Abstract
Globally, the textile industry is considered as a major contributor to the development of the country. However, the improper disposal of colored wastewater in the ecosystem leads to various environmental and health-related problems. Color removal, mainly from the textile wastewater effluent, has been the biggest challenge from the past decade. Various physiochemical, biotechnological, and nanotechnology methods have been used to overcome these challenges. However, no such method has been reported for effective and economical treatment for textile wastewater. Effluent from textile processes such as dyeing, manufacturing, and finishing processes contains a high concentration of chemicals, including acids, binders, salts, etc., which are hazardous to environment and ecosystem. Furthermore, various chemicals used for the sizing, softening, and brightening of the fabric are also present in the wastewater. Therefore, textile wastewater effluent needs an eco-friendly and economically viable method for effective treatment. This book chapter provides a critical review on the advancement in treatment technologies available for decolorization, degradation, and mineralization of the textile wastewater and also suggested an effective and economically viable alternative for textile effluent.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 Introduction
The environmental problems and toxic pollutants produced by industrial processes are one of the major challenges faced worldwide. Among the industries, the textile industry has been increasing consistently with a significant contribution to global development. However, textile manufacturing yields a large amount of colored and highly toxic wastewater, which leads to hazardous effects on the environment (Jilani 2015). The usage of synthetic dye has been increased subsequently in the textile and dyeing industry due to their cost-effectiveness, high stability to light, temperature, detergent, and microbial attack as compared to natural dye (El-kassas 2014). Almost 10,000 different synthetic dyes are currently manufactured mainly azo dyes and are frequently used by the textile and various other industries for dyeing due to more economical stability than natural dyes (Xu et al. 2007b). Azo dyes are the largest class of synthetic dye, which are characterized by the presence of azo (-N=N-) groups in their structure. These dyes are toxic and highly persistent to the environment, and their metabolic products are mutagenic and carcinogenic in nature (Xu et al. 2007a). The presence of these toxic and carcinogenic products leads to the serious environmental pollution. A large amount of these textile dyes and pigments are lost during the finishing process and discharged as industrial effluents at various stages. The presence of azo and vat dyes, sodium silicates, formaldehyde-based dye fixing agents, softeners and non-biodegradable agents, soaps and detergents, and heavy metals is a continuous threat to all living beings. It is reported that nearly 10–15% of these dyes are directly discharged into open water bodies, such as rivers and lakes during the manufacturing process (Mani and Bharagava 2018).
In India, due to high demand for cotton and polyester, the textile industries consume around 80% of the total production of 1,30,000 tons of dyestuff. These dyes in wastewater affect aquatic life due to the occurrence of toxic metal components. Presently, heterocyclic and aromatic are widely used by the textile industry. The complex and stable structure of dye is poising a greater difficulty in degrading the textile wastewater but also in any kind of complex matrix (Holkar et al. 2016). The use of synthetic dyes has tremendously increased in the textile processes, and its exposure to human has led to health concerns and serious ecological consequences. Therefore, there is an urgent need for treatment of textile effluent before being discharged to the open environment.
The treatment of textile effluent involves physical, chemical, and biological methods such as coagulation, adsorption, filtration, and using chemical flocculates. However, many technologies have been developed by the scientific community such as bioremediation of azo dye using bacteria, fungi, and plant extract (Lavanya 2014). Moreover, the enzymatic remediation of azo dye seems to be the most promising remediation method. However, isolation and characterization of the various potential enzymes for azo dye degradation is a time-consuming and expensive process. To overcome the disadvantage of chemical and physical methods, the researchers have focused on the development of the nanoparticles. Nanotechnology is the alternative to the conventional physicochemical approaches as it is clean and eco-friendly to the environment (Khatoon N and Sardar M 2017).
The aim of this chapter is to provide an overview of the different wet processing steps in the textile industry. This chapter also explains the current treatment technologies for degradation of the textile wastewater.
16.2 Processing Steps in the Textile Industry
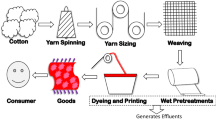
The manufacturing steps in textile industries involve different processes from the processing of the raw material to the final product development. It starts in collecting and arranging the fibers, then transforming fibers into yarn, and altering the yarn into fabric, and then this fabric goes into various wet processing steps. The major steps involved in the manufacturing process of textile industries include yarn formation, fabric formation, fabric/wet processing, and textile fabrication (Madhav et al. 2018). The wet processing steps of textile fabrics are described in details as follows (Fig. 16.1):
16.2.1 Sizing
In this process, the protruding loose fibers are removed by the action of heat or direct flame. The sizing agents are selected based on the type of textile material, cost-efficacy, etc. This step utilizes the cellulosic- and protein-based starch derivatives such as methylcellulose and oxyethlycellulose, glue, and gelatin as sizing agents for the initial texture smoothing of the textile material. Moreover, carboxymethylcellulose (CMC) and polyvinyl alcohol (PVA) are widely used as a secondary sizing agent for cotton yarns (Madhav et al. 2018). The other similar synthetic anionic groups such as modified polyesters, glycols, sulfonic acids, and polyurethane anionomer are used in the sizing of the textile industries (Regan 1962).
16.2.2 De-sizing
In the de-sizing process, the threads are attached together, preventing them from breakages in the loom, where the warps are strengthened with mechanical stresses and friction with the help of de-sizing agents (Regan 1962). The de-sizing agents are originally enzymes produced from syrup extracts such as amylase, maltase, cellulose, etc. The enzymatic-based de-sizing agents are widely used in wet textile processing because it produced little wastewater and is eco-friendly to fabric texture (Chatha et al. 2017).
16.2.3 Scouring
The term scouring in textile industry implies removing the impurities without causing damage to the fabric. The impurity includes dirt, surfactants, soaps, fats, gums, pectin, oils, and waxes along with nonfibrous impurities. In this process, both natural and added impurities are removed with hydrophobic functionalities as completely as possible. In textile processing, consumption of extensive alkaline-based treatment leads to formation of various by-products in the wastewater effluent and poses severe damage to cellulose content of the fabric (Chatha et al. 2017). To overcome such problems, a wide range of industrially relevant enzyme such as cellulases, pectinase, proteases, and lipases along with the mix of the consortium has been investigated (Tzanov et al. 2001).
16.2.4 Bleaching
The purpose of the bleaching of the textile fibers is to remove the remaining stains and attached sizing, de-sizing, or scouring agents used in the previous steps and increase the dyeing efficiency of the textiles. The widely used bleaching agents in the textile industry include oxidizing agents such as H2O2, O3, Ca(OCl)Cl, Ca(OCl)2, NaOCl, K2Cr2O7, etc. and reducing agents such as FeSO4, H2S, Na2SO4, NaHSO4, etc. Among various bleaching agents, the hypochlorite, hydrogen peroxide, oxalic acid, and sodium silicate are widely used (Tanapongpipat et al. 2008).
16.2.5 Mercerizing
After the bleaching process, the mercerizing in textile industry is utilized to improve the tensile strength, durability, luster, and dyeing efficiency of the fabrics. In the mercerizing process, the cotton fabric is treated with a high concentration (about 18–24% by weight) of sodium hydroxide. After that, the fabric goes through the longitudinal shrinkage during impregnation in the sodium hydroxide solution. The most commonly used mercerizing agents for the cotton fiber are caustic soda and sodium hydroxide, which change the crystalline nature of the cellulose and cotton fibers (Ghosh et al. 2004; Duchemin et al. 2012). The excessive mercerizing agent is removed by washing 1–3 min while holding the cotton fabric under stress.
