Abstract
Textile industries are considered as one of the major contributors for water pollution. Textile wastewater primarily contains organic dye molecules along with heavy metal ions and some polymeric waste material which causes adverse effects on aquatic system and human health. Insufficient and incomplete treatment of textile wastewater is a major concern especially when it is discharged directly into the water bodies. Removal of dye molecules from wastewater has been addressed by several researchers using various physical, chemical, and biological processes. This chapter highlights applications and challenges with conventional and advanced treatment processes. Advanced oxidation process using various combinations of oxidants and energy sources has emerged as a significant technique. Applications of nanomaterials and polymers have also been explored significantly for the degradation of pollutants present in textile wastewater. Integration of suitable technologies is proposed to achieve complete degradation of pollutants discharged in textile wastewater.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
India is one of the largest producers and exporters of textiles and garments in the world. In India, the textile industry plays a significant role towards the economic growth of the country and offers mutual exchange of technology between the countries. In addition to these benefits, the textile industry is also considered to be highly polluting industry and leading consumer of water [1]. The textile waste is inevitable outcome of industrialization. It uses more than 200 L of water/kg of textile product along with numerous other chemicals and discharge huge amount of wastewater to water bodies [2]. It is estimated that about 3 × 106 L of wastewater is reproduced after processing about 20,000 kg of textiles per day. India is not the only country to face challenges in addressing the issues of textile wastewater management. China produces approximately 170 million tons of municipal solid wastes annually where the contribution of textile waste is 10–15% [3]. According to US EPA, the contribution of textile waste is 9–10% in the total municipal solid waste generated in the United States [4].
In addition to textile industry, other industries such as pharmaceutical, leather, and plastic industry also discharge approximately 50,000 tons of organic dyes every year into water bodies [5]. Textile wastewater includes (a) suspended solid material, (b) mineral oils, (c) surfactants, (d) Phenols and resins, (e) halogenated organics produced, (f) dyes and polymeric substances, and (g) heavy metals such as lead, mercury, chromium, copper, and zinc. The heavy metals have tendency to accumulate in the biological tissues and are not easily degradable [6]. The disposal of these waste generates serious governance issues, thus there is a need to recycle them in sustainable manner [7].
It has been reported that processing of polyester and cotton requires approximately 100–350 kg of water per kg of fabric which contribute up to 80% total wastewater load [8]. Dyeing and finishing processes utilize large quantities of chemicals and release complex organic compounds and heavy metals to wastewater thus poses major threats [9]. In the wet processing of textile fibers, after dyeing the fibers are repeatedly rinsed, and treated with numerous surfactants, salts, recalcitrant and toxic chemicals which eventually discharged in water bodies [10].
In the dyeing process, the usage of reactive dyes is increasing exponentially due to high demand of bright colored fabric. These reactive dyes contain various reactive groups such as halotriazine, sulfone, pyrimidine, and dichloro-fluoropyrimidine [11]. These dyes are generally used to dye cotton fabric however their fixation rate is about 60–90% only. Thus, unfixed residual is discharged as the textile effluent along with other alkali, acids, organic dyes, and heavy metals [12]. The synthetic cationic and anionic dyes such as methylene blue, methyl orange, and rhodamine B are also not degraded easily thus adversely affect soft tissues and cause genetic changes [13]. Prolonged exposure to these toxicants leads to nervous system disorders, thyroid dysfunction, and may result as lethal as well [14]. In addition, due to the presence of various dyes and heavy metals, it is highly carcinogenic in nature and causes severe adverse effects [15].
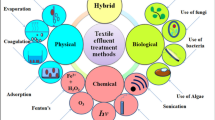
Textile wastewater contains a wide range of organic pollutants with different chromophores and auxochromes (Fig. 1). Therefore, it is not possible to treat a wide range of pollutants ranging from nanoparticles to polymers, metallic to non-metallic, and organic to inorganic using single treatment methodology. In addition, textile wastewater has highly fluctuating pH, with high chemical oxygen demand, biological oxygen demand, and suspended solids.
Thus, it is imperative to design and adopt a sustainable and eco-friendly model of textile industry which reduces and overcomes all challenges of waste minimization and disposal [16]. It is needed to design effective techniques to understand the pathways of pollutant degradation and formation of intermediates or products. The methodologies should not only meet international standards, but also be able to protect the biodiversity and to ensure the availability of pure water for future generations. The Textile and Apparel industry in India contributes to 14% of total industrial production, 4% of gross domestic product, and 15% of total export earnings (Textile Ministry, Make in India, TechSci Research, 2017). There are various treatments available for treating textile wastewater such as adsorption, photochemical oxidation, catalytic oxidation, flocculation, coagulation, and membrane filtration; however, these methods are compromised with certain limitation (Table 1). The major problem is sludge generation in large quantities which further needs to be treated thereby increasing the cost of treatment process [17].
This chapter will focus upon discharge of heavy metals and polymeric fibers in textile effluent and their possible remediation. The chapter will also address modifications in the conventional methods and advanced technologies to address the removal of various organic and inorganic pollutants from textile wastewater.
2 Heavy Metals in Textile Effluent
Textile effluents are rich in organic and inorganic contents along with heavy metals and other toxicants. Heavy metal discharge to water bodies is of serious concern as they are nonbiodegradable in the environment [15]. Among all heavy metals lead, copper, cadmium, chromium, zinc, and manganese are widely used for the production of textile dyes and other materials in the dyeing process [18].
Several researchers have investigated the concentration of heavy metals in textile discharge and emphasized their adverse effects on aquatic system and living organisms [19]. Chromium pollution by textile industries is highly prevalent, as chromium is one of the main elements of metal-based dyes in textile industries [20]. Few essential metals such as iron, copper, zinc, and manganese are required in minute quantities to maintain normal cellular growth and functioning, however, excess quantity beyond permissible limit impose adverse effects on human health.
Direct and indirect discharge of heavy metals into the environment causes environmental pollution at various levels and poses threat to aquatic and human lives on prolonged exposure [21]. To propose possible solutions for the remediations of heavy metals it is needed to have complete understanding of their permissible limit, toxicological profile, biotransformation, and distribution in abiotic and biotic environment. Heavy metals are known to cause disturbances in neurological function, neurotoxicity, hepatotoxicity and nephrotoxicity in humans and animals, bone degeneration, damage to soft tissues, impaired heme synthesis, carcinoma to multiple organs eventually lead to cellular death [22, 23]. In addition, heavy metals also inhibit microbial growth, their activities and various biological processes such as nitrogen fixation, decomposition of organic matter in soil. Lead and chromium possess the ability to generate reactive radicals, which cause oxidative imbalance, resulting to cellular and DNA damage [24]. These metals also bioaccumulate in aquatic plants and animals and further enter to ecological cycle [25]. The heavy metals possess electron-sharing affinities and form covalent bond with sulfhydryl groups of proteins thereby inhibiting activity of antioxidant enzymes. Studies associated with the assessment of occupational exposure to textile wastewater have reported increased risk of hepatic, renal, and cardiovascular disorders [26]. Utilization of textile waste water for irrigation leads to accumulation of various heavy metals in crops which further enters to food chain undergoes biomagnification [27]. Thus, the toxins and pollutants discharged in water bodies by industries persist in the environment and decrease the quality of life. This necessitates the requirement of effective techniques for the removal of toxicants from industrial effluents prior to their discharge into water bodies.
3 Microplastic Pollution by Textile Effluent
Textile industry was earlier considered to release only dye containing wastewater along with some other organic and inorganic pollutants. However, in the recent past, it has been studied that it has also contributed significantly towards microplastic and polymeric pollution. During finishing process of textile, some urea-based resins such urea–formaldehyde, dimethylol ethylene urea are used for imparting anti-crease finish to final product which ultimately discharge formaldehyde to wastewater. Formaldehyde blocks respiratory tracts and causes serious respiratory ailments. Copolymers of acrylic acid such as acrylate and methacrylate containing free carboxyl groups are also released into water bodies which are resistant to biological attack. Plastic particles smaller than 5 mm in length are referred to as microplastics [28]. It has become a growing concern in recent years owing to its small size [29]. In order to propose effective solution for microplastics, it is imperative to evaluate possible sources of its pollution. These particles have derived from a wide range of industries such as polymer, personal care products, textile, and processing industries [30].
