Abstract
The environment of our planet is severely affected by the depletion of natural resources and the hazardous wastes generated by anthropogenic activities. Water pollution by industrial effluents is one such area whereby technological intervention is an utmost necessity for achieving sustainable solutions. Textile effluents are laden with various organic dyes which impart toxicity to the aquatic ecosystems they are discharged into. For removal of these toxic dyes, various technologies have been adopted by researchers and scientists. Use of novel materials as adsorbents has also been used widely. Among the adsorbents used polymeric materials have also found usage in this regard. Thus use of various polymers and their composites in dye removal is an important area of study for dye removal purposes. These polymeric materials possess huge potential for wastewater treatment purposes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The utilization and regeneration of the sustainable resources for the development of industrial technology has become a great concern worldwide. Water pollution has become a major worldwide environmental issue harming the sustainability of the environment [4, 27]. Water pollution disrupts the supply chain in industrial belts posing a serious threat to the environmental feasibility [53]. During last few decades, there has been a lot of research for the minimization of toxic pollutants from the environment to make it healthy and sustainable. Highly toxic compounds have been released into the environment directly or indirectly. These compounds maybe dyes, pesticides, polycyclic aromatic hydrocarbons, radionuclides, and heavy metals [14]. They are widely used in industries like paper mills, leather, textile, pharmaceuticals, and cosmetics [44, 57, 58].
It has been reviewed that the main causes of water pollution are caused by the excessive discharge of textile wastewater which can pose harmful effects on the human mankind like cancer, skin allergies, headaches, different mutations, etc. due to its complex aromatic structure [17]. The removal of organic dyes from the ecosystem is found to be a challenging task, so effective approaches are developed to mitigate the destruction of natural resources. The minimization of dye wastes can reduce the risk of water pollution [42]. Due to the complex molecular structure of dyes, they can resist degradation caused by light, physical or biological pathways using micro-organisms [8]. More than 10,000 different dyes have been utilized in pharmaceutical, leather, cosmetics, textile, and paper industries [38]. Approximately 2% of these textile industry dyes directly end up as effluent in water sources. Therefore, the amount of dye concentration estimated in wastewater varies in the range of 5-200 mg/L [2].
Different techniques are available for the removal of dye which includes microbial as well as physical methods like advanced oxidation (by photodegradation), coagulation, flocculation, membrane separation, adsorption, microbial degradation [7, 18].
The complete depletion of dye molecule can be readily obtained by the process of oxidation, either by photochemical process or simple chemical method [20]. The main goal of any advanced oxidation technique is to synthesize the hydroxyl molecule (HO*) for better interaction through hydrogen bonding and utilizing it as a strong oxidizing agent to degrade the contaminant in the aqueous solution. Photocatalytic degradation method is one of the most attractive and promising techniques among the various approaches due to its reliability on the utilization of irradiation energy, generation to less harmful by-products, simplicity, and eco-friendly. Coagulation and flocculation are the processes utilized in water treatment to remove pollutants like oil, grease, dyes, metals maintaining COD, and water concentration. This can lead to development of highly cost-effective polymer composites [65]. Membrane separation process is widely used nowadays to remove the contaminants in a physical manner by the utilization of a membrane thus yielding a high environmental stability and the removal capacity is also high by involving high pressure-driven methods such as ultrafiltration, nanofiltration, reverse osmosis [32]. The stability of the membrane is at high permeation flux with 99% in neutral or alkaline environments [52]. Adsorption process is the most widely used technology for the dye removal process among all these methods due to its cost-effectiveness, high stability, low toxicity [6, 13].
The second important aspect is designing and commercial utilization of affordable and novel material for the industrial wastewater treatment has gained a significant growth in a few years. Nanocomposite materials have proven as a suitable alternative in order to overcome the drawbacks of monolithic and micro composites [48].
Nano-structured polymer composites help in improving the water quality thus enhancing environmental stability [56, 66]. Research on polymer composites helps in effective enhancement of wastewater treatment removing the micropollutants and focusing on the challenges of future research. Research on the Polymer composite was done due to their attractive properties like better adsorption and removal characteristics of metal ions, dyes, and other toxic pollutants [10]. Polymer composites consist of organic or inorganic compounds to provide allied advantageous properties such as stiffness, low density, high thermal resistance, chemical stability [40].
Polymer composites blended with natural fibers like cellulose obtained from different Agro waste like sugarcane bagasse, peanut shell proved to be an attractive option for the researchers. The polymer composites blended with graphene, carbon, or clay-based were majorly used in water treatment as well as desalination technologies [33, 59]. The development of a reinforced composite polymer by the addition of different linked monomers into it, which are derived from easily available and user-friendly agricultural wastes makes it productive and enhances sustainability [26]. The generation of the polymer reinforced composites in wastewater remediation and involving it in dye removal techniques is gaining worldwide interest for researchers and young scientists. Utilization of the industrial wastes used for the preparation of polymer composites reinforced with polymer, clay, cellulose/chitin, and carbon can functionalize the fiber surfaces and reduce the absorption of moisture making them compatible [29].
In this chapter, we have discussed various types of polymer composites that have been used for dye removal purposes. Recent works in this field have also been discussed here.
2 Various Types of Polymer Composites in Dye Removal
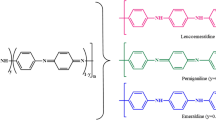
Different types of Polymers and polymeric composites are used for dye removal purposes (Fig. 1). The polymer composites are filled with fillers like clay, metal, carbon, or fibers.