16.2.6 Dyeing and Printing
Dyeing is the process in which the fabric or yarn is treated with a dye to impart color. The auxochrome groups such as amine, carboxyl, sulphonate, hydroxyl, and chromophore groups like azo, carbonyl, nitro, quinoid groups in the dye are responsible for the color (Waring and Hallas 2013). There are several types of dyeing agents such as direct dyes, acid dyes, basic dyes, mordant dyes, and vat dyes for dyeing different kinds of fibers, etc. The different synthetic dyes have their own affinity toward a particular type of fiber (Rippon and Evans 2012). These dyes are also responsible for water pollution by rendering unacceptable color in the textile wastewater.
The process of printing is similar to dyeing, except in a way that printing is intended to impart color to the specific area of the textile material in the form of solution, while in dying, dye is applied in the form of thick paste to prevent its spread. The printing process also generates wastewater similar to dyeing effluent (Holkar et al. 2016; Manekar et al. 2014).
16.2.7 Finishing
In this process, fabrics are exposed to several types of finishing process like washing, drying, pressing, and conditioning. Specific properties such as softening, waterproofing, antibacterial, and UV protective are imparted to the fabric during the finishing process (C.R. Holkar et al. 2016). The list of pollutant compounds that are produced at different stages of wet processing is shown in Table 16.1.
16.3 Current Treatment Technology
16.3.1 Bio-based Technologies for Degradation and Mineralization
Recent decades have witnessed several physical- and chemical-based methods, which have been used for the decolorization of azo dye (Fig. 16.1). But these methods are costly, producing a huge amount of sludge after the treatment and required safe disposal (Uday et al. 2016: Liu et al. 2019). Physical method for the treatment such as adsorption and membrane filtration of textile effluent needs further treatment, so it has also been a time-consuming process (Uday et al. 2016). Apart from the recent development, biological treatment methods are most appropriate for degradation of effluent and also cost-effective and eco-friendly in nature (Song et al. 2003: Chen 2006). Most of the bio-based bioremediation is usually done by the use of microorganisms (algae, fungus, and bacteria) to remove the dye contained from the effluent. In this approach, microorganisms have the ability to adapt themselves in toxic wastes or dye effluent and develop naturally new resistant strain (Saratale et al. 2011). However, such strain effective for the dye removal transforms toxic dye/chemicals to less toxic or harmful. The treatment of dye has several advantages and disadvantages with different treatment methods and a wide range of applicability (Fig. 16.2).
16.3.1.1 Microalgae-Based Biodegradation of Textile Effluent
Treatment of textile effluent using microalgae is the most important and effective process. Bio-based treatment process using the microalgae has potential to bio-transforming, biodegrading, and adsorbing the dye which is present in natural wastewater (Aragaw and Asmare 2018). However, the rate of degradation was higher when using the microalgae compared to bacteria and fungus and subsequently eliminates the pollution in particulate wastewater (Aragaw and Asmare 2018). In most of the cases, green microalgae were used for the treatment of dye effluent, and such algae are present in freshwater and saltwater ecosystem (Gupta et al. 2014; Devi et al. 2016). Algae grown on the surface of waste water have large surface area which leads to high biosorption potential. Hence, the electrostatic force of attraction towards contamination present in wastewater is high. Several reports about harmful metabolites present in the wastewater such as PO43, RCOO−, -NH2, and –OH are absorbed by the algae present in the wastewater surface (Al-Fawwaz and Abdullah 2016).
Mechanism of decolorization of the dye by algae is different as compared to fungus or bacteria. In the first stage, the algae biomass is converted into carbon dioxide and water. Second stage algae participate in the conversion of chromophore material to the nonchromophore material; after that final stage, chromophore is adsorbed by the algal biomass (Alvarez et al. 2015). Many studies have been reported (Table 16.2) showing the effective use of the algae against azo dye degradation by producing the azoreductase enzyme for decolorization. Other studies have reported that algae species utilize azo dyes as C and N source for their growth.
16.3.1.2 Fungal-Based Biodegradation of Textile Effluent
Fungus-based biological methods for the treatment of dye were widely studied in white-rot fungus; such organism has the ability to degrade plant lignin and other polymers found in the plant cell wall (Bar and Aust 1994). However, not only white-rot fungus participates in decolorization of textile effluent, there are also different fungus strains reported which can decolorize or biosorb various dye or dye effluents (Przystas et al. 2015). According to the mode of action and surrounding environment, fungus can be classified in two types: live cell, which can either decolorize or biosorb the dye, and the dead cell (fungus biomass), which only adsorbs the dye. Several studies of different fungal strains and fungal-based degradations of dye have been reported for treating the wastewater discharge, and these studies successfully replace the physical- and chemical-based treatment methods (Table 16.3) (Lade et al. 2015a).
The biodegradation or bioremediation of effluent from textile, pulp, and paper industries is mainly carried out by using fungus. They are producing extracellular lignin-modifying enzymes, such as lignin peroxidase (Lip), manganese peroxidase (MnP), and laccase (Singh and Singh 2010). However, the participation of theses enzymes for degradation is different from each fungus (Lade et al. 2015b). For bioremediation, as compared to bacteria, different fungal cultures were used due a large amount of biomass, hyphal spectra and filamentous fungi are successfully degrade under certain niches due to large surface area and cell to cell communication (Singh et al. 2012; Joutey et al. 2013).
16.3.1.3 Bacterial-Based Biodegradation of Textile Effluent
The microbial population is already present in the dye effluent or wastewater due to complex feed substance in the wastewater and transforms them into the simplest form, improving treatment process. The treatment of wastewater is a common and reliable technique these days. Several reports already related to treatments of textile effluent, and a large number of numerous bacterial species participate in the degradation of dye and complex mineralization of different sorts of dyes. The huge advantages of such a process are low running costs, nontoxic end products, and being inexpensive. The process of degradation may be aerobic, anaerobic, and combination of both; most of the bacterial species are using the aerobic process for the treatment of dyes. Different types of dyes are degraded by several bacteria, but not all dye degradation by bacteria have been identified. In the framework of bioremediation, several reports have been conducted on numerous assessments on microorganism having the ability to catabolize organic pollutants.
Microorganisms are playing an important role in remediating several organic contaminates which can be used as pollution indicators for different toxic compounds (Everts and Kanwar 1994; Hruby et al. 2016). Using the bacterial culture for degradation is of main advantage as they can grow in a short period of time compared to other microorganisms. The manipulation of genes at genetic level can enhance the dye degradation ability of bacteria. The microorganisms present in the organic pollutants are able to catabolize chlorinated and aromatic hydrocarbon as an energy sources and can be used in the degradation of sulfur-based textile dyes (Yang et al. 2014; Nguyen et al. 2016). Different studies and identification of bacteria show how azo dyes are degraded in a short period of time. Several bacterial enzymes have been reported which participate in the breakdown of the azo bonds under both aerobic and anaerobic conditions (Wang et al. 2017). The intermediate metabolite products of dye should further be catabolized by other bacterial enzymes such as hydroxylase and oxygenase (Elisangela et al. 2009). The microbial consortium is widely used for the degradation of textile effluent due to its combined action of several enzymes. Table 16.4 showed the various bacterial strains and different dyes used for the degradation and few strains capable of decolorizing various types of dye which are structurally different.
