Abstract
In this study, a series of quaternary ammonium-modified hydrochars was fabricated using rice straw as raw material. The physicochemical properties of the hydrochars before and after modification were characterized by FTIR, XPS and CHN element analysis, and an adsorption mechanism was proposed. Results showed the quaternary ammonium groups were successfully grafted onto the hydrochar. The modified hydrochar showed favorable capacity for methyl orange (MO) elimination from aqueous solution with a maximum value of 849 mg/g, which was an order of magnitude higher than that of pristine hydrochar (49 mg/g). Besides, the MO adsorption of all the adsorbents fitted well with the pseudo-second-order and Langmuir models, and the adsorption process was exothermal. Analysis of the adsorption mechanism showed that the decontamination process was primarily controlled by electrostatic attractions and ion exchange.
Graphical abstract
Utilization of rice straw to produce quaternary ammonium-functionalized hydrochars for the efficient removal of methyl orange.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice straw is one of the cheapest and resourceful agricultural by-products. Over 730 million tons of rice straw are generated worldwide (Sewu et al. 2017), and the number is still growing rapidly. Most of the rice straw is incinerated directly, which results in the release of toxic air pollutants and particulate matter that cause severe effects on human health (Gadde et al. 2009). Hence, numerous research works have been carried out to make full use of rice straw.
A potential processes for crop straw utilization is to translate it into functional materials, which can be served as biosorbents (Foo et al. 2012; Sarker et al. 2017), catalysts (Liu et al. 2015), briquettes (Brand et al. 2017) and pulp (Kaur et al. 2017). Biochar, derived from thermal-treated crop straws, normally has high surface area and porosity (Chen et al. 2018) and has been broadly investigated as biosorbent for the removal of metal ions (Amin et al. 2016; Tan et al. 2017), inorganic salts (Takaya et al. 2016) and emerging contaminants (Chen et al. 2019). Biochar could be obtained by pyrolysis or hydrothermal treatment of waste crop straws (Li et al. 2018c). Compared with conventional pyrolysis, the hydrothermal treatment possesses significant advantages, such as mild reaction condition, high yield of solid and minimal emission (Ning et al. 2017). Particularly, hydrochar obtained from hydrothermal carbonization holds abundant active oxygen functional groups (carboxylic, lactone and phenolic hydroxyl group), much lower ash content, and it has been broadly served as an alternative low-cost adsorbent for the elimination of wastewater contaminants, such as heavy metal ions and cationic dyes (Li et al. 2019). At the same time, the application of hydrochar in the elimination of anionic dyes is still in its infancy and further experimental studies are required. Unfortunately, due to minor surface area, negatively charged surface and lack of strong binding sites, pristine hydrochar has limited elimination capacity for concentrated anionic dyes (Li et al. 2018a).
Therefore, modification of hydrochar is extremely necessary. Many methods have been applied for tailoring the physicochemical properties to enhance the adsorption capacity, including thermal treatment, acids/alkalis treatment and surface modification (Fang et al. 2018). Although the thermal treatment and acids/alkalis treatment could enhance the porosity, degree of aromatization and oxygenated functional groups content, improving the adsorption performance of hydrochar toward anionic dyes remains still a crucial challenge, because of the repulsion effect. Surface chemical modification has been proved to be an effective route to increase adsorption capacity of hydrochar (Li et al. 2019). The surface structure of hydrochar can be tailored with various auxiliary ingredients to enhance the removal efficiency of wastewater pollutants. For instance, Fe–Mn binary oxide-functionalized hydrochar exhibited higher adsorption ability compared with pristine hydrochar (Ning et al. 2017). Polyethyleneimine-modified biomass hydrochar also served as an adsorbent for effluent treatment, and the maximum adsorption capacity of the functionalized hydrochar was 2.65 times higher than that of the original hydrochar (Shi et al. 2018). Nevertheless, the cationicity of hydrochar needs to be further increased to enhance the affinity toward anionic dyes. Quaternary ammonium group, with positive charges, shows high affinity toward anionic pollutions in a wide pH range and has been used for modifying adsorbents for the elimination of emerging anionic contaminations (Ferrero and Periolatto 2012). Therefore, combining the advantages of hydrochar and quaternary ammonium groups for the adsorption of anionic dyes has great significance. However, to the best of our knowledge, the quaternary ammonium group-functionalized hydrochar has not been exploited and used as adsorbent for the removal of anionic dyes in aqueous media. The existing research gap is how the surface properties of the modified hydrochar would affect the adsorption performance of anionic dyes. As a typical acidic anionic azo dye, methyl orange (MO) is commonly used in research laboratory, textile, leather, pharmaceutical, paper and ink industries (Subbaiah and Kim 2016). Moreover, MO is easily soluble, highly toxic, nonbiodegradable and resistant to light and oxidizing agents. Based on these considerations, MO was chosen as a model pollutant for assessing and comparing the adsorption performances of the modified hydrochars.
