Abstract
High altitude sickness is associated with increased levels of reactive oxygen species (ROS) that leads to oxidative stress in cells. At elevated mountains, hypoxia induces several signaling pathways that contribute to imbalance in cellular homeostasis and oxidative stress. This can further lead to altered redox balance and accumulation of free radicals resulting in numerous pathophysiological disorders including pulmonary asthma, cardiovascular and metabolic disorders. Balanced ratio of pro- and antioxidants is essential for healthy life. Thus, antioxidant supplements are usually given at high altitudes to maintain cellular homeostasis. Use of antioxidant supplements can strengthen the surpassed levels of ROS in respond to oxidative stress and the supply of antioxidants is enhanced by using nanotechnology. The recent advancement in nanotechnology has ensued sustained delivery of drugs and more efficient results in curing various metabolic disorders. Emergence of nanotechnology has provided researchers with enhanced solubility, bioavailability, stability with less toxicity and conventional side effects. This chapter highlights the relation amid high altitudes, increased oxidative stress, and how antioxidant supplements and nanomaterial conjugated antioxidants provide shield to cells and ameliorates the oxidative damage in high altitude related sickness.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- High altitude sickness
- Reactive oxygen species
- Antioxidants
- Nano-medicine
- Cardiovascular
- Pulmonary disorders
13.1 Introduction
High range of foothills has always been a good source of captivation and motivation for populaces. The tranquility and quietness of mountains magnetize individuals to explore the environment and trap people to inhabit the areas of even higher spreads of mountains. Investigators have proposed the additional antioxidant supplements for the eminent levels of oxidative stress which could be particularly advantageous in this situation. There is a very précised research done, which suggests that antioxidant supply is beneficial for acute mountain sickness, reducing muscle soreness and improving cell membrane fluidity. The antioxidant supplementation challenge at altitude deceits in defining the perfect amalgamation and concentration of antioxidant nutrients, which controls excess oxidative stress allowing adaptations to hypoxia. Vitamin C and E, β-carotene, selenium are rich antioxidants which have proven beneficiary effects in AMS, muscle soreness, and oxygenation of peripheral tissues. A balanced antioxidants supply can counteract this oxidative stress maintaining ROS levels in the body. The antioxidant supplementation system is enhanced via nanotechnology.
Nanotechnology comprehends advanced technology combined with biological processes comprising manufacturing of new drugs and delivery, reformative medicine, and a defensible environment (Bowman and Hodge 2007; Roco 2003; Sahoo et al. 2007). However, the originality behind the term is, an emerging family of various technologies involving a broad range of nanoscience and nanotechnology, enables the manipulation of matter at atomic levels (Bowman and Hodge 2007; Ramsden 2016). The National Nanotechnology Initiative (NNI) marked the Global emergence of nanotechnology in January 2000 (Roco 2003). This technology reassures re-construction of the world made by human itself by an expansion of uprising products from machineries to medicines (Sachan and Gupta 2015). Hence aid of nanotechnology in high altitude sickness is the major advancement in the treatment of the related disorders. The following chapter elaborates about the benefits of antioxidant therapy and nano-medicine in acute mountain sickness.
13.2 From Physiological to Molecular: Change of Perspectives
Reviewing the existence of different molecular events, it is clearly very evident that all these patho-physiologies might be interconnected to each other. Individuals with acute high altitude sicknesses express the presence of fluids rushed into the extracellular spaces, in brain or in lungs. There lies molecular dynamics in the existence of hypobaric hypoxia and related disorders. These molecular factors have an imperative part in regulating the production of ROS and also they have a key role in release of antioxidants to suppress the possessions of generated reactive oxygen species.
13.3 Stabilization of HIF in Signaling of ROS During Hypoxic Conditions
HIF-1 substitutes a heterodimer, HIF-1α and HIF-1β oxygen and non-oxygenic regulated. The oxygen dependent HIF complex has prolyl hydroxylases family (PHDs) like PHD1, PHD2, and PHD3. In aerobic condition these PHDs degrade α subunit of HIF, whereas in oxygen deficient condition it results in dimerization and stabilization of HIF. At higher altitudes due to lessened PHD2 activity HIF-1α increases which represents HIFs as the major player of the responses to cells due to limited O2 supply. Though all the forms of PHD play an essential role in HIF regulation, but it is found that suppression of PHD2 increases the level of HIF where HIF-2α level can be enhanced by the suppression of PHD3 (Appelhoff et al. 2004). To stabilize HIF-1α shushing of PHD2 with siRNAs is necessary in case of normoxic human cells, while no effect on the stability of HIF-1α was observed in epistating the role of PHD1, 3 in both normal and hypoxic conditions (Berra et al. 2003). Hence marking PHD2 as potential oxygen sensor. As already discussed mitochondria are the major site for ROS production which further increases during the hypoxic conditions leading to the redox changes resulting in participation in other transcriptional responses. For the binding activity of HIF-1α DNA, mitochondrial ROS is required (Chandel et al. 1998), specifically complex III stabilizes both the subunits of HIF linking ROS and HIF stabilization.
