA/EPA ratio has been considered an index to determine the optimal levels for these fatty acids in the growth and development of fish larvae (Koven et al. 1993; Tocher et al. 1997; Rodriguez et al. 1998). Since a deficiency or excess of DHA and EPA in the diet can affect survival and malformation of fish larvae (Sargent et al. 1999b), it is necessary to provide an adequate amount of these essential fatty acids through dietary manipulation (Palmtag et al. 2006).
Temperature is vital for the larval fish early development, because it can adjust the fish metabolism and feeding behavior (Ma 2014; Kestemont and Baras 2001). In aquaculture, temperature directly influences the size of fish larvae at hatching, the age and size at yolk absorption, growth, survival, feeding, and digestion in fish larvae (Martinez-Palacios et al. 1996; Rombough 1996; Jobling 1997; Fielder et al. 2005; Bustos et al. 2007). Besides, several studies have demonstrated that high mortality and abnormality of fish larvae are attributed to inappropriate temperature (Ørnsrud et al. 2004; Lein et al. 1997; Ludwig and Lochmann 2009). Furthermore, the way yolk energy is utilized varies between incubation temperatures. In many species, if the temperature is higher than the optimal level, the size at the onset of exogenous feeding can be smaller than in other temperatures, thereby contributing to complication in larval fish rearing, especially in species with small larvae. Within the temperature range of fish tolerance, the increase of temperature accelerates ontogenetic development, but a high temperature may reduce fish survival. At high temperature, yolk-sac absorption of fish larvae is fast, but the period of endogenous feeding is short (Dou et al. 2005; Bustos et al. 2007; Ma 2014). Therefore, choosing the appropriate temperature is essential to improve the growth and survival of fish larvae in hatcheries.
Golden pompano Trachinotus ovatus has been used as a new candidate species for aquaculture. Although the life cycle of golden pompano has been closed and several key aspects such as food and feeding, development of the larval digestive system, and the weaning protocols have been successfully explored (Ma et al. 2014a, b, d), high malformation of golden pompano during the early development stage has severely reduced the production efficiency (Ma et al. 2014c). Chapter 4 has identified the position, type, and frequency of skeletal and jaw malformations in hatchery rearing of larval golden pompano. Nonetheless, factors causing malformations are still unclear in this fish. This chapter discusses the nutritional enhancement and ambient temperature on the rearing performance of golden pompano under a hatchery culturing condition. These chapter results provide better understanding on how nutritional enhancement and temperature regulate larval fish development. This chapter contributes to developing management strategies to improve this fish and other similar species in the hatchery production efficiency.
5.2 Nutritional Enhancement Regulates Fish Growth and Survival
Feed of DHA and EPA in larval fish is vital for the growth of fish (Rezek et al. 2010). As dietary DHA levels increased, improved fish growth has been observed in striped jack Caranx vinctus (Takeuchi et al. 1996), yellowtail Seriola quinqueradiata (Furuita et al. 1996), and Japanese flounder Paralichthys olivaceus (Izquierdo et al. 1992). The growth response of fish larvae to different enrichment products varies among species. For instance, the larval growth of striped bass Morone saxatilis and gilthead seabream Sparus aurata is not affected by feeding Artemia nauplii enriched with Algamac 2000 or PL-Cr (DHA-rich phospholipid extract of Crypthecodinium sp.), but the growth of halibut Hippoglossus hippoglossus larvae fed Artemia nauplii enriched with DHA Seleco is slower than those fed with PL-Cr (Harel et al. 2002). In golden pompano, fish growth was enhanced when fish larvae were fed with Artemia nauplii enriched with Algamac 3080 or Nannochloropsis (Fig. 5.1). Fatty acid composition of enriched and unenriched Artemia nauplii is shown in Table 5.1.
Specific growth rate and RNA/DNA of golden pompano in different nutrient enhancements (Ma et al. 2016)
Fish treated with Algamac 3080 had the best SGR, which is consistent with the higher dietary DHA levels in the treated live feed. As a sensitive growth and nutritional condition indicator (Islam and Tanaka 2005; Zehra and Khan 2013), the RNA/DNA ratio indicates that better growth performance occurred in the treatment of Algamac 3080. However, the fish growth in the treatments of Nannochloropsis and Spirulina is not consistent with their RNA/DNA ratios and dietary DHA levels (Fig. 5.1). As Faulk et al. (2005) suggested, such inconsistency may be possibly due to the difference in the protein content or amino acid profiles of live prey fed with different enrichment formulas.
Highly unsaturated fatty acids, especially DHA, EPA, and ARA, are necessary to marine fish growth, development, and survival (Cahu et al. 2003a; Sargent et al. 1999b; Rezek et al. 2010). In order to develop lipid-enriched food of fish larvae, the requirements of essential fatty acids of fish larvae have been extensively researched by using live bait enriched with different oils and micronutrients, and the aim is to enhance the content of essential fatty acids of live bait (Takeuchi 1997; Sargent et al. 1997; Izquierdo et al. 2000). However, excessive content of lipid or unbalanced composition of lipid classes leads to low growth and skeletal malformation in species such as gilthead seabream Sparus aurata (Salhi et al. 1999), Atlantic cod Gadus morhua (Kjørsvik et al. 2009), yellowtail kingfish Seriola lalandi (Ma and Qin 2014), and Atlantic halibut Hippoglossus hippoglossus (Olsen et al. 2000). Enrichment did not change the DHA/ARA ratio, but Artemia nauplii enriched with Algamac 3080 resulted in a higher DHA/EPA ratio (0.36:1, Table 5.1). The high DHA/EPA ratio treated with Algamac 3080 resulted in rapid growth but low survival. In contrast, the unenriched and Nannochloropsis treatments showed better survival where the DHA/EPA ratio was 0.07:1–0.22:1. Low fish survival rate of Algamac 3080 treatment supports the claim of a previous study that a high DHA/EPA ratio and a high DHA content may reduce the survival of larval fish (Planas and Cunha 1999) as the composition of unbalanced lipid classes in the diet affects the digestion and absorption of fatty acids in fish larvae (Salhi et al. 1997, 1999).