16.3.1.4 Degradation Based on Biosorption
Biosorption is also effective using a full process where the biologically active material metabolites are participating in the absorption of textile dyes. Several researches have reported different bacterial species for the treatment of textile effluent which participate in the degradation of dye (Joshi et al. 2019). In the past few years, few reports were available, and researchers tried to use agricultural waste material such as wheat straw, cotton, leaves of pine trees, sugarcane bagasse, shells of cashew nut, fungal and yeast cells, bacterial biomass, and algal biomass (Robinson et al. 2002; Tunc et al. 2009; Deniz and Karaman 2011; Kalaiarasi et al. 2012; Khataee et al. 2013; Ferreira et al. 2015). Using such process is cost-effective and minimal steps are required for processing (Vitorand Corso 2008). The limitation of biosorption process is because of the presence of heteropolysaccharide and lipid segments of the cell wall in the microorganism, which interfere with the adsorption process. The viability of biosorption depends upon several biotic and abiotic factors such as pH, temperature, ionic quality, contact time, concentration of dye, adsorbent dose, and structure of dye is much more important due to adherence in the microbial surface and type of microorganism (Deniz and Karaman 2011).
16.3.2 Nanotechnology-Based Approach
The nanotechnology is a branch of science which deals with the control, integration, and manipulation of various atoms and molecules at the nanoscale to form different materials (Homyak et al. 2008). Nanotechnology opens a new platform to treat wastewater more efficiently due to its higher surface-area-to-volume ratio, high absorption, interaction, and reaction capabilities (Kumar et al. 2017). The removal of hazardous compounds from wastewater with the help of nanomaterials is an eco-friendly and cost-effective technique. The nanomaterials are a small particle with few nanometers and synthesized in different forms such as nanowires, nanotubes, films, quantum dots, and colloids (Raman and Kanmani 2016). Based on the nature of nanomaterials, it may be classified into three main types: nanoadsorbents, nanocatalyst, and nanomembranes (Anjum et al. 2016).
16.3.2.1 Nanoadsorbents
Nanoadsorbents are nanoscale particles synthesized from organic or inorganic materials which have higher affinity towards adsorbing elements. Sorption is a process in which a material referred to as “sorbate” gets adsorbed on another substance, called sorbent, by physical or chemical interaction (Kumar et al. 2017). Sorbents are widely used for the removal of organic pollutants generated from textile effluent. The steps involved in sorption process is presented in (Fig. 16.3) (Kumar et al. 2017).
Nanoparticles possess higher specific surface areas than the bulk particles. They have the ability to functionalize with different chemical groups and thus improves their affinity towards the target contaminants. These two properties make them very effective as sorbents. Moreover, nanosorbents possess nanosized pores which aids in the sorption of contaminants. The reversible nature of adsorbents can be regenerated by suitable desorption processes for multiple uses with a low maintenance cost, high efficiency, and ease of operation (Kumar et al. 2017). Therefore, the adsorption process has emerged as one of the major techniques for removal of pollutants from wastewater (Hua et al. 2012). Some of the nanosorbents are listed below:
Carbon Nanosorbents
Carbon-based nanomaterials are well-known for the adsorption of various organic and inorganic pollutants in wastewater due to its nontoxicity and high sorption capacities. Primarily, activated carbon is used as sorbents, but it is difficult to remove organic and inorganic contaminants and heavy metals at parts per billion (ppb) levels. With the development of nanotechnology carbon nanotubes (CNTs), fullerene and graphene are synthesized and used as nanosorbents (Khajeh et al. 2013). The structure of CNTs, at the atomic scale is well-defined and uniform which makes them different from activated carbons (ACs). In ACs the parameters like pore diameter and adsorption energy are needed to quantify the adsorption whereas,in CNTs, one can deal directly with well-defined adsorption sites present on the adsorbed molecules (Kumar et al. 2017).
Carbon Nanotubes
Carbon nanotubes (CNTs) are used for the removal of dyes and its intermediates coming from textile industry effluent. Active sites of the CNT surface play an important role in dye adsorption on CNTs. Their thermal, mechanical, electrical, and physical properties make them ideal for work on single-walled nanotubes (SWNT) and multi-walled nanotubes (MWNT). The main mechanisms of absorbing different dye compounds by CNTs depend on nature of the dyes that is cationic or anionic (Rajabi et al. 2017). The surface area to volume ratio of CNTs is greater than other common adsorbents which makes them more effective as compared to other commonly available adsorbents. The high contact surface ensures effective collisions between the molecules of the dyes and the surface of the carbon nanotubes, which in turn leads to an improvement in the efficiency of the adsorbent (Machado et al. 2012). Adsorption capacities of dye on CNTs can be enhanced by modifying CNTs with a functional group. Adsorption rate is further dependent on various other factors like pH, temperature, contact time, and initial concentration. For adsorption of cationic dye, high value of pH is favorable, and for anionic dye low value of pH is preferred (Machado et al. 2012). CNTs are also effective in removing polycyclic aromatic compounds (Hayati et al. 2011) and hence find special application in the textile industry as the dyeing process releases the maximum amount of aromatic compounds into the effluent stream. Activated carbon fibers synthesised by electrospinning of CNTs, which are nanoporous in nature showed increased affinity towards the organic sorption for benzene, toluene, xylene, and ethylbenzene (which are used as solvents in the textile industry) than granular activated carbon (Abou et al. 2015).
16.3.2.2 Metal Oxide Nanosorbents
Nanosized metal oxides are gaining attention for removal of heavy metals from textile wastewater compared to other conventional techniques like precipitation, ion exchange, and membrane filtration because of their fast kinetics, high activities, large surface areas, adsorption capacity, and selectivity. Some of the nanosorbents are used for wastewater treatment such as iron oxide, aluminum oxide, titanium oxide, manganese oxide, zinc oxide. Nano CuO adsorbent has been studied for removal of heavy metal ions such as Cd2+ and Fe3+ from wastewater by the adsorption mechanism (Taman et al. 2015). Removal of several organic pollutants present in wastewater using Fe oxide nanosorbents has been studied. The Fe oxides exhibit excellent adsorption capacity for heavy metal ions due to magnetic properties (Kumar et al. 2017). Hua et al. (2012) reported that titanium oxide (TiO2) nanoparticles were able to remove multiple metals (Zn, Cd, Pb, Ni, Cu) from effluent. Studies reveal that removal of various heavy metal ions from wastewaters using nano alumina coated with sodium dodecyl sulfate on which 2,4-dinitrophenylhydrazine was immobilized found that the resultant adsorbent possesses high adsorption capacity for Cd2+, Cr3+, and Pb2+ (Afkhami et al. 2010). Nano-aluminum oxyhydroxides are considered and applied for the extraction of methyl violet from wastewaters and found to be successful (Kerebo et al. 2016). Iron oxide-alumina mixed nanocomposite fiber was prepared by means of electrospinning method, and its sorption nature was studied toward Cu2+, Pb2+, Ni2+, and Hg2+; the percentage removal was found to be in order Hg2+ > Ni2+ > Pb2+ > Cu2+ with adsorption capacities (Mahapatra et al. 2013). Manganese oxide (MnOs) nanoparticle is widely used for the removal of various heavy metals such as arsenic from wastewater. Adsorption of various heavy metals such as Pb2+, Cd2+, and Zn2+ on HMOs usually happens due to the inner-sphere formation mechanism (Anjum et al. 2016). Zinc oxide (ZnO) can absorb heavy metals due to their porous micro-nanostructure with the high surface area. ZnO are widely used as nanoadsorbent for the removal of Co2+, Ni2+, Cu2+, Cd2+, Pb2+, Hg2+, and As3+ heavy metals from wastewater in the form of nano-paltes, nano-assemblies and nano-sheets. Microporous nano-assemblies show high affinity towards the adsorption of mercury, lead and arsenic due to their electropositive nature (Gupta et al. 2015).