13.4 Association of PGC-1 α and Sirtuins with HIF-1α in Signaling of ROS
Hypoxia causes the self-destruction of mitochondrial cells via the process of autophagy, reducing ROS and providing a sufficient amount of oxygen to the remaining mitochondrial cells. In muscle cells there is a key relation between the activity of HIF-1α and PGC-1α. It is observed that increase in PGC-1α levels rises up the mitochondrial biogenesis, leading to increased oxygen consumption and HIF-1α stabilization. Sirtuins (SIRT) are dependent on ratio of NAD to NADH, where increased NAD levels activate sirtuins and elevated NADH levels overpower SIRT activity. SIRT1 downregulates the activity of HIF-1α by its deacetylation during hypoxia. Another classes of Sirtuin (SIRT1, SIRT6) downregulates HIF-1 alpha mediated transcription by chromatin binding on HRE (Zhong et al. 2010). Similarly, overexpression of SIRT3 downregulates HIF stabilization and ROS production. Kinases, Sirtuins, and PGC-1alpha are some factors that are essentially regulated by the ROS generated by physical exercises for mitochondrial biogenesis. The modulation occurs by changes in the redox state of the body. PGC-1α reduces ROS production either by activation of antioxidant system or by elevating the number of mitochondria.
13.5 Nuclear Erythroid-Related Factor 2
Nuclear erythroid-related factor 2 is a transcriptional factor which legalizes antioxidant, anti-inflammatory, and observes the redox homeostasis. KEAP1 (Kelch-like erythroid cell-derived protein1) is released via Nrf2 during stress conditions (Oh and Jun 2017; Huang et al. 2002). Once released it is transported to the nucleus, activating genes conferring resistance to various oxidative stress related neurodegenerative molecules. It is well established that fluctuations in cellular oxygen due to hypoxia generated oxidative stress affects HIF-1α and Nrf2. Adenocarcinoma cell line (A549) of lungs has elevated NOX1 levels which are required for amplified ROS accumulation during sporadic hypoxia inducing HIF-1α and Nrf2. Inhibition of endogenous NOX1 hinders the expression of Nrf2 and Trx1, whereas its overexpression causes an upsurge in Nrf2 and Trx1. The twin upregulation of these factors upsurges HIF-1α signaling. Hence, Trx1tends to appear as a link between Nrf2 and HIF-1α. Nrf2 is a transcriptional factor which controls the genes of antioxidant and detoxification system (Malec et al. 2010). There has been a reduction in mitochondrial components among the cells surviving H2O2 treatment which was prohibited by overexpression of Nrf2 preventing mitochondrial-related morphological changes.
13.6 Oxidative Stress Markers at High Altitude
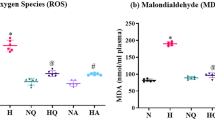
Increased oxidative stress damages the cellular molecules imposing toxicological implications. To mark the existence of ROS produced due to oxidative stress different indicators are studied. These are called “biomarkers,” measuring the normal and pathogenic processes (Biomarkers Definitions Working Group 2001). Injuries to aerobic cell due to ROS production majorly affect the membrane oxidation called as lipid peroxidation (Debevec et al. 2017). This membrane damage causes elevation in exhalation of pentane gas. During hypoxia, polyunsaturated fatty acids (PUFAs) in membranes get highly affected, which alters the normal functions of cells (Magalhães et al. 2005). Another cellular damage is the changes in fluidity of red cell membrane due to vitamin E/depletion in the same (Simon-Schnass 1994).
Training at high altitude influences the antioxidant defense system (SOD and CAT) of RBCs (Güzel et al. 2000). Along with this the increased thiobarbituric acid reactive substances (TBARS) in blood plasma due to lipid peroxidation are linked with the injury in muscle cell membranes (Wozniak et al. 2001; Ramazan et al. 2000; Bernabucci et al. 2002; Vani et al. 2010). Intermittent hypoxia activates HIF with augmented levels of endothelin causing vasoconstriction, inflammatory cytokines, and irregular lipid metabolism (Friedman et al. 2014; Gangwar et al. 2020). There is no inconsistency about the reduction of glutathione reductase and increased glutathione oxidase during hypoxic condition. A potent indicator for oxidative stress is glutathione sulfide (GSSG) as it increases at high altitude (Magalhaes et al. 2004). Also reduced glutathione peroxidase, cytochrome c oxidase, and superoxide dismutase in the lungs appear to be wise indicators for oxidative stress (Lemoine et al. 2018).
13.7 Physiological Consequences of Oxide Generated Stress
Formation of free radical is an important characteristic involved in the complicated patho-physiology of high altitude ailment (Purkayastha et al. 1999). It is reported that exercise before few hours of being exposed to high range marks the increase in sincerity of altitude sickness (Roach et al. 2000). Moller et al. (2001a, b) deduced that workout at high altitude proliferates the breaking of DNA strands which results in hypoxia and depletion in an antioxidant system capability to endure the affront of oxidative stress. Apart from mountain sickness the increased oxidative stress can also be responsible for impairment in oxygen consumption, functioning of muscle, and ultimately contributing to chronic diseases. Figure 13.1 explains the physiology behind hypoxic hypobaric.
13.8 Antioxidant Therapy for High Altitude Sickness
Higher ROS levels are related with exercise at moderate levels, reducing antioxidant potentiality and increased oxidative stress. The overproduction of ROS, in antioxidant defense systems, damages lipids, proteins, and DNA impairing cell and function of immune system (Fig. 13.2). Fascinatingly, increase in oxidative stress is the consequence of normobaric hypoxia than hypobaric hypoxia. At moderate altitudes, studies have represented inflammation and disease in association with increased oxidative stress. Exogenous antioxidants counterbalance the free radicals, it is rational to theorize that supplementation of antioxidants would be a well-intentioned therapy to battle oxidative stress induced due to high altitude. Though early surveys have shown that supplement of antioxidant modulates the effects on oxidative stress and symptoms of altitude sickness. With the present crucial role of RONS in endurance training, hypoxia and activated antioxidant defenses together, there is no adequate confirmation which recommends that a single dose of antioxidant supplement is sufficient to reduce the oxidative stress generated. Together, there is no adequate confirmation which recommends that single dose of antioxidant supplement is appropriate to attenuate induced stress.