5.3 Malformation
Skeletal malformation on fish in marine aquaculture is a recurrent issue (Ma and Qin 2014; Ma et al. 2014c), and skeletal malformation negatively affects fish quality in commercial production via suppressing fish growth and survival (Andrades et al. 1996; Boglione et al. 2001). The abnormalities in fish larvae can have sublethal (Barahona-Fernandes 1982; Cobcroft et al. 2001) or lethal effects on fish larvae (Boglione et al. 2013) as the distorted mouth shape would affect the efficiency of food ingestion (Pittman et al. 1989), while notochord anomalies in newly hatched larvae can severely affect fish swimming (Boglione et al. 2013). Jaw malformations are a common type of skeletal malformation, and there are many different forms (Cobcroft et al. 2001) that have been frequently found in both wild-caught and artificially reared marine fish (Boglione et al. 2001). Izquierdo et al. (2010) believed that PUFA play a key role in bone formation, and the composition of fatty acid composition in bone and cartilage can also be affected by dietary lipids (Watkins et al. 1997; Kokkinos et al. 1993; Liu et al. 2004). As the dietary lipids are primarily from live feeds, enrichment on live feeds may affect jaw malformation. In golden pompano, enrichment significantly affected jaw malformation. Fish fed Artemia nauplii enriched with Algamac 3080 or Spirulina showed two times lower in jaw malformation than those fed unenriched Artemia nauplii or Artemia nauplii enriched with Nannochloropsis (Fig. 5.2). The results of low jaw malformation treated by Algamac 3080 and Spirulina may indicate that such nutrient enrichment is sufficient to meet the needs of jaw development during this stage.
Jaw malformation of golden pompano fed with Artemia nauplii enriched with different nutrient enhancement (Ma et al. 2016)
Up to present, the relationship between the deficiency of essential fatty acids and the development of skeletal anomalies is poorly understood (Boglione et al. 2013).
Hamre et al. (2002) suggest that insufficient intake of n-3 HUFA in Artemia nauplii may cause abnormal development of fish larvae. Recent evidence has demonstrated that fatty acids, such as EPA, DHA, and ARA, play a key role in the development of bone.
For example, a 50% reduction in deformed fish was found in diet supplement with higher levels of DHA (Izquierdo et al. 2010), and changes in dietary ARA/EPA may indirectly affect osteoblast development and bone metabolism (Berge et al. 2009). In golden pompano, skeletal malformation was improved in the treatment of Algamac 3080, and this result is in line with high levels of DHA in feed. The results of this study indicate that 2.56% DHA level may be suitable for the larval golden pompano’s skeletal development. Vertebral column malformation and caudal complex malformation are the most frequently reported body deformity in commercially cultured species (Negm et al. 2013). Up to present, little is known on the causes triggering the deformity in the caudal complex (Haga et al. 2011). In the literature, vertebral deformities are often associated with swim bladder abnormality (Daoulas et al. 1991; Chatain 1994), but vitamin A deficiency can also induce vertebral column deformities (Negm et al. 2013). In golden pompano, the highest vertebral column (Vco) malformation (60.9%) and epural (Ep) malformation (75.1%) were observed in the fish fed Artemia enriched with Nannochloropsis, and the lowest Vco malformation (7.7%) and Ep malformation (0.7%) were found in the treatment of Spirulina enrichment. Significantly higher hypural malformation (61.0%) was observed in the treatment of Algamac 3080 enrichment than in other treatments (Fig. 5.3). This result may suggest that nutrient enhancement in Artemia nauplii affects vertebral deformities, and the impact of nutritional components in Artemia nauplii on larval fish development warrants further study in future.
The vertebral column (Vco), caudal vertebra (Vca), hypural (Hy), and epural (Ep) malformations and vertebral malformation of larval golden pompano of four enrichment treatments on 27 DPH (Ma et al. 2016)
5.4 Temperature Affects Fish Performance
Temperature affects fish growth, metabolism, and food intake, and the effects of temperature on body growth have been recorded in many larval fish species including nase Chondrostoma nasus (Keckeis et al. 2001), striped trumpeter Latris lineata (Choa et al. 2010), haddock Melanogrammus aeglefinus (Martell et al. 2005), Australian snapper Pagrus auratus (Fielder et al. 2005), yellowtail kingfish Seriola lalandi (Ma 2014), and Atlantic halibut Hippoglossus hippoglossus (Lein et al. 1997). In golden pompano, the newly hatched golden pompano grow slowly in the first 9 days. After 12 DPH, the size of the different temperature treatment of fish larvae of differences began to emerge. Temperature has an effect on fish growth on 18 DPH, which accelerates when the temperature increases from 29 to 33 °C (Fig. 5.4).