3 Polymer Composites
Polymer-polymer composites are mainly used in the process of dye removal since it has many attractive and advantageous properties like mechanical strength, high thermal stability, flexibility, durability, and high surface area with high surface-to-volume ratio. These properties make the polymer capable of doping with the other polymer composites by the synthesis involving functionalization by the method of crosslinking and blending with different fillers. The fillers maybe comprised of different Agrowaste like Sugarcane bagasse, peanut shells, rice husk ash, etc. The polymer-polymer composites have attracted the scientists and researchers all over the world because of their properties. So, these composites are well utilized in the purpose of wastewater treatment and in the desalination methods. The main advantage of these types of composites is wider range of pollutants can be utilized for the Bioremediation treatment, but the disadvantage is high production cost [11].
Polymers are often used as fillers in polymers, or one or more polymers are synthesized to form a new polymeric material where different fillers are reinforced to form a composite system. Elkady and co-workers developed a copolymer were developed using Styrene and acrylonitrile by the in-situ polymerization method and using nanofibers as filler and used it for dye removal (by basic Crystal violet dye) through the adsorption batch process. The polymer composite was produced by the technique of electrospinning [19]. The composite morphology is shown in (Fig. 2).

(reproduced from [20], open-access article)
SEM micrographs of a poly (Acrylonitrile-Styrene copolymer (AN-co-ST)) nanofibers; and b modified poly (AN-co-ST) nanofibers; c effect of initial dye concentration on dye removal capacity and percentage decolorization onto the chemically modified nanofiber
The surface morphological characteristics were identified by the SEM technique indicating a uniformity for the carboxylated nanofibers. The adsorptivity of the process was also been affected by the structural modifications with the isolated microbes. The adsorption capacity was noted at about 30 min as 67.11 mg/g. This is because of the toxicological and structural changes interpreted in the copolymer with the functional groups of carboxyl acid and amino acid bringing about immunological changes in the polymer by the high adsorptive power in the removal of basic dyes like Crystal violet and Methylene blue dye [34]. Synthesis of cyclodextrin composites also proved to be an exceptional group of polymer-polymer composites with cost-effective power, high feasibility, and effectiveness in the process of dye removal due to their good physicochemical properties [31, 68]. The efficacy for dye removal was increased by crosslinking the cyclodextrins with the polydopamine for the high thermal stability and structural feasibility immobilized with starch to utilize it in wastewater and biodegradation treatment using fungi and cyanobacteria due to their microporous nature. The \(\beta\) cyclodextrin plays a significant role in the adsorption process of dye removal using microbes due to the intraparticle diffusion and interlinking forces of attraction in dyes with the polymer doped composites [63].
Similarly, polymer composites like PVA/Cellulose, PVA/chitin were generated from the fillers combined with the monomers. The fillers were utilized as the natural fibers like cellulose. Cellulose was extracted from the Agrowaste like Sugarcane bagasse, Peanut shell waste. The cellulose was also grafted with 2-acrylamido-methylpropane generating efficient polymer composites for dye and heavy metal removal involving micro-organisms like Aspergillus sp. For the preparation of PVA/chitosan or PVA/Cellulose polymer, initially, PVA polymeric membrane was produced by adding 4 g PVA with 100 mL water, and continuously stirred for two hours. After the solution turns transparent, some amount of chitosan or cellulose in a 1:2 ratio is added to form a homogenous solution by the process of ultra-sonification. Finally, the polymer composites were obtained. And then the weights were measured, analyzed for the dye removal with Methyl orange dye solution under dark conditions with UV irradiation and the adsorption-desorption study was evaluated through UV-vis spectroscopy. Then the dye-loaded composites were used for the Biodegradation treatment using microbes like fungi and bacteria maintaining an optimum alkaline pH and temperature of about 35 °C [47]. Also, polymer composites with diethylenetriamine with polyacrylonitrile were produced by the membrane separation and electrospinning technique. The composites were characterized by SEM and TGA. SEM confirmed the presence of roughness in the structure due to the incorporation of diethylamine with the presence of microbes which affected the dye removal process [3]. The advantages and disadvantages of Polymer doped polymer composites are represented in (Table 1).
As mentioned earlier various types of fillers are reinforced in polymer matrices to form polymer composites which can be used in wastewater treatment. Some of these types of filler reinforced composites are discussed in detail in the following sections.
4 Polymer-Carbon Composites
Carbon when effectively involved with Polymer composites has been significantly used in the water treatment involving Biodegradation processes and adsorption with dye removal process exists in different molecular properties with advantages like high mechanical and chemical properties, high thermal stability, and cost-effective technique. Polymer doped carbon composites are generated with different forms of carbon like carbon nanotubes (CNT), Activated carbon, Graphene and can be utilized in various industrial and commercial applications. The advantages of the composites enable them for the high removal efficiency of varied micropollutants like dye, heavy metals from wastewater. The polymer doped carbon composites have attracted the scientists due to the excellent properties of high surface area and high solubility persists. The generation process of polymer-carbon composites is represented in (Fig. 3).