13.9 Effects on Antioxidant System at High Altitude
For normalizing the reactive oxygen/nitrate species effects, an enzymatic and non-enzymatic system has been established by aerobic cells. This system comprises MnSOD, CuSOD which converts superoxide to less empowering hydrogen peroxide species. Along with this glutathione peroxidase and catalase decompose hydrogen species to water. The other non-enzymatic system is complex and consists of non-enzymatic antioxidants. A study reported alternating disclosure to high range at 4000 m, which ensued decrease in protein content activity of mitochondria (Radak et al. 1994). Nakanishi et al. 1995 found that at high altitude the activity of glutathione peroxidase (GPX) decreases in liver signifying the sensitivity of liver to oxidative stress at higher ranges. In other study the action of GPX was compared in the blood serum of highlander’s native (Imai et al. 1995). This suggests that GPX activity strongly depends upon the state of thiol system. Glutamyl cycle continuously synthesizes most essential antioxidant, i.e. glutamyl cysteinyl glycine (Fig. 13.3). The level of reduced glutathione decreases as the altitude range increases, while the level of oxidized glutathione increases with increased altitude range (Ilavazhagan et al. 2001; Joanny et al. 2001). All these studies represent that there is a heavy decrease in the capacity of enzymatic systems at higher altitudes. Schmidt et al. (2002) applied an antioxidant combination for the reduction in oxidative stress due to altitude. This combination was found to be very active and reduced the damage caused by increased oxidative stress. Few rats were shortly exposed to an altitude range of 8000 m, later it was found that melatonin levels were increased in their blood serum (Kaur et al. 2002). This melatonin acts as an antioxidant, in which exposure after 4 days reported the decrease in mitochondrial number of pinealocytes which suggested that there is another source apart from pinealocytes which also produce melatonin. There are some pointers which suggest that antioxidant supplementation thwarts the oxidative damage due to high altitude to macromolecules.
13.10 Status of Antioxidant Defense System in Body
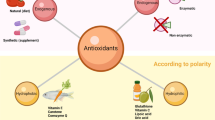
For the survival of all forms of life specifically aerobic in nature, detoxification of ROS is an essential factor to pay attention to. In order to enhance the deleterious effects produced by oxidant species, the human system is armed with collection of antioxidants (Table 13.1), which are characterized as enzymatic and non-enzymatic, supplied exogenously via food. These supplements actively produce free radical scavengers by contributing electrons to ROS (Kunwar and Priyadarsini 2011; Shinde et al. 2012; Birben et al. 2012).
The antioxidant system works differently in the body: (1) formation of ROS at minimum level, (2) scavenging of reactive species via catalytic molecule, (3) repair and removal of damaged molecules (Sies 1986). This system holds the capacity to develop antioxidants on adaptation to stress environment, in an appropriate concentration. Hence, it is essential for a chain reaction to occur completely in order to stabilize the generated radicals via stearic hindrance. This determines the importance of antioxidant’s efficacy. It is witnessed that antioxidant defense system deteriorates at high range, which can be conquered by supplying antioxidants as food add-ons to the body (Poljsak et al. 2013; Halliwell 2011).
13.11 Antioxidant Therapy: Prevention of High Altitude Sickness
Altered endovascular permeability due to excessive ROS generation results in the pathophysiological conditions as AMS, HAPE, and HACE hindered by the supplementation of antioxidants, respectively. These antioxidants could be descent oxygen or a combinatory therapy in severe illness. The extract of Ginkgo biloba consists of antioxidant properties and showed a protective effect on rats with condition of HACE. It was detected that subjects treated with this extract showed diminished MDA levels along with an increased SOD and GSH concentration (Botao et al. 2013). Patir et al. (2012) explained the part quercetin plays in reduction of hypoxia-induced cerebral edema (HACE) against rats. The development of AMS at an altitude of 5000 m is directly associated with increased serum hydro-peroxides level. Supplementation of antioxidants as vitamins majorly reduces the oxidative stress, enhances total GSH content during high altitude hypoxia (Magalhaes et al. 2004; Araneda et al. 2005). It was analyzed that supplementation of antioxidants tends to be a better alternative to acetazolamide, a pharmacological drug, which is used to inhibit symptoms of AMS protecting hypoxic tissues with no after effects (Gertsch et al. 2004). Hereafter, it can be said that there are few benefits of these non-pharmacological mediators, but with defined dosages only in order to battle these patho-physiologies at high altitude (Table 13.2).
13.12 Nano-Medicine in Acute Mountain Sickness
13.12.1 Beginning of Nanotechnology
Modern science of nanotechnology has been in use for centuries as the nano-materials were utilized to decorate the cathedral windows in medieval times. Not limiting the use of nano-formulations, Chinese used the nano-formulations of gold for introducing red color to ceramic porcelains, acting as an inorganic dye (Pokropivny et al. 2007). In ancient period a process known as Ayurvedic Bhasma was generally used for the preparation of active nanoparticles which involved the melting of metals and then refrigerating them in appropriate media as phyto-herbal juices for definite time. In order to transform these metals into biologically active nanoparticles the above process should be performed numerous times to obtain bhasma (incinerated metals) (Kumar Pal 2015; Sharma and Prajapati 2016). In 1974 Norio Taniguchi coined the term nanotechnology (Allhoff et al. 2010; Krukemeyer et al. 2015), at the University of Tokyo. According to him he described nanotechnology as a resource of yielding particles with accuracy and ultrafine dimensions (Allhoff et al. 2010) (Fig. 13.4). The term nanotechnology is a Greek derived word where “nano” means dwarf. The particle with this technology should have at least one dimension in nanometer (nm) range. Hence, nanotechnology is carved as “technology at nanoscale (1–100 nm),” involving the design and applied materials by regulating their structure and properties (Fakruddin et al. 2012; Ramsden 2016). This field of technology has given promising results in numerous sectors, from health care industry to microelectronics. Specifically in medication, it has shown groundbreaking possibilities in delivering drugs therapies in the areas of research and development (Jena et al. 2017; Roco 2003; Safari and Zarnegar 2014).