Specific growth rate of standard length and RNA/DNA ratio of golden pompano larvae reared at 23, 26, 29, and 33 °C (Yang et al. 2016)
The rapid growth of golden pompano at high temperature may be related to the improvement of larval feeding and digestive function after 15 DPH, as Ma et al. (2014b) found that the gastric glands and goblet cells appeared in the intestines of larval golden pompano after 15 DPH at the temperature of 27–29 °C. As the Florida pompano Trachinotus carolinus (Riley et al. 2009), the mouth gape height of 1.05 mm should allow larval golden pompano to ingest Artemia nauplii and other similar particle sizes by 12 DPH.
Therefore, the significant differences in fish size between thermal treatments after 18 DPH may be also related to the use of more energetic food from 9 DPH onward. Riley et al. (2009) found that the growth trajectories of T. carolinus could vary substantially between progenies, with some progenies exhibiting slower growth and others much faster growth.
The RNA/DNA ratio has been proven as a sensitive indicator of growth and nutritional condition in fish larvae and juveniles (Buckley et al. 1999; Islam and Tanaka 2005; Zehra and Khan 2013). Previous studies have demonstrated that water temperature and food availability can affect the RNA/DNA ratio of fish larvae (Goolish et al. 1984; Mathers et al. 1992). In golden pompano, the RNA/DNA ratio of fish significantly dropped when temperature increased from 29 to 33 °C at 18 DPH (Fig. 5.4). Reduction in RNA/DNA ratio can be regarded as a result of nutrient deficiency in fish larvae (Tanaka et al. 2008). The reduction of RNA to DNA ratio of fish at 33 °C may indicate a slow growth status of fish larvae at 18 DPH. At the end of this study, the high SGR of a few larger fish may be due to cannibalism. However, the reason for the lower RNA/DNA ratio at 33 °C is not clear.
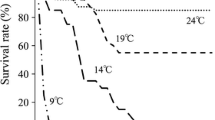
Fish mortality frequently occurs at the key period of nutritional transition from endogenous to exogenous nutrition (Ma et al. 2012; Otterlei et al. 1999). During the period of transitional feed, when food supply and photoperiod are within the range of fish requirements, the temperature can strongly impact fish survival (Gardeur et al. 2007; McGurk 1984; Kamler 1992; Ma 2014). For instance, mortality is closely related to temperature in the larvae and juveniles of Pangasianodon hypophthalmus (Baras et al. 2011), Seriola lalandi (Ma 2014), Glyptocephalus cynoglossus (Bidwell and Howell 2001), and Inimicus japonicus (Wen et al. 2013). Ma (2014) suggests a temperature-sensitive period where mortality is likely to occur during the early ontogeny of golden pompano. In golden pompano, survivals of fish larvae were low in all the temperature treatments (Fig. 5.5). There could be many reasons why survival rates were so low, including egg quality, the inadequacy of feeding schedules, or cannibalism (May 1974; Baras and Jobling 2002; Baras et al. 2011; Ma et al. 2012). Nevertheless, there is some clear-cut tendency here that the survival of golden was highest at 29 °C and declined at lower or warmer temperatures, with 23 °C being unsuitable for their rearing.
Survival rate of golden pompano larvae at 23, 26, 29, and 33 °C (Yang et al. 2016). (The letters of “temperature” in the x-axis is crowding)
As one of the primary physical factors, the water temperature can affect early morphological abnormalities during the ontogenetic development of fish larvae (Seikai et al. 1980, 1986; Klimogianni et al. 2004). Evidence indicates that high water temperature can increase the percentage of malformation during the ontogenetic development in fish species such as tilapia Oreochromis mossambicus (Wang and Tsai 2000), Senegalese sole Solea senegalensis (Dionisio et al. 2012), and gilthead seabream Sparus aurata (Georgakopoulou et al. 2010). The present study demonstrates a significant temperature effect on the vertebral column and caudal vertebra malformation in golden pompano. This result is consistent with similar findings in Dicentrarchus labrax (Sfakianakis et al. 2006). In golden pompano, temperature associated abnormity in the vertebral column of the larval golden pompano was the most prevalent malformation (Fig. 5.6). Additionally, the V-shaped malformation accounted for >50% of the whole deformations. A previous study indicates that the absence of swim bladder or its malfunction could account for over 50% of V-shaped deformations in fish (Daoulas et al. 1991). Hemal lordosis can also result from the exposure to high flow velocities (Divanach et al. 1997) or turbulent flows (Kihara et al. 2002; Kranenbarg et al. 2005). In the present study, deformities frequently occurred at all temperatures, suggesting that other factors may also prevail in the present study causing deformity. Future research is required to further identify these factors.
Malformation of golden pompano larvae reared at 23, 26, 29, and 33 °C. Vco the vertebral column malformation, Vca caudal vertebra malformation, Hy hypural malformation, Ep epural malformation (Yang et al. 2016). You should indicate the meaning of bar diagram and scattered diagram (i.e., jaw malformation vs. without deformity) in the right diagram
The caudal fin complex is one of the most sensitive parts of the skeletal system in fish, and deformities can occur even in the range of normal rearing temperature (Takeuchi et al. 1998; Haga et al. 2002, 2011). Rates of deformities can increase with increasing temperature, but this is not consistent in gilthead seabream Sparus aurata (Fernández et al. 2008; Georgakopoulou et al. 2010). In golden pompano, however, the proportion of fish with deformed caudal fin complex did not vary significantly with water temperature (Fig. 5.6). Jaw malformation is a significant concern in fish culture because it affects fingerling quality (Von Westernhagen 1988). Frequently, as the temperature of water rises, the proportion of larval fish exhibiting jaw deformities increases (Alderdice and Velsen 1971; von Westernhagen 1974; Bolla and Holmefjord 1988; Lein et al. 1997), and we observed the same pattern in golden pompano. This temperature-dependent pattern is often attributed to higher oxygen (Rombough 1997) and nutritional requirements at high temperatures, which may not be met unless feed with higher energy or protein contents is provided (Cahu et al. 2003a, b; Ma 2014).