In 2016, textile washing processes generated approximately 5.4 million tons of synthetic fibers worldwide [31]. Among various types of microplastic, synthetic fibers are of most dominant type detected in water, sediments, and living organisms [32]. Over 90% of microplastics reaches global coastal environments via synthetic fibers [33]. Industrial and domestic textile laundry is considered as major sources of synthetic fibers, however, till date there are not many reports highlighting the impact of textile industries towards microplastic pollution. At industrial scale production of textile, fiber requires large amount of water which ultimately discharge into water bodies. The wastewater discharged by textile industries contains various toxic compounds, such as phenols, thiazoles, and phthalates, thus, considered as a primary source of water pollution source [34].
It has been estimated that a household washing machines release thousands of synthetic fibers after every wash cycle [35] and due to release of textile wastewater it adds approximately 160,000,000 microplastic particles per day to water bodies [36]. In wastewater treatment plants these particles undergo stress force, and mechanical mixing which further decreases their particle size and acts as carrier for various contaminates to food chain [37, 38].
Microplastics have been classified into five categories on the basis of their shape as films, fragments, fibers, foam, and pellets [39]. Among all the categories, the most harmful is microfiber due to its size specification. Usually, the length of this fiber ranges between 100 μm and 5 mm and width nearly 1.5 orders of magnitude shorter in comparison to length [40]. Various toxic chemicals such as heavy metals, organic dyes, poly aromatic hydrocarbons, and polychlorinated biphenyl are adsorbed over these fibers and easily enter food chain. Due to small size, they are easily ingested by aquatic animals and cause deleterious effects [41]. In Textile industries, a well-defined wastewater treatment plant removes various organic and inorganic impurities; however, the current available technologies retain major part of microplastics which are being continuously discharged to the water bodies. According to some reports, majorities of microfibers are removed in treatment stage before the aeration process only still a large amount enters the aquatic system because the quality of wastewater discharged to water bodies is very high [42]. Bioavailability of microfibers largely depends upon their shape and size therefore their accumulation is different in comparison to chemical pollutants.
In the last one-year, COVID-19 pandemic has created havoc worldwide and World Health Organization prescribed mandatory usage of face masks as one of the safety measures to prevent spread of in human beings. Thus, the usage of face masks and other accessories such as gloves, face shields, protective shoes, and suits increased thereby their manufacturing as well. All these materials are manufactured with various synthetic polymeric materials such as nylon, polyester, and copolymer of polyether–polyurea [43]. Thus, these types of textile industries have also contributed significantly to the polymeric pollution [44].
4 Advanced Oxidation Process (AOP) for Removal of Heavy Metals and Organic Compounds
Advanced Oxidation process is defined as a chemical treatment process for the removal of organic pollutants from wastewater by oxidation process using generation of highly reactive hydroxyl radical \(\left( {{\text{OH}}^{ \cdot } } \right)\). If industrial wastewater contains nondegradable organic and inorganic compounds such as various dyes, polymeric substance, heavy metals, and their compounds, it is difficult to remove those pollutants by simple chemical or biological methods. Conventional oxidation techniques are not able to completely oxidize complex structure of organic compounds as they are resistant to oxidation. To efficiently remove such complex organic compounds from water bodies, more aggressive treatment methods are needed [45]. At high temperature the rate of oxidative degradation is limited owing to lesser availability of oxygen while at low temperature, though the availability of oxygen remains high however, degradation rate remains less due to limited activity of microorganisms. In addition, reducing chemicals discharged in wastewater also absorbs dissolved oxygen thereby retarding process of pollutant degradation. Thus, if there is sufficient supply of oxygen in terms of oxidizing radicals, it would help to enhance degradation of pollutants.
4.1 Fenton’s Reagent
AOP is one of the most promising techniques to degrade organic pollutant from wastewater. In this method, highly reactive hydroxyl radical \(\left( {{\text{OH}}^{ \cdot } } \right)\) are generated by using primary oxidants either O2 or H2O2 (Fig. 2).
In this process, all organic compounds are degraded to carbon dioxide (CO2), H2O, and inorganic salts [46]. This technique can remove persistent organic pollutants such as textile dyes, pharmaceuticals, polymeric substances, microplastics and serves as an effective treatment step in water purification or final stage before water discharge to water bodies [47]. Hydroxyl radical is highly reactive towards oxidative degradation, however, the major drawback is its non-selectivity. Along with the targeted molecules, it reacts with many other nontarget compounds present in the water matrix. Thus, demand of \({\text{OH}}^{ \cdot }\) increases to complete the process of degradation.
On the basis of generation of \({\text{OH}}^{ \cdot }\) Radical, the process can be defined as homogeneous and heterogeneous (Fig. 3). Homogeneous process requires chemical reagents (O2, O3, H2O2) while heterogeneous process works with energy sources such as UV light source or photocatalyst.
Homogeneous Fenton and Photo-Fenton processes have few limitations as [48]:
-
These reactions operate at pH less than 3 when Fe exist in soluble form
-
Concentration of reagents need to be maintained optimum to avoid scavenger effects
-
Sludge is produced as by-product thus disposal needs to be maintained
-
As Fe salts are used for the supply of Fe thus, in the treated water Fe remains is present in traces which may adversely affect soft tissues
-
Textile wastewater contains acidic radicals such as chloride (Cl−), sulfate (SO4−2), phosphates (PO4−3), which may react with \({\text{OH}}^{ \cdot }\) and decrease the efficiency of the oxidation process.
Fenton’s reagents (H2O2 and Fe2+) and ozone (O3) under AOP have been used extensively to treat textile wastewater to achieve the best results. Fenton's oxidation was used to degrade various reactive dyes such as Remazol Yellow, Remazol Black 5, Remazol Blue and Remazol Red, and was found significantly effective in their decolorization (>99%) [49]. This process is also effective for the degradation of aromatic amines as well under acidic conditions. This oxidation process can be used to reduce the organic matter present in textile effluent. In this process, concentration of H2O2 serves as a limiting factor, i.e., higher concentration of H2O2 produces more \({\text{OH}}^{ \cdot }\) radical; however, increasing H2O2 concentration above a certain level may further reduce the rate of oxidation due to self-decomposition and generation of less reactive free radical [50].
In addition, excess of H2O2 may react with \({\text{OH}}^{ \cdot }\) radical and compete with the degradation and reducing the efficiency of treatment process [51].
4.2 Ozone (O3)
Ozone is a potent oxidizing agent known for treating wastewater. Ozone gets easily dissolved in water and oxidize organic compounds and pollutants in two different ways:
-
Direct oxidation as molecular ozone
-
Indirect oxidation via formation of secondary oxidants like \({\text{OH}}^{ \cdot }\).
Ozone due to its strong oxidizing property and high oxidizing potential (2.07) has been used for wastewater treatment since 1970s [52]. Germicidal efficacy of ozone is 100 times in comparison to hypo chloric acid disinfection property is 3,125 times faster. Ozone due to its highly unstable nature should always be generated on site. Oxidation potential of O3 is −2.07 V which is greater than that of other potent oxidizing agents such as hypochlorite acid (−1.49 V) and chlorine (−1.26 V) (Table 2). With the help of ozone treatment, many nonbiodegradable products can also be decomposed. The challenges in using ozone treatment are the limited life span which is 20 min in water, and its reduction in the alkaline water [53]. Ozone function in two ways (a) a powerful disinfecting agent (b) a strong oxidizing agent and eliminate color, odor, and toxic organic compounds [54]. Ozone has been reported to decolorize all dyes, except insoluble vat and disperse dyes because these dyes have slow reaction rate which becomes a limiting factor in the reaction [55]. Decomposition of ozone produces free radical \({\text{OH}}_{{2}}^{ \cdot }\) and \({\text{OH}}^{ \cdot }\) which oxidizes various organic and inorganic impurities such as heavy metals and their salts, organic matter, and biological matter.
Ozone reacts via three specific reactions for the oxidation of organic molecules and polymeric substances: electrophilic addition of ozone to the Carbon–Carbon double bond: Ozone readily adds on to aliphatic unsaturated compounds, such as olefin and causes the oxidative cleaving of the alkene or alkyne.
Ozonolysis: Ozone gives addition reaction with alkenes to form ozonide. The reaction takes place in non-aqueous solvents, as presence of traces of water as well will hydrolyze ozone to other products before the reaction.

Substitution reaction: In this reaction, one atom or functional group is replaced with other groups. Ozone leads to cleavage of carbon–carbon bonds to generate fragmented organic compounds via inserting oxygen atom between the ring carbon and hydrogen atom [54].