Synthesis process of polymer-carbon composites, a generalized scheme using polyvinyl alcohol (PVA) as matrix and activated carbon as filler [9]
Carbon nanotubes (CNT) are the cylindrical structure of carbon materials with a one-dimensional graphitic structure having high surface area making it promizable to be utilized in Bioremediation process involving dye and oil removal. They are of two types multiwalled carbon tubes and single-walled carbon nanotubes. But the carbon nanotubes possess a low solubility technique due to the absence of surface functional groups which resulted in low adsorption performance. The carbon nanotubes show an excellent functionalization property for the degradation process. Graphene when interlinked with the multiwalled nanotube structure grafted with a PVA polymer enhances the functional property due to crosslinking by sp2 hybridization [50]. The polymer composites in the combination of activated carbon and Graphene possess a high stability for the bioremediation by the utilization of advanced treatment technologies like Photodegradation and ozonation (in presence of ozone) and micro-organisms. Activated Carbon preparation from the agricultural wastes has foreseen the environmental problems and thus increases the mechanical stability of the carbon nanocomposites with the reinforcement of activated carbon derived from Agrowaste like Sugarcane bagasse, Rice husk ash, Peanut, Coconut waste, Tea waste, etc.
The composite research on carbon nanotubes attracted attention due to the improved characteristics by surface modifications in the adsorption performance involving dye removal enhancing the thermal and mechanical stability. Fabrication of carbon nanotubes by surface functionalization is a facile approach. A recent literature study was done where carbon nanotubes were grafted with poly (sodium-p-styrene sulfonate) where the CNT was initially coated with a layer of dopamine and then polymerized to utilize in water treatment involving biodegradation by Azolla cyanobacteria then in the removal of a basic Methylene blue dye. The enhanced dye removal showed a great adsorptive capacity at 25 min with 174 mg/g [61]. Polyaniline when encapsulated with multiwalled CNT’s by the in-situ polymerization process possesses a high removal capacity of about 884.84 mg/g with alizarin yellow dye [60]. This suggested that the adsorption study interpreted pseudo second-order Langmuir isotherm and thus the polymer doped carbon CNT composite may be hence utilized as a cost-effective adsorbent for the removal of various dyes.
PVA/AC polymer doped composite is synthesized by the process of 4 g of PVA (Polyvinyl Alcohol) powder was mixed with 100 mL of distilled water and kept for stirring for 2 h to form a transparent solution [47]. Then 2 g of activated carbon was generated from Agrowaste Sugarcane bagasse and carbonized in muffle furnace to obtain the activated carbon then was added in 10 ml of prepared PVA solution and stirred for about 1 h. Hence, the obtained solution was ultrasonicated for 30 min. Then cast on a sterile petri dish by the solution casting polymerization technique and utilized in dye removal process and finally dye-loaded composites were used in biodegradation using bacterial strains [47].
Polymer doped graphene composites have gained a lot of interest due to their effective physicochemical properties and their three-dimensional structure which enhances the porous nature, high thermal resistance, and high electrical conductivity. Polymer-Graphene composites can be structured and fabricated onto membranous films to enhance the bioremediation and dye removal process, also may be used in ultrafiltration membrane separation techniques [28]. Sodium alginate beads were grafted with Graphene to form a composite with excellent selectivity and permeavity [12]. In a study Poly (N, N-2-ethyl aminoethyl methacrylate) was cast with graphene oxide for the removal of methylene orange dyes [15]. A nanoporous fibrous membranous composite was developed using multiwalled carbon nanotube encapsulated with polymer for dye removal by Methylene blue and Congo red because of its low permeation flux and low pressure-driven process carried out by electrospinning and spraying technique [51]. The polymer composites doped with graphene possess good adsorption properties focusing on magnetic adsorbents with biopolymers. Biopolymers were used where iron oxide played a significant role in the magnetic property. The dye removal by polymer-graphene composites was subjected to photocatalytic processes by the isolated micro-organisms of fungi [69]. The polymer doped graphene composite is most commonly used composite because of its high adsorption and removal property due to the functionalization and high porosity which enables it in the utilization of Bioremediation process. The advantages and Disadvantages of Polymer doped Carbon composites are interpreted in (Table 2).
5 Polymer-Clay Composites
Some treatment technologies for the water treatment processes involving Bioremediation and Adsorption process are favored due to their attractive properties such as high effectiveness in the removal process, easy operation technique, and high efficiency. Polymer doped clay composites are one of them which are emerging technologies utilized in the wastewater processes with flexible nature and nature of adsorbents. Clays constitute natural materials with low cost used as natural efficacious adsorbents for the removal of many micropollutants like oil, heavy metals, and dyes. The advantage of these types of composites is their high porosity and high surface-to-volume ratio. But their disadvantage is their wettability process is very poor and slow. Polymer-clay composites can be produced by different associative methods like flocculation, exfoliation, coating, and intercalation (explained in Fig. 4).