This study aimed to investigate the preparation of various hydrochars modified by different types and proportions of quaternary amine groups for the adsorption of anionic dyes. The adsorption process was studied with emphasis on contact time, adsorption temperature, initial pH of MO and the physicochemical properties of adsorbents. Adsorption kinetics, adsorption isotherms and the corresponding adsorption mechanism were also investigated.
Methods
Reagents
The rice straw (RS) was stemmed from Nanchong, Sichuan Province, China. It was smashed and selected with 100 mesh sieve. All chemical reagents including iron chloride hexahydrate, ferrous sulfate heptahydrate, sodium hydroxide, epichlorohydrin, N, N-dimethylformamide (DMF), triethylamine and MO were of analytical reagent grade and supplied by local Chemical Reagent Company (Guangzhou, China). All reagents were used directly without any treatment.
Preparation and characterization of hydrochar-based adsorbents
Preparation of rice straw hydrochar
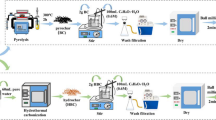
The hydrochar was prepared via hydrothermal treatment of rice straw with ferric salts. Briefly, 2.6 g RS, 0.15 mol/L ferrous sulfate and 0.3 mol/L ferric chloride were mixed and dispersed in 80 mL of deionized water. Next, 30 mL of 2.0 mol/L NaOH solution was added into the mixture under continued stirring to obtain a black suspension in dropwise. Lastly, the mixture was sealed in a Teflon-lined autoclave and then reacted at 180 °C for 6 h. The collected product was washed with deionized water and dried in an oven at 60 °C. The sample was named HRS.
Preparation of modified hydrochar adsorbents
The quaternary amine group-modified hydrochar adsorbents were synthesized by loading different amounts of quaternary ammonium groups onto the hydrochar through etherification–amination reaction. The procedure was as follows: 0.20 mol epichlorohydrin and 16 mL DMF were mixed with the HRS and underwent reaction at 100 °C for 1 h. Then, 0.05 mol pyridine was added to the mixture and underwent reaction for another 1 h. Afterward, 0.02, 0.04 and 0.06 mol trimethylamine were added and the reaction was continued for 3 h. The final products were separated by external magnetic field and washed immediately with 0.1 M NaOH, 0.1 M HCl, ethanol and deionized water to purify the adsorbents. The wet samples were dried at 60 °C for 12 h. The corresponding products were obtained after drying, and sieving procedures, named MHRS-0.5, MHRS-1.0, MHRS-1.5. For comparison, another adsorbent was synthesized without trimethylamine, which was named MHRS-Py.
Characterization of the adsorbents
Fourier transform infrared spectroscopy (FTIR, Bruker VERTEX 33, Germany) and X-ray photoelectron spectroscopy (XPS, Type K-Alpha, Thermofisher Scientific Company) were used to analyze the functional groups of adsorbents. Nitrogen content was measured by an element analyzer with CHNS pattern (Elementar Vario EL III, Germany).