The oxidation reaction using ozone is affected by various factors like pH, temperature, total organic carbon, and chemical oxygen demand. It has been reported that at acidic pH ozone reacts as intact O3 molecule and the reaction is slow while at alkaline pH the reaction is rapid due to decomposition of ozone into hydroxyl free radicals. Thus, alkaline pH ranging from 8 to 10 is optimum for oxidation of organic molecules by ozone [54].
4.3 Ozone (O3)/Ultraviolet (UV)/Ultrasound
Combination of two or more AOPs may provide synergistic effects for oxidative degradation by generating more hydroxyl radicals. The various combinations can be
-
UV radiation + Ozone
-
UV radiation + Hydrogen Peroxide
-
Ozone + Ultrasound energy + Photochemical/photocatalytic oxidation.
The efficacy of combined system will depend upon the extent of synergism, number of generated hydroxyl radicals, and efficacy of contact between free radicals and pollutant molecules [56].
Treatment of organic compounds with ozone does not provide complete oxidation to CO2 and H2O in many cases. The intermediate remained in water after incomplete oxidation may be same or more toxic that initial toxicant. Thus, combination of O3 with UV is more effective in comparison to simple ozonation. Photons of UV light activate ozone molecules, thus promote generation of hydroxyl radicals at a quicker rate thus increasing the efficacy of oxidation reaction [57]. For efficient ozone photolysis maximum radiation output of 254 nm from the UV lamp must be used. While proposing a combination of ultrasound with O3, the operating frequency should not be more than 500 kHz which plays as one of the significant factors [56].
4.4 Hydrogen Peroxide (H2O2)/Ultraviolet (UV) Radiation
Hydrogen peroxide alone is not effective for oxidation of organic toxicants at both acidic and alkali pH [58], however, when H2O2 is irradiated with UV radiations it forms two hydroxyl radicals \({\text{2OH}}^{ \cdot }\) that react with organic contaminants present in textile effluent [59]. The combination of H2O2 + UV is also highly effective to the scavenging effect of carbonate ions at alkaline pH. In the combination of H2O2 with UV radiation, the peroxide is activated by UV light. The extent of activation primarily depends upon concentration of H2O2, strength of UV radiation, pH of medium, and composition of dye. Acidic dyes are most easily decomposed by this system having accumulative number of azo groups; however, it is not effective for degradation of pigments [60]. This method has various advantages over other oxidation process and the most important is no sludge formation at any stage of process.
The process is effective at acidic pH; however, at alkaline pH H2O2 under UV radiation oxidizes alkalis to produce oxygen and water rather in place of hydroxyl radicals. Concentration of H2O2 also largely affects the reaction. At high concentration, it competes with the dye molecules for reaction with hydroxyl radicals and decreases the rate of oxidation. In addition, at high concentration, \({\text{OH}}^{ \cdot }\) radicals dimerize to form H2O2 [61]. Thus, it is essential to optimize concentration of H2O2 to have maximum efficacy of the reaction. The rate of dye degradation increases with the increase in intensity of UV light [62]. It has been found that increasing the power intensity of UV radiation from 18 to 54 W increased degradation efficiency from 90.69 to 100% due to excessive generation of \({\text{OH}}^{ \cdot }\) [63].
Advantages of H 2 O 2 /UV process are as follows:
-
No Sludge formation
-
Reaction takes place at ambient temperature
-
Oxygen generated as by-product can be used for biological decay
-
Rate of reaction depends upon pH, concentration of H2O2, and intensity of UV radiation.
4.5 Ozone/Ultraviolet/Hydrogen Peroxide
Among all AOPs, the combination of O3/UV/H2O2 has been reported to be best for the purification of dye wastewater including degradation of polyester fiber, and degradation of dye molecules [64]. The addition of H2O2 to O3/UV system enhances the rate of \({\text{OH}}^{ \cdot }\) generation by promoting decomposition of ozone and acting as a catalyst for the reaction [65]. In addition, at alkaline pH H2O2 reacts with O3 to produce \({\text{OH}}_{{2}}^{ \cdot }\) radical which dissociates O3 more effectively than \({\text{OH}}^{ \cdot }\); however, at acidic pH the reaction is slow. This method is termed as peroxone method. At alkaline pH, H2O2 itself dissociates into \({\text{OH}}_{{2}}^{ - }\) ions which also initiate ozone decomposition more effectively than \({\text{OH}}^{ - }\) ion [66].
5 Advanced Techniques for the Degradation of Metallic and Polymeric Compounds
5.1 Photocatalysis
The principle of photocatalyst lies in the generation of electron as reducing agents and hole as oxidizing agents. The mode of action is described in Fig. 4. In photocatalysis, the design of photocatalytic reactor is also very important in order to have effective contact between the toxicants and photocatalyst and adequate absorption of photons. Thus, for an ideal photoreactor it must have high specific surface area, high mass transfer, and direct light irradiation of catalyst surface [67]. This process has various advantages as (a) It completely oxidize organic pollutants to CO2, water and mineral acids, (b) moderate temperature and pressure is needed, (c) no sludge is generated thus free from sludge disposal problem.
Generation of free radicals in photocatalysis (1) With the help of UV light the electrons are excited, (2) electrons excited from valence band to conduction band generating behind positively charged holes (h+) which act as oxidizing agent, (3) electrons are quenched by scavengers to prevent their combination with holes, and (4) electrons act as reducing agents and generate free radicals
Titanium dioxide (TiO2) is the most commonly used photocatalyst owing to its high nontoxic nature, inertness towards biological and chemical reagents and high photocatalytic activity [68]. Photocatalytic degradation of various organic substances has been assessed using TiO2 since the last six decades [69]. TiO2 has been reported to degrade wide range of pollutants including dyes, phenols, plasticizers, polychlorinated biphenyls, surfactants, and dioxins [70]. The major challenge in using TiO2 as a photocatalyst is its bandgap width (3.2 eV), which allows absorption of wavelengths lower than 380 nm only, i.e., in UV region. Various techniques such as dye sensitization, modification of surface, and doping have been used by researchers to enhance the activity of TiO2 [71]. TiO2 has been doped with copper, cobalt, molybdenum, and many other metals to increase its light absorption capacity to visible region [72]. TiO2 doped with zinc showed better photocatalytic activity and reduced bandgap energy in visible light [73]. However using heavy metals for doping TiO2 may produce toxic intermediates and create heavy metal pollution [74].
TiO2 photocatalytic systems can be used as either photoreactors or immobilized on solid support system (Table 3). In photoreactors, the suspension system is maintained for the reaction. In suspension system, the catalyst has to be separated from treated wastewater and recycled before discharge which is a cost intensive and time-taking process. In addition, absorptions of UV light by catalyst particles limit its effective penetration to the desired depth thereby limiting complete oxidation [75]. This problem can be addressed by using immobilized photocatalyst over some suitable support system. However, immobilization of catalyst also decreases the rate of reaction due to availability of reduced surface area of catalyst for reaction. Researchers have studied degradation of Acid Blue 25 dye using immobilized titania nano photocatalysis and reported lower generation of hydroxyl radicals with increased concentration of dye [76]. At high concentration of dye, a smaller number of photons hit the surface of catalyst resulting in decreased generation of hydroxyl radicals [77]. Complete degradation of dye converts all carbon, nitrogen, and Sulfur atoms to CO2, nitrate, and sulfate ions, respectively [78]. Immobilization of TiO2 on polymeric sheets is one of the efficient methods for the photocatalytic implementations at large scale owing to easy availability and handling, flexibility, cost effective, and light weight of polymer [79]. The photocatalytic activity of TiO2 is largely affected by various cations, anions, and organic compounds as they can be adsorbed over TiO2 surface and decrease the activity [80, 81]. Photocatalytic degradation technique cannot be implemented for field applications in treating large-scale contaminated sites.
5.2 Metallic Nanoparticles
Metals play a very important role in removal of dyes from textile wastewater. One of the areas is by using magnetic nanoparticle as adsorbents treatment [88]. In the recent past, many researchers have used various magnetic materials for the removal of dyes from wastewater [89]. Usually, applications of nanoparticles are limited for the removal of anionic dyes due to similar charges both on dye and surface of nanoparticle there for several modifications were done to change surface properties of these particles (Table 4).
Jabbar et al. [93] synthesized cobalt aluminate (CoAl2O4) nanoparticles by pyrolysis for the dissociation of Novacron Deep Night S-R dye in combination with UV radiation. They reported that in the presence of H2O2 the dye degraded 67% within 50 min. Several metal oxides have also been reported for the photocatalytic degradation of organic dyes and pollutants [94]. Spinel oxides are stable semiconductors which have sufficient band gap energies suitable for photocatalytic degradation of organic dyes [95]. The catalytic efficiency of such metal oxides depends on their specific surface area, morphology, crystallinity, particle size, and chemical composition [96]. Several routes of metal oxide preparation and their benefits are presented in Fig. 5.