A polymer cationic clay composite was grafted with polyvinyl pyridine (polymer) and montmorillonite (cationic clay) as a novel adsorbent for the removal of dyes like Methylene blue with an effective pH response. The high elimination of organic pollutants has been due to the high presence of leachate ions and high zeta potential. At an increase of pH level, the desorption of pollutants takes place using these types of composites [21]. A similar study was conducted using montmorillonite and sodium 2-acrylamido-2-methylpropane sulfonate and N isopropylacrylamide with a graft polymerization using a sulfate free technique. This enhanced the dye removal property by Methylene blue dye and the bioremediation process with these dye-loaded composites. The composites have been so efficient that they can be reused three to four times with no removal loss [5]. The dye has an adsorption capacity of 1875 mg/g representing the highest removal involving mercuric ions due to its highly porous nature and chelation property [64]. Also, a polymer-clay composite produced from montmorillonite with polyvinyl alcohol and poly (4-styrenesulfonic acid-comaleic acid having highly efficient and cost-effective adsorbents [36]. A hydrogel composite with polymer doped clay composite comprising polymers with montmorillonite was exfoliated using bis [2-(methacryloyloxyethyl] phosphate as a crosslinker used for the elimination of methyl red, methylene blue, and crystal violet from wastewater with maximum adsorption capacity of 113, 155, and 176 mg/g. The desorption technique was exhibited in the Ethanol solution [39]. From the recent literature survey, anionic clay-based composite of magnesium aluminum coated with polydopamine was produced for the desalination and water treatment for the bioremediation purposes by using microbes. Also, utilizing removal of copper ions as well as dye removal by Methylene blue and Methyl orange with an adsorption capacity of 198.73 mg/g [11]. The good adsorption capacity was due to the interaction of hydroxyl molecules through hydrogen bonding with removal of dye from wastewater enhancing the adsorption and thus this composite can be effectively used for the bioremediation purposes too [16, 62]. The schematic representation of synthesis of acrylamide and N-isopropyl acrylamide-montmorillonite composite and its use for methylene blue elimination from aqueous media is represented in (Fig. 5).

(reproduced from Berber et al. [11], open-access article)
A schematic representation of synthesis of acrylamide and N-isopropyl acrylamide-montmorillonite composite, and its use for methylene blue elimination from aqueous media
The advantages and disadvantages of Polymer-clay composites are discussed in (Table 3).
6 Fiber-Reinforced Polymer Composites
Fiber-reinforced composites have been nowadays the most prominent option to be used in case of water treatment since they are very lightweight and have high tensile strength due to the reinforcement of fillers into them. The fillers maybe of metal, ceramic, carbon, and natural fibers like cellulose. These composites are a heterogeneous mixture of two materials, one embedded into the other. Fiber-reinforced polymer composites have high performance due to their crosslinking nature of cellulosic fibers with excellent structural properties. These properties make the polymer capable of reinforcing with fillers by the synthesis involving functionalization by the method of crosslinking and blending. The fillers maybe comprised of cellulose extracted from different Agrowaste like Sugarcane bagasse, peanut shells, rice husk ash, etc. The synthesis of fiber-reinforced polymer composite has been interpreted in (Fig. 6).
Synthesis of fiber-reinforced polymer composite membrane, a pictorial representation with nanocellulose as filler and PVA as matrix [37]
Polyvinyl composites reinforced with nanocellulose, chitin, carbon were synthesized. These composites were cost-effective, eco-friendly, and reusable. The crosslinking component was the maleic acid to generate the biopolymer composite film. The composite film was evaluated for the dye removal process involving Methylene blue dye, and the effect of parameters like pH, temperature, concentration was studied for the batch process. The adsorption capacity was extremely high as 467.5 mg/g, confirmed from the FTIR and SEM analysis. SEM analysis showed a rough surface after the incorporation of methylene blue dye into it. FTIR evaluated structural bonds linked to the fillers with different functional characteristics [37].
PVA/Chitin and PVA/nanocellulose was prepared by initially preparing PVA film, dissolving 10 g of PVA granules with 70 g of water, and kept stirring until the solution becomes homogenous at 85 °C for about 2 h. Then nanocellulose was prepared by ultrasonicating the prepared cellulose mixture for 15 min. Now, finally, the chitin or nanocellulose is mixed with maleic acid and PVA in a weight ratio of 1:5 and stirring it for about 3 h. at 500 rpm. HCL was added to the mixture of maleic acid since it behaves as a catalyst. After the solution turns homogenous, pour into a sterile petri dish and keep it under a low heat for the generation of the fiber-reinforced polymeric composite membrane [22]. Finally, the polymer composites were utilized for the bioremediation process involving dye removal and the dye-loaded composites can also be used for the biodegradation process. The isotherm and adsorption studies were done to investigate the adsorption-desorption, and kinetic parameters at an alkaline pH range about 8.5 so that the composites can be regenerated again for further use [43]. The advantages and disadvantages of Fiber-reinforced polymer composites are discussed in (Table 4). Various properties of all these polymer reinforced composites have been explained in (Table 5).
7 Dye Removal Properties for the Different Polymer Composites Used in the Study
See Table 5.
8 Recent Works of Dye Removal Using Polymer Composites
In the recent literature survey, for the purpose of photocatalytic process, a hybrid composite was produced. Polyoxometalate reinforced with polymer was prepared at optimum temperature conditions by the photocatalysis technique. The polyoxometalate was reinforced into the polymeric resin and then was studied under light intensity to interpret the dye removal capacity. The prepared composites reinforced into the polymer matrix revealed high adsorption properties, and the photocatalytic ability was studied under UV irradiation on the Eosin dye [25]. It was noted that the removal efficiency was about 98 and 93%, respectively using these polyoxometalate hybrid composites, whereas it was 94% using silicon reinforced with polyoxometalate composites. The photocatalytic characteristics were studied to increase the quantum yield for the dye decolorization which is crosslinked with the ligands of functional groups occupying oxygen molecules [45].
