Abstract
Environmental concerns gain importance with the increase in the lack of sources on the world. Especially, the textile industry has many contaminating effects on the environment, and the most important one is wastewater problems related to the high chemical load that is coming from textile processes and high water consumption. To eliminate the adverse results of textile wastewater discharges and high usages, wastewater must refine before discharging and must reuse in suitable processes. Refining processes are very valuable for decreasing the contaminants in wastewater, but these processes need extra cost, time, production place, and investments. Multifunctional agents as adsorbing composites can provide many advantages to refining processes and alternative reuse strategies and environmental conservation. In this chapter, the needs for refinement and types of refining processes are evaluated first. Then, adsorbent materials and their composites are detailed with properties, production stages, and usages. Finally, this chapter offers an alternative adsorbent composite production and usage study based on clay and modified chitosan.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Textile wastewater
- Refining processes
- Dyestuff adsorbing composites
- Clay
- Chitosan
- Cellulose derivatives
- Biochar
- Biopolymers
1 Introduction
Water sources are decreasing day by day with various reasons as increasing human population, global warming, uncontrolled water consumption, water contamination, etc. The human population increase and global warming are indirect reasons and more related to the balance of nature. Water consumption and contamination are direct reasons for source decrease, and they are related to human behavior directly and can be controlled or limited by environmental awareness. Water consumption was 1041 billion liters per day for surface water sources and 3002 billion liters per day for groundwater, and these values are very high for the future of the earth [1]. Water contamination is the most unfortunate reason for lack of water because water is wasted knowingly or unknowingly without consuming it, and huge amounts of water become unusable because of the lack of water quality or becoming harmful.
Many sectors affect water contamination with different roles, and the major effect is discharging wastewater of their industrial procedures. In the case of industrial wastewater, the textile industry has the maximum amount of water usage as using 200 L water during pretreatment, coloration, and finishing processes (see Fig. 1) applied for producing 1 kg fabric [2]. Among wet processes, dyeing and finishing operations use around 50% of the water and generate 80% of wastewater [3]. The wastewater of the textile industry which includes high color and toxicity has a very huge amount of contaminant units in it; approximately, 8000 chemicals can be in wastewater, and most of them belong to dyeing [3]. These contaminants can be dyestuffs (acidic, basic, reactive, azo, metal-complex, etc.), finishing agents, bleaching and cleaning agents, lubricants, alkaline and acid auxiliary chemicals, surfactants, metal ions, and fiber coming from textile materials, organic or inorganic compounds, and all of them vary widely in their physical and chemical characters. Wastewater of a desized product can include carboxymethyl cellulose, starch, polyvinyl alcohol, waxes, fats, resins, and glucose [4] with high biological oxygen demand (BOD) which is 35–50% of BOD total, but polyvinyl alcohol and carboxymethyl cellulose are not harmful and do not affect the BOD amount. If a product is bleached, then this process adds hydrogen peroxide, hypochlorite, acids, caustic soda, chlorine like chemicals [5, 6], and the water characteristic is alkaline with 5% of BOD. For mercerized product, caustic soda can be seen with the high amount, and its characteristics turn to strongly alkaline [7] with low BOD. In wastewater of dyeing processes, dyestuff, reducing agents (hydrosulfide, sulfides, soap, acetic acid), and mordants [8, 9] are seen, and it is strongly colored with fairly high BOD which is 6% of the total BOD. Printing adds dyestuff or pigments, gums, starch, mordants, china clay, oil, metallic salts, and acids into wastewater, and its characteristic can be defined as highly colored and oily with fairly high BOD. When the last process of finishing is applied to the product, chemicals like tallow, Glauber salt, traces of starch, common salt, and special agents [10, 11] can be seen in wastewater that is slightly alkaline with low BOD [3].
Dyestuffs cause the color and toxicity of the wastewater. Especially, cationic dyestuffs that include benzidine or aromatic compounds can be carcinogenic or mutagenic for water creatures and human beings. Anionic and reactive dyestuffs have persistent behavior to be eliminated because of being water soluble and become harmful to nature. Dyestuffs in water act like a photocatalytic material and do not let sunlight to penetrate the water supply; thus, water living habitat gets harm with the change of the oxygen balance; eutrophication which is uncontrolled reproduction of algae in a small water supply is seen and some other species extinct due to suppression of algae [3]. Dyestuff presence in water supplies decreases biodiversity.