Zeolites
Zeolite is an effective and economical option for the removal of basic dye from textile wastewater because of its low cost, high surface area, and high ion-exchange capability. More than 40 different types of zeolites occur in nature (Kumar et al. 2017). The higher adsorption capacity of zeolite is due to high porosity (Chunfeng et al. 2009). Linoptilolite natural zeolite is readily available in nature, and it is commonly used as adsorbent (Kandisa et al. 2016). MCM-22 is a novel nanoporous zeolite as it holds unusual framework topology, high thermal stability, large surface area, and good adsorption capacity, rendering this zeolite very interesting for adsorption and catalysis. It has been proved as an effective adsorbent to remove some basic dyes like methylene blue, crystal violet, and rhodamine B from aqueous solution (Wang et al. 2006). For removing of heavy metals, two natural zeolites were pre-treated with NaOH; it was found that the modified zeolites have high adsorption capacity for lead (Pb) and cadmium (Cd) with a metal removal efficiency of more than 99%.
Dendrimers
Dendrimers are a class of macromolecules classified by a highly branched structure of great regularity with compact shape, and a large number of (reactive) end groups and spaces between the branches for taking up target molecules. Modified dendrimers have been applied to extract dyes either in liquid-liquid systems or liquid-solid systems (B. Hayati et al. 2011). Polyamidoamine (PAMAM) are polymer dendrimers synthesized from a monomer of ethylenediamine. Large internal cavities, high solubility, low viscosity, and the diversification of structure design are key features of PAMAM dendrimers. The surface of PAMAM dendrimer has –NH2 groups which entrap the contaminants and get adsorbed in its internal cavities which makes them prominent feature for degradation of dyes (Zhou et al. 2013). Three-dimensional PAMAM dendrimer-based polymeric network has been applied for extraction of azo dye (extraction of methyl orange dye) from aqueous solution (Ghosh 2008). Taleb et al. (2015) studied the adsorption of Isma acid fast yellow G dye using PAMAM/copper sulfide (CuS)/AA nanocomposite to determine the dye sorption character. The kinetics data indicates that the adsorption of dye was followed by pseudo-second-order kinetics with dye dosage of 25 and 100 mg/L. Also, Sadeghi-kiakhani et al. (2012) investigated chitosan-polypropylene imine (CS-PPI) as a biocompatible adsorbent biopolymer for the removal of textile dyes, namely, Reactive Black 5 (RB5) and Reactive Red 198 (RR198), and resulted in 97 and 99% of dye removal efficiency under optimal condition.
Zerovalent
Zerovalent iron refers to the zero charges carried by each Fe atom. Nano zerovalent iron (nZVI) particle acts as a reducing agent which reduces Fe2+ and Fe3+, and during the reduction process, the hydroxyl or hydrogen ions are generated which react with dye molecules and lead to breakage of chromophore (-N=N-) bond (Raman and Kanmani 2016). The degradation of textile dyes using nZVI particles depends on pH, nZVI dose, initial dye concentration, and dye volume (Samagata and Shah 2014). The efficiency of nZVI reactivity is improved, if the nZVI technique is integrated with other treatments like microwave radiation, ultrasonic irradiation, H2O2 oxidation, and UV illumination (Chen et al. 2011; Satapanajaru et al. 2011).
16.3.2.3 Nanocatalyst
Nanoparticles in the form of nanocatalyst have been enormously used in wastewater treatments as it possesses high catalytic efficiency, high surface area, mass transfer effect, effective enzyme storage, high surface reaction activity, and small size (1–100 nm) (Sharma et al. 2017). The various nanoparticles used for the treatment of textile effluent are tabulated in Table 16.5. A nanostructured catalyst medium which is sensitive to exposure of light is mainly employed to degrade textile pollutants present in wastewater. Such nanocatalyst works on the principle of photocatalysis. High photoactivity, nontoxicity, photostability, cost-effectiveness, and biologically and chemically inert are the properties of an ideal photocatalyst. Photocatalytic activity is produced by illuminating a semiconductor material to photons with energies equal to or larger than the semiconductors bandgap. This generates electron-hole pairs, which produces free radicals (e.g., OH−) to undergo further reactions (Zheng et al. 2011). Photocatalysis is a surface phenomenon, and its general mechanism is explained in Fig. 16.4.
Nanostructured semiconductor photocatalyst usually used includes zinc oxide (ZnO), titanium dioxide (TiO2), zinc sulfide (ZnS), ferric oxide (Fe2O3), and cadmium sulfide (CdS). Key areas in which photocatalysis can be applied for the treatment of textile effluent include the elimination of:
-
Organic contaminants.
-
Inorganic contaminants.
-
Heavy metals.
Removal of Organic Contaminants
Photocatalysis is used for the decomposition of harmful organic contaminants present in wastewater into harmless substances. The organic contaminants, like carboxylic acids, alcohols, phenolic derivatives, and chlorinated aromatic compounds, can be removed from textile wastewater using photocatalysis (Kumar et al. 2017). In this regard, semiconductor metal oxides structured as nanophotocatalyst such as TiO2 and ZnO are proved to be successful. Silver nanocomposites have been successfully prepared by green route using two medicinal plant leaf extract, i.e., Solanum nigrum and Cannabis sativa. These biosynthesized silver nanocomposites were used for the removal of textile dyes (Khatoon and Sardar 2017).
Removal of Inorganic Contaminants
It includes chemicals such as halide ions, cyanide, thiocyanate, ammonia, nitrates, and nitrites that can be effectively decomposed by photocatalytic reaction (Mills et al. 1982). The photocatalytic removal of toxic CH3Hg(II)Cl Hg(II)Cl and has been investigated using TiO2 nanoparticles (Serpone et al. 1987).
Removal of Heavy Metals
Major heavy metals present in wastewater from textile industries include copper, arsenic, lead, cadmium, mercury, and chromium. It has been reported that nickel oxide nanocatalyst is considered as a useful catalyst for the treatment of wastewater containing lead (Mahmoud et al. 2015). TiO2 catalyst has been studied to recover gold (III), platinum (IV), and rhodium (III) chloride salts from wastewater effluent. A combination of activated carbon obtained from sewage sludge along with TiO2 nanoparticles has been used to reduce Hg2+ ions followed by recovery of metallic Hg (P. Senthil Kumar et al. 2017).