5.5 Conclusion
In summary, Artemia nauplii enriched with Algamac 3080 can enhance fish growth performance and reduce malformation. Fish fed Artemia nauplii enriched with Nanochloropsis showed high survival but with high jaw malformation. Nutritional enhancement in Artemia nauplii can significantly affect the performance of golden pompano larvae. The water temperature of 26–29 °C enhanced growth and survival, while 23 °C was too low for both. The deformity of the jaw, vertebral column, and caudal vertebra significantly increased at 33 °C. Therefore, the temperature range of 26–29 °C is optimal, and temperature >33 °C and <23 °C may have adverse impacts on fish performance. Future study should focus on refining the optimum dietary enrichment and rearing temperature in golden pompano larvae to improve growth and survival and decrease boney malformation of fish larvae.
References
Afonso JM, Montero D, Robaina L, Astorga N, Izquierso MS, Gines R (2000) Association of a lordosis-scoliosis-kyphosis deformity in gilthead seabream (Sparus aurata) with family structure. Fish Physiol Biochem 22:159–163
Alderdice DF, Velsen FPJ (1971) Some effects of salinity and temperature on early development of Pacific herring (Clupea pallasi). J Fish Res Board Can 28:1545–1562
Andrades JA, Becerra J, Fernández-Llebrez P (1996) Skeletal deformities in larval, juvenile and adult stages of cultured gilthead sea bream (Sparus aurata L.). Aquaculture 141:1–11
Barahona-Fernandes MH (1982) Body deformation in hatchery reared European sea bass Dicentrarchus labrax (L). Types, prevalence and effect on fish survival. J Fish Biol 21:239–249
Baras E, Jobling M (2002) Dynamics of intracohort cannibalism in cultured fish. Aquac Res 33:461–479
Baras E, Raynaud T, Slembrouck J, Caruso D, Cochet C, Legendre M (2011) Interactions between temperature and size on the growth, size heterogeneity, mortality and cannibalism in cultured larvae and juveniles of the Asian catfish, Pangasianodon hypophthalmus (Sauvage). Aquac Res 42:260–276
Bell JG, Sargent JR (2003) Arachidonic acid in aquaculture feeds: current status and future opportunities. Aquaculture 218:491–499
Berge GM, Witten PE, Baeverfjord G, Vegusdal A, Wadsworth S, Ruyter B (2009) Diets with different n-6/n-3 fatty acid ratio in diets for juvenile Atlantic salmon, effects on growth, body composition, bone development and eicosanoid production. Aquaculture 296:299–308
Bidwell DA, Howell WH (2001) The effect of temperature on first feeding, growth, and survival of larval witch flounder Glyptocephalus cynoglossus. J World Aquacult Soc 32:373–384
Boglione C, Gagliardi F, Scardi M, Cataudella S (2001) Skeletal descriptors and quality assessment in larvae and post-larvae of wild-caught and hatchery-reared gilthead sea bream (Sparus aurata L. 1758). Aquaculture 192:1–22
Boglione C, Gisbert E, Gavaia P, Witten PE, Moren M, Fontagne S, Koumoundouros G (2013) Skeletal anomalies in reared European fish larvae and juveniles. Part 2: Main typologies, occurrences and causative factors. Rev Aquac 5:S121–S167
Bolla S, Holmefjord I (1988) Effect of temperature on development of Atlantic halibut. Aquaculture 74:355–358
Buckley LJ, Caldarone EM, Ong TL (1999) RNA-DNA ratio and other nucleic acid based indicators for growth and condition of marine fishes. Hydrobiologia 401:265–277
Bustos CA, Landaeta MF, Bay-Schmith E, Lewis R, Moraga X (2007) Effects of temperature and lipid droplet adherence on mortality of hatchery-reared southern hake Merluccius australis larvae. Aquaculture 270:535–540
Cahu C, Zambonino Infante J, Takeuchi T (2003a) Nutritional components affecting skeletal development in fish larvae. Aquaculture 227:245–258
Cahu CL, Infante JLZ, Barbosa V (2003b) Effect of dietary phospholipid level and phospholipid: neutral lipid value on the development of sea bass (Dicentrarchus labrax) larvae fed a compound diet. Br J Nutr 90:21–28
Castro J, Pino A, Hermida M, Bouza C, Chavarrias D, Merino P, Sanchez L, Martinez P (2007) A microsatellite marker tool for parentage assessment in gilthead sea bream (Sparus aurata). Aquaculture 272:S210–S216
Chatain B (1994) Abnormal swimbladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus). Aquaculture 119:371–379
Choa BY, Carter CG, Battaglene SC (2010) Effects of temperature regime on growth and development of post-larval striped trumpeter (Latris lineata). Aquaculture 305:95–101
Cobcroft JM, Battaglene SC (2009) Jaw malformation in striped trumpeter Latris lineata larvae linked to walling behavior and tank color. Aquaculture 289:274–282
Cobcroft J, Battaglene S (2013) Skeletal malformations in Australian marine finfish hatcheries. Aquaculture 396–399:51–58
Cobcroft JM, Pankhurst PM, Sadler J, Hart PR (2001) Jaw development and malformation in cultured striped trumpeter Latris lineata. Aquaculture 199:267–282