Total dissolved solids (TDS) are one of the problems of wastewater, and the reason for this is common salt and Glauber’s salt. Even it is at low level, TDS damages the balance of the water habitat because the salt amount changes and osmotic balance is damaged; thus, water creatures can get harm due to dehydration or excess water penetration. Also, the water supply becomes useless for human activities [3].
Oil, grease, clay, silt, and gritty materials can be categorized under suspended solids, and they form a barrier on the surface of the water; thus, oxygen and sunlight inlet are prevented, and the temperature of water habitat decreases. As a result, water creatures get serious harm from these impurities [3].
Toxic effects can be seen for all living things in case of drinking, eating, and respiration, in case of exposure to heavy metals as manganese, copper, arsenic, cadmium, mercury, chromium, zinc, lead, etc., with amounts more than the limitations [3].
Furthermore, impurities can accumulate in pipes and results with clogging unless the wastewater is refined with suitable methods. This can add an economic problem to maintain it. Besides the economic problems, water scarcity and some illnesses such as haemorrhage, dermatitis, an ulcer on the skin, vomiting gastritis, nausea, skin irritation and risk of bladder cancer can be seen due to lack of clean and enough water [12].
These contaminants must be eliminated at refining procedures before discharging not to contaminate the nature and harm all living things. Refining is also needed for reusing wastewater; thus, water consumption is also decreased with reuse policy. This reuse policy provides water, chemical and energy saving, and decreasing waste production.
In this study, general refining methods for wastewater treatment, textile wastewater characteristics, and selection of refining processes depending on wastewater characteristics are explained, and alternative composite structures applicable as well as needs for these alternatives are discussed. Finally, a case study on a novel dyestuff adsorbing nanocomposite is evaluated.
2 Wastewater Refining
Refining is impurity elimination from waste to turn it into more suitable forms. In the case of a wastewater refining, it is clarifying wastewater, which is a result of an industrial process, by removing undesired particles from the process environment, end products, and by-products. Refining is very important for both discharging and saving according to regulations and standards because it is a preliminary stage of both of them. Quality of wastewater must be increased; contaminants, color, and odor of the water must be removed, and it is turned into suitable levels. Refinement is a series of clearing processes; refinement wastes as in mud or liquid form must be disposed of suitably, and this needs an extra disposing strategy. There are certain types of refining, but the application of those changes according to characteristics of the wastewater. There are three main types of refining methods as physical refining, oxidative refining, and biological refining. According to the wastewater characteristics, some methods can be applied or all of them can be used in a combination. In a refining step, material elimination starts from bigger particles to tiny particles and chemicals. Generally, first, solid particles are eliminated by using different sizes of grids; then, oxidative/chemical and biological treatments are carried to eliminate small contaminants, color, and odor [13].
Textile wastewater includes many textile fibers, chemicals, and dyestuff, and it is more complex to refine; thus, different refining combinations are applied, and it brings a huge refining process with long time consumption. All these different impurities produce huge chemical loads, and they must be removed carefully for yielding more clear wastewater to discharge in limits or reuse in more suitable applications. According to applied textile processes and characteristics of the resultant wastewater, one or more refining processes that are mentioned in Fig. 2 can be selected, and in these steps, using more functional agents as dye adsorbing composites could decrease the total number of refining steps demanded and time consumption with the increase of efficiencies.
Classification of refining methods [13]
2.1 Physical Refining Methods
Physical refining is eliminating the particles in the wastewater by using different sizes of physical equipment. It is generally the primary refining step because, firstly, bigger particles must be removed as fabric pieces, fibers, some metallic particles, and floccules. Physical methods can be classified into three groups as filtration, flocculation, and adsorption.