16.3.2.4 Nanomembrane
Membranes play an important role in treatment of textile dyes present in waste water system includes microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO). The selection of membrane for the treatment depends upon the pore size of the membrane. The main advantage of membrane-based technology is no requirement for the addition of chemicals. Suspended particles, debris, colloids, and many biological molecules, like sulfur, vat, and azoic dyes, are removed by means of MF and UF membrane at significantly low pressure. Organic and inorganic impurities from textile effluent are removed by RO, as it rejects more than 95% of ionic species (Amini et al. 2011; Thamaraiselvan and Noel 2014). Various nanofiltration membranes, at neutral pH, have negatively charged surface. NF membranes, pore size up to 2 nm, enable separation of salt solutions containing monovalent cations, divalent salts, low molecular weight organic compounds (200–1000 Da), and anions from the dye wastewater (Bruggen et al. 1999; All et al. 2006). The charges present on the surface of the membrane play an important role in permeation and rejection during membrane separation. This enables recovery and reuses tons of salts like NaCl used in dyeing industries. Molecular charge and polarity also have an effect on dye rejection (Bruggen et al. 1999). It has been studied that, in whatever proportion the reactive dye and NaCl used, the hydrolyzed reactive dyes and dye by-products were retained approximately 98% and the mineral salt recovery reached a rate of 98% (All et al. 2006). It has been reported that modified PSf membranes, using sodium p-styrene sulfonate monomer, were applied to treat colored textile effluents exhibiting acceptable performance in terms of flux and rejection. Dye rejection was higher than 96%, and hydraulic permeability was 0.48–0.56 m3 m−2 day−1 at 0.4 MPa (Akbari et al. 2002, 2006). Amini et al. 2011 demonstrate the dye removal ability from colored textile wastewater by using polysulfone ultrafiltration membrane modified with acrylic acid and resulted 86–99.9% dye rejection and 7.6 L m−2 h−1 hydraulic permeability. Polyamide nanomembranes are applied and evaluated for removal of five different fiber reactive dyes found in wastewater, namely, reactive yellow 145, reactive blue 15, reactive black 5, reactive orange 16, and reactive red 194. The flux for all the samples ranged between 7.8 and 9.2 ml/cm2 s (Taylor et al. 2014b). It was observed that the maximum dye rejection was up to 95–99% by using nanofiltration membrane NF45 and DK1073 (Lopes et al. 2005). Nanomembranes can be fabricated with nanoparticles to enhance the remediation efficiency in the aspect of permeability, catalytic reactivity, and fouling resistance. In recent times, high-temperature membrane distillation is also being investigated for dye wastewater treatment as part of zero liquid discharge (ZLD) strategy (Thamaraiselvan and Noel 2014). Though the nanofiltration is advantageous, membrane fouling is a drawback of filtration technique. Dyes introduce the undesirable flux decline by forming a colloidal fouling layer. With the help of feed pre-treatment modification and membrane cleaning, the process of membrane fouling can be prevented (P. Senthil Kumar et al. 2017).
16.4 Conclusions
The demand for better quality processed textile fabric has increased due to the modernization in the recent times across the globe. Therefore, there was an increasing load of waste generated from the textile-based industries. The textile industries waste and effluents are complex mixture in nature due to the presence of synthetic dyes, pigments, heavy metal compounds, and other uncharacterized metal salt complexes formed in the intermediate manufacturing processes. The increasing difficulty and complexity in treating textile wastes have led to a constant search for new techniques that are effective, eco-friendly, and economically viable. However, with the combination of nanotechnology, it become possible to remove both toxic properties and color of the dyes released into the environment.
References
Abou MF, El-trass A, El-sigeny S (2015) Synthesis of polyamidoamine dendrimer (PAMAM/CuS/AA) nanocomposite and its application in the removal of Isma acid fast yellow G Dye. 29. https://doi.org/10.1002/pat.3517
Afkhami A, Saber-tehrani M, Bagheri H (2010) Simultaneous removal of heavy-metal ions in wastewater samples using. J Hazard Mater 181(1–3):836–844. https://doi.org/10.1016/j.jhazmat.2010.05.089
Akbari A, Desclaux S, Remigy JC, Aptel P (2002) Treatment of textile dye effluents using a new photografted nanofiltration membrane. Desalination 149:101–107
Akbari A, Desclaux S, Rouch JC, Aptel P, Remigy JC (2006) New UV-photografted nanofiltration membranes for the treatment of colored textile dye effluents. 286:342–350. https://doi.org/10.1016/j.memsci.2006.10.024
Al-Fawwaz AT, Abdullah M (2016) Decolorization of methylene blue and malachite green by immobilized Desmodesmus sp. isolated from North Jordan. Int J Environ Sci Dev 7:95
Ali SAM, Akthar N (2014) A study on bacterial decolorization of crystal violet dye by Clostridium perfringens, Pseudomonas aeruginosa and Proteus vulgaris. Res Article Biol Sci 4:89–96
All C, Moulin P, Maisseu M, Charbit F (2006) Treatment and reuse of reactive dyeing effluents. 269:15–34. https://doi.org/10.1016/j.memsci.2005.06.014
Alvarez MS, Rodriguez A, Sanroman MA, Deive FJ (2015) Microbial adaptation to ionic liquids. RSC Adv 5(23):17379–17382
Amini M, Arami M, Mohammad N, Akbari A (2011) Dye removal from colored textile wastewater using acrylic grafted nanomembrane. Desalination 267(1):107–113. https://doi.org/10.1016/j.desal.2010.09.014
Anjum M, Miandad R, Waqas M, Gehany F, Barakat MA (2016) Remediation of wastewater using various nano- materials. Arab J Chem. https://doi.org/10.1016/j.arabjc.2016.10.004
Aragaw TA, Asmare AM (2018) Phycoremediation of textile wastewater using indigenous microalgae. Water Pract Technol 13(2):274–284
Babu BR, Parande AK, Raghu S, Kumar TP (2007) Cotton textile processing: waste generation and effluent treatment. Text Technol 11:141–153
Barr DP, Aust SD (1994) Mechanisms the white rot fungi use to degrade pollutants. Environ Sci Technol 28:79A–87A
Caparkaya D, Cavas L (2008) Biosorption of methylene blue by a brown alga Cystoseirabarbatula Kützing. Acta Chim Slov 55(3):547–553