Adsorption experiments
The adsorption experiments were tested on the constant temperature oscillator with a agitate speed of 160 rpm. In the adsorption isotherms trails, 0.1 g of the modified or unmodified hydrochars was added into 0.1 L aqueous solution with MO initial concentration ranging from 200 to 1000 mg/L at natural pH (not being adjusted using additional acids or alkalis, and the pH varied from 6.20 to 6.70 with the increased initial concentration) and 30 °C, 40 °C and 50 °C for 24 h. In the adsorption kinetic experiments, 0.1 g of the modified hydrochars was added into a 0.1 L initial MO concentration of 1000 mg/L. After a period of time, samples were taken to analyze the concentration of MO in the solution. In the pH experiment, 0.02 g of the modified hydrochars was added into 0.05 L of MO solution with an initial concentration of 500 mg/L at different pH levels for 6 h. The concentration of MO was determined by UV–Vis spectrophotometer method (UV-2450, Shimadzu). The adsorption capacity was calculated as follows:
where Qe (mg/g) is the adsorption capacity at equilibrium; C0 and Ce (mg/L) are the initial and equilibrium concentrations of MO; V (L) was the volume of the experimental solution; and m (g) is the weight of adsorbent.
Results and discussion
Synthesis and characterization of adsorbents
The modified hydrochar adsorbents were synthesized by the combination of etherification and amination reaction, as shown in Fig. S1. Hydrochar was obtained from hydrothermal treatment RS with ferrite, epichlorohydrin was used as the etherifying agent, and DMF was used as solvent to enhance the sensitivity of the epoxide ring (Orlando et al. 2002). The hydroxyl groups in cellulose were attacked by epichlorohydrin to produce hydroxyl cellulose ethers. The hydroxy cellulose ethers were cyclized under the catalysis of pyridine to obtain the epoxy cellulose ethers. Meanwhile, pyridine underwent reaction with haloalkane to get pyridinium onium salt (Hossein Beyki et al. 2017; Naushad et al. 2018). Lastly, the quaternary ammonium-modified hydrochars were obtained through the amination reaction between epoxy ether and triethylamine.
FTIR technique was applied to identify the functional groups in the hydrochar and modified hydrochars (Fig. 1). The wide absorption peak nearby 3440 cm−1 was ascribed to the hydroxyl stretching vibration of HRS. The other characteristic peaks at 2920 cm−1, 1643 cm−1 and 899 cm−1 were related to C–H stretching vibration, bending mode of the adsorbed water, deformation of glycoside bonds caused by ring vibration and hydroxyl bending deformation, respectively (Wu et al. 2016). Compared with pristine hydrochar, two new peaks appeared at 1636 cm−1 and 1385 cm−1, which were allocated to the C=N and C=C adsorption peak of pyridine ring in MHRS-Py, respectively (Hossein Beyki et al. 2017; Ma et al. 2015). These phenomena meant that pyridine might partially react with alkyl chloride. After the introduction of triethylamine, a sharp absorption peak that appeared at 774 cm−1 was attributed to the N–H stretching vibration of free amino groups. The peak at around 1488 cm−1 was ascribed to the stretching vibration of the C–N bond (Cao et al. 2016; Karachalios and Wazne 2013). These results confirm that amine groups were successfully modified onto the hydrochar surface. Additionally, the intensity of the absorption peak at 1488 cm−1 and 774 cm−1 was enhanced from MHRS-0.5 to MHRS-1.5, in response to the increase in amine group contents.
XPS analysis was conducted to analyze the protonated amine groups on the backbone of hydrochar, because they were difficult to distinguish through FTIR (Cao et al. 2016). Figure 2 shows that the N 1s spectra of MHRS-Py were deconvoluted into two peaks at 398.3 eV and 400.8 eV, which were assigned to the free amino groups and the protonated pyridinic nitrogen, respectively (Xiao et al. 2017). The Cl 2p spectra also could be deconvoluted into two peaks at 198.0 and 196.4 eV, which belonged to the alky C–Cl groups and the pyridinic N–Cl groups, respectively (Ma et al. 2015; Song et al. 2016). These indicated that pyridine not only acted as a catalyst, but reacted with epichlorohydrin to form pyridinium onium salts (Hossein Beyki et al. 2017; Naushad et al. 2018), which benefit enhancing the ion exchange capacity of the adsorbents. After the introduction of triethylamine in the process, two new peaks appeared at around 401.8 for N 1s and 197.8 eV for Cl 2p, which were attributed to the peak of alkyl quaternary ammonium groups and [N+(C2H5)4]Cl− groups, respectively (Cao et al. 2016). With the increase in trimethylamine amount content, the relative content of quaternary ammonium salt based on peak area ratio increased, whereas the peak area of alky C–Cl groups declined, suggesting that protonated amino groups were successfully gratified onto hydrochar via amination reaction.