In filtration, different sizes of sieves and filters are used. According to the ingredient and temperature of the effluent that will be refined, the pore size of sieves and filters alter, and a combination of these with various sizes can be combined. A physical refining system starts from coarser grids to finer filter, and by using in a sequence, particles that can be seen are removed one by one until the only particles that can pass from the finest filter in the refining system. Filtration can be ultrafiltration, nanofiltration, and reverse osmosis [13]. In ultra- and nanofiltration, effluent passes through a filter without any force or effect, and the number of impurities in it is decreased. However, in reverse osmosis, a membrane is used, and the opposite behavior of osmosis mechanism is used. In osmosis, water directly penetrates through the membrane from the diluted region to a more concentrated region without any force, while in reverse osmosis, effluent passes from concentrated region to diluted region by applying pressure on the effluent, and effluent is cleaned and collected at the diluted region. In this sequence of filtration, particle sizes that are removed decrease from ultrafiltration to reverse osmosis and impurities removed step by step.
Flocculation is the precipitation of particles thanks to their density differences or forming precipitation units by using additives to make it faster. Particles collected at the bottom of flocculation pools by waiting certain times and cleaner wastewater are collected from the upper part of the pool. It can be used for disperse dyestuffs, and it has low efficiency at reactive and vat dyestuffs, but it has limited usage and high mud production.
Adsorption is holding impurities on the surface of the adsorbent; thus, particles are removed from wastewater and stay on the adsorbent. When adsorbent is removed, wastewater is cleaned. This method has high discoloration efficiency even at low color concentrations and low cost [13]. Adsorbent materials and their details will be discussed in the further part of this chapter.
2.2 Oxidative Refining Methods
Oxidative methods use generally oxidative chemicals, and this class can be known as chemical methods. Different radicals are used to eliminate impurities by oxidizing them. These methods can be applied easily; thus, they are the most commonly used class. This class has two subtitles as advanced oxidative method (AOM) and chemical oxidative methods.
The advanced oxidative method uses hydroxyl radicals which are very strong oxidative, and the generated radicals eliminate organic and inorganic impurities. This method is very useful for textile wastewater and fast [13]. This method can be supported by ultrasonic or hydrodynamic cavitation. Also, photocatalytic oxidation and Fenton reaction which is between Fe3+ ions and H2O2 can be in AOM-type refining. In Fenton reaction, a Fe2+ ion mixture which has pH 2–3 as the acid is prepared, and H2O2 is added to form hydroxyl radicals [14]. Other Fenton’s reagents can be used, but iron salts are the most common ones. Mud formation is a disadvantage of the Fenton method because reagent and dyestuff form a complex molecule and produce floccules [13].
The chemical oxidative method is another method for oxidative methods. It uses oxidative agents as ozone and hydrogen peroxide that attacks to the conjugated double bonds and functional groups at chromophore region of dyestuffs. In this method, effluent is taken into the tank, and the oxidant is added in the reactor, and finally, caustic is added for pH adjustment. In the case of ozone that needs good stabilization conditions as pH and temperature, it removes toxic ingredients, hydrocarbons, phenols, etc., and decreases chemical oxygen demand (COD) of the effluent. For hydrogen peroxide, pH, temperature, peroxidase usage, and strength are parameters that must be paid attention to for suitable removal of dyestuff, especially for acid dyes. To improve these methods, hydrogen peroxide and UV light are used for oxygen. Another chemical is NaOCl thanks to its Cl− component for breaking amine bond. Also, coagulation is another option in chemical methods for wastewater that cannot form floccules naturally by waiting as in the flocculation method. Particles coagulate with coagulant chemicals, and coagulated particles are eliminated [14]. If the coagulation occurs by using reaction with ions that are released as Al(OH)3 and Fe(OH)3 from aluminum and iron electrodes, it is known as electrocoagulation [15].
2.3 Biological Refining Methods
Biological refining is eliminating impurities by decomposing them in a natural cycle of microorganisms. These methods are very advantageous because they are environmentally-friendly, relatively lower cost, having lower water consumption, and including non-hazardous metabolites. Efficiency depends on pH, temperature, oxygen concentration, amount of microorganisms, and dyestuff because living things are used, and their metabolism is affected by environmental conditions; thus, it needs attention. Biological refining methods are classified into two groups as methods done by microorganisms and enzymes [13]. If the enzymes such as ligninase (toxicity removal), cellulase (breaking cellulose particles into glucose), peroxidase (removal of color and peroxides), and dehalogenase (dechlorination and removal of chlorophenols) that are used by microorganisms or can degrade the impurities in the wastewater are directly added into the refining baths, then it is enzyme-type methods. Enzymes are specific to their substrates and only deal with them, and if the conditions as temperature, pH, etc., are suitable, they cannot be deformed, and they can be used more than once [16]. If the microorganisms directly used in refining and enzymes that have in their metabolic reactions are used, then it is microorganism-type refining. Also, refining methods with microorganisms can be divided into two subtitles as aerobic and anaerobic refining. For example, bacterial refining can be aerobic or anaerobic, while fungi refining is aerobic generally [17].