Copeman LA, Parrish CC, Brown JA, Harel M (2002) Effects of docosahexaenoic, eicosapentaenoic, and arachidonic acids on the early growth, survival, lipid composition and pigmentation of yellowtail flounder (Limanda ferruginea): a live food enrichment experiment. Aquaculture 210:285–304
Daoulas C, Economou AN, Bantavas I (1991) Osteological abnormalities in laboratory reared sea-bass (Dicentrarchus labrax) fingerlings. Aquaculture 97:169–180
Dionisio G, Campos C, Valente LMP, Conceicao LEC, Cancela ML, Gavaia PJ (2012) Effect of egg incubation temperature on the occurrence of skeletal deformities in Solea senegalensis. J Appl Ichthyol 28:471–476
Divanach P, Papandroulakis N, Anastasiadis P, Koumoundouros G, Kentouri M (1997) Effect of water currents during post larval and nursery phase on the development of skeletal deformities in sea bass (Dicentrarchus labrax L.) with functional swim bladder. Aquaculture 156:145–155
Dou SZ, Masuda R, Tanaka M, Tsukamoto K (2005) Effects of temperature and delayed initial feeding on the survival and growth of Japanese flounder larvae. J Fish Biol 66:362–377
Faulk CK, Holt GJ (2003) Lipid nutrition and feeding of Cobia Rachycentron canadum larvae. J World Aquacult Soc 34:368–378
Faulk CK, Holt GJ, Davis DA (2005) Evaluation of fatty acid enrichment of live food for yellowtail snapper Ocyurus chrysurus larvae. J World Aquacult Soc 36:271–281
Ferguson MM, Danzmann RG (1998) Role of genetic markers in fisheries and aquaculture: useful tools or stamp collecting? Can J Fish Aquat Sci 55. https://doi.org/10.1139/f98-096
Fernández I, Hontoria F, Ortiz-Delgado JB, Kotzamanis Y, Estévez A, Zambonino-Infante JL, Gisbert E (2008) Larval performance and skeletal deformities in farmed gilthead sea bream (Sparus aurata) fed with graded levels of Vitamin A enriched rotifers (Brachionus plicatilis). Aquaculture 283:102–115
Fielder DS, Bardsley WJ, Allan GL, Pankhurst PM (2005) The effects of salinity and temperature on growth and survival of Australian snapper, Pagrus auratus larvae. Aquaculture 250:201–214
Furuita H, Takeuchi T, Watanabe T, Fujimoto H, Sekiya S, Imaizumi K (1996) Requirements of larval yellowtail for eicosapentaenoic acid, docosahexaenoic acid, and n-3 highly unsaturated fatty acid. Fish Sci 62:372–379
Gardeur JN, Mathis N, Kobilinsky A, Brun-Bellut J (2007) Simultaneous effects of nutritional and environmental factors on growth and flesh quality of Perca fluviatilis using a fractional factorial design study. Aquaculture 273:50–63
Georgakopoulou E, Katharios P, Divanach P, Koumoundouros G (2010) Effect of temperature on the development of skeletal deformities in Gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture 308:13–19
Goolish EM, Barron MG, Adelman IR (1984) Thermoacclimatory and response of nucleic acid and protein content of carp muscle tissue: influence of growth rate and relationship to glycine uptake by scales. Can J Zool 62:2167–2170
Haga Y, Takeuchi T, Seikai T (2002) Influence of all-trans retinoic acid on pigmentation and skeletal formation in larval Japanese flounder. Fish Sci 68:560–570
Haga Y, Du SJ, Satoh S, Kotani T, Fushimi H, Takeuchi T (2011) Analysis of the mechanism of skeletal deformity in fish larvae using a vitamin A-induced bone deformity model. Aquaculture 315:26–33
Hamre K, Opstad I, Espe M, Solbakken J, Hemre G-I, Pittman K (2002) Nutrient composition and metamorphosis success of Atlantic halibut (Hippoglossus hippoglossus, L.) larvae fed natural zooplankton or Artemia. Aquac Nutr 8:139–148
Harel M, Koven W, Lein I, Bar Y, Behrens P, Stubblefield J, Zohar Y, Place A (2002) Advanced DHA, EPA, and ArA enrichment materials for marine aquaculture using single cell heterotrophs. Aquaculture 213:347–362
Hattori M, Sawada Y, Kurata M, Yamamoto S, Kato K, Kumai H (2004) Oxygen deficiency during somitogenesis causes centrum defects in red sea bream, Pagrus major (Temminck et Schlegel). Aquac Res 35:850–858
Islam MS, Tanaka M (2005) Nutritional condition, starvation status and growth of early juvenile Japanese sea bass (Lateolabrax japonicus) related to prey distribution and feeding in the nursery ground. J Exp Mar Biol Ecol 323:172–183
Izquierdo MS, Arakawa T, Takeuchi T, Haroun R, Watanabe T (1992) Effect of n-3 HUFA levels in Artemia on growth of larval Japanese flounder (Paralichthys olivaceus). Aquaculture 105:73–82
Izquierdo MS, Socorro J, Arantzamendi L, Hernandez-Cruz CM (2000) Recent advances in lipid nutrition in fish larvae. Fish Physiol Biochem 22:97–107
Izquierdo MS, Socorro J, Roo J (2010) Studies on the appearance of skeletal anomalies in red porgy: effect of culture intensiveness, feeding habits and nutritional quality of live preys. J Appl Ichthyol 26:320–326
Jobling M (1997) Temperature and growth: modulation of growth rate via temperature change. In: Wood CM, McDonald DG (eds) Global warming: implications for freshwater and marine fish. Cambridge University Press, Cambridge, pp 225–253