Celia MP, Suruthi S (2016) Textile dye degradation using bacterial strains isolated from textile mill effluent. Int J Appl Res 2:337–341
Cengiz S, Cavas L (2008) Removal of methylene blue by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Bioresour Technol 99(7):2357–2363
Chairin T, Nitheranont T, Watanabe A, Asada Y, Khanongnuch C, Lumyong S (2013) Biodegradation of bisphenol A and decolorization of synthetic dyes by laccase from white-rot fungus, Trametes polyzona. Appl Biochem Biotechnol 169(2):539–545
Chatha SA, Asgher M, Iqbal HM (2017) Enzyme-based solutions for textile processing and dye contaminant biodegradation—a review. Environ Sci Pollut Res 24(16):14005–14018
Chen H (2006) Recent advances in azo dye degrading enzyme research. Curr Protein Pept Sci 7(2):101–111
Chen Z, Jin X, Chen Z, Megharaj M, Naidu R (2011) Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J Colloid Interface Sci 363(2):601–607. https://doi.org/10.1016/j.jcis.2011.07.057
Choo HK, Choi JS, Hwang DE (2007) Effect of coagulant types on textile wastewater reclamation in a combined coagulation/ultrafiltration system. Desalination 202(1–3):262–270
Chunfeng W, Jiansheng LI, Lianjun W, Xiuyun SUN (2009) Adsorption of dye from wastewater by zeolites synthesized from fly ash: kinetic and equilibrium studies. Chin J Chem Eng 17(3):513–521. https://doi.org/10.1016/S1004-9541(08)60239-6
Correia VM, Stephenson T, Judd JS (1994) Characterisation of textile wastewaters – a review. Environ Technol 15(10):917–929
Daneshvar N, Ayazloo M, Khataee AR, Pourhassan M (2007) Biological decolorization of dye solution containing Malachite Green by microalgae Cosmarium sp. Bioresour Technol 98(6):1176–1182
Deniz F, Karaman S (2011) Removal of an azo-metal complex textile dye from colored aqueous solutions using an agro-residue. Microchem J 99(2):296–302
Devi S, Murugappan A, Kannan RR (2016) Textile dye wastewater treatment using freshwater algae in packed-bed reactor: modeling. Desalin Water Treat 57(38):17995–18002. https://doi.org/10.1080/19443994.2015.1085910
Diller BG, Mogahzy YE, Inglesby MK, Zeronian SH (2016) Effects of scouring with enzymes, organic solvents, and caustic soda on the properties of hydrogen peroxide bleached cotton yarn. Text Res J 68(12):920–929
Duchemin B, Thuault A, Vicente A, Rigaud B, Fernandez C, Eve S (2012) Ultrastructure of cellulose crystallites in flax textile fibres. Cellulose 19(6):1837–1854
El-kassas HY (2014) Bioremediation of the textile waste effluent by Chlorella vulgaris. Egypt J Aquat Res 40:301–308
El-safty SA, Shahat A, Ismael M (2012) Mesoporous aluminosilica monoliths for the adsorptive removal of small organic pollutants. J Hazard Mater 201–202:23–32. https://doi.org/10.1016/j.jhazmat.2011.10.088
Elisangela F, Andrea Z, Fabio DG, de Menezes Cristiano R, Regina DL, Artur C-P (2009) Biodegradation of textile azo dyes by a facultative Staphylococcus arlettae strain VN-11 using a sequential microaerophilic/aerobic process. Int Biodeterior Biodegrad 63(3):280–288
Everts CJ, Kanwar RS (1994) Evaluation of rhodamine WT as an adsorbed tracer in an agricultural soil. J Hydrol 153:53–70. https://doi.org/10.1016/0022-1694(94)90186-4
Ezhilarasu A (2016) Textile industry dye degrading by bacterial strain Bacillus sp. Int J Adv Res Biol Sci 3(3):211–226
Falavarjani ER, Khorasani AC, Ghoreishi SM (2012) Microbial reduction of monoazo and diazo-linked dyes by Pseudomonas aeruginosa and Pseudomonas putida. J Pure Appl Microbiol 6:1559–1570
Ferreira BC, Teodoro FS, Mageste AB, Gil LF, de Freitas RP, Gurgel LV (2015) Application of a new carboxylate-functionalized sugarcane bagasse for adsorptive removal of crystal violet from aqueous solution: kinetic, equilibrium and thermodynamic studies. Ind Crop Prod 65:521–534
Ghaly AE, Ananthashankar R, Alhattab MVVR, Ramakrishnan VV (2014) Production, characterization and treatment of textile effluents: a critical review. J Chem Eng Process Technol 5:1–18
Ghosh S (2008) Extraction of azo dye molecules from aqueous solution using polyamidoamine dendrimer based polymeric network. J Chem Res 2008:419–421
Ghosh P, Samanta AK, Basu G (2004) Effect of selective chemical treatments of jute fibre on textile-related properties and processibility. Ind J Fibre Text Res 29(1):85–99
Gulati D, Jha I (2014) Microbial decolorization of dye reactive blue 19 by bacteria isolated from dye effluent contaminated soil. Int J Curr Microbiol App Sci 3:913–922
Gupta VK, Bhushan R, Nayak A, Singh P, Bhushan B (2014) Biosorption and reuse potential of a blue green alga for the removal of hazardous reactive dyes from aqueous solutions. Biorem J 18(3):179–191
Gupta VK, Tyagi I, Sadegh H, Shahryari-ghoshekandi R (2015) Nanoparticles as adsorbent; a positive approach for removal of noxious metal ions: a review. 34(3):195–214. https://doi.org/10.3923/std.2015.195.214
Harane RS, Adivarekar RV (2017) Sustainable processes for pre-treatment of cotton fabric. Text Cloth Sustain 2:2. https://doi.org/10.1186/s40689-016-0012-7
Hayati B, Mahmoodi NM, Arami M, Mazaheri F (2011) Dye removal from colored textile wastewater by poly(propylene imine) dendrimer: operational parameters and isotherm studies. 39(7):673–679. https://doi.org/10.1002/clen.201000182
Hemapriya J, Vijayanand S (2014) Ecofriendly bioremediation of a triphenylmethane dye by textile effluent adapted bacterial strain vp-64. Int J Curr Microbiol App Sci 3(9):983–992
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB (2016) A critical review on textile wastewater treatments : possible approaches. J Environ Manag 182:351–366. https://doi.org/10.1016/j.jenvman.2016.07.090
Homyak GJL, Dutta J, Tabbals HF, Rao A (2008) Introduction to nanoscience. CRG Press, Boca Raton, FL
Hruby CE, Soupir ML, Moorman TB, Shelley M, Kanwar RS (2016) Effects of tillage and poultry manure application rates on Salmonella and fecal indicator bacteria concentrations in tiles draining Des Moines Lobe soils. J Environ Manag 171:60–69
Hu ZG, Zhang J, Chan WL, Szeto YS (2006) The sorption of acid dye onto chitosan nanoparticles. 47:5838–5842. https://doi.org/10.1016/j.polymer.2006.05.071
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. 212:317–331. https://doi.org/10.1016/j.jhazmat.2011.10.016
Imran M, Arshad M, Asghar HN, Asghar M, Crowley DE (2014) Potential of Shewanella sp. strain IFN4 to decolorize azo dyes under optimal conditions. Int J Agric Biol 16:578–584