Aerobic biological refining methods degrade or convert impurities into less harmful materials by using oxygen with a microorganism, especially at low-strength wastewater which has lower COD than 1000 mg/L [17]. Aerobic refining is very effective, especially at decolorization of dyestuffs by using microorganisms as Pseudomonas fluorescens, Bacillus fusiformis, Staphylococcus aureus, Enterococcus gallinarum, Brevibacillus laterosporus, etc. [18]. These methods are very good at less odor production and efficient removing, but oxygen need, mud production, clogging, and costs can be adverse sides of aerobic refining methods.
Anaerobic biological treatment does not need oxygen in the degradation of impurities at high strength wastewater which has higher COD than 4000 mg/L. Producing natural gas like methane, having low mud production, and cost can be favorable sides of anaerobic refining methods [17]. With the light of this information, if there is high-strength water, firstly, anaerobic methods are applied, and then, aerobic methods can be added, after the organic material amount of the wastewater is suitable for aerobic microorganisms, to yield the best sides of both methods.
All types of refining methods have some advantages and disadvantages compared to each other, but using alternative environmentally-friendly refining processes that decrease the process amount, chemical, and refining waste, refining cost and time are very valuable and important for total production and environmental concern. It provides an increase in the application of refining with better and more desired options. If refining is easy and economical or it has any advantages over the classical processes, then the refining gains importance, and more companies would like to attend the environmental concern. In most cases, methods or multifunctional materials that can offer all these benefits in a single step are the needs of future applications with growing technologies and keeping fast fashion cycles in a good manner. For these reasons, many studies are concentrated on the development of novel refining processes and multifunctional materials for refining.
3 Adsorbent Materials
Adsorbent material usage is one of the alternative refinement methods. They show promise for holding dyestuff and chemical agents. The holding ability of materials depends on the adsorption capacity and ionic characteristics. Thanks to the suitable interaction between adsorbed chemical and adsorbent, the chemical is removed from wastewater and kept in the adsorbent. Adsorbent materials can be natural or polymer-based, i.e., more synthetic ones, but behaviors are the same in principle.
3.1 Properties
The physical properties of adsorbents can be changed according to their raw materials and desired conditions of end usage. First, physical property can be the shape of adsorbent as pellet, rod, molding, or monolith, while the other one is size such as 0.25 and 5 mm of the radius, but pore amount and size are also indicative. To be an effective adsorbent, it must have high stability for thermally and mechanically to withstand the process conditions and showing the same behavior without any loss, structure with pores that make surface area higher, and high capacity to adsorb enough material [19].
3.2 Working Mechanism
Collecting a material or chemical in the interface of a host material is known as adsorption. The substance that is collected is called adsorbate, while the collecting material is adsorbent, and both of them can be solid, liquid, or gas [20]. This collection mechanism changes according to the interaction between adsorbent and adsorbate as chemical or physical. Due to the interaction type, the type of adsorption changes. Physisorption is done by using physical interactions which is the result of energy and entropy decrease as Van der Waals forces, surface adsorption, hydrophobic interactions, hydrogen bond, diffusion into the material network, and these types of adsorptions can be reversible. Chemisorption is done by chemical interactions as ionic or covalent bonding, complexation (coordination), proton displacement, chelation, electrostatic interactions, oxidation/reduction, inclusion complex formation, and it is irreversible, and it has a high range of heat because of strong chemical bonding. Adsorptions are affected by temperature, the concentrations of adsorbate and adsorbent [20,21,22].
3.3 Advantages and Disadvantages
Using adsorbent materials in wastewater treatment processes has some disadvantages such as waste production and weak selectivity, but these can be eliminated with various enhancements or reuse strategies. If the waste adsorbent can be reused in some other processes with different forms as filler, dyestuff source, etc., waste products can be decreased or zeroed, and some modifications on adsorbent can enhance the selectivity of the adsorbent. However, adsorbent usage at refining of wastewater has more advantages than disadvantages as high performance, low cost, wide pH range for process conditions, and ease of operation for treatment [23].