Kamler E (1992) Early life history of fish: an energetics approach. Chapman and Hall, London
Keckeis H, Kamler E, Bauer-Nemeschkal E, Schneeweiss K (2001) Survival, development and food energy partitioning of nase larvae and early juveniles at different temperatures. J Fish Biol 59:45–61
Kestemont P, Baras E (2001) Environmental factors and feed intake: mechanisms and interactions. In: Houlihan D, Boujard T, Jobling M (eds) Food intake in fish. Blackwell Science, Cornwall, pp 131–156
Kihara M, Ogata S, Kawano N, Kubota I, Yamaguchi R (2002) Lordosis induction in juvenile red sea bream, Pagrus major, by high swimming activity. Aquaculture 212:149–158
Kjørsvik E, Olsen C, Wold P-A, Hoehne-Reitan K, Cahu CL, Rainuzzo J, Olsen AI, Øie G, Olsen Y (2009) Comparison of dietary phospholipids and neutral lipids on skeletal development and fatty acid composition in Atlantic cod (Gadus morhua). Aquaculture 294:246–255
Klimogianni A, Koumoundouros G, Kaspiris P, Kentouri M (2004) Effect of temperature on the egg and yolk-sac larval development of common pandora, Pagellus erythrinus. Mar Biol 145:1015–1022
Kokkinos PP, Shaye R, Alam BS, Alam SQ (1993) Dietary lipids, prostaglandin E2 levels, and tooth movement in alveolar none of rats. Calcif Tissue Int 53:333–337
Koumoundouros G, Divanach P, Kentouri M (1999) Osteological development of the vertebral column and of the caudal complex in Dentex dentex. J Fish Biol 54:424–436
Koumoundouros G, Divanach P, Kentouri M (2001) The effect of rearing conditions on development of saddleback syndrome and caudal fin deformities in Dentex dentex (L.). Aquaculture 200:285–304
Koumoundourous G, Oran G, Divanach P, Stefanakis S, Kentouri M (1997) The opercular complex deformity in intensive gilthead sea bream Sparus aurata L. larviculture. Moment of apparition and description. Aquaculture 149:215–226
Koven WM, Tandler A, Sklan D, Kissil GW (1993) The association of eicosapentaenoic and docosahexaenoic acids in the main phospholipids of different-age Sparus aurata larvae with growth. Aquaculture 116:71–82
Kranenbarg S, Waarsing JH, Muller M, Weinans H, van Leeuwen JL (2005) Lordotic vertebrae in sea bass (Dicentrarchus labrax L.) are adapted to increased loads. J Biomech 38(6):1239–1246
Kusuda R, Sugiyama A (1981) Studies on the characters of Staphylococcus epidermidis isolated from diseased fishes, 1: On the morphological, biological and biochemical properties. Fish Pathol 16:15–24
Lein I, Holmefjord I, Rye M (1997) Effects of temperature on yolk sac larvae of Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 157:123–135
Liang Q, Xie Y, Fang Z (2012) Individual and joint toxicity of Zn2+ and Cd2+ during the early embryonic development of zebrafish (Danio rerio). J Fish Sci China 19:283–292
Liu D, Veit HP, Denbow DM (2004) Effects of long-term dietary lipids on mature bone mineral content, collagen, crosslinks, and prostaglandin E2 production in Japanese quail. Poult Sci 83:1876–1883
Liu Z, Zhang S, Yang J (2012) Toxic effects of chlorobenzene on embryonic development and larva of zebrafish. Environ Sci Technol 35:25–28
Ludwig GM, Lochmann SE (2009) Effect of temperature on larval sunshine bass growth and survival to the fingerling stage. N Am J Aqualcult 71:260–266
Ma Z (2014) Food ingestion, prey selectivity, feeding incidence, and performance of yellowtail kingfish Seriola lalandi larvae under constant and varying temperatures. Aquac Int 22:1317–1330
Ma Z, Qin JG (2014) Replacement of fresh algae with commercial formulas to enrich rotifers in larval rearing of yellowtail kingfish Seriola lalandi (Valenciennes, 1833). Aquac Res 45:949–960
Ma Z, Qin JG, Nie Z (2012) Morphological changes of marine fish larvae and their nutrition need. In: Pourali K, Raad VN (eds) Larvae: morphology, biology and life cycle. Nova Science Publishers, Inc., New York, pp 1–20
Ma Z, Guo H, Zhang D, Hu CQ, Jiang S (2014a) Food ingestion, consumption, and selectivity of pompano, Trachinotus ovatus (Linnaeus 1758) under different rotifer densities. Aquac Res. https://doi.org/10.1111/are.12413
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Qin JG, Zhang D (2014b) Ontogenetic development of digestive functionality in golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem 40:1157–1167
Ma Z, Zheng P, Guo H, Zhang N, Jiang S, Zhang D, Qin JG (2014c) Jaw malfromation of hatchery reared golden pompano Trachinotus ovatus (Linnaeus 1758) larvae. Aquac Res. https://doi.org/10.1111/are.12569
Ma Z, Zheng P, Guo H, Zhang N, Wang L, Jiang S, Qin JG, Zhang D (2014d) Effect of weaning time on the performance of Trachinotus ovatus (Linnaeus 1758) larvae. Aquac Nutr 21:670–678
Ma Z, Zheng P, He D, Jiang S, Qin JG (2016) Effect of feeding Artemia nauplii enriched with different enhancement products on larval performance of golden pompano Trachinotus ovatus (Linnaeus, 1758). Indian J Fish 63(2):62–69