C, H and N contents in HRS and MHRS were determined, and the results are listed in Table S1. The N content of the original HRS was extremely low (0.19 wt%), but it increased to 5.48 wt% after chemical modification, indicating that the amine groups were successfully loaded onto the HRS surface. The key factor total exchange capacity (TEC) from the equation (TEC (mEq/g) = N wt%/1.4) was varied from 2.48 to 3.91 mEq/g, manifesting that the modified hydrochar would possess great potential for MO uptake.
Influence of contact time on MO adsorption
To study the influence of contact time on the MO removal performance and determine the adsorption equilibrium time, the adsorption capacities at intervals time were recorded and are plotted in Fig. 3. All four adsorbents showed the same trends in adsorption capacity, and the adsorption process could be divided into three parties. Taking MHRS-Py for example, the removal of MO occurred at a rapid speed in the initial period (0–90 min). The MO adsorption rate decelerated and became stable at the second stage (90–360 min). At the last section (over 360 min), the MO adsorption was designed as the equilibrium state. At the initial stage, many unoccupied active sites and high adsorbate concentration favored the MO adsorption (Zhang et al. 2017). With the adsorption that proceeds, increasingly more adsorption sites were occupied. The remaining active adsorption sites became more sensitive for MO adsorption, and reaching equilibrium took a longer time to (Lafi and Hafiane 2016). Compared with MHRS-Py, the second stage and equilibrium time of the other three adsorbents were relatively long. These could be because more reactive alkyl quaternary ammonium groups were implanted, leading to higher adsorb ability of the adsorbents, but restricting the diffusion rate, which was consistent with the rate constant described in Table S3 (Shi et al. 2018).
Effect of temperature on MO adsorption
The effect of temperature on the uptake capacity of prepared adsorbents was carried out at 30 °C, 40 °C and 50 °C. The adsorption performance curves of MO on the four adsorbents are shown in Fig. 4. The adsorption capacity of MHRS-1.5 steeply rose and gradually reached the maximum. The equilibrium adsorption capacity of MO increased from 198 to 849 mg/g with the increase in the initial MO concentrations (from 200 to 1000 mg/L), implying that the adsorbent could be saturated with high concentration MO molecules (Tanhaei et al. 2015). Higher temperature led to lower MO adsorption capacity of MHRS, and the maximum MO uptakes (Qm,exp) of MHRS-1.5 were 849.8, 837.6 and 819.7 mg/g at 30 °C, 40 °C and 50 °C, respectively. In addition, the adsorption isotherms of MHRS-1.0, MHRS-0.5 and MHRS-Py with different temperatures exhibited the same trends, indicating that the removal process of MO by the modified hydrochar was mainly exothermic, which was consistent with the related reports (Li et al. 2016; Tanhaei et al. 2015). However, the adsorption capacity for both adsorbents did not change significantly at different temperatures, indicating that the chemical adsorption was in dominating station, which was slightly affected by temperature (Zhang et al. 2012).
Effect of pH on MO adsorption
In the adsorption process, pH played an important role and could change the surface charge of the adsorbents and the degree of ionization of MO. The MO adsorption capacity of four adsorbents under different pH conditions is presented in Fig. S2. MO was positively charged at lower pH due to the protonated amine groups, whereas the MO molecular was negatively charged at higher pH (Yang et al. 2017). MO would become turbid at pH < 4.0. Hence, the initial solution pH was selected from 4.5 to 10.0. At pH 4.5, all four adsorbents exhibited higher capacity toward MO. With the increase in pH, the removal capacity showed a downward trend. In alkaline condition, the amine groups of MO were unprotonated and high concentrations of hydroxide ions competed with MO anions, reducing the adsorption capacity. On the other hand, the greater the content of amine groups on the hydrochars loaded, the higher MO removal capacity obtained at the same pH, which was similar to the discussion above mentioned. All these phenomena indicated that the force of the modified hydrochars to adsorb MO might be electrostatic interactions and ion exchange.
Effect of hydrochar physicochemical properties on MO removal
In the past few years, quaternary ammonium group has been applied for modifying adsorbents. However, the quaternary ammonium group-functionalized hydrochar has rarely been exploited and used for the removal of anionic dyes. Meanwhile, it is urgent need to investigate the effects of the type and proportion of quaternary ammonium groups on the improvement in the adsorption capacity. Herein, the effect of surface properties of the modified hydrochar on MO adsorption performance was systematically studied.