Jilani S (2015) Bioremediation application for textile effluent treatment. 23(1):26–34. https://doi.org/10.5829/idosi.mejsr.2015.23.01.9227
Joshi N, Rathod M, Vyas D, Kumar R, Mody KH (2019) Multiple pollutants removal from industrial wastewaters using a novel bioflocculant produced by Bacillus licheniformis NJ3. Environ Prog Sustain Energy 38:S306–S314
Joutey NT, Bahafid W, Sayel H, Ghachtouli EN (2013) Biodegradation: involved microorganisms and genetically engineered microorganisms. In: Biodegradation-life of science. InTech, Rijeka
Kalaiarasi K, Lavanya A, Amsamani S, Bagyalakshmi G (2012) Decolourization of textile dye effluent by non-viable biomass of Aspergillus fumigatus. Braz Arch Biol Technol 55(3):471–476
Kandisa RV, Kv NS, Shaik KB, Gopinath R (2016) Bioremediation & biodegradation dye removal by adsorption: a review. 7(6). https://doi.org/10.4172/2155-6199.1000371
Karacakaya P, Kılıç NK, Duygu E, Dönmez G (2009) Stimulation of reactive dye removal by cyanobacteria in media containing triacontanol hormone. J Hazard Mater 172(2–3):1635–1639
Kerebo A, Desta A, Duraisamy R (2016) Removal of methyl violet from synthetic wastewater using nano aluminium oxyhydroxide. Int J Eng Res Dev 12(8):22–28
Khajeh M, Laurent S, Dastafkan K (2013) Nanoadsorbents: classification, preparation, and applications (with Emphasis on Aqueous Media). https://doi.org/10.1021/cr400086v
Khataee AR, Vafaei F, Jannatkhah M (2013) Biosorption of three textile dyes from contaminated water by filamentous green algal Spirogyra sp.: kinetic, isotherm and thermodynamic studies. Int Biodeterior Biodegrad 83:33–40. https://doi.org/10.1016/j.ibiod.2013.04.004
Khatoon N, Sardar M (2017) Efficient removal of toxic textile dyes using silver nanocomposites. J Nanosci: Curr Res 2(3):2–6
Kiliç NK, Dönmez G (2012) Remazol blue removal and EPS production by Pseudomonas aeruginosa and Ochrobactrum sp. Pol J Environ Stud 21(1)
Kochher S, Kumar J (2011) Microbial decolourization of crystal violet by Bacillus subtilis. Biol Forum Int J 3:82–86
Kumar KV, Sivanesan S, Ramamurthi V (2005) Adsorption of malachite green onto Pithophora sp., a fresh water algae: equilibrium and kinetic modelling. Process Biochem 40(8):2865–2872
Kumar PS, Narayan AS, Dutta A (2017) Nanochemicals and effluent treatment in textile industries. https://doi.org/10.1007/978-981-10-2188-6
Lade H, Govindwar S, Paul D (2015a) Low-cost biodegradation and detoxification of textile azo dye CI reactive blue 172 by Providencia rettgeri strain HSL1. J Chem
Lade H, Kadam A, Paul D, Govindwar S (2015b) Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J 14:158–174
Lavanya C (2014) Degradation of toxic dyes: a review. Int J Curr Microbiol App Sci 3(6):189–199
Liu L, Bilal M, Duan X, Iqbal HM (2019) Mitigation of environmental pollution by genetically engineered bacteria—current challenges and future perspectives. Sci Total Environ. 444–454 doi.org/https://doi.org/10.1016/j.scitotenv.2019.02.390
Lopes CN, Petrus JCC, Riella HG (2005) Color and COD retention by nanofiltration membranes. 172:77–83. https://doi.org/10.1016/j.desal.2004.07.030
Machado FM, De Pelotas UF, Bergmann CP, Adebayo M, State O (2012) Adsorption of a textile dye from aqueous solutions by carbon nanotubes adsorption of a textile dye from aqueous solutions by carbon nanotubes, (December). https://doi.org/10.1590/S1516-14392013005000204
Madhav S, Ahamad A, Singh P, Mishra PK (2018) A review of textile industry: wet processing, environmental impacts, and effluent treatment methods:31–41. https://doi.org/10.1002/tqem.21538
Mahapatra A, Mishra BG, Hota G (2013) Electrospun Fe 2 O 3 – Al 2 O 3 nanocomposite fibers as efficient adsorbent for removal of heavy metal ions from aqueous solution. J Hazard Mater:258, 116–259, 123. https://doi.org/10.1016/j.jhazmat.2013.04.045
Mahmoud AM, Ibrahim FA, Shaban SA, Youssef NA (2015) Adsorption of heavy metal ion from aqueous solution by nickel oxide nano catalyst prepared by different methods. Egypt J Pet. https://doi.org/10.1016/j.ejpe.2015.02.003
Manekar P, Patkar G, Aswale P, Mahure M, Nandy T (2014) Detoxifying of high strength textile effluent through chemical and bio-oxidation processes. Bioresour Technol 157:44–51
Mani S, Bharagava RN (2018) Textile industry wastewater: environmental and health hazards and treatment approaches, (December)
Marungrueng K, Pavasant P (2007) High performance biosorbent (Caulerpa lentillifera) for basic dye removal. Bioresour Technol 98(8):1567–1572
Mills A, Davies RH, Worsley D (1982) Water purification by semiconductor photocatalysis
Modi S, Pathak B, Fulekar MH (2015) Microbial synthesized silver nanoparticles for decolorization and biodegradation of azo dye compound. J Environ Nanotechnol 4:37–46
Moon B, Park Y, Park K (2011) Fenton oxidation of Orange II by pre-reduction using nanoscale zero-valent iron. Desalination 268(1–3):249–252. https://doi.org/10.1016/j.desal.2010.10.036
Moussavi G, Mahmoudi M (2009) Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. 168:806–812. https://doi.org/10.1016/j.jhazmat.2009.02.097
Nguyen AK, Fu CC, Juang SK (2016) Biosorption and biodegradation of a sulfur dye in high-strength dyeing wastewater by Acidithiobacillus thiooxidans. J Environ Manag 182:265–271
Oak U, Ghattargi V, Pawar S, Bhole B (2016) Degradation of Drimarene Red, a reactive textile dye by an extremophilic Bacillus sp. isolated from fresh water. Int J Appl Pure Sci Agric 2:105–113
Ozer A, Akkaya G, Turabik M (2006) The removal of acid red 274 from wastewater: combined biosorption and biocoagulation with Spirogyra rhizopus. Dyes Pigments 71(2):83–89
Pokharia A, Ahluwalia SS (2016) Decolorization of Xenobiotic Azo Dye-Black WNN by immobilized Paenibacillus alvei MTCC 10625. Int J Environ Bioremed Biodegrad 4:35–46
Prasad A, Rao KVB (2011) Physicochemical analysis of textile effluent and decolorization of textile azo dye by Bacillus endophyticus strain VITABR13. IIOAB J 2(2):55–62
Przystas W, Zablocka-Godlewska E, Grabinska-Sota E (2015) Efficacy of fungal decolorization of a mixture of dyes belonging to different classes. Braz J Microbiol 46(2):415–424
Qin Q, Sun T, Yin W, Xu Y (2017) Rapid and efficient removal of methylene blue by freshly prepared manganese dioxide. Cogent Eng 7:1–10. https://doi.org/10.1080/23311916.2017.1345289
Rajabi M, Mahanpoor K, Moradi O (2017) Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotube functional groups : critical review. RSC Adv:47083–47090. https://doi.org/10.1039/c7ra09377b.