Martell DJ, Kieffer JD, Trippel EA (2005) Effect of temperature during early life history on embryonic and larval development and growth in haddock. J Fish Biol 66:1558–1575
Martinez-Palacios CA, Chavez-Sanchez MC, Ross LG (1996) The effects of water temperature on food intake, growth and body composition of Cichlasoma urophthalmus (Günther) juveniles. Aquac Res 27:455–461
Mathers EM, Houlihan DF, Cunningham MJ (1992) Nucleic acid concentration and enzyme activities as correlates of growth rate of the saithe Pollachius virens: growth rate estimates of open-sea fish. Mar Biol 112:363–369
May RC (1974) Larval mortality in marine fishes and the critical period concept. In: Blaxter JHS (ed) The early life history of fish (Conference: International symposium on the early life history of fish). Springer, Berlin, pp 3–19
McEvoy LA, Naess T, Bell JG, Lie O (1998) Lipid and fatty acid composition of normal and malpigmented Atlantic halibut (Hippoglossus hippoglossus) fed enriched Artemia: a comparison with fry fed wild copepods. Aquaculture 163:237–250
McGurk MD (1984) Effects of delayed feeding and temperature on the age of irreversible starvation and on the rates of growth and mortality of Pacific herring larvae. Mar Biol 84:13–26
Morais S, Conceicao LEC, Ronnestad I, Koven W, Cahu C, Infante JLZ, Dinis MT (2007) Dietary neutral lipid level and source in marine fish larvae: effects on digestive physiology and food intake. Aquaculture 268:106–122
Negm RK, Cobcroft JM, Brown MR, Nowak BF, Battaglene SC (2013) The effects of dietary vitamin A in rotifers on the performance and skeletal abnormality of striped trumpeter Latris lineata larvae and post larvae. Aquaculture 404–405:105–115
Okamoto T, Kurokawa T, Gen K, Murashita K, Nomura K, Kim S-K, Matsubara H, Ohta H, Tanaka H (2009) Influence of salinity on morphological deformities in cultured larvae of Japanese eel, Anguilla japonica, at completion of yolk resorption. Aquaculture 293:113–118
Olsen AI, Attramadal Y, Reitan KI, Olsen Y (2000) Food selection and digestion characteristics of Atlantic halibut (Hippoglossus hippoglossus) larvae fed cultivated prey organisms. Aquaculture 181:293–310
Ørnsrud R, Gil L, Waagbø R (2004) Teratogenicity of elevated egg incubation temperature and egg vitamin A status in Atlantic salmon, Salmo salar L. J Fish Dis 27:213–223
Otterlei E, Nyhammer G, Folkvord A, Stefansson SO (1999) Temperature- and size-dependent growth of larval and early juvenile Atlantic cod (Gadus morhua): a comparative study of Norwegian coastal cod and Northeast Arctic cod. Canadian J Fish Aquat Sci 56:2099–2111
Owen MG, Eynon B, Woodgate S, Davies SJ, Fox S (2012) Increased water current induces micro-architectural changes to the vertebral bone of juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 344–349:141–146
Palmtag MR, Faulk CK, Holt GJ (2006) Highly unsaturated fatty cid composition of rotifers (Brachionus plicatilis) and Artemia fed various enrichments. J World Aquacult Soc 37:126–131
Pittman K, Skiftesvik AB, Harboe T (1989) Effect of temperature on growth rates and organogenesis in the larvae of halibut (Hippoglossus hippoglossus L.). Rapports et Proces-verbaus du Conseil international pour I’Exploration de la Mer 191:421–430
Planas M, Cunha I (1999) Larviculture of marine fish: problems and perspectives. Aquaculture 177:171–190
Rezek TC, Watanabe WO, Harel M, Seaton PJ (2010) Effects of dietary docosahexaenoic acid (22:6n-3) and arachidonic acid (20:4n-6) on the growth, survival, stress resistance and fatty acid composition in black sea bass Centropristis striata (Linnaeus 1758) larvae. Aquac Res 41:1302–1314
Riley KL, Weirich CR, Cerino D (2009) Development and growth of hatchery-reared larval Florida pompano (Trachinotus carolinus). Fish Bull 107:318–328
Rodriguez C, Perez JA, Badia P, Izquierdo MS, Fernandez-Palacios H, Lorenzo-Hernandez A (1998) The n-3 highly unsaturated fatty acids requirements of gilthead seabream (Sparus aurata L.) larvae when using an appropriate DHA/EPA ratio in the diet. Aquaculture 169:9–23
Rombough PJ (1996) The effects of temperature on embryonic and larval development. In: Wood CM, McDonald DG (eds) Society for Experimental Biology seminar series 61: Global warming implications for freshwater and marine fish. Cambridge University Press, Cambridge, pp 177–223
Rombough PJ (1997) The effects of temperature on embryonic and larval development. In: Wood CM, McDonald DG (eds) Global warming. Implications for freshwater and marine fish. Cambridge University Press, Cambridge, pp 177–223
Salhi M, Izquierdo MS, Hernandez-Cruz CM, Socorro J, Fernandez-Palacios H (1997) The improved incorporation of polyunsaturated fatty acids and changes in liver structure in larval gilthead seabream fed on microdiets. J Fish Biol 51:869–879