Oxidation process by using Zero Valent iron has added advantages as it involves various pathways comprising of reduction and oxidation reactions which are specific for the nature of pollutant and reaction conditions [97]. The generation of free radicals occurs in three stages:
Nanotechnology is playing an imperative role in environmental remediation of organic and inorganic pollutants since the last decade [98]. Several researchers have reported efficacy of nano zero-valent iron towards degradation of organic textile pollutants [99]. It has been observed that specific area of nano zero-valent iron is much larger (29 m2/g) in comparison to bulk particles (0.2 m2/g) which improves the rate of oxidation and degradation of organic pollutants [100]. The degradation of various organic and inorganic pollutants by using nano zero-valent iron is summarized in Fig. 6. In this process, pH of the reaction media, dose of nanoparticle, and initial concentration of dye play a very important role in degradation process of organic pollutants. If pH is alkaline, Fe2+ ions and OH− ions may precipitate as Fe (OH)2 on the surface of zero-valent iron and occupying the reactive sites [101]. At alkaline pH, the reducing pathway is prevalent which is less effective in comparison to oxidation process. Aggregation of these particles is a major challenge in this process as due to high surface area these particles are highly reactive and aggregate at the faster rate. These particles get oxidized and aggregated in the presence of atmospheric air thus are highly unstable [102].
Various solid supports can be used to prevent aggregation of nano zero-valent iron and increase their efficiency for dye degradation (Fig. 7). The solid support system maintains nanoparticles in dispersed phase, protects them from air oxidation, and increases contact area, however, reduced their activity towards degradation of textile dyes. Few supports such as biochar, kaolin and rectorite, rather than degrading the pollutants simply adsorb them and transfer them to the surrounding environment. Hence, degradation or oxidation of pollutants is recommended instead of their adsorption.
Incorporation of heavy metals as a support system for the synthesis of nanoparticles may further introduce these toxic substances to the environment. Thus, researchers proposed the synthesis of nano zero-valent iron using various green support materials such as extracts of green tea, eucalyptus leaf, grape leaf, and other tree leaf extracts. These extracts provide stabilization to nanoparticles and enhance their efficacy as well for the degradation of textile dyes and other organic pollutants [103]. Zhang et al. [104] reported removal of Pb(ii) by using nano zero-valent iron supported over kaolin while Ali et al. [105] synthesized nano zero-valent iron using porous cation exchange resin as a solid support reported the efficacy for the reduction of Cr(vi) to Cr(iii) in aqueous solution.
The process of pollutant degradation is based on reducing nature of these particles. Nano zero-valent iron particles are potential reducing agent (electron donors) while organic dye molecules are brilliant oxidizing agent (electron acceptors). Therefore, they reduce Fe2+ ions, and generate hydroxyl ions which further dissociate chromophore bond and auxochrome bond thereby decolorize the dye molecule. The intermediates on complete mineralization produce CO2, H2O, and inorganic ions. The efficiency and reactivity of nanoparticles can be improved, by integrating with other treatments such as irradiation with microwave, UV, or ultrasonic irradiation and using H2O2 oxidation [106]. Partial removal (up to 55%) of organic pollutants using nano zero-valent iron has been reported, however, by using additional oxidizing agent H2O2 and integration of UV technique the removal increases up to 90.0% [107]. Mao et al. [106] reported decolorization of reactive yellow and solvent blue dyes up to 60.0% and 94.0%, respectively, in only 5 min using nano iron along with microwave radiations. Studies are being conducted using scrap zero-valent iron as well for the treatment of wastewater as a method to propose reutilization of by-product from machinery industries [108]. These methods are one of the promising and cost-effective way for treating textile wastewater.
5.3 Natural and Modified Polymers
Natural polymers plant or animal-based serves as an efficient method for treating textile wastewater and dye removal. Removal of organic and inorganic pollutants using natural adsorbents is considered to be economic, and sustainable alternative over other physical and chemical processes [109]. Biosorption is a passive process in which metal ions present in the textile wastewater interact with the functional groups on biological material's surface. This process ensures various advantages such as cost effectiveness, reduced sludge generation, easy regeneration of adsorbent, and higher removal capacity [110]. The specific chemical structure and composition of the polymer play a significant role towards effective removal of dyes from textile wastewater. Natural polymers are synthesized from various plant sources which then further subjected to chemical and physical treatment to give modified natural polymer with enhanced efficacy for the removal of textile dyes (Fig. 8).
The natural polymers contain various anionic groups such as hydroxyl (OH−), phosphate (PO4−), and carboxyl (COO−). These groups show affinity towards cationic dye molecules present in wastewater by using electrostatic force of attraction [111]. Usually, these polymers are effective in acidic environment, however, removal of methylene blue dye is reported in alkaline conditions (pH-8) as well [112]. Seeds of nirmali show excellent binding properties for dye molecules due to the presence of OH− groups along with galactomannan and the galactan molecular chains. Polymers differ in molecular weights based on their molecular structure. High molecular weight polymers carryout removal of organic dye molecules more effectively due to charge neutralization, bridging, and electrostatic attraction. Chemical modifications have been done in cellulose with sulfur-bearing functional groups such as thiols, dithiocarbamates, dithiophosphates, and xanthates for metal bonding as these groups have higher affinities for heavy metals ions and least affinities for other light metals [113]. Xanthates offer high stability with heavy metal ions, in addition they are easily prepared and cost effective as well. The sludge formed using xanthate metal complexes is easy to settle down thereby can be disposed of with minimal efforts. On the other hand, the precipitates as metal hydroxide may release metals ions into the environment thus, they need to be treated before disposal and can be pretreated using solidification or stabilization [114].
In recent years researchers have focused on the extraction of microbial polymers owing to their low cost and easy processing. The microbes which have been used are Pseudomonas pseudoalcaligenes, Pseudomonas plecoglossicida, and Staphylococcus aureus. These polymers have been effectively used for those dyes which are resistant towards decolorization due to their acidic nature such as fawn dyes, mediblue, mixed dyes, and whale dyes [115].
5.4 Nanocomposites
Metal oxide nanoparticles and nanocomposites show excellent adsorption properties for heavy metal ions present in textile effluent [116]. Magnesium-based nanoparticles with different morphologies have been used for the removal of various heavy metal ions and organic pollutants. The mechanism of adsorption using Mg-based nanoparticles includes electrostatic attraction and surface complexation between the dye molecule and hydroxyl groups which are present at the surface of the adsorbent. These nanoparticles carry out oxidative degradation of organic molecules by breaking down P–S or P–O bond of organophosphates and other organic dye molecules [117]. Magnesium oxide (MgO) embedded fiber-based substrate have been used successfully as an adsorbent for the removal of toxic dyes from textile water [118]. MgO nanoparticles in combination with carbon nanofibers have been used as a significant adsorbent for the removal of heavy metal ions from textile effluent [118]. Adsorption of cadmium (Cd2+) ions have been studied using polyacrylonitrile-based carbon nanofibers using MgO as adsorbent [119]. Reinforcement of MgO nanoparticles increases the adsorption capacity of carbon nanofibers. Hybrid nanofibers of MgO with polypropylene glycol have been also prepared for the removal of heavy metals [120]. These nanofibers had specific surface area of 185 m2/g and found to be effective for the removal of Pb, Cu, and Cd at pH of 7.5. In addition, regeneration experiments showed that efficacy remained high even after seven cycles.
5.5 Graphene and Its Composites
Graphene is a covalently bonded two-dimensional lattice having specific properties. Graphene is oxidized to form graphene oxide which can be further chemically modified to have several functional groups such as epoxies, carboxyl, and hydroxyl [121]. Presence of such functional groups increases negative charge on graphene oxide surface and helps to interact with dye molecules through H-bonding. Graphene oxide due to its conjugated structure has been studied as a potent option for the adsorption of dye molecules from textile effluent [122].