13.12.2 Nano-Formulations
For the application of nanotechnology there has been a consistent development among the formulations of different nano-platforms as nano-biomaterials of a specific surface properties, essential for the interaction of biological compounds and their substantial beneficial effects (Fig. 13.5) (Prasad et al. 2018). Natural and synthetic biomaterials interacting with biological systems have been extensively used in the field of pharmacology as bone grafts, drug transport, tissue engineering. However the advancement in expansion of novel technology has been accomplished by combining the aids of nanotechnology and nano-biomaterials (Lee and Kim 2014). These nano-biomaterials include nanoscaled materials used in the arena of biomedical as delivery of drugs, bio-imaging, tissue engineering, and biosensor (Ali et al. 2013; Sitharaman 2016; Shen 2006; Yang et al. 2011). Lately, the development of nano-formulations for medicinal drugs has fascinated the attention of several researchers, specifically for delivering drugs and to enhance properties of conventional site directed drugs (Jeevanandam et al. 2016). Dendrimers, nanoparticles synthesized of polymers, liposomes, and micelles are some of the common nano-formulations, attaining significance in the pharmaceutical business for improved drug delivery (Singh et al. 2016).
13.12.3 Hypoxic Nano-Formulations
Low oxygen availability with substantial effects on cells is a condition of hypoxia (Semenza 2015). The affected tissues exist with various conditions when in hypoxic situation (Airley et al. 2000), as disruptive sleep, cerebral disorders, and several cardiac disorders (Bhatia et al. 2017). Hypoxic conditions come with inflammatory responses as rheumatoid arthritis, bowel disease, and ischemic reperfusion injury (Airley et al. 2000). The condition of hypoxia (increased ROS levels) has become a threat to life. It can be fatal for both healthy individuals and individuals with cardiovascular, respiratory and hemolytic diseases (Sun et al. 2016). The application of nanotechnology is to develop nano-formulations to overpower the resistance of delivering conventional drugs and herbal formulations for various pathophysiological conditions including respiratory, cardiac myotrophy, malignancy, and high altitude induced conditions.
13.12.4 Hypobaric Hypoxia and Nano-Formulations
As discussed earlier, hypobaric hypoxia fails to adapt the exposure of high altitudes which in turn is allied with different physiological disorders as pulmonary edema (HAPE) and cardiac hypertrophy (HACE) (Wilkins et al. 2015). For the upgrading of treatment for the mountain illness few investigators have been working in the arena of nanotechnology for the development of nano-therapeutics against high altitude physiologies. Such therapeutic nano-formulations include nano-curcumin and nano-ceria.
13.12.5 Nano-Curcumin for Hypobaric Hypoxia
Though native curcumin has many pharmacological properties, but still there exhibits certain restrictions on its properties of being an effective pharmacological drug. To overcome these restrictions different nano-formulations have been developed, demonstrating permeability, protracted blood circulation, better constancy, and précised discharge of a dose at site to be targeted (Gera et al. 2017). There exist two specifics about both hypoxia and curcumin, i.e. cardiomyocyte hypertrophy induced by hypoxia and curcumin with antioxidant and anti-hypertrophic effects to combat the same. The pharmacological ability of curcumin is limited due to its low bioavailability. Nehra et al. (2015) conducted a study to analyze severity of nano-curcumin against hypertrophy and apoptosis induced by hypoxia and compared it to the naked curcumin targeting the same. Consequences of this research revealed that nano-curcumin expressively countered the hypoxia-induced hypertrophy and apoptosis via downregulating the activation of several factors essential for reducing the production of ROS. This study concluded that nano-curcumin can potentially cure cardiac pathologies induced via hypoxia by restoration of oxidative balance (Nehra et al. 2015). Moreover, improvement in the cardiac damage due to chronic hypobaric hypoxia can be done by using nano-curcumin as compared to nano-curcumin, Nehra et al. (2016b). High altitude acquaintance frequently leads to accretion of fluids in lungs, resulting in high altitude-induced pulmonary edema (HAPE) (Sagi et al. 2014). Thus, the above study underlined the defensive worth of nano-curcumin in lungs at high altitudes (Nehra et al. 2016a).
13.12.6 Nanoceria for Hypobaric Hypoxia
Nanoparticles customized of cerium oxide have a quenching effect against reactive oxygen species (ROS) both in vitro and in vivo as well. Although their efficiency in protecting lungs during oxidative stress due to hypobaric hypoxia was left undiscovered until 2013. So, using microemulsion method spherical nanoparticles (7–10 nm) were produced for lung protection during hypobaric hypoxia. Arya et al. (2014) found that nanoceria decreases ROS content by lowering the amount of cellular calcium. Impaired memory and cognitive dysfunction occur when an individual exposed to high altitude ranges develops reactive nitrogen and oxygen species in the cortex and hippocampus area of brain. Nanoceria coated with polyethylene glycol (PEG-CNPs) were proficiently localized in the brain of rodent resulting in reduced oxidative stress and related damage during. Consequently, demonstrating the promising act of nanoceria as therapeutic agent in metabolic diseases (Arya et al. 2016).