4 Adsorbent Composite Production
Adsorbent materials have many advantages compared to each other; however, more properties can be needed to maintain the desired conditions, or sometimes, abilities of the adsorbents must be improved for different cases. In these cases, composite structure can come to screen to yield the abilities of at least two materials or to enhance the functions. With composite production stability, applicability, ease of use, working conditions, cost, end-use, and reusability after waste treatment can be improved. Also, the composite structure can offer lower costs and reduction of process time. Composite structures are yielded by combining at least one layered material such as silica balls and nanotubes and one or two polymer-based materials.
The composite structure can be classified into two groups as micro- and nanocomposites. If polymer component covers around the layered structure without any separation of layers, then it is called microcomposite. Nanocomposites are produced when layered structure deforms by enlarging or separation. If the gap between layers enlarge and polymer component penetrates the enlarged gaps, then it is called intercalated nanocomposites. If the layers are totally separated from each other and polymer component covers individual layers thanks to even distribution of layers, then it is named as exfoliated composite. All types of composites can be used according to end uses, characteristics of the wastewaters, and needed removal amounts, but more uniform composites are produced with exfoliated structures [24].
Structures of composite types are given in Fig. 3, and advantages and disadvantages of them are given in Table 1.
There are three main composite production methods with layered units and polymer units as solution intercalation, melt intercalation, and in situ polymerization.
In situ polymerization method uses a layered unit as clay and monomer units. They are putting in together, and after swelling of layers, monomers are located around the separated or enlarged layers thanks to surface energy and polarity of monomers. Then, curing chemicals are added to turn monomers into polymers. When polymerization occurs, then in situ polymerized composite is formed and layers catch up more polar monomers inside. For this type of composite structure, polymers as polyamide 6 is suitable [25].
Solution intercalation uses a solution to swell the layered units, and dissolved polymers are added to penetrate gaps of layers. When the solution is evaporated, layers catch up the polymer to form intercalated composites [25].
Melt intercalation is a method for layered units and thermoplastic polymers as polyamide, polystyrene, etc. Layered units and melted polymer are mixed, and penetration of polymer is done thanks to the compatibility of polymer and layers. Due to interaction between layers and polymer, the gap enlarging and penetration increases; thus, both intercalated and exfoliated composites can be formed after annealing under glass transition temperature. The advantage of this method is applicability at the extrusion of melt spinning techniques [25].
The advantages and disadvantages of composite production methods are given in Table 2.
5 Raw Materials for Natural Adsorbents
Adsorbent materials can be natural or polymer-based, i.e., more synthetic ones. Natural ones offer further benefits for both the environment and human health. Also, recycling is done by using natural sources to produce adsorbents. For these purposes, various alternatives can be used as agricultural wastes [26], clay minerals, cellulose derivatives, active carbon forms from different wastes [27, 28], and their composites. In this case, natural adsorption capacities can be increased by modifying them or combining them as a composite.
Especially, clay is a very useful material as a layered substance. It has a sandwich-like structure made of clay minerals, and the gap between these layers can be increased, and adsorption abilities can be enhanced. Also, inserting a compatible polymer structure into these gaps can enhance both adsorption capabilities and gives additional physical, chemical, thermal, or mechanical new properties to the clay. Synthetic [29] or natural polymers can be used, but using natural or more biodegradable polymers can be more environmentally-friendly, and it also promotes reuse of the final product in the preceding production steps. These natural polymers can be chitosan [30], carboxymethyl cellulose [31], and other polymers derivatives as well as activated biochars [26].