Rajendran R, Prabhavathy P, Karthiksundaram S, Pattabi S, Dinesh Kumar S, Santhanam P (2015) Biodecolorization and bioremediation of denim industrial wastewater by adapted bacterial consortium immobilized on inert polyurethane foam (PUF) matrix: a first approach with biobarrier model. Pol J Microbiol 64(4):329–338
Ramalingam S, Ponnusamy KS (2015) Novel adsorbent from agricultural waste (cashew NUT shell) for methylene blue dye removal: optimization by response surface methodology. Water Resources Ind 11:64–70
Raman CD, Kanmani S (2016) Textile dye degradation using nano zero valent iron : a review. J Environ Manag 177:341–355. https://doi.org/10.1016/j.jenvman.2016.04.034
Regan I (1962) Enzymes and their application in textile processing, especially desizing. J Soc Dye Colour 78(11):533–542
Rippon JA, Evans DJ (2012) Improving the properties of natural fibres by chemical treatments. In: Handbook of natural fibres. Oxford, Woodhead Publishing, pp 63–140
Robinson T, Chandran B, Nigam P (2002) Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Res 36:2824–2830
Rott U, Minke R (1999) Overview of wastewater treatment and recycling in the textile processing industry. Water Sci Technol 40(1):137–144
Sadaf S, Bhatti HN, Bibi I (2013) Efficient removal of disperse dye by mixed culture of Ganoderma lucidum and Coriolus versicolor. Pak J Agr Sci 50:261–266
Sadeghi-kiakhani M, Arami M, Gharanjig K (2012) Dye removal from colored-textile wastewater using chitosan-PPI dendrimer hybrid as a biopolymer : optimization , kinetic , and isotherm studies:1–13. https://doi.org/10.1002/app.37615
Samagata S, Shah P (2014) Application of nanoscale zero-valent iron for wastewater treatment. Int Conf Multidiscip Res Pract I(VII):342–344
Saratale RG, Saratale GD, Chang JS, Govindwar SP (2011) Bacterial decolorization and degradation of azo dyes: a review. J Taiwan Inst Chem Eng 42(1):138–157
Sarayu K, Sandhya S (2010) Aerobic biodegradation pathway for Remazol Orange by Pseudomonas aeruginosa. Appl Biochem Biotechnol 160(4):1241–1253
Satapanajaru T, Chompuchan C, Suntornchot P, Pengthamkeerati P (2011) Enhancing decolorization of reactive black 5 and reactive red 198 during nano zerovalent iron treatment. Desalination 266(1–3):218–230. https://doi.org/10.1016/j.desal.2010.08.030
Senthilkumar S, Perumalsamy M, Prabh HJ (2014) Decolourization potential of white-rot fungus Phanerochaete chrysosporium on synthetic dye bath effluent containing Amido black 10B. J Saudi Chem Soc 18:845–853
Serpone N, Ah-You YK, Tran TP, Harris R, Pelizzetti E, Hidaka R (1987) AM1 simulated sunlight photoreduction and elimination of Hg(II) and CH3Hg(II) chloride salts from aqueous suspensions of titanium dioxide. Sol Energy 39(6):491–498
Sethi S, Malviya MM, Sharma N, Gupta S (2012) Biodecolorizationof azo dye by microbial isolates from textile effluent and sludge. Univers J Environ Res Technol 2(6):582–590
Shah M (2014) Efficacy of Rhodococcus rhodochrous in microbial degradation of toludine dye. J Pet Environ Biotechnol 5(4):187
Sharma N, Bhatnagar P, Chatterjee S, John PJ, Soni IP (2017) Bio nanotechnological intervention : a sustainable alternative to treat dye bearing waste waters. Ind J Pharm Biomed Sci (IJPBR) 5(1):17–24
Sharma R, Sharma S (2015) Biosorption of Alizarin by Burkholderia sp. Int J Curr Microbiol App Sci 4:112–122
Shinkafi MS, Mohammed IU, Audu AA (2015) Degradation and decolourization of textile dyes effluents. Eur J Biotech Biosci 3:06–11
Singh L, Singh VP (2010) Microbial degradation and decolorization of dyes in semi-solid medium by the fungus–Trichoderma harzianum. Environ We Int J Sci Tech 5:147–153
Singh AK, Singh R, Soam A, Shahi SK (2012) Degradation of textile dye orange 3R by Aspergillus strain (MMF3) and their culture optimization. Curr Disc 1:7–12
Sinha S, Nigam S, Singh R (2015) Potential of Nostocmuscorum for the decolorisation of textiles dye RGB-red. Int J Pharm Bio Sci 6:1092–1100
Song ZY, Zhou JT, Wang J, Yan B, Du CH (2003) Decolorization of azo dyes by Rhodobactersphaeroides. Biotechnol Lett 25(21):1815–1818
Tahir H, Sultan M, Jahanzeb Q (2008) Removal of basic dye methylenblue byusing bioabsorbents Ulva lactuca and Sargassum. Afr J Biotechnol 7:2649–2655
Taleb M, El-Trass A, El-Sigeny S (2015) Synthesis of polyamidoamine dendrimer (PAMAM/CuS/AA) nanocomposite and its application in the removal of Isma acid fast yellow G Dye. Polym Adv Technol 2015(26):994–1002
Tanapongpipat A, Khamman C, Pruksathorm K, Hunsom M (2008) Process modification in the scouring process of textile industry. J Clean Prod 16(1):152–158
Taylor P, Khaloo SS, Fattahi S (2014a) Enhancing decolorization of Eriochrome Blue Black R during nano-size zero-valent iron treatment using ultrasonic irradiation. Desalin Water Treat:37–41. https://doi.org/10.1080/19443994.2013.801322
Taylor P, Rashidi HR, Sulaiman NMN, Hashim NA, Hassan CRC, Ramli MR (2014b) Synthetic reactive dye wastewater treatment by using nano-membrane filtration. Desalin Water Treat:37–41. https://doi.org/10.1080/19443994.2014.912964
Thamaraiselvan C, Noel M (2014) Membrane processes for dye wastewater treatment; recent progress in fouling control:37–41. https://doi.org/10.1080/10643389.2014.900242
Thanunchanok C, Thitinard N, Akira W, Yasuhiko A, Chartchai K, Saisamorn L (2014) Purification and characterization of the extracellular laccase produced by WR710-1 under solid-state fermentation. J Basic Microbiol 54(1):35–43
Tunc O, Tanacı H, Aksu Z (2009) Potential use of cotton plant wastes for the removal of Remazol Black B reactive dye. J Hazard Mater 163:187–198
Tzanov T, Calafell M, Gübitz G, Cavaco-Paulo A (2001) Bio-preparation of cotton fabrics. Enzym Microb Technol 29:357–362
Uday US, Bandyopadhyay TK, Bhunia B (2016) Bioremediation and detoxification technology for treatment of Dye (s) from textile effluent. In: Textile wastewater treatment. InTech, Rijeka
Van Der Bruggen B, Schaep J, Wilms D, Vandecasteele C (1999) Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J Membr Sci 156:29
Vitor V, Corso CR (2008) Decolorization of textile dye by Candida albicans isolated from industrial effluents. J Ind Microbiol Biotechnol 35(11):1353–1357
Wang N, Chu Y, Wu F, Zhao Z, Xu X (2017) Decolorization and degradation of Congo red by a newly isolated white rot fungus, Ceriporia lacerata, from decayed mulberry branches. Int Biodeterior Biodegrad 117:236–244
Wang S, Li H, Xu L (2006) Application of zeolite MCM-22 for basic dye removal from wastewater. 295:71–78. https://doi.org/10.1016/j.jcis.2005.08.006
Waring RD, Hallas G (2013) The chemistry and application of dyes. Springer Science & Business Media, Berlin
Xu M, Guo J, Kong X (2007a) Fe (III)-enhanced Azo reduction by Shewanella decolorationis S12:1342–1349. https://doi.org/10.1007/s00253-006-0773-z
Xu M, Guo J, Sun G (2007b) Biodegradation of textile azo dye by Shewanella decolorationis S12 under microaerophilic conditions:719–726. https://doi.org/10.1007/s00253-007-1032-7
Yang YH, Jia BR, Chen B, Li L, (2014) Degradation of recalcitrant aliphatic and aromatic hydrocarbons by a dioxin-degrader Rhodococcus sp. strain p52. Environmental Science and Pollution Research 21 (18):11086–11093.
Zheng H, Ou JZ, Strano MS, Kaner RB, Mitchell A (2011) Nanostructured tungsten oxide – properties, synthesis, and applications. Adv Funct Mater 21(12):2175–2196
Zhou SL, Li J, Hong G, Chang C (2013) Dendrimer modified magnetic nanoparticles as adsorbents for removal of dyes:6814–6819. https://doi.org/10.1166/jnn.2013.7784
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pandit, P.R. et al. (2020). Bio-nano Approaches: Green and Sustainable Treatment Technology for Textile Effluent Challenges. In: Shah, M., Banerjee, A. (eds) Combined Application of Physico-Chemical & Microbiological Processes for Industrial Effluent Treatment Plant. Springer, Singapore. https://doi.org/10.1007/978-981-15-0497-6_16
Download citation
DOI: https://doi.org/10.1007/978-981-15-0497-6_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0496-9
Online ISBN: 978-981-15-0497-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)