Recently, many researchers have given much consideration to the polymeric materials to be used as nano-adsorbents. The polymers such as polyaniline, polystyrene, and polypyrrole can be coated over the surface of nanoparticles to enhance their photoelectrical properties environmental suitability. Polymeric nanocomposites of graphene have been reported to be effective for the degradation of inorganic pollutants and organic dye molecules [123]. Noreen et al. [124] synthesized a range of nanocomposites of graphene oxide with polyaniline, polypyrrole, and polystyrene to achieve a higher removal rate of Actacid orange-RL dye. Composite of graphene oxide with polyaniline has been reported as potential adsorbent for the removal of Hg2+ ion and dye removal owing to its loose porous structure [125]. However, the applications of these nanocomposites are limited for the removal of heavy metals due to their expensive and complex synthesis and troublesome recycling [126]. In addition, graphene oxide due to its high clustering tendency is less effective in the adsorption process [127]. Thus, a sustainable adsorbent should be cost effective, show high adsorption efficacy, ease of separation and high reusability.
Natural polysaccharide, Chitosan has also been used to improve mechanical strength and adsorption ability of nanocomposites. The free –NH2 group in chitosan reacts with other metal/nonmetal oxides and provides chemical stability [128]. The –COOH group in graphene oxide easily interact with highly reactive –NH2 group of chitosan to give a stable nanocomposite with improved adsorption capacity [129]. Combination of biopolymer chitosan with graphene oxide enhances thermal and mechanical stability, improves hydrophilic property of composite, and reduces entangling tendency during separation [130].
Poly vinyl alcohol (PVA) is a biodegradable polymer, with hydrophilic nature and high fiber-forming ability. It can easily cross-link with graphene oxide/chitosan nanocomposite to improve its adsorption efficacy for dye removal [131, 132]. The separation of graphene oxide/chitosan–PVA biopolymer from treated water is very simple and can be completed with limited technology. Das et al. [133] studied effective removal of Congo red dye using this nano-polymer composite. Under acidic condition, the amino group (–NH2) in chitosan get protonated and then ionized further to ammonium ion thereby increasing swelling properties of GO/Chitosan–PVA polymer resulting in increased weight of polymer [133]. However, at high pH swelling decreased due to deprotonation. At high concentration of dye, removal efficiency decreases due to competitive adsorption on active sites of adsorbent [134]. At low pH due to increased ionization, electrostatic attraction between negatively charged dye molecules and positively charged polymer molecules increases which accelerates adsorption of dye molecules [135].
5.6 Waste Textile Fiber Copolymer
The prime component of textile industry is fiber, either natural or synthetic which can be converted to branched copolymer by free radical or ionic polymerization of chemical groups. The cellulose molecules in textile fiber show strong interaction with adjacent molecules due to the presence of –OH and develop hydrophilicity, degradability, and absorption ability [136]. Conversion of waste textile into adsorbent material is a novel approach which can be achieved via two ways (a) incorporation of metal-binding groups to the fiber backbone (b) Graft copolymerization (Fig. 9).
Incorporation of metal-binding functionalities into textile fiber produces heavy metal adsorbents [137]. In order to form branched copolymer, the monomers are grafted to fiber backbone [138]. Heating at specific temperature enhances the rate of graft copolymerization thus, among available heating processes the microwave heating is considered as effective and reliable method. Zhou et al. [139] used both microwave and UV radiation and converted waste textiles fiber to an adsorbent by grafting acrylic acid. Cerium(iv) ammonium nitrate was selected as free radical initiator and adsorption was carried out at high pH. Under acidic conditions, H+ ions compete with metal ions for the adsorption and decrease the efficiency of process [140]. However, at alkaline pH deprotonation takes place and the surface of waste textile fiber becomes negative, which further enhances adsorption of cationic species. [141], prepared graft copolymer of textiles waste by the reaction with poly-acrylic acid by the process of free radical polymerization. The chelating properties were developed by the reaction polymer with diamine solution. They studied the removal of Pb(ii) and Cr(vi) ions and reported removal as 11.81 mg/g and 2.19 mg/g, respectively. The advantage of such polymers is they can be easily regenerated and adsorbed metals can be recovered by elution.
6 Conclusion
Enormous number of toxicants have been generated and released into the environment due to insufficient treatment techniques adopted for the treatment of textile wastewater. The challenge is to design a process which is cost effective, easy to operate, less time consuming and able to degrade a wide range of pollutants ranging from nano to polymer and inorganic to organic level. Advanced oxidation process using potent oxidants and high energy radiations either alone or in combination is one of the promising methods. In addition, due to the advancement of nanotechnology, the incorporation of nanoparticle and nanocomposites have provided significant solutions to this problem. Various modified polymers of natural and synthetic origin provide high removal efficacy of pollutants. However, it can be proposed that the best design for textile wastewater treatment can be a hybrid technology which utilizes principles of advanced oxidation process and materials ranging from metallic nanoparticles to polymers.
References
Chavan R (2001) Indian textile industry-environmental. Indian J Fibre Text Res 26:11–21
Vajnhandl S, Valh JV (2014) The status of water reuse in European textile sector. J Environ Manag 141:29–35. https://doi.org/10.1016/j.jenvman.2014.03.014
Sala M, Carmen M (2012) Electrochemical techniques in textile processes and wastewater treatment. Environ Photocatal 2012:1–12. https://doi.org/10.1155/2012/629103
U.S. EPA (2013) Report on the 2013 U.S. Environmental Protection Agency (EPA) international decontamination research and development conference. Research Triangle Park, NC, November 05–07, 2013. U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-14/210, 2014
Johann F, Osma José L, Toca-Herrera S-C (2018) Uses of laccases in the food industry. Enzym Res 2010:1–8. https://doi.org/10.4061/2010/918761
Xiang Y, Xiang Y, Wang L, Li X (2018) Effects of sewage sludge modified by coal gasification slag and electron beam irradiation on the growth of Alhagi sparsifolia Shap and transfer of heavy metals. Environ Sci Pollut Res 25:11636–11645
Haule LV, Carr CM, Rigout M (2016) Preparation and physical properties of regenerated cellulose fibres from cotton waste garments. J Clean Prod 112:4445–4451
Yao L (2014) An evaluation of the carbon footprint and carbon reduction measures of textile raw material stage. Tianjin Polytech Univ J 33:71–76
Mustafa I, Delia TS (2006) Biological treatment of acid dyeing wastewater using a sequential anaerobic/aerobic reactor system. Enzym Microb Technol 38:887–892. https://doi.org/10.1016/j.enzmictec.2005.05.018
Hasanbeigi A, Price L (2015) A technical review of emerging technologies for energy and water efficiency and pollution reduction in the textile industry. J Clean Prod 95:34–48. https://doi.org/10.1016/j.jclepro.2015.02.079
Tehrani-Bagha AR, Amini FL (2010) Decolorization of a reactive dye by UV-enhanced ozonation. Prog Color Color Coat 3:1–8
Körbahti BK, Aktaş N, Tanyolaç A (2007) Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. J Hazard Mater 5:83–90. https://doi.org/10.1016/j.jhazmat.2007.02.005
Ullah A, Farooq M, Nadeem F, Rehman A, Hussain M, Nawaz A, Naveed M (2020) Zinc application in combination with zinc solubilizing Enterobacter sp. MN17 improved productivity, profitability, zinc efficiency, and quality of desi chickpea. J Soil Sci Plant Nutr 20:2133–2144