5.1 Clay
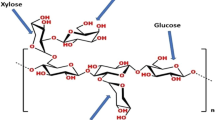
According to AIPEA, clay is a natural material that is made up of fine-grained minerals that pass to plastic state in the appropriate water content and became rigid when it is heated or dried [32]. Clay mineral particles, especially the ones that are smectite, are not exactly at a crystalline state. Smectite crystal is better than normal smectite at bringing the silicate layers together. If the montmorillonite particles are examined under an electron microscope, it will be seen that it does not have a regular shape like real crystalline, but it does have an irregular shape like a ripped paper. The core of the particles is covered with irregular silicate layers, bent, and have worn edges [32]. Between layer stacks, the particles have a weak contact at lots of points. At these ‘breaking points,’ the particles can be easily crashed, or the rheological attitudes may be affected by the mechanic forces [33]. Layered silicates which are generally used at nanocomposites are members of 2:1 structural pyrosilicate family. Crystalline cape structure of 2:1 phyllosilicates is made of a structure that is compressed between tetrahedral layers that join the sides of octahedral layers of magnesium or aluminum hydroxides. The layer thickness is 1 nm, and the lateral diameter varies between 300 (A) and several microns. These layers get into stack form with the interlayers that are formed between the layers, or the Van der Waals spaces which are known as gates. The isomorphic replacement of Si+4 with Al+3 at tetrahedral layers and Al+3 with Mg+2 at the octahedral layer generates an excessive negative charge. This negative charge is balanced with the Ca+2 and Na+ cations which are placed between the layers. This type of layered silicates is characterized by a middle-level surface charge which can be named as cation exchange capacity (CEC), and this charge varies from one layer to another (see Fig. 4) [32].
Because of the water absorbency property of silicate layers, these layers and water-repellent polymer matrices cannot make good interaction. To avoid this, the clay surfaces should be modified. To modify the clay, there are many types of mechanisms which are getting into reaction with acids, the attachment of organic and inorganic ions, ion exchange using organic and inorganic cations, plasma, ultrasound, burning, etc. Modified clays are also used as rheological control factor, at removing organic pollutants from water, air, and oil with adsorption, and at cosmetics and dyeing fields [34]. The process which makes clays organophilic is done with changing inorganic alkali cations at the clay surface with the desired organic cation. To make the middle layers more organophilic, hydrate cations at the layers are changed with surface-active agents such as alkali ammonium and alkali phosphonium. The clays that are organically modified, organophilic, and have less surface energy are more compatible with organic polymers so these polymers can get into the middle layers. When the inorganic changeable cations are changed by the organic onium ions which are at the middle layer surfaces, the polarity of the polymer pairs and the surface polarity of the clay and the middle layers of the clays enlarge. Thereby, when organo-modified silicates (organoclays) and ions that have long alkali chains are used, dyestuffs, polymers, and other such chemicals can penetrate more decent [35].
The primary change of metallic cations with organic cations takes place in the aqueous medium. The hydrophobic organic tails going away tendency from the aqueous medium and the tendency to hydration of little inorganic cations in water make a repulsive power for the reaction. The neutralization of the negatively charged layers occurs when the positively charged ions settle inside gaps of the inner layers. Van der Waals force is formed between oxygen and alkali chain. As a result of the primary adsorption of ammonium cations, three cases take place at the inner layer of the silicate. Molecules can penetrate inner layers due to breaking the water in the inner layer by cations, and it is called hydrophobic adsorption [36]. Van der Waals or hydrophobic interaction can be seen with ammonium cation alkali chains and adsorbed nonpolar molecules at gaps of the inner layer. When organic cations are placed at inner layers, they enlarge layer gaps [37].
Secondary adsorption occurs with Van der Waals forces which are between adsorbed organic molecules and aliphatic chains of changeable cations. That is why the secondary adsorption between molecules that have a low polarity or nonpolar molecules and the long-chained ammonium montmorillonite complexes increases with the increment of the basal gaps and primary adsorbed organic ions amount [38].
At the experiments that are done with alkali ammonium ions that have different alkali chain lengths which are used as a surface-active agent, it is seen that the longer chained alkali ammonium ions (C18-C20) increase the distance between the silicate layers more than the shorter chained alkali ammonium ions (C9-C10). Also, between unary, binary, trilogy, and quart onium ions, quart alkali ammonium ions are the most effective ions that increase one layer distance to another one best [39]. The reason for this is quart ammonium ions make a strong bond with MMT. This force increases with the increment of the displaced components of the ammonium cations [40].
The sum of equivalent mass number of changeable cations (meg) at 100 g of the mineral is called the cation exchange capacity (CEC), and it is a significant parameter to nominate the most suitable clay type for defined end-use [41].