13.12.7 Nano-Formulations Forthcoming for Hypobaric Hypoxia
In patients with various metabolic diseases, hypoxia is commonly witnessed, promoting multiple organ failure frequently (Sun et al. 2016). The developed nano-therapeutics are appreciated in the ailment of patho-physiologies induced by high altitude. Attempts have already been made to stabilize HIF-1α, involved in the reaction to hypoxia virtually (Arachchige et al. 2015). Self-assembled hypoxia responsive nanoparticles (HR-NPs) encapsulating doxorubicin (DOX) were developed by Thambi et al. (2014). HR-NPs can successfully supply DOX into human carcinoma cell line under hypoxia. In vivo biodistribution study established that HR-NPs were gathered specifically at the site of hypoxic tumor cells. This study accentuated the possibility of HR-NPs as nano-carriers for targeted delivery in treatment of hypoxic diseases (Thambi et al. 2014). Some instances of drugs conjugated with nano-formulations are listed in Table 13.3, which tend to regulate and prevent high altitude hypoxic physiologies.
13.13 Conclusion
Increment in ROS production on exposure to high altitude disrupts the efficiency of antioxidant system, resulting in damaging macromolecules oxidatively. Supplementation of antioxidant supplementation has advantageous effects that can diminish the oxidative damage allied with high altitude. Surplus levels of ROS majorly affect cell to cell signaling upsetting overall physiology of individuals exposed to high ranges. Appropriate quantity of antioxidant supplementation is an important way to provide control on excessively produced ROS for one to adapt in hypoxic condition.
Nanoscience is an emerging technology with different applications of targeted delivery which is further utilised for the better treatment of human related disorders. Patho-physiologies related to high altitude are a menace to human physiology under hypoxic environment, and when in contact with UV. A few pharmacological intrusions are being utilized for the management of these physiologies under these harsh environmental conditions. It would be more profitable if nano-based products are utilized in this framework, because nano-formulations improve the pharmacokinetics, conserving value of allopathic and herbal drugs. This chapter presented readers, with the concepts of molecular mechanisms behind prevalence of high altitude sickness, antioxidant therapy for counteracting hypobaric hypoxia and nano-formulations enhancing the effects of antioxidants. This chapter is essential for improved conception of high altitude related patho-physiologies and the other tactics applied for their supervision.
Abbreviations
- AMS:
-
Acute mountain sickness
- CAT:
-
Catalase
- DOX:
-
Doxorubicin
- GSSG:
-
Glutathione disulfide
- HACE:
-
Hypoxia-induced cerebral edema
- HAPE:
-
Hypoxia-induced pulmonary edema
- HIF:
-
Hypoxia inducible factor
- HRE:
-
Hypoxia responsive elements
- HR-NPs:
-
Hypoxia responsive nanoparticles
- NOX:
-
Nitrogen oxides
- Nrf:
-
Nucleoid related factor
- PEG-CNPs:
-
Polyethylene glycol ceria nanoparticles
- PGC:
-
Pparg coactivator
- PHD:
-
Prolyl hydroxylases family
- PUFA:
-
Polyunsaturated fatty acid
- ROS/RNS:
-
Reactive oxygen species/reactive nitrogen species
- SOD:
-
Superoxide dismutase
- TBAR:
-
Thiobarbituric acid reactive substances
References
Airley RE, Monaghan JE, Stratford IJ (2000) Hypoxia and disease: opportunities for novel diagnostic and therapeutic prodrug strategies. Pharm J 264(7094):66
Ali SH, Almaatoq MM, Mohamed AS (2013) Classifications, surface characterization, and standardization of nanobiomaterials. Int J Eng Technol 2(3):187
Allhoff F, Lin P, Moore D (2010) What is nanotechnology and why does it matter? From science to ethics. Wiley-Blackwell, Chichester
Anselmo AC, Mitragotri S (2016) Nanoparticles in the clinic. Bioeng Transl Med 1(1):10–29
Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia inducible factor. J Biol Chem 279:38458–38465
Arachchige MC, Reshetnyak YK, Andreev OA (2015) Advanced targeted nanomedicine. J Biotechnol 202:88–97
Araneda OF, García C, Lagos N, Quiroga G, Cajigal J, Salazar MP, Behn C (2005) Lung oxidative stress as related to exercise and altitude. Lipid peroxidation evidence in exhaled breath condensate: a possible predictor of acute mountain sickness. Eur J Appl Physiol 95:383–390
Arya A, Sethy NK, Das M, Singh SK, Das A, Ujjain SK, Sharma RK, Sharma M, Bhargava K (2014) Cerium oxide nanoparticles prevent apoptosis in primary cortical culture by stabilizing mitochondrial membrane potential. Free Radic Res 48(7):784–793
Arya A, Gangwar A, Singh SK, Roy M, Das M, Sethy NK, Bhargava K (2016) Cerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK-PKC-CBP signaling cascade. Int J Nanomedicine 11:1159–1173
Bernabucci U, Ronchi B, Lacetera N, Nardone A (2002) Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J Dairy Sci 85:2173–2179
Berra E, Benizri E, Ginouvès A, Volmat V, Roux D, Pouysségur J (2003) HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1a in normoxia. EMBO J 22:4082–4090