Without any modification, the pristine hydrochar exhibited a low capacity for the MO (49 mg/g), manifesting the lack of surface functional groups. Hence, the modification of hydrochar was essential. Pyridine reportedly could not only induce the hydroxy cellulose ether cyclized but also react with haloalkane to get pyridinium onium salt, which could decontaminate anion pollutions (Gao et al. 2009). Nevertheless, the adsorption performance of pyridinium onium salt-modified materials has been seldom reported (Hossein Beyki et al. 2017). In this study, MHRS-Py exhibited MO capacity as high as 386 mg/g, which might be ascribed to the electrostatic interaction between the pyridine onium salt and MO. For the MHRS-0.5, the adsorption capacity was increased to 643 mg/g. Compared with MHRS-Py, the nitrogen content of MHRS-0.5 was slightly increased (about 1 wt %, see Table S1), whereas the adsorption performance was increased by 66% (257 mg/g), which meant that the alkyl quaternary ammonium salt was more likely to enrich MO than the pyridine onium salt. Similar results showed that cetyltrimethylammonium bromide-modified tea waste exhibited better adsorption performance of MO compared with cetylpyridinium bromide-modified tea waste (Foroughi-dahr et al. 2015). Moreover, the adsorbability for MO removal showed a strong dependence on the quaternary ammonium loading contents in the hydrochar composites. Increasing quaternary ammonium loading content facilitated much greater percentage of MO removal, and the adsorbability toward MO was 805 mg/g and 849 mg/g for MHRS-1.0 and MHRS-1.5, respectively, indicating functional improvement in MO adsorption of the hydrochar by a protonated amine. In addition, the implantation of protonated amine groups greatly enhanced the affinity toward MO, and the adsorption isotherms of the four adsorbents both were the H-type, in which MO was almost fully adsorbed under low concentration and the isotherm showed vertical and steep increase to reach a plateau trend (Giles et al. 1974).
Boehm titration method was used to determine the amount of surface oxygen groups present on the hydrochar surface, and the results are shown in Table S2. The phenolic hydroxyl content in the raw hydrochar was high, and it decreased in the modified hydrochars, indicating that the phenolic hydroxyl may participate in the etherification reaction during the modification of hydrochar (Wahlström et al. 2017). However, there was no obvious correlation between the amount of acid/basic groups and the adsorption capacities of these hydrochar adsorbents, suggesting that the quaternary ammonium group played the leading role during the adsorption process.
Adsorption kinetic models
The pseudo-first-order and pseudo-second-order models were applied to describe the experimental datum, and the corresponding parameters are shown in Table S3. Due to the relatively high fitting coefficient, the MO adsorption procedure conformed to the pseudo-second-order kinetic model, which manifested that chemical process with ion exchange was the controlling mechanism for the MO removal (Song et al. 2016). Moreover, the rate constant of MMRS-Py was higher than that of the other three adsorbents. As the loading amount of alkyl quaternary ammonium groups increased, the rate constant increased. More reactive alkyl quaternary ammonium groups were implanted, leading to higher adsorption capacity of the adsorbents, but restricting the diffusion rate. Compared with adsorption capacity, the value of k2 did not change significantly after alkyl quaternary ammonium group modification, indicating that alkyl quaternary ammonium group modification was also favorable for the removal of MO (Shi et al. 2018).
Adsorption isotherms
In this study, the experimental data were described by Langmuir isotherm model (Niu et al. 2017):
and Freundlich isotherm model (Zhao and Zhou 2016):
where Qe (mg/g) and Ce (mg/L) are the MO uptake capacity and the adsorption equilibrium concentration of MO, respectively. Qmax (mg/g) is theoretical maximal uptake capacity. KL is Langmuir adsorption constant at equilibrium, which describes the affinity between MO and the adsorbents. Kf is Freundlich adsorption constant at equilibrium, which shows the uptake capacity; n describes the adsorption intensity (Yargıç et al. 2015; Zhao and Zhou 2016).