Bentonite which has a general formula of Al4Si8O20(OH)4.nH2O is clay that shows the strong colloidal property, has high plasticity, has a soft handle, and can be easily shaped. Also, it is an important ion exchanger that swells when it gets into the water, can be activated by acid, darkens the drilling muds, and has wide surface area [42, 43]. Inside the bentonite, there are natural clay minerals such as kaolin and illite and non-clay minerals such as gypsum, quartz, rutile, calcite, dolomite, and volcanic ash. They are found in nature in many colors such as white, gray, green, yellow, and pink [42].
The most distinguishing property of bentonites is holding water inside their structure as presented in Fig. 5. Taking water in its structure and widening its crystalline structure is called swelling. Swelling is directly proportional to the type and amount of cations between the 2:1 segments. At these segments, generally, there are Na+ and Ca+2 cations, and while the Na+/Ca+2 equimolar ratio increases, the swelling also increases [44].
The bentonite which can swell five times when in contact with water is accepted as good in trade, but better bentonites can swell 10–20 times. The swelling property of bentonite is lost after a particular temperature. The bentonite which has high ionization density has a dry density of 2.7–2.8 g/cm3, and the dust form has a density of 1.6–1.7 g/cm3 [42].
Intercalation of dye molecules (especially organic dyes) in between layers of inorganic clays are done with different purposes and applications. Bergman et al. reported the metachromatic behavior of methylene blue that is cationic dye stuff on clay which is Wyoming montmorillonite as first [38]. After that study, interaction between organic molecules and clay particles is researched with many studies.
Bannani Karim et al. used Morocco clay which is characterized by diffraction as adsorbent material for searching the adsorption kinetic of basic red (BR46) with isotherm and thermodynamic parameter at different dye at different dye concentrations, adsorbent weight, and pH. Chemical composition of clay was identified by using XRF as 53.11% SiO2, 16.95% Al2O3, 5.94% Fe2O3, 3.51% CaO, 2.51% MgO, 0.2% SO3, 4.64% K2O, 0.26% Na2O, and 0.09% P2O5. After the experiments increasing at adsorption amount was observed with increasing initial pH and dye concentrations and with 40 mg adsorbent maximum removal was seen [45].
Vimonses et al. worked with three different natural clays: bentonite, kaolin and zeolite, and a material that is produced by burning of the clays with its effects for removing anionic dyes from aqueous solutions. Experiments were analyzed with Brunauer–Emmett–Teller (BET), differential temperature analysis (DTA), thermogravimetric analysis (TGA), and UV-visible spectrophotometer. According to results, mixtures 2, 6, and 9 had the most suitable removing capacity. High dye removing efficiency was yield with burning at 300 °C for 1.5 h. Increasing the ratio of removing dye was observed with increasing initial dye concentration [46].
Li et al. studied with the purpose of understanding principles of swelling clays (montmorillonite SWy-2, Na montmorillonite, SHCa-1) at removing methylene blue, explaining the mechanism of methylene blue adsorption to verify the usage of methylene blue for specific surface area (SSA) and cation exchange capacity (CEC) determination. With the use of UV-visible spectrophotometer, X-ray diffraction (XRD), chromatography, FTIR, and TG-DTG analyses, it is observed that load intensity was a limiting factor for MB adsorption and swelling clays with high CEC adsorbed more MB [47].
Ten and Li evaluated adsorption of methylene orange52 (C14H14N3NaO3S) by using raw and activated montmorillonite with HCl. With using BET and XRD at experiments, it was examined that adsorption capacity of modified clay with HCl because of substitution of Fe2+ and Al3+ ions at montmorillonite with H+ ions as a result of activation with HCl, the distance between layers of clays are decreased, and crystallization degree of raw clay is more than activated clay with HCl [48].
Karagözoğlu et al. worked on kinetic, isotherm, and thermodynamic parameters of Astrazon Blue (FGRL) adsorption from aqueous solutions with different concentrations between 100 and 300 mg/L, temperature (303–323 °K) and adsorbent dosages by using sepiolite, flying clay, and the carbonated seed of apricot and analyzed by using Perkin Elmer UV-visible spectrophotometer. It was observed that the adsorbed dyestuff amount for each clay increased when initial dye concentration and adsorbent amount increase. With the use of thermodynamic parameters, it also came to light that adsorption amount increased when the temperature was increased, and adsorption processes are endothermic [49].