The characteristics of the polymer were evaluated by the FTIR analysis. After the photopolymerization process, the photocatalytic properties of the prepared polyoxometalate reinforced with the polymeric resin were reserved thus enabling efficient dye removal using Eosin dye. The composites were generated due to the immobilization process studied under UV light. The composite efficiency was interpreted utilizing UV spectroscopy with a wavelength of 405 nm maintaining a pH of 8. The development of these composites leads to high yield and offers new possibilities to remove contaminants from wastewater enhancing the photocatalytic efficacy. Thus, these polymer composites can be regenerated, recycled, and utilized for bioremediation removing pollutants like dye and for other purposes to enhance environmental sustainability.
The development of polymer composites of clay with the green composite polyethylene was done for removing methylene blue dye from wastewater. The different optimum parameters were maintained during the experimental process such as pH (9), concentration of the dye at 1 × 10−5 M with an agitation speed of 1440 rpm. The removal of methylene blue dye from the polymer composites was due to the interaction of the molecules through hydrogen bonding increasing the surface area and porosity [23]. The adsorption and kinetic parameters like temperature, pH, and dye concentrations were investigated for the composites having high adsorptive capacity stated that the adsorption process depicted pseudo second-order.
The three different concentrations were maintained as 5 × 10−6, 10 × 10−6, and 25 × 10−6 M, with room temperature and the values of pH were 5.5, 7, and 9, respectively. The sample was used for the adsorption experiments evaluated through UV-vis spectroscopy and was characterized by SEM, TGA. The literature survey revealed from the results increasing methylene blue dye concentration enhances the adsorption capacity maintaining a pH value of 5.5–9. Due to the molecular interaction and hydrogen bonding, the kinetic energy increases. The adsorption isotherm models for the best fit curve were obtained by pseudo first-order, pseudo second-order, mass transfer diffusion, and intraparticle diffusion. The activation energies, isotherm studies evaluating kinetic as well as thermodynamic parameters were discussed. The Gibbs’s free energy was found to be − 70.64 kJ/mol, Entropy − 70.64 J/mol/K, and the activation energy as 12.37 kJ/mol maintaining a room temperature. The results revealed that the reaction was spontaneous and exothermic and thus this type of composites can be utilized in water treatment and providing green, sustainable environment [49]. Formation of the polymer-clay composite and its dye removal characteristics has been shown in (Figs. 7 and 8).

(reproduced from [49], open-access article)
Formation of GCPf (clay polyethylene composite) and its removal of methylene blue from water solution

(reproduced from [49], open-access article)
Removal of methylene blue by clay polyethylene composites from water in the laboratory
The polymer composites of polyaniline reinforced with hexaferrite and PVA was prepared and examined for dye removal analysis by Reactive black dye. Composite hydrogels were produced by the process of oxidation of aniline hydrochloride mixed with ammonium sulfate with 5% PVA in the presence of hexaferrite particles. These composites show a good adsorptive and magnetic power due to the presence of ferrite molecules with crosslinking of functional bonds incorporated into the hydrogel composites. The mixture prepared was initially kept for thawing at − 5 °C for 5 days for the polymerization to take place [54]. After the thawing process, the composites were produced by the lyophilization process. The innovation for the electromagnetic property of the polymer composites attracted different researchers and scientists worldwide. These polymer composites were prepared due to their advantageous properties such as high chemical strength, high thermal stability, cost-effectiveness, high electrical conductivity, and environmental feasibility.
The porosity of the composites becomes high due to the presence of hexaferrite molecules thus enabling high efficacy for dye removal utilizing the hydrogels [11]. The freezing attitude of the hydrogels reinforced with polyaniline was incorporated because of the magnetic hexaferrite molecules which exhibit easy separation of adsorbents from the fluid. The adsorptive removal capacity was about 99% by Reactive black dye, this is due to the reason of cohesive forces present in the hydrogels by the reinforcement of polyaniline and hexaferrite in presence of PVA, thus can be utilized in bioremediation and water treatment. The deformation of composites results in effective magnetic anisotropy and low coerciveness [46].
An eco-friendly bio adsorbent polymer composite was generated by the process of graft polymerization with high removal capacity of azo dyes from solutions. 1,1-diallyl-4-carboxypiperidin-1-ium bromide was incorporated into biomaterials like cellulose, chitosan. Different parameters which affected the adsorption property of dye are temperature, pH, adsorbent dosage, and solution concentration. The biopolymer composites served as efficient adsorbents with excellent amphoteric approach which enabled the composites in the bioremediation, desalination, and water treatment study. The bio adsorbent composite served as a pH-responsive material for the removal of azo dyes such as indigo and Congo red from the solutions [35, 67]. The polymer composites helped in the decontamination of dye-containing water solutions. The surface morphological properties and functionalization characteristics were determined by SEM, FTIR, TGA, and VSM which revealed the roughness nature of the composites after dye degradation. The adsorption isotherm models like Langmuir isotherm and pseudo second-order kinetic model were investigated to obtain the best fit model for the experimental analysis and use it further. The polymer composites were regenerated with a basic solution of HCL or NaOH [41]. The adsorption capacity for the azo dyes was 840.33 mg/g and 909.1 mg/g, respectively. This is due to the entrapment and immobilization of dye molecules into the polymer bio adsorbents with cost-effective materials as an environment-friendly technique that enables the sustainability with regeneration, and ease in magnetic separation for the polymer composites.