The parameters of the two isotherm models are listed in Table S4. Based on the results, the correlation coefficients of the Langmuir model were all above 0.999, exhibiting a better match with experimental data than the Freundlich model. According to the Langmuir isotherms, the calculated maximum uptake capacity (Qm,cal) for MO was 854.7 mg/g, 840.3 mg/g and 826.4 mg/g at 30 °C, 40 °C and 50 °C, respectively, which were approximate with the experimental data. The essential factor RL, from the equation \(R_{L} = \frac{1}{{1 + K_{L} *C_{0} }}\) (where C0 and KL are same as mentioned above), manifested that the adsorption process at all tested temperatures were beneficial because the values of RL were in the range of 0–1 (Lafi and Hafiane 2016).
Comparison with other adsorbents
The comparison of the maximum uptake ability for MO on the modified and unmodified rice straw hydrochars with other amine-based adsorbents recorded in the literature is shown in Table 1. Amine groups, such as primary amine and secondary amine, could be easily protonated in the acidic solution and were implanted into the adsorbents for the segregation of anionic dyes via strong electrostatic attraction (Jiang et al. 2018). However, the adsorption performance of this kind of adsorbent was greatly affected by the solution pH, and the adsorption efficiency dropped sharply with the increase in the solution pH (Subbaiah and Kim 2016). Nitrogen-containing surfactant-modified adsorbents were also applied for the removal of MO (Lafi and Hafiane 2016). The main adsorption mechanism was electrostatic attraction and hydrophobic–hydrophobic interactions. However, the weak hydrophobic interactions contributed less to the adsorption process, whereas the limited loading amount of surfactant caused the surface of the adsorbent to be less positively charged, leading to low adsorption capacity. Compared with the two kinds of adsorbents, quaternary ammonium group-modified adsorbents have elicited great interests for the elimination of emerging anionic contaminations, because they could capture anionic species via the electrostatic force in acidic and alkaline solution (Zhang et al. 2012). Most previous studies exploited the single quaternary ammonium salt to functionalize the adsorbents and showed high capacity (Li et al. 2018b). In our study, the two kinds of quaternary ammonium groups applied for modifying the hydrochar presented a relatively high adsorption capacity toward MO, which may be due to the synergy effect of the pyridine onium salts and alkyl quaternary ammonium salts.
Regeneration and reuse study
Regeneration and reuse are important indicators for evaluating adsorption performance and are significant for the practicality of these adsorbents. Given the best adsorption performance, MHRS-1.5 was adopted for further investigation on its regeneration and reuse performance. The adsorption–desorption experiment was performed with 1000 mg/L MO solution on MHRS-1.5. After adsorption, MO-loaded MHRS-1.5 was eluted by 0.5 mol/L HCl solution. After desorption completed, the adsorbent was washed with deionized water repeatedly and dried for reuse in the subsequent experiments. The succeeding adsorption cycle experiments were performed with the same conditions, and the results are shown in Fig. 5. After four recycles, MHRS-1.5 still held 74% of the original capacity (628 mg/g). This indicated that MHRS-1.5 could be economical and efficient adsorbent and repeatedly used for the removal of anionic dyes from sewage.
Adsorption mechanism
The mechanism of MO adsorption onto MHRS-1.5 was analyzed through FTIR and XPS (Fig. 1f and Fig. S3). Two new peaks at around 1605 and 1516 cm−1 were attributed to the aromatic skeleton vibration and the elastic vibration of –N=N–. The peaks at around 1039 and 1195 cm−1 were ascribed to the S=O stretching vibration peak, manifesting that the R-SO3− groups of MO were included in the removal process. XPS analysis showed no detectable peak for S 2p before adsorption, and an apparent peak of S 2p was identified after MO adsorption. The S 2p spectra were also deconvoluted into two peaks, which were attributed to the interaction of alkyl quaternary ammonium groups and pyridinium onium salt groups with R-SO3−, respectively. Moreover, compared with the spectrum before adsorption, the intensity of [N(C2H5)4]+Cl− significantly reduced and the peak at around 197.8 eV disappeared after MO adsorption. These implied that the adsorption mechanism of MO by MHRS-based adsorbent was the ion exchange between Cl− and R-SO3− and the electrostatic attraction between quaternary ammonium salts and R-SO3− (Fig. 6).