Gürses et al. examined changes of remaining methylene blue which is a cationic dyestuff from aqueous solutions by using montmorillonite and nontronite clay according to pH, temperature, initial dye concentration, mixture ratio, and adsorbent amount. Firstly, the chemical composition of clay was determined. In this study, it was understood that adsorption tends to be a balance of approximately 60 min. Maximum dye removing with 100 mg/L initial concentration was 58.2 mg/g. Increasing in dye concentration increased the adsorption capacity. Adsorption capacity increased at the range of 20–40 °C, while it decreased at the range of 40–60 °C. At pH 3 and 7, minimum adsorption value was observed, while the maximum adsorption value was seen at pH 1 and 5.6 [50].
Weng and Pan searched on methylene blue adsorption characteristics of active clay on the mud that was formed from a waste of an oil producer for food. The structure of clay was determined by SEM. Removing of methylene blue was increased with increased pH. Adsorption of methylene blue was increased with increased temperature. The maximum adsorption capacity of active clay which is treated under high pressure is 2.44 × 10–4 mol/g, pH 5.5, and 25 °C [51].
5.2 Cellulose and Its Derivatives
Cellulose is a widely used polymer around the world in various forms because it is a very common polymer, and it is easy to reach work on. It has many types, and all modifications can be done according to the usage.
Carboxymethyl cellulose (CMC) is anionic water-soluble cellulose ether that is used in various industries including personal care, food, chemicals, pharmaceuticals, textile, drilling fluids as well as paper. Carboxymethyl cellulose (CMC) is nearly always present in the market as the sodium salt of carboxymethyl cellulose (Na-CMC), and it is water-soluble and prefered to use in this form, a property enhanced by its ionic nature. When it is acidic, it is insoluble. It has an average degree of substitution (DS) of 0.75, and 4000 cP is its maximum viscosity for 1% solution. The viscosity of CMC increases below pH 4, due to the presence of sodium salt of weak acid groups, when free acid is formed. If all COOH groups of CMC have been reacted for salt formation, its pH would be 8.25. Na-CMC has higher moisture absorption than other cellulose ethers, directly proportional to its DS, thanks to its ionic character [34]. Thanks to its strong anionic character, Na-CMC is also used for removal of cationic dyestuff from textile dyeing effluents, and its usefulness for removal of various dyestuffs is presented [52, 53].
The first step is treating cotton fiber with sodium hydroxide. In this step, negatively charged oxygen atom is produced by removing the hydrogen atom from the hydroxyl group, and a reactive form of cellulose is produced which is called alkali or soda cellulose. These sites of cellulose are used for reaction of chloroacetate. Secondly, soda cellulose is treated with the sodium salt of chloroacetic acid under alkaline conditions. As shown in Fig. 6, hydroxyl groups of cellulose chain are using for reaction of chloroacetate to produce an ether linkage, and hydrochloric acid is formed at the end of the reaction [54, 55]. The first substitution occurs on the primary alcohols, and then, the second substitution is seen on the secondary alcohols. The substitution will occur more rapidly in the regions where crystallinity is lower. Moreover, very inhomogeneous carboxymethyl group distribution is seen in the early stages of the reaction. Carboxymethyl group distribution becomes more homogeneous when the reaction time is extended [56].
The resulting material which is carboxymethyl cellulose is very swellable and has highly acidic carboxyl groups that enhance the values in elongation at break, moisture regain, soil release, water retention, and tensile strength [57, 58]. CMC that is suitable for being an adsorbent for dye removal from aqueous solution in a broader pH range has low DS value, and it is water insoluble [59].
Salama and his friends examined the removal of methyl orange dye from aqueous solutions by using Carboxymethyl cellulose-g-poly(2-(dimethylamino) ethyl methacrylate) (CMC-g-PDMAEMA) in hydrogel form as an adsorbent and finding the adsorption kinetics of CMC-g-PDMAEMA. At the study, high viscosity CMC sodium salt is used, and FTIR spectroscopy is done to observe the structural changes of CMC-g-PDMAEMA and