9 Future Perspectives
The chapter foresees the generation of different types of polymer composites and their application in dye removal as well as for bioremediation in water treatment and desalination. The demand for clean water has been increased drastically in past decades due to increased marine pollution. Different methods were being employed for the treatment of above problems [1, 24]. The removal processes mainly involved adsorption techniques and separation processes (bioremediation or phytoremediation).
A vast variety of polymer reinforced composites for dye removal have been exhibited and utilized in lab-scale experimentation techniques, therefore exhibiting a positive outcome. Thus, challenges can be overcome by employing more applications to these composites in removing dyes and other micropollutants. Elimination of dye and weight loss of the composites results due to the polymerization techniques and electrochemical processes [30, 55].
10 Conclusion
As discussed in this chapter, the polymer reinforced composites made of all synthetic and natural polymers have remarkable, and attractive properties of adsorption and bioremediation when combined with different adsorbents like polymer, clay, cellulose, and carbon. The recent works related to the dye removal study utilizing the polymer composites have also been discussed in this chapter. These composites are prepared for the sustainability of the environment since they are cost-effective, and environment friendly. The production of these polymers yields a high cost, but they have easy fabrication, have high chemical and thermal strength, and application process. Using Agro waste in the preparation of natural fibers as a filler in developing the polymer-polymer composites and in the generation of activated carbon for polymer-carbon composites exhibit high efficacy. A lot of research has been done to make the polymers durable, highly efficient, and have high adsorption properties to be utilized in dye removal from industrial wastewaters.
References
Abdullah N, Tajuddin M, Yosuf H (2018) Carbon based polymer nanocomposites for dye removal:32493262
Ahmad R, Kumar R (2010) Adsorption studies of hazardous malachite green onto treated ginger waste. J Environ Manage 91(4):1032–1038
Almasian A, Olya ME, Mahmoodi NM (2015) Preparation and adsorption behavior of diethylenetriamine/polyacrylonitrile composite nanofibers for a direct dye removal. J Fib Polym 16(9):1925–1934
Ao C, Zhao J, Xia T, Huang B, Wang Q, Gai J, Chen Z, Zhang W, Lu C (2021) Multifunctional La (OH)3@cellulose nanofibrous membranes for efficient oil/water separation and selective removal of dyes. Sep Purif Technol 254:117603
Atta AM, Al-Lohedan H, AL Othman HL, Abdel AA (2015) Characterization of reactive amphiphilic montmorillonite nanogels and its application for removal of toxic cationic dye and heavy metals water pollutants. J Ind Eng Chem 31:374–384
Bagha AT, Nikkar H, Mahmoodi N, Markazi M, Menger FM (2011) The sorption of cationic dyes onto kaolin: kinetic, isotherm and thermodynamic studies. Desalination 266(1–3):274–280
Barka N, Qourzal S, Assabbane A, Nounah A, Ait-Ichou Y (2010) Photocatalytic degradation of an azo reactive dye, reactive yellow 84, in water using an industrial titanium dioxide coated media. Arab J Chem 3(4):279–283
Bedin KC, Souza IPAF, Cazetta AL, Spessato L, Ronix A, Almeida VC (2018) CO2 spherical activated carbon as a new adsorbent for methylene blue removal: kinetic, equilibrium and thermodynamic studies. J Mol Liq 269:132–139
Behera SK, Kim JH, Guo X, Park HS (2008) Adsorption equilibrium and kinetics of polyvinyl alcohol from aqueous solution on powdered activated carbon. J Hazard Mater 153:1207–1214
Berber MR (2020) Current advances of polymer composites for water treatment and desalination. J Chem 19:7608423
Bober P, Zasońska BA, Humpoliček P, Kucekova Z, Varga M, Horak D, Babayan V, Kazantseva N, Prokeš J, Stejskal J (2020) Polyaniline-maghemite based dispersion: electrical, magnetic properties and their cytotoxicity. J Synth Met 214:23–29
Cao K, Jiang K, Zhao J (2014) Enhanced water permeation through sodium alginate membranes by incorporating graphene oxides. J Mem Sci 469:272–283
Crini G. Non-conventional low-cost adsorbents for dye removal: a review. Biores Technol 97(9):1061–1085
Diez MC (2010) Biological aspects involved in the degradation of organic pollutants. J Soil Sci Pl Nutri 10(3):244–267
Dong L, Fan W, Tong X, Zhang H, Chen M, Zhao Y (2018) A CO2-responsive graphene oxide/polymer composite nanofiltration membrane for water purification. J Mat Chem A 6(16):6785–6791
Dou J, Huang Q, Huang H (2019) Mussel-inspired preparation of layered double hydroxides-based polymer composites for removal of copper ions. J Coll Inter Sci 533:416–427