Bhatia A, Shard P, Chopra D, Mishra T (2011) Chitosan nanoparticles as carriers of immuno-restoratory plant extract: synthesis, characterization, and immuno-restoratory efficacy. Int J Drug Deliv 3(2):381–385
Bhatia D, Ardekani MS, Shi Q, Movafagh S (2017) Hypoxia and its emerging therapeutics in neurodegenerative, inflammatory and renal diseases. In: Hypoxia and human diseases. InTech, Rijeka, Croatia
Bilati U, Allemann E, Doelker E (2005) Nanoprecipitation versus emulsion-based techniques for the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues. AAPS PharmSciTech 6(4):E594–E604
Biomarkers Definitions Working Group (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69(3):89–95. https://doi.org/10.1067/mcp.2001.113989
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Botao Y, Ma J, Xiao W, Xiang Q, Fan K, Hou J, Wu J, Jing W (2013) Protective effect of ginkgolide B on high altitude cerebral edema of rats. High Alt Med Biol 14:61–64
Bowman DM, Hodge GA (2007) A small matter of regulation: an international review of nanotechnology regulation. Columbia Sci Technol Law Rev 8(1):1–36
Bulbake U, Doppalapudi S, Kommineni N, Khan W (2017) Liposomal formulations in clinical use: an updated review. Pharmaceutics 9(2):12
Cabrales P, Han G, Roche C, Nacharaju P, Friedman AJ, Friedman JM (2010) Sustained release nitric oxide from long-lived circulating nanoparticles. Free Radic Biol Med 49(4):530–538
Chakraborty K, Shivakumar A, Ramachandran S (2016) Nanotechnology in herbal medicines: a review. Int J Herb Med 4(3):21–27
Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci 95:11715–11720
Debevec T, Millet GP, Pialoux V (2017) Hypoxia-induced oxidative stress modulation with physical activity. Front Physiol 8:84
Fakruddin M, Hossain Z, Afroz H (2012) Prospects and applications of nanobiotechnology: a medical perspective. J Nanobiotechnol 1(10):1–8
Friedman JK, Nitta CH, Henderson KM, Codianni SJ, Sanchez L, Ramiro-Diaz JM, Howard TA, Giermakowska W, Kanagy NL, Gonzalez Bosc LV (2014) Intermittent hypoxia-induced increases in reactive oxygen species activate NFATc3 increasing endothelin-1 vasoconstrictor reactivity. Vascul Pharmacol 60(1):17–24
Gangwar A, Paul S, Ahmad Y, Bhargava K (2020) Intermittent hypoxia modulates redox homeostasis, lipid metabolism associated inflammatory processes and redox post-translational modifications: benefits at high altitude. Sci Rep 10:7899
Gera M, Sharma N, Ghosh M, Lee SJ, Min T, Kwon T, Jeong DK (2017) Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget 8(39):66680–66698
Gertsch JH, Basnyat B, Johnson EW, Onopa J, Holck PS (2004) Randomised, double blind, placebo controlled comparison of Ginkgo biloba and acetazolamide for prevention of acute mountain sickness among Himalayan trekkers: the prevention of high altitude illness trial (PHAIT). BMJ 328:797
Güzel NA, Sayan H, Erbas D (2000) Effects of moderate altitude on exhaled nitric oxide, erythrocytes lipid peroxidation and superoxide dismutase levels. Jpn J Physiol 50:187–190
Halliwell B (2011) Free radicals and antioxidants-quo vadis? Trends Pharmacol Sci 32:125–130
Hu L, Xing Q, Meng J, Shang C (2010) Preparation and enhanced oral bioavailability of cryptotanshinone-loaded solid lipid nanoparticles. AAPS PharmSciTech 11(2):582–587
Huang HC, Nguyen T, Pickett CB (2002) Phosphorylation of Nrf2 at Ser40 by protein kinase C regulates antioxidant response element mediated transcription. J Biol Chem 277:42769–42742
Ilavazhagan G, Bansal A, Prasad D, Thomas P, Sharma SK, Kain AK, Kumar D, Selvamurthy W (2001) Effect of vitamin E supplementation on hypoxia-induced oxidative damage in male albino rats. Aviat Space Environ Med 72:899–903
Imai H, Kashiwazaki H, Suzuki T, Kabuto M, Himeno S, Watanabe C, Moji K, Kim SW, Rivera JO, Takemoto T (1995) Selenium levels and glutathione peroxidase activities in blood in an Andean high-altitude population. J Nutr Sci Vitaminol (Tokyo) 41:349–361
ISO (2008). ISO 6709:2008(en) preview. www.iso.org. Accessed 8 June 2016
Jeevanandam J, San Chan Y, Danquah MK (2016) Nanoformulations of drugs: recent developments, impact, and challenges. Biochimie 128(129):99–112
Jena M, Mishra S, Jena S, Mishra SS (2017) Nanotechnology future prospect in recent medicine: a review. Int J Basic Clin Pharmacol 2(4):353–359
Joanny P, Steinberg J, Robach P, Richalet JP, Gortan C, Gardette B, Jammes Y (2001) Operation Everest III: the effect of simulated sever hypobaric hypoxia on lipid peroxidation and antioxidant defense systems in human blood at rest and after maximal exercise. Resuscitation 49:307–314
Kaur C, Srinivasan KN, Singh J, Peng CM, Ling EA (2002) Plasma melatonin, pinealocyte morphology, and surface receptors/antigen expression on macrophages/microglia in the pineal gland following a high-altitude exposure. J Neurosci Res 67:533–543
Kimura S, Egashira K, Chen L, Nakano K, Iwata E, Miyagawa M, Tsujimoto H, Hara K, Morishita R, Sueishi K, Tominaga R (2009) Nanoparticle-mediated delivery of nuclear factor κB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension 53(5):877–883