Korniłłowicz-Kowalska T, Rybczyńska K (2014) Anthraquinone dyes decolorization capacity of anamorphic Bjerkandera adusta CCBAS 930 strain and its HRP-like negative mutants. World J Microbiol Biotechnol 30:1725–1736
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress and its possible reversal by chelation therapy. Indian J Med Res 128:501–530
Robinson BH, Brooks R, Howes AW, Kirkman JH, Gregg PEH (1997) The potential of the high-biomass nickel hyperaccumulator Berkheya coddii for phytoremediation and phytomining. J Geochem Explor 60:115–126
Domínguez A, Couto SR, Sanromán MÁ (2005) Dye decolorization by Trametes hirsuta immobilized into alginate beads. World J Microbiol Biotechnol 21:405–409. https://doi.org/10.1007/s11274-004-1763-x
Correia VM, Stephenson T, Judd SJ (1994) Characterisation of textile wastewaters—a review. Environ Technol 15:917–929
Nahar K, Chowdhury MAK, Chowdhury MAH (2018) Heavy metals in handloom-dyeing effluents and their biosorption by agricultural byproducts. Environ Sci Pollut Res 25:7954–7967. https://doi.org/10.1007/s11356-017-1166-9
Banat IM, Nigam P, Singh D, Marchant R (1996) Microbial decolorization of textile-dye containing effluents: a review. Bioresour Technol 58:217–227
Sharifuzzaman SM, Rahman H, Ashekuzzaman SM, Islam MM, Chowdhury SR, Hossain MS (2016) Heavy metals accumulation in coastal sediments. In: Environmental remediation technologies for metal-contaminated soils. Springer, Tokyo, pp 21–42
Gautam RK, Mudhoo A, Lofrano G, Chattopadhyaya MC (2014) Biomass-derived biosorbents for metal ions sequestration: adsorbent modification and activation methods and adsorbent regeneration. J Environ Chem Eng 2:239–259
Mittal M, Flora SJS (2006) Effects of individual and combined exposure to sodium arsenite and sodium fluoride on tissue oxidative stress, arsenic and fluoride levels in male mice. Chem Biol Interact 162:128–139
Mittal M, Chatterjee S, Flora SJS (2018) Combination therapy with vitamin C and DMSA for arsenic–fluoride co-exposure in rats. Metallomics 10:1291–1306
Mathur N (2005) Mutagenicity assessment of effluents from textile/dye industries of Sanganer, Jaipur (India): a case study. Ecotoxicol Environ Saf 61:105–113. https://doi.org/10.1016/j.ecoenv.2004.08.003
Akhtar MF, Ashraf M, Ahmad AA, Javeed A, Sharif A, Saleem A, Akhtar B (2016) Textile industrial effluent induces mutagenicity and oxidative DNA damage and exploits oxidative stress biomarkers in rats. Environ Toxicol Pharmacol 41:180–186. https://doi.org/10.1016/j.etap.2015.11.022
Ahmed S, Rasul MG, Martens WN (2011) Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: a review. Water Air Soil Pollut 215:3–29. https://doi.org/10.1007/s11270-010-0456-3
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, Mcgonigle D, McConigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304:838–838
Galafassi S, Nizzetto L, Volta P (2019) Plastic sources: a survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci Total Environ 693:133499. https://doi.org/10.1016/j.scitotenv.2019.07.305
Mahon AM, O’Connell B, Healy MG, O’Connor I, Officer R, Nash R, Morrison L (2017) Microplastics in sewage sludge: effects of treatment. Environ Sci Technol 51:810–818. https://doi.org/10.1021/acs.est.6b04048
Carr SA (2017) Sources and dispersive modes of micro-fibers in the environment. Integr Environ Assess Manag 13:466–469
Abbasi S, Soltani N, Keshavarzi B, Moore F, Turner A, Hassanaghaei M (2018) Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 205:80–87
Barrows APW, Cathey SE, Petersen CW (2018) Marine environment microfiber contamination: global patterns and the diversity of microparticle origins. Environ Pollut 237:275–284
Avagyan R, Luongo G, Thorsén G, Östman C (2015) Benzothiazole, benzotriazole, and their derivates in clothing textiles—a potential source of environmental pollutants and human exposure. Environ Sci Pollut Res 22:5842–5849
Napper IE, Thompson RC (2016) Release of synthetic microplastic plastic fibres from domestic washing machines: effects of fabric type and washing conditions. Mar Pollut Bull 112:39–45. https://doi.org/10.1016/j.marpolbul.2016.09.025
Magni S, Binelli A, Pittura L, Avio CG, Della Torre C, Parenti CC, Gorbi S, Regoli F (2019) The fate of microplastics in an Italian wastewater treatment plant. Sci Total Environ 652:602–610. https://doi.org/10.1016/j.scitotenv.2018.10.269
Enfrin M, Lee J, Gibert Y, Basheer F, Kong L, Dumée LF (2020) Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J Hazard Mater 384:121393
Li J, Zhang K, Zhang H (2018) Adsorption of antibiotics on microplastics. Environ Pollut 237:460–467. https://doi.org/10.1016/j.envpol.2018.02.050
Miller RZ, Ajr W, Winslow BO, Galloway TS, Apw B (2017) Mountains to the sea: river study of plastic and nonplastic microfiber pollution in the northeast USA. Mar Pollut Bull 124:245–251
Fischer EK, Paglialonga L, Czech E, Tamminga M (2016) Microplastic pollution in lakes and lake shoreline sediments—a case study on Lake Bolsena and Lake Chiusi (central Italy). Environ Pollut 213:648–657
Dris R, Gasperi J, Rocher V, Tassin B (2018) Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris megacity: sampling methodological aspects and flux estimations. Sci Total Environ 618:157–164
Lares M, Ncibi MC, Sillanpää M, Sillanpää M (2018) Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res 133:236–246
Shruti VC, Pérez-Guevara F, Elizalde-Martínez I, Kutralam-Muniasamy G (2020) First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks-future research and environmental considerations. Sci Total Environ 726:138580
Kutralam-Muniasamy G, Pérez-Guevara F, Elizalde-Martínez I, Shruti VC (2020) Review of current trends, advances and analytical challenges for microplastics contamination in Latin America. Environ Pollut 115463
El-Shahawi MS (2010) An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 80:1587–1597. https://doi.org/10.1016/j.talanta.2009.09.055
Deng Y, Zhao R (2015) Advanced oxidation processes (AOPs) in wastewater treatment. Curr Pollut Rep 1:167–176
Zangeneh H, Zinatizadeh AAL, Feizy M (2014) A comparative study on the performance of different advanced oxidation processes (UV/O3/H2O2) treating linear alkyl benzene (LAB) production plant’s wastewater. J Ind Eng Chem 20:1453–1461
Lucas MS, Peres JA (2007) Degradation of reactive black 5 by Fenton/UV-C and ferrioxalate/H2O2/solar light processes. Dye Pigment 74:622–629
Meriç S, Selçuk H, Belgiorno V (2005) Acute toxicity removal in textile finishing wastewater by Fenton’s oxidation, ozone and coagulation–flocculation processes. Water Res 39:1147–1153
GilPavas E, Dobrosz-Gómez I, Gómez-García MÁ (2019) Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Sci Total Environ 651:551–560
Ghanbari F, Moradi M (2015) A comparative study of electrocoagulation, electrochemical Fenton, electro-Fenton and peroxi-coagulation for decolorization of real textile wastewater: electrical energy consumption and biodegradability improvement. J Environ Chem Eng 3:499–506
Koch M, Yediler A, Lienert D, Insel G, Kettrup A (2002) Ozonation of hydrolyzed azo dye reactive yellow 84 (CI). Chemosphere 46:109–113
Arslan I, Balcioglu IA (2001) Advanced oxidation of raw and biotreated textile industry wastewater with O3, H2O2/UV-C and their sequential application. J Chem Technol Biotechnol Int Res Process Environ Clean Technol 76:53–60
Langlais B, Reckhow DA, Brink DR (1991) Ozone in water treatment––application and engineering. Lewis Publishers, Michigan
Rajeswari R, Kanmani S (2009) Degradation of pesticide by photocatalytic ozonation process and study of synergistic effect by comparison with photocatalysis and UV/ozonation processes. J Adv Oxid Technol 12(2):208–214
Saharan VK, Pinjari DV, Gogate PR, Pandit AB (2014) Advanced oxidation technologies for wastewater treatment: an overview. Elsevier, Butterworth, Heinemann, UK, pp 141–191
Al-Kdasi A, Idris A, Saed K, Guan CT (2004) Treatment of textile wastewater by advanced oxidation processes—a review. Glob NEST Int J 6:222–230