Du JJ, Yuan YP, Sun JX, Peng FM, Jiang X, Qiu LG, Xie AJ, Shen YH, Zhu JH (2011) New photocatalysts based on MIL-53metal–organic frameworks for the decolorization of methylene blue dye. J Hazard Mater 190:945–951
Elahmadi MF, Bensalah F, Gadri A (2009) Treatment of aqueous wastes contaminated with Congo red dye by electrochemical oxidation and ozonation processes. J Hazar Mat 168(2–3):1163–1169
Elkady M, El-Assar M, Hassan H (2016) Adsorption profile of basic dye onto novel fabricated carboxylated functionalized co-polymer nanofibers. Polymers 8(5):177
Forgacs E, Cserháti T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Gardi I, Mishael YG (2018) Designing a regenerable stimuli responsive grafted polymer-clay sorbent for filtration of water pollutants. Sci Technol Adv Mat 19(1):588–598
Gatabi MP, Moghaddam HM (2016) Point of zero charge of maghemite decorated multiwalled carbon nanotubes fabricated by chemical precipitation method. J Mol Liq 216:117–125
Ghaedi M, Hossainian H, Montazerozohori M, Shokrollahi A, Shojaipour F, Soylak M, Purkait MK (2011) A novel acorn-based adsorbent for the removal of brilliant green. Desalination 281:220–233
Ghaemi N, Madaeni SS, Daraei P, Rajabi H, Shojaeimehr T, Rahimpour F (2015) PES mixed matrix nanofiltration membrane embedded with polymer wrapped MWCNT: fabrication and performance optimization in dye removal by RSM. J Hazard Mater 298:111–121
Ghali M, Brahmi C, Benltifa M, Dumur F, Duval S (2019) New hybrid polyoxometalate/polymer composites for photodegradation of eosin dye. J Poly Sci 57(14):1538–1549
Ghosh SK, Pal S, Ray S (2013) Study of microbes having potentiability for biodegradation of plastics. Int J Environ Sci Pollut 20:4339–4355
Halder JN, Islam MN (2015) Water pollution and its impact on the human health. J Environ Hum 2:36–46
Jayakaran P, Nirmala GS, Govindarajan L (2019) Qualitative and quantitative analysis of graphene-based adsorbents in wastewater treatment. Int J Chem Eng:9872502:17
Kalia S, Vasishta S, Kaith BS (2011) Cellulose nanofibers reinforced bioplastics and their applications, handbook of bioplas bio computer engineering application. Wiley-Scrivener Publishing, NY, USA
Li Z, Li Y, Zhu RS (2015) Synthesis and characterization of mesoporous carbon nanofibers and its adsorption for dye in wastewater. Adv Powder Technol 27:591–598
Li Y, Zhou Y, Lei J, Pu S (2019) Cyclodextrin modified filter paper for removal of cationic dyes/Cu ions from aqueous solutions. Wat Sci Tech 78(12):2553–2563
Lin AG, Liu PY, Liu G, Zhang GZ (2006) Ind Water Treat 26:5–8
Liu X, Ma R, Wang X (2019) Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: a review. Environ Pollut 252:62–73
Liu Q, Li Y, Chen H (2020). Superior adsorption capacity of functionalized straw adsorbent for dyes and heavy-metal Ions. J Hazard Mats 382:121040
Mahida VP, Patel MP (2016) Removal of heavy metal ions from aqueous solution by superabsorbent poly (NIPAAm/DAPB/AA) amphoteric nanohydrogel. Desalin Water Treat 57:13733–13746
Medhat F, Pakizeh M (2018) Preparation and characterization of a nano clay/PVA/PSf nanocomposite membrane for removal of pharmaceuticals from water. Appl Clay Sci 162:326–338
Mok CF, Ching YC, Muhamad F, Osman NAA, Dai Hai N, Hassan CRC (2020) Adsorption of dyes using poly (vinyl alcohol) (PVA) and PVA-based polymer composite adsorbents: a review. J Polym Env 28:775–793
Mondal S (2008) Methods of dye removal from dye 399 house effluent—an overview. Environ Eng Sci 25(3):383–396
Nakhjiri MT, Bagheri GM, Kurdtabar M (2018) Effect of bis [2-(methacryloyloxy)ethyl] phosphate as a crosslinker on poly (AAm-co-AMPS)/Na-MMT hydrogel nanocomposite as potential adsorbent for dyes: kinetic, isotherm and thermodynamic study. J Poly Res 25(11):244
Pang H, Wu H, Wang X, Hu B, Wang X (2019) Recent advances in composites of graphene and layered double hydroxides for water remediation: a review. Chem Asian J 14(15):2542–2555
Patel SR, Patel R, Patel MP (2020) Eco-friendly bio adsorbent-based polymer composites as a pH-responsive material for selective removal of anionic and azo dyes from aqueous solutions, J Macromol Sci Part A:1827957
Paulino AT, Guilherme MR, Reis AV, Campese GM, Muniz EC, Nozaki J (2006) Removal of methylene blue dye from an aqueous media using superabsorbent hydrogel supported on modified polysaccharide. J Colloid Interface Sci 301:55–62
Pirbazari A, Pargami N (2015) Surfactant-coated tea waste: preparation, characterization and its application for methylene blue adsorption from aqueous solution. J Environ Anal Toxicol 5(5):1
Raffi F, Hall JD, Cernigila CE (1997) Mutagenicity of azo dyes used in foods, drugs and cosmetics before and after reduction by clostridium species from the human intestinal tract. Food Che Toxicol 35:897–901
Rajeshwar K, Osugi ME, Chanmanee W, Chenthamarakshan CR, Zanoni MV, Kajitvichyanukul P, Krishnan-Ayer J (2008) J Photochem Photobiol C Photchem Rev 9(4):171