Koryagin AS, Mochalova AE, Salomatina EV, Eshkova OY, Smirnova LA (2013) Adaptogenic effects of chitosan-gold nanocomposites under simulated hypoxic conditions. Inorg Mater Appl Res 4(2):127–130
Krukemeyer MG, Krenn V, Huebner F, Wagner W, Resch R (2015) History and possible uses of nanomedicine based on nanoparticles and nanotechnological progress. J Nanomed Nanotechnol 6(6):1–7
Kumar Pal S (2015) The Ayurvedic Bhasma: the ancient science of nanomedicine. Rec Pat Nanomed 5(1):12–18
Kunwar A, Priyadarsini KI (2011) Free radicals, oxidative stress and importance of antioxidants in human health. J Med Allied Sci 1:53–60
Lee H, Kim YH (2014) Nanobiomaterials for pharmaceutical and medical applications. Arch Pharm Res 37(1):1–3
Lemoine AJ, Revollo S, Villalpando G, Valverde I, Gonzales M, Laouafa S, Soliz J, Joseph V (2018) Divergent mitochondrial antioxidant activities and lung alveolar architecture in the lungs of rats and mice at high altitude. Front Physiol 9:311
Magalhaes J, Ascensao A, Viscor G, Soares J, Oliveira J, Marques F, Duarte J (2004) Oxidative stress in humans during and after 4 hours of hypoxia at a simulated altitude of 5500 m. Aviat Space Environ Med 75:16–22
Magalhães J, Ascensão A, Marques F, Soares JM, Ferreira R, Neuparth MJ, Duarte JA (2005) Effect of a high-altitude expedition to a Himalayan peak (Pumori, 7,161 m) on plasma and erythrocyte antioxidant profile. Eur J Appl Physiol 93:726–732
Malec V, Gottschald OR, Li S, Rose F, Seeger W, Hänze J (2010) HIF1 alpha signaling is augmented during intermittent hypoxia by induction of the Nrf2 pathway in NOX1-expressing adenocarcinoma A549 cells. Free Radic Biol Med 48:1626–1635
Moghaddam AZ, Hamzekolaei MM, Khajali F, Hassanpour H (2017) Role of selenium from different sources in prevention of pulmonary arterial hypertension syndrome in broiler chickens. Biol Trace Elem Res 180(1):164–170
Moller P, Loft S, Lundby C, Olsen NV (2001a) Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J 15:1181–1186
Moller P, Loft S, Lundby C, Olsen NV (2001b) Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative damage in humans. FASEB J 15:1181–1186
Morgan MT, Nakanishi Y, Kroll DJ, Griset AP, Carnahan MA, Wathier M, Oberlies NH, Manikumar G, Wani MC, Grinstaff MW (2006) Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer Res 66(24):11913–11921
Nagpal K, Singh SK, Mishra DN (2013) Formulation, optimization, in vivo pharmacokinetic, behavioral, and biochemical estimations of minocycline loaded chitosan nanoparticles for enhanced brain uptake. Chem Pharm Bull 61(3):258–272
Nakanishi K, Tajima F, Nakamura A, Yagura S, Ookawara T, Yamashita H, Suziki K, Taniguchi N, Ohno H (1995) Antioxidant system in hypobaric-hypoxia. J Physiol 489:869–876
Nehra S, Bhardwaj V, Kalra N, Ganju L, Bansal A, Saxena S, Saraswat D (2015) Nanocurcumin protects cardiomyoblasts H9c2 from hypoxia-induced hypertrophy and apoptosis by improving oxidative balance. J Physiol Biochem 71(2):239–251
Nehra S, Bhardwaj V, Bansal A, Saraswat D (2016a) Nanocurcumin accords protection against acute hypobaric hypoxia induced lung injury in rats. J Physiol Biochem 72(4):763–779
Nehra S, Bhardwaj V, Kar S, Saraswat D (2016b) Chronic hypobaric hypoxia induces right ventricular hypertrophy and apoptosis in rats: therapeutic potential of nanocurcumin in improving adaptation. High Alt Med Biol 17(4):342–352
Oh YS, Jun HS (2017) Effects of glucagon-like peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci 19:E26
Patir H, Sarada SKS, Singh S, Mathew T, Singh B, Bansal A (2012) Quercetin as a prophylactic measure against high altitude cerebral edema. Free Radic Biol Med 53:659–668
Pokropivny V, Lohmus R, Hussainova I, Pokropivny A, Vlassov S (2007) Introduction to nanomaterials and nanotechnology. Tartu University Press, Tartu
Poljsak B, Suput D, Milisav I (2013) Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013:1–11
Prasad M, Lambe UP, Brar B, Shah I, Manimegalai J, Ranjan K, Rao R, Kumar S, Mahant S, Khurana SK, Iqbal HM (2018) Nanotherapeutics: an insight into health care and multidimensional applications in medical sector of the modern world. Biomed Pharmacother 97(2018):1521–1537
Purkayastha SS, Sharma RP, Ilavaahagan G, Siridharan K, Ranganathan S, Selvamurthy W (1999) Effect of vitamin C and E in modulating peripheral vascular response to local cold stimulus in man at high altitude. Jpn J Physiol 49:159–167
Radak Z, Lee K, Choi W, Sunoo S, Kizaki T, OhIshi S, Suzuki K, Taniguchi N, Ohno H, Asano K (1994) Oxidative stress induced by intermittent exposure at a simulated altitude of 4000 m decreases mitochondrial superoxide dismutase content in soleus muscle of rats. Eur J Appl Physiol 69:392–395
Ramazan MS, Ekeroglu SH, Dulger H, Algun E (2000) The effect of dietary treatment on erythrocyte lipid peroxidation, superoxide dismutase, glutathione peroxidase, and serum lipid peroxidation in patients with type 2 diabetes mellitus. Clin Biochem 33:669–666