Ebrahiem EE, Al-Maghrabi MN, Mobarki AR (2017) Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab J Chem 10:S1674–S1679
Crittenden JC, Hu S, Hand DW, Green SA (1999) A kinetic model for H2O2/UV process in a completely mixed batch reactor. Water Res 33(10):2315–2328
Andreozzi R, Caprio V, Marotta R (2001) Oxidation of benzothiazole, 2-mercaptobenzothiazole and 2-hydroxybenzothiazole in aqueous solution by means of H2O2/UV or photo assisted Fenton systems. J Chem Technol Biotechnol Int Res Process Environ Clean Technol 76:196–202
Aleboyeh A, Moussa Y, Aleboyeh H (2005) The effect of operational parameters on UV/H2O2 decolorization of acid blue 74. Dyes Pigm 66:129–134
Shen YS, Wang DK (2002) Development of photoreactor design equation for the treatment of dye wastewater by UV/H2O2 process. J Hazard Mater 89:267–277
Yang Y, Wyatt II, Travis D, Bahorsky M (1998) Decolorization of dyes using UV/H2O2 photochemical oxidation. Text Chem Color 30:27–35
Azbar NURI, Yonar T, Kestioglu K (2004) Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 55:35–43
Singh R, Verma RS (2010) Advance oxidation processes for textile waste water treatment-At a glance. Curr World Environ 5:317
Glaze WH, Kang JW (1989) Advanced oxidation processes. Description of a kinetic model for the oxidation of hazardous materials in aqueous media with ozone and hydrogen peroxide in a semibatch reactor. Ind Eng Chem Res 28:1573–1580
Oliveira DF, Batista PS, Muller PS Jr, Velani V, França MD, De Souza DR, Machado AE (2012) Evaluating the effectiveness of photocatalysts based on titanium dioxide in the degradation of the dye Ponceau 4R. Dye Pigment 92:563–572
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B Environ 49:1–14
Horikoshi S, Serpone N (2020) Can the photocatalyst TiO2 be incorporated into a wastewater treatment method? Background and prospects. Catal Today 340:334–346
Singla P, Pandey OP, Singh K (2016) Study of photocatalytic degradation of environmentally harmful phthalate esters using Ni-doped TiO2 nanoparticles. Int J Environ Sci Technol 13:849–856
Šuligoj A, Štangar UL, Ristić A, Mazaj M, Verhovšek D, Tušar NN (2016) TiO2–SiO2 films from organic-free colloidal TiO2 anatase nanoparticles as photocatalyst for removal of volatile organic compounds from indoor air. Appl Catal B Environ 184:119–131
Janczarek M, Wei Z, Endo M, Ohtani B, Kowalska E (2016) Silver-and copper-modified decahedral anatase titania particles as visible light-responsive plasmonic photocatalyst. J Photonics Energy 7:012008
Singla P, Sharma M, Pandey OP, Singh K (2014) Photocatalytic degradation of azo dyes using Zn-doped and undoped TiO2 nanoparticles. Appl Phys A 116:371–378
Manivel A, Naveenraj S, Kumar S, Selvam P, Anandan S (2010) CuO-TiO2 nanocatalyst for photodegradation of acid red 88 in aqueous solution. Sci Adv Mater 2(1):51–57
Ray AK, Beenackers AA (1998) Development of a new photocatalytic reactor for water purification. Catal Today 40:73–83
Mahmoodi NM, Arami M (2009) Degradation and toxicity reduction of textile wastewater using immobilized titania nanophotocatalysis. J Photochem Photobiol B Biol 94:20–24
Mahmoodi NM, Arami M, Limaee NY, Tabrizi NS (2006) Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J Colloid Interface Sci 295:159–164
Mahmoodi NM, Borhany S, Arami M, Nourmohammadian F (2008) Decolorization of colored wastewater containing azo acid dye using photo-Fenton process: operational parameters and a comparative study. J Color Sci Technol 2:31–40
Essawy AA, Aleem SAE (2014) Physico-mechanical properties, potent adsorptive and photocatalytic efficacies of sulfate resisting cement blends containing micro silica and nano-TiO2. Constr Build Mater 52:1–8
Abdullah M, Low GK, Matthews RW (1990) Effects of common inorganic anions on rates of photocatalytic oxidation of organic carbon over illuminated titanium dioxide. J Phys Chem 94:6820–6825
Parent Y, Blake D, Magrini-Bair K, Lyons C, Turchi C, Watt A, Prairie M (1996) Solar photocatalytic processes for the purification of water: state of development and barriers to commercialization. Sol Energy 56:429–437
Thanekar P, Lakshmi NJ, Shah M, Gogate PR, Znak Z, Sukhatskiy Y, Mnykh R (2020) Degradation of dimethoate using combined approaches based on hydrodynamic cavitation and advanced oxidation processes. Process Saf Environ Prot 143:222–230
Pant HR, Pant B, Pokharel P, Kim HJ, Tijing LD, Park CH, Kim CS (2013) Photocatalytic TiO2–RGO/nylon-6 spider-wave-like nano-nets via electrospinning and hydrothermal treatment. J Membr Sci 429:225–234
Wang JC, Lou HH, Xu ZH, Cui CX, Li ZJ, Jiang K, Shi W (2018) Natural sunlight driven highly efficient photocatalysis for simultaneous degradation of rhodamine B and methyl orange using I/C codoped TiO2 photocatalyst. J Hazard Mater 360:356–363
EL-Mekkawi DM, Abdelwahab NA, Mohamed WA, Taha NA, Abdel-Mottaleb MSA (2020) Solar photocatalytic treatment of industrial wastewater utilizing recycled polymeric disposals as TiO2 supports. J Clean Prod 249:119430
Pinho LX, Azevedo J, Brito A, Santos A, Tamagnini P, Vilar VJ, Boaventura RA (2015) Effect of TiO2 photocatalysis on the destruction of Microcystis aeruginosa cells and degradation of cyanotoxins microcystin-LR and cylindrospermopsin. Chem Eng J 268:144–152
Thomas M, Naikoo GA, Sheikh MUD, Bano M, Khan F (2016) Effective photocatalytic degradation of Congo red dye using alginate/carboxymethyl cellulose/TiO2 nanocomposite hydrogel under direct sunlight irradiation. J Photochem Photobiol A Chem 327:33–43
Ambashta RD, Sillanpää M (2010) Water purification using magnetic assistance: a review. J Hazard Mater 180:38–49
Qadri S, Ganoe A, Haik Y (2009) Removal and recovery of acridine orange from solutions by use of magnetic nanoparticles. J Hazard Mater 169:318–323
Peng X, Yan Z, Cheng X, Li Y, Wang A, Chen L (2019) Quaternary ammonium-functionalized rice straw hydrochar as efficient adsorbents for methyl orange removal from aqueous solution. Clean Technol Environ Policy 21:1269–1279
Kamran S, Tavallali H, Azad A (2014) Fast removal of reactive red 141 and reactive yellow 81 from aqueous solution by Fe3O4 magnetic nanoparticles modified with ionic liquid 1-octyl-3-methylimidazolium bromide. Biquarterly Iran J Anal Chem 1:78–86
Mahmoodi NM, Abdi J, Bastani D (2014) Direct dyes removal using modified magnetic ferrite nanoparticle. J Environ Health Sci Eng 12(1):1–10
Jabbar EI, ElHafdi M, Benchikhi M, El Ouatib R, Er-Rakho L, Essadki A (2019) Photocatalytic degradation of navy blue textile dye by nanoscale cobalt aluminate prepared by polymeric precursor method. Environ Nanotechnol Monit Manag 12:100259
Rahimi-Nasrabadi M, Pourmohamadian V, Karimi MS, Naderi HR, Karimi MA, Didehban K, Ganjali MR (2017) Assessment of supercapacitive performance of europium tungstate nanoparticles prepared via hydrothermal method. J Mater Sci Mater Electron 28:12391–12398
Tatarchuk T, Al-Najar B, Bououdina M, Ahmed MA (2019) Catalytic and photocatalytic properties of oxide spinels. In: Handbook of ecomaterials. Springer International Publishing, New York, pp 1701–1750
Abdel-Khalek EK, Rayan DA, Askar AA, Maksoud MA, El-Bahnasawy HH (2021) Synthesis and characterization of SrFeO3−δ nanoparticles as antimicrobial agent. J Sol Gel Sci Technol 97:27–38
Katsoyiannis IA, Ruettimann T, Hug SJ (2008) pH dependence of Fenton reagent generation and As(iii) oxidation and removal by corrosion of zero valent iron in aerated water. Environ Sci Technol 42:7424–7430
Kamat PV, Meisel D (2003) Nanoscience opportunities in environmental remediation. Comptes Rendus Chim 6:999–1007
Tan C, Dong Y, Fu D, Gao N, Ma J, Liu X (2018) Chloramphenicol removal by zero valent iron activated peroxymonosulfate system: kinetics and mechanism of radical generation. Chem Eng J 334:1006–1015
Shih YH, Tso CP, Tung LY (2010) Rapid degradation of methyl orange with nanoscale zerovalent iron particles. Nanotechnology 7:7
Chen JL, Al-Abed SR, Ryan JA, Li Z (2001) Effects of pH on dechlorination of trichloroethylene by zero-valent iron. J Hazard Mater 83:243–254