Sanchez PA, Stolbov OV, Kantorovich SS, Raikher YL (2019) Modeling the magnetostriction effect in elastomers with magnetically soft and hard particles. Soft Matter 15:7145–7158
Sarkhel R, Sengupta S, Das P, Bhowal A (2020) Comparative biodegradation study of polymer from plastic bottle waste using novel isolated bacteria and fungi from marine source. J Poly Res 27(16)
Schmidt D, Shah D, Giannelis EP (2002) New advances in polymer/layered silicate nanocomposites. Curr Opin Solid State Mater 6(3):205–212
Sen F, Demirbas O, Calimli MH, Aygun A, Alma MH, Nas MS (2018) The dye removal from aqueous solution using polymer composite films. J Appl Wat Sci 8:206
Serp P, Corrias M, Kalck P (2003) Carbon nanotubes and nanofibers in catalysis. Appl Catal A 253(2):337–358
Shi J, Wu T, Teng K, Wang W, Shan M, Xu Z (2016) Simultaneous electrospinning and spraying toward branch-like nanofibrous membranes functionalized with carboxylated MWCNTs for dye removal. Mater Lett 26(9):166
Song CW, Wang TH, Pan YQ (2006) Sep Purif Technol 51:80–84
Soni S, Bajpai PK, Mittal J, Arora C (2020) Utilization of cobalt doped iron based MOF for enhanced removal and recovery of methylene blue dye from wastewater. J Mol Liq 314:113642
Stejskal J, Bober P, Trchova M, Kovalcik A, Hodan J, Hromadkova J, Prokeš J (2017) Polyaniline cryogels supported with poly (vinyl alcohol): soft and conducting. Macromolecules 50:972–978
Sun Y, Wang G, Dong Q, Qian B, Meng Y, Qiu J (2014) Electrolysis removal of methyl orange dye from water by electrospun activated carbon fibers modified with carbon nanotubes. J Chem Eng 253:73–77
Takka S, Gürel A (2010) Evaluation of chitosan/alginate beads using experimental design: formulation and in vitro characterization. AAPS Pharm Sci Tech 11:460–466
Vandeviveri PC, Bianchi R, Verstraete W (1998) Treatment and reuse of wastewater from the textile wet-processing industry: review of emerging technologies. J Chem Tech Bio 72:289–302
Verma P, Madamwar D (2003) Decolorization of synthetic dyes by a newly isolated strain of Serratia marcescens. World J Microbi Biotech 19:615–618
Wang X, Chen L, Wang L (2019) Synthesis of novel nanomaterials and their application in efficient removal of radionuclides. Sci China Chem 62(8):933–967
Wu K, Yu J, Jiang X (2018) Multi-walled carbon nanotubes modified by polyaniline for the removal of alizarin yellow from aqueous solutions. Adsorp Sci Tech 36(1–2):198–214
Xie Y, He C, Liu L (2015) Carbon nanotube-based polymer nanocomposites: biomimic preparation and organic dye adsorption applications. RSC Adv 5(100):82503–82512
Yang XJ, Zhang P, Li P (2019) Layered double hydroxide/polyacrylamide nanocomposite hydrogels: green preparation, rheology and application in methyl orange removal from aqueous solution. J Mol Liq 280:128–134
Yue X, Jiang F, Zhang D, Lin H, Chen Y (2017) Preparation of adsorbent based on cotton fiber for removal of dyes. J Fib Polym 18(11):2102–2110
Zeng H, Wang L, Zhang D, Wang F, Sharma VK, Wang C (2019) Amido-functionalized carboxymethyl chitosan/montmorillonite composite for highly efficient and cost-effective mercury removal from aqueous solution. J Coll Int Sci 554:479–487
Zeng YB, Yang CZ, Zhang JD, Pu WH (2007) J Hazard Mater 147:991–996
Zhang P, Lo I, O’Connor D, Pehkonen S, Cheng H, Hou D (2017) High efficiency removal of methylene blue using SDS surface modified ZnFe2O4 nanoparticles. J Colloid Interface Sci 508:39–48
Zhen Y, Ning Z, Shaopeng Z, Yayi D, Xuntong Z, Jiachun S, Weiben Y, Yuping W, Jianqiang C (2015) A pH-and temperature-responsive magnetic composites adsorbent for targeted removal of nonylphenol. ACS Appl Mater Interfaces 7:24446–24457
Zhou Y, Hu Y, Huang W, Chen G, Cui C, Lu J (2018) A novel amphoteric β-cyclodextrin-based adsorbent for simultaneous removal of cationic/anionic dyes and bisphenol a. J Chem Eng 341:47–57
Zinadini S, Zinatizadeh AA, Rahimi M, Vatanpour V, Zangeneh H (2014) Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J Membr Sci 453:292–301
Acknowledgment
The author acknowledges people of Jadavpur University for their valuable guidance and support at each level of the research. The work was supported by RUSA 2.0 (financial assistance) from Jadavpur University.
Conflict of Interest
There is no conflict of interest by the authors.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sarkhel, R., Sengupta, S., Das, P., Bhowal, A. (2022). Dye Removal Using Polymer Composites as Adsorbents. In: Khadir, A., Muthu, S.S. (eds) Polymer Technology in Dye-containing Wastewater. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-19-0886-6_4
Download citation
DOI: https://doi.org/10.1007/978-981-19-0886-6_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0885-9
Online ISBN: 978-981-19-0886-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)