Ramsden J (2016) Nanotechnology: an introduction, William Andrew
Reddy MK, Wu L, Kou W, Ghorpade A, Labhasetwar V (2008) Superoxide dismutase-loaded PLGA nanoparticles protect cultured human neurons under oxidative stress. Appl Biochem Biotechnol 151(2–3):565–577
Roach RC, Maes D, Sandoval D, Robergs RA, Icenogle M, Hinghofer-Szalky HH, Lium D, Loeppky JA (2000) Exercise exacerbates acute mountain sickness at simulated high altitude. J Appl Physiol 88:581–585
Roco MC (2003) Nanotechnology: convergence with modern biology and medicine. Curr Opin Biotechnol 14(3):337–346
Sachan AK, Gupta A (2015) A review on nanosized herbal drugs. Int J Pharm Sci Res 6(3):961–970
Safari J, Zarnegar Z (2014) Advanced drug delivery systems: nanotechnology of health design: a review. J Saudi Chem Soc 18(2):85–99
Sagi SSK, Mathew T, Patir H (2014) Prophylactic administration of curcumin abates the incidence of hypobaric hypoxia induced pulmonary edema in rats: a molecular approach. J Pulm Respir Med 4:1000164
Sahoo SK, Parveen S, Panda JJ (2007) The present and future of nanotechnology in human health care. Nanomedicine 3(1):20–31
Sahu A, Bora U, Kasoju N, Goswami P (2008) Synthesis of novel biodegradable and self-assembling methoxy poly (ethylene glycol)–palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater 4(6):1752–1761
Semenza GL (2015) AJP-cell theme: cellular responses to hypoxia. Am J Physiol Cell Physiol 309(6):C349
Schmidt MC, Askew EW, Roberts DE, Prior RL, Ensign WY Jr, Hesslink RE Jr (2002) Oxidative stress in humans training in a cold, moderate altitude environment and their response to a phytochemical antioxidant supplement. Wilderness Environ Med 13:94–105
Sharma R, Prajapati P (2016) Nanotechnology in medicine: leads from Ayurveda. J Pharm Bioallied Sci 8(1):80–81
Shen JC (2006) Nanobiomaterials. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 28(4):472–474
Shinde A, Ganu J, Naik P (2012) Effect of free radicals & antioxidants on oxidative stress: a review. J Dent Allied Sci 1:63–66
Si HY, Li DP, Wang TM, Zhang HL, Ren FY, Xu ZG, Zhao YY (2010) Improving the antitumor effect of genistein with a biocompatible superparamagnetic drug delivery system. J Nanosci Nanotechnol 10(4):2325–2331
Sies H (1986) Biochemistry of oxidative stress. Angew Chem Int Ed Engl 25:1058–1071
Simon-Schnass I (1994) Risk of oxidative stress during exercise at high altitude. In: Sen CK, Packer L, Hanninen O (eds) Exercise and oxygen toxicity. Elsevier, Amsterdam, pp 191–210
Singh K, Ahmad Z, Shakya P, Ansari VA, Kumar A, Zishan M, Arif M (2016) Nano formulation: a novel approach for nose to brain drug delivery. J Chem Pharm Res 8(2):208–215
Sitharaman B (ed) (2016) Nanobiomaterials handbook. CRC, Hoboken
Sun K, Zhang Y, D’Alessandro A, Nemkov T, Song A, Wu H, Liu H, Adebiyi M, Huang A, Wen YE, Bogdanov MV (2016) Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nature 7:12086
Thambi T, Deepagan VG, Yoon HY, Han HS, Kim SH, Son S, Jo DG, Ahn CH, Suh YD, Kim K, Kwon IC (2014) Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 35(5):1735–1743
Vani R, Reddy CS, Asha Devi S (2010) Oxidative stress in erythrocytes: a study on the effect of antioxidant mixtures during intermittent exposures to high altitude. Int J Biometeorol 54:553–562
Wilkins MR, Ghofrani HA, Weissmann N, Aldashev A, Zhao L (2015) Pathophysiology and treatment of high-altitude pulmonary vascular disease. Circulation 131(6):582–590
Wozniak A, Drewa G, Chesy G, Rakowski A, Rozwodowska M, Olszewska D (2001) Effect of altitude training on the peroxidation and antioxidant enzymes in sportsmen. Med Sci Sports Exerc 33:1109–1113
Wu TH, Yen FL, Lin LT, Tsai TR, Lin CC, Cham TM (2008) Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int J Pharm 346(1):160–168
Xu X, Yu J, Tong S, Zhu Y, Cao X (2011) Formulation of silymarin with high efficacy and prolonged action and the preparation method thereof. US Patent 20110201680. 18 August 2011. Jiangsu University
Yang L, Zhang L, Webster TJ (2011) Nanobiomaterials: state of the art and future trends. Adv Eng Mater 13(6):B197–B217
Yen FL, Wu TH, Lin LT, Cham TM, Lin CC (2008) Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen-induced hepatotoxicity in rats. Food Chem Toxicol 46(5):1771–1777
Zheng X, Kan B, Gou M, Fu S, Zhang J, Men K, Chen L, Luo F, Zhao Y, Zhao X, Wei Y (2010) Preparation of MPEG–PLA nanoparticle for honokiol delivery in vitro. Int J Pharm 386(1):262–267
Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140:280–293
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mudgal, P., Paliwal, S. (2022). Antioxidant Therapy for High Altitude Sickness and Nano-Medicine. In: Sharma, N.K., Arya, A. (eds) High Altitude Sickness – Solutions from Genomics, Proteomics and Antioxidant Interventions. Springer, Singapore. https://doi.org/10.1007/978-981-19-1008-1_13
Download citation
DOI: https://doi.org/10.1007/978-981-19-1008-1_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1007-4
Online ISBN: 978-981-19-1008-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)