Tiraferri A, Chen KL, Sethi R, Elimelech M (2008) Reduced aggregation and sedimentation of zero-valent iron nanoparticles in the presence of guar gum. J Colloid Interface Sci 324:71–79
Machado S, Pacheco JG, Nouws HPA, Albergaria JT, Delerue-Matos C (2015) Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci Total Environ 533:76–81
Zhang X, Lin S, Chen Z, Megharaj M, Naidu R (2011) Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: reactivity, characterization and mechanism. Water Res 45(11):3481–3488
Ali SW, Mirza ML, Bhatti TM (2015) Removal of Cr(vi) using iron nanoparticles supported on porous cation-exchange resin. Hydrometallurgy 157:82–89
Mao H, Qiu Z, Shen Z, Huang W (2015) Hydrophobic associated polymer based silica nanoparticles composite with core–shell structure as a filtrate reducer for drilling fluid at utra-high temperature. J Pet Sci Eng 129:1–14
Shu HY, Chang MC, Chang CC (2009) Integration of nanosized zero-valent iron particles addition with UV/H2O2 process for purification of azo dye acid black 24 solution. J Hazard Mater 167:1178–1184
Wang D, Ma W, Han H, Li K, Hao X (2017) Enhanced treatment of Fischer-Tropsch (FT) wastewater by novel anaerobic biofilm system with scrap zero valent iron (SZVI) assisted. Biochem Eng J 117:66–76
Ishak SA, Murshed MF, Md Akil H, Ismail N, Md Rasib SZ, Al-Gheethi AAS (2020) The application of modified natural polymers in toxicant dye compounds wastewater: a review. Water 12:2032
Vilar VJ, Botelho CM, Boaventura RA (2007) Methylene blue adsorption by algal biomass based materials: biosorbents characterization and process behaviour. J Hazard Mater 147:120–132
Gunatilake SK (2015) Methods of removing heavy metals from industrial wastewater. Methods 1:14
Aisyah SI, Norfariha S, Azlan M, Norli I (2014) Comparison of synthetic and natural organic polymers as flocculant for textile wastewater treatment. Iran J Energy Environ 5:436–445
Janaki V, Vijayaraghavan K, Oh BT, Ramasamy AK, Kamala-Kannan S (2013) Synthesis, characterization and application of cellulose/polyaniline nanocomposite for the treatment of simulated textile effluent. Cellulose 20:1153–1166. https://doi.org/10.1007/s10570-013-9910-x
Othmani A, Kesraoui A, Boada R, Seffen M, Valiente M (2019) Textile wastewater purification using an elaborated biosorbent hybrid material (luffa–cylindrica–zinc oxide) assisted by alternating current. Water 11:1326
Buthelezi SP, Olaniran AO, Pillay B (2012) Textile dye removal from wastewater effluents using bioflocculants produced by indigenous bacterial isolates. Molecules 17:14260–14274
Gao SL, Mäder E, Plonka R (2008) Nanocomposite coatings for healing surface defects of glass fibers and improving interfacial adhesion. Compos Sci Technol 68:2892–2901
Lange LE, Obendorf SK (2012) Effect of plasma etching on destructive adsorption properties of polypropylene fibers containing magnesium oxide nanoparticles. Arch Environ Contam Toxicol 62:185–194. https://doi.org/10.1007/s00244-011-9702-y
Woo DJ, Obendorf SK (2014) MgO-embedded fibre-based substrate as an effective sorbent for toxic organophosphates. RSC Adv 4:15727–15735
Othman FEC, Yusof N, Jaafar J, Ismail AF, Abdullah N, Hasbullah H (2016) Adsorption of cadmium(ii) ions by polyacrylonitrile-based activated carbon nanofibers/magnesium oxide as its adsorbents. Malays J Anal Sci 20:1467–1473
Almasian A, Giahi M, Fard GC, Dehdast SA, Maleknia L (2018) Removal of heavy metal ions by modified PAN/PANI-nylon core-shell nanofibers membrane: filtration performance, antifouling and regeneration behavior. Chem Eng J 351:1166–1178
Banerjee P, Mukhopadhyay A, Das P (2019) Graphene oxide–nanobentonite composite sieves for enhanced desalination and dye removal. Desalination 451:231–240
Guo H, Jiao T, Zhang Q, Guo W, Peng Q, Yan X (2015) Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res Lett 10:1–10
Jiao T, Liu Y, Wu Y, Zhang Q, Yan X, Gao F, Li B (2015) Facile and scalable preparation of graphene oxide-based magnetic hybrids for fast and highly efficient removal of organic dyes. Sci Rep 5:1–10
Noreen S, Tahira M, Ghamkhar M, Hafiz I, Bhatti HN, Nadeem R, Younas F (2021) Treatment of textile wastewater containing acid dye using novel polymeric graphene oxide nanocomposites (GO/PAN, GO/PPy, GO/PSty). J Mater Res Technol 14:25–35
Guo R, Jiao T, Li R, Chen Y, Guo W, Zhang L, Peng Q (2018) Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS Sustain Chem Eng 6:1279–1288
Zhang H, Hines D, Akins DL (2014) Synthesis of a nanocomposite composed of reduced graphene oxide and gold nanoparticles. Dalton Trans 43:2670–2675
Petosa AR, Jaisi DP, Quevedo IR, Elimelech M, Tufenkji N (2010) Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions. Environ Sci Technol. https://doi.org/10.1021/es100598h
Fan H, Wang L, Zhao K, Li N, Shi Z, Ge Z, Jin Z (2010) Fabrication, mechanical properties, and biocompatibility of graphene-reinforced chitosan composites. Biomacromolecules 11:2345–2351. https://doi.org/10.1021/bm100470q.
Chang X, Wang Z, Quan S, Xu Y, Jiang Z, Shao L (2014) Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl Surf Sci 117:43–56. https://doi.org/10.1016/j.apsusc.2014.07.202
Banerjee P, Barman SR, Mukhopadhayay A, Das P (2017) Ultrasound assisted mixed azo dye adsorption by chitosan–graphene oxide nanocomposite. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2016.10.009
Sharma R, Singh N, Gupta A, Tiwari S, Tiwari SK, Dhakate SR (2014) Electrospun chitosan-polyvinyl alcohol composite nanofibers loaded with cerium for efficient removal of arsenic from contaminated water. J Mater Chem A. https://doi.org/10.1039/c4ta02363c
Xu X, Tian M, Qu L, Zhu S (2016) Graphene oxide/chitosan/polyvinyl-alcohol composite sponge as effective adsorbent for dyes. Water Environ Res. https://doi.org/10.2175/106143016x14609975746127
Das L, Das P, Bhowal A, Bhattachariee C (2020) Synthesis of hybrid hydrogel nano-polymer composite using graphene oxide, chitosan and PVA and its application in waste water treatment. Environ Technol Innov 18:100664. https://doi.org/10.1016/j.eti.2020.100664
Santhi T, Manonmani S, Smitha T (2010) Removal of methyl red from aqueous solution by activated carbon prepared from the Annona squamosa seed by adsorption. Chem Eng Res Bull 14:11–18
Mahmoodi NM, Abdi J, Taghizadeh M, Taghizadeh A, Hayati B, Shekarchi AA, Vossoughi M (2019) Activated carbon/metal-organic framework nanocomposite: preparation and photocatalytic dye degradation mathematical modeling from wastewater by least squares support vector machine. J Environ Manag 233:660–672
Wang S, Lu A, Zhang L (2016) Recent advances in regenerated cellulose materials. Prog Polym Sci 53:169–206
Singh V, Tiwari A, Tripathi DN, Sanghi R (2006) Microwave enhanced synthesis of chitosan-graft-polyacrylamide. Polymer 47:254–260
Littunen K, Hippi U, Saarinen T, Seppala J (2013) Network 611 formation of nanofibrillated cellulose in solution blended 612 poly(methyl methacrylate) composites. Carbohydr Polym 91:183–190
Zhou G, Byun JH, Oh Y, Jung BM, Cha HJ, Seong DG, Chou TW (2017) Highly sensitive wearable textile-based humidity sensor made of high-strength, single-walled carbon nanotube/poly(vinyl alcohol) filaments. ACS Appl Mater Interface 9:4788–4797
Zhu Y, Hu J, Wang J (2014) Removal of Co2+ from radioactive wastewater by polyvinyl alcohol (PVA)/chitosan magnetic composite. Prog Nucl Energy 71:172–178
Racho P, Waiwong W (2020) Modified textile waste for heavy metals removal. Energy Rep 6:927–932
Acknowledgements
Author thanks Prof. (Dr.) I. K. Bhat Vice chancellor Manav Rachna University for providing all kinds of support for writing this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bansal, M. (2022). Textile Wastewater Treatment Using Sustainable Technologies: Advanced Oxidation and Degradation Using Metal Ions and Polymeric Materials. In: Muthu, S.S., Khadir, A. (eds) Advanced Oxidation Processes in Dye-Containing Wastewater. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-19-0987-0_9
Download citation
DOI: https://doi.org/10.1007/978-981-19-0987-0_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0986-3
Online ISBN: 978-981-19-0987-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)