Conclusion
Protonated amine group-modified rice straw hydrochars were successfully synthesized. Batch adsorption experiments suggested the modified hydrochars exhibited much higher affinity toward MO compared with pristine hydrochar. The decontamination capacity of the modified hydrochars exhibited a strong dependence on the loading contents of quaternary ammonium groups, and the maximum adsorption ability (849 mg/g) was obtained by MHRS-1.5 at 30 °C owing to the synergy effect of the pyridine onium and alkyl quaternary ammonium groups. The adsorption process was exothermic. Langmuir isotherm model and pseudo-second-order kinetic model with high correlation coefficients represented matched very well with the adsorption experimental data. The uptake mechanism of MO on the protonated amine-modified hydrochar was mainly controlled by the electrostatic attraction and the ion exchange. The present work shows that the quaternary ammonium-modified rice straw hydrochar obtained will be a promising adsorbent for the removal of anionic pollutants.
References
Amin FR et al (2016) Biochar applications and modern techniques for characterization. Clean Technol Environ Policy 18:1457–1473
Brand MA et al (2017) Production of briquettes as a tool to optimize the use of waste from rice cultivation and industrial processing. Renew Energy 111:116–123
Cao W et al (2016) 13C NMR and XPS characterization of anion adsorbent with quaternary ammonium groups prepared from rice straw, corn stalk and sugarcane bagasse. Appl Surf Sci 389:404–410
Chen Q et al (2018) Classical theory and electron-scale view of exceptional Cd(II) adsorption onto mesoporous cellulose biochar via experimental analysis coupled with DFT calculations. Chem Eng J 350:1000–1009
Chen Q et al (2019) Insights into sulfamethazine adsorption interfacial interaction mechanism on mesoporous cellulose biochar: coupling DFT/FOT simulations with experiments. Chem Eng J 356:341–349
Fang J et al (2018) Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J Ind Eng Chem 57:15–21
Ferrero F, Periolatto M (2012) Functionalized fibrous materials for the removal of dyes. Clean Technol Environ Policy 14:487–494
Foo LPY et al (2012) Potential Malaysia agricultural waste materials for the biosorption of cadmium(II) from aqueous solution. Clean Technol Environ Policy 14:273–280
Foroughi-dahr M et al (2015) Experimental study on the adsorptive behavior of Congo red in cationic surfactant-modified tea waste. Process Saf Environ Prot 95:226–236
Gadde B et al (2009) Air pollutant emissions from rice straw open field burning in India, Thailand and the Philippines. Environ Pollut 157:1554–1558
Gao B-Y et al (2009) Preparation and characteristics of quaternary amino anion exchanger from wheat residue. J Hazard Mater 165:461–468
Giles CH et al (1974) A general treatment and classification of the solute adsorption isotherm part. II. Experimental interpretation. J Colloid Interface Sci 47:766–778
Hossein Beyki M et al (2017) Magnetic cellulose ionomer/layered double hydroxide: an efficient anion exchange platform with enhanced diclofenac adsorption property. Carbohyd Polym 157:438–446
Jiang Y et al (2018) Cross-linked chitosan/β-cyclodextrin composite for selective removal of methyl orange: adsorption performance and mechanism. Carbohyd Polym 182:106–114
Karachalios A, Wazne M (2013) Nitrate removal from water by quaternized pine bark using choline based ionic liquid analogue. J Chem Technol Biotechnol 88:664–671
Kaur D et al (2017) Prospects of rice straw as a raw material for paper making. Waste Manag 60:127–139
Lafi R, Hafiane A (2016) Removal of methyl orange (MO) from aqueous solution using cationic surfactants modified coffee waste (MCWs). J Taiwan Inst Chem Eng 58:424–433
Li K et al (2016) Efficient adsorption of both methyl orange and chromium from their aqueous mixtures using a quaternary ammonium salt modified chitosan magnetic composite adsorbent. Chemosphere 154:310–318
Li B et al (2018a) Sorption of methyl orange from aqueous solution by protonated amine modified hydrochar. Biores Technol 268:454–459
Li P et al (2018b) Highly selective adsorption of dyes and arsenate from their aqueous mixtures using a silica-sand/cationized-starch composite. Microporous Mesoporous Mater 263:210–219