Salhi M, Hernández-Cruz CM, Bessonart M, Izquierdo MS, Fernández-Palacios H (1999) Effect of different dietary polar lipid levels and different n-3 HUFA content in polar lipids on gut and liver histological structure of gilthead seabream (Sparus aurata) larvae. Aquaculture 179:253–263
Sandel E, Nixon O, Lutzky S, Ginsbourg B, Tandler A, Uni Z, Koven W (2010) The effect of dietary phosphatidylcholine/phosphatidylinositol ratio on malformation in larvae and juvenile gilthead sea bream (Sparus aurata). Aquaculture 304:42–48
Sargent JR, McEvoy LA, Bell JG (1997) Requirements, presentation and sources of polyunsaturated fatty acids in marine fish larval feeds. Aquaculture 155:117–127
Sargent J, Bell G, McEvoy L, Tocher D, Estevez A (1999a) Recent developments in the essential fatty acid nutrition of fish. Aquaculture 177:191–199
Sargent J, McEvoy L, Estevez A, Bell G, Bell M, Henderson J, Tocher D (1999b) Lipid nutrition of marine fish during early development: current status and future directions. Aquaculture 179:217–229
Seikai T, Tanangonan NJB, Tanaka M (1980) Temperature influence on larval growth and metamorphosis of the Japanese flounder Paralichthys olivaceus in the laboratory. Bull Jpn Soc Sci Fish 52:407–424
Seikai T, Tanangonan JB, Tanaka M (1986) Temperature influence on larval growth and metamorphosis of the Japanese flounder Paralichthys olivaceus in laboratory. Bull Jpn Soc Sci Fish 52:977–982
Sfakianakis DG, Koumoundouros G, Divanach P, Kentouri M (2004) Osteological development of the vertebral column and of the fins in Pagellus erythrinus (L. 1758), temperature effect on the developmental plasticity and morpho-anatomical abnormalities. Aquaculture 232:407–424
Sfakianakis DG, Georgakopoulou E, Papadakis IE, Divanach P, Kentouri M, Koumoundouros G (2006) Environmental determinants of haemal lordosis in European sea bass, Dicentrarchus labrax (Linnaeus, 1758). Aquaculture 254:54–64
Takeuchi T (1997) Essential fatty acid requirements of aquatic animals with emphasis on fish larvae and fingerlings. Rev Fish Sci 5:1–25
Takeuchi Y, Masuda R, Ishizaki Y, Watanabe T, Kanematsu M, Imaizumi K, Tsukamoto K (1996) Determination of the requirement of larval striped jack for eicosapentaenoic acid and docosahexaenoic acid using eniched Artemia nauplii. Fish Sci 62:760–765
Takeuchi T, Dedi J, Haga Y, Seikai T, Watanabe T (1998) Effect of vitamin A compounds on bone deformity in larval Japanese flounder (Paralichthys olivaceus). Aquaculture 169:155–165
Tanaka Y, Satoh K, Yamada H, Takebe T, Nikaido H, Shiozawa S (2008) Assessment of the nutritional status of field-caught larval Pacific bluefin tuna by RNA/DNA ratio based on a starvation experiment of hatchery-reared fish. J Exp Mar Biol Ecol 354:56–64
Tocher DR, Mourente G, Sargent JR (1997) The use of silages prepared from fish neural tissues as enrichers for rotifers (Brachionus plicatilis) and Artemia in the nutrition of larval marine fish. Aquaculture 148:213–231
von Westernhagen H (1974) Incubation of garpike eggs (Belone belone Linne) under controlled temperature and salinity conditions. J Mar Biol Assoc UK 55:945–957
Von Westernhagen H (1988) Sublethal effects of pollutants on fish eggs and larvae. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic, San Diego
Wang L, Tsai C (2000) Effects of temperature on the deformity and sex differentiation of tilapia, Oreochromis mossambicus. J Exp Zool 286:534–537
Watanabe T (1993) Importance of docosahexaenoic acid in marine larval fish. J World Aquacult Soc 24:152–161
Watkins BA, Shen CL, Memurtry JP, Xu H, Bain SD (1997) Dietary lipids modulate bone prostaglandin E2 production, insulin-like growth factor-I concentration and formation rate in chicks. J Nutr 127:1084–1091
Wen W, Huang X, Chen Q, Feng L, Wei L (2013) Temperature effects on early development and biochemical dynamics of a marine fish, Inimicus japonicus. J Exp Mar Biol Ecol 442:22–29
Yang Q, Ma Z, Zheng P, Jiang S, Qin JG, Zhang Q (2016) Effect of temperature on growth, survival and occurrence of skeletal deformity in the golden pompano Trachinotus ovatus larvae. Indian J Fish 63(1):74–82
Zehra S, Khan MA (2013) Dietary lysine requirement of fingerling Catla catla (Hamilton) based on growth, protein deposition, lysine retention efficiency, RNA/DNA ratio and carcass composition. Fish Physiol Biochem 39:503–512
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 China Agriculture Press
About this chapter
Cite this chapter
Yang, Q., Yu, G., Qin, J.G., Ma, Z. (2022). Nutrition and Temperature Regulate Rearing Performance of Golden Pompano Trachinotus ovatus Larvae. In: Ma, Z., Yu, G., Qin, J.G. (eds) Ontogenetic development of pompano Trachinotus ovatus. Springer, Singapore. https://doi.org/10.1007/978-981-19-1712-7_5
Download citation
DOI: https://doi.org/10.1007/978-981-19-1712-7_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1711-0
Online ISBN: 978-981-19-1712-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)