Li Y et al (2018c) Hydrochars from bamboo sawdust through acid assisted and two-stage hydrothermal carbonization for removal of two organics from aqueous solution. Biores Technol 261:257–264
Li B et al (2019) The polyaminocarboxylated modified hydrochar for efficient capturing methylene blue and Cu(II) from water. Biores Technol 275:360–367
Liu W-J et al (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285
Ma F et al (2015) Preparation of pyridinium-functionalized magnetic adsorbent and its application for nitrate removal from aqueous solution. Water Air Soil Pollut 226:212
Naushad M et al (2018) Efficient removal of toxic phosphate anions from aqueous environment using pectin based quaternary amino anion exchanger. Int J Biol Macromol 106:1–10
Ning Q et al (2017) Fabrication of hydrochar functionalized Fe-Mn binary oxide nanocomposites: characterization and 17[small beta]-estradiol removal. RSC Adv 7:37122–37129
Niu Y et al (2017) Novel recyclable adsorbent for the removal of copper(II) and lead(II) from aqueous solution. Biores Technol 229:63–68
Orlando US et al (2002) Preparation of agricultural residue anion exchangers and its nitrate maximum adsorption capacity. Chemosphere 48:1041–1046
Sarker TC et al (2017) Sugarcane bagasse: a potential low-cost biosorbent for the removal of hazardous materials. Clean Technol Environ Policy 19:2343–2362
Sewu DD et al (2017) Highly efficient adsorption of cationic dye by biochar produced with Korean cabbage waste. Biores Technol 224:206–213
Shi Y et al (2018) Polyethylene imine modified hydrochar adsorption for chromium (VI) and nickel (II) removal from aqueous solution. Biores Technol 247:370–379
Song W et al (2016) Adsorption–desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater. Bioresource Technol 210:123–130
Subbaiah MV, Kim D-S (2016) Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: kinetics, isotherms, and thermodynamic studies. Ecotoxicol Environ Saf 128:109–117
Takaya CA et al (2016) Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 145:518–527
Tan Z et al (2017) Cadmium removal potential by rice straw-derived magnetic biochar. Clean Technol Environ Policy 19:761–774
Tanhaei B et al (2015) Preparation and characterization of a novel chitosan/Al2O3/magnetite nanoparticles composite adsorbent for kinetic, thermodynamic and isotherm studies of Methyl Orange adsorption. Chem Eng J 259:1–10
Wahlström R et al (2017) Lignin cationization with glycidyltrimethylammonium chloride aiming at water purification applications. Ind Crops Prod 104:188–194
Wu Y et al (2016) Functionalized agricultural biomass as a low-cost adsorbent: utilization of rice straw incorporated with amine groups for the adsorption of Cr(VI) and Ni(II) from single and binary systems. Biochem Eng J 105, Part A:27–35
Xiao P et al (2017) Comparison with adsorption of Re(VII) by two different γ-radiation synthesized silica-grafting of vinylimidazole/4-vinylpyridine adsorbents. J Hazard Mater 324:711–723
Yang D et al (2017) Rational design and synthesis of monodispersed hierarchical SiO2@layered double hydroxide nanocomposites for efficient removal of pollutants from aqueous solution. Chem Eng J 323:143–152
Yargıç AŞ et al (2015) Assessment of toxic copper(II) biosorption from aqueous solution by chemically-treated tomato waste. J Clean Prod 88:152–159
Zhang W et al (2012) Adsorption of anionic dyes from aqueous solutions using chemically modified straw. Biores Technol 117:40–47
Zhang B et al (2017) Enhanced adsorption capacity of dyes by surfactant-modified layered double hydroxides from aqueous solution. J Ind Eng Chem 49:208–218
Zhao S, Zhou T (2016) Biosorption of methylene blue from wastewater by an extraction residue of Salvia miltiorrhiza Bge. Biores Technol 219:330–337
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant Nos. 21676095, 21736003) and the Science and Technology Program of Guangzhou, China (201804020014). The authors would also gratefully acknowledge the support from the Guangdong Provincial Laboratory of Green Chemical Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peng, X., Yan, Z., Cheng, X. et al. Quaternary ammonium-functionalized rice straw hydrochar as efficient adsorbents for methyl orange removal from aqueous solution. Clean Techn Environ Policy 21, 1269–1279 (2019). https://doi.org/10.1007/s10098-019-01703-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-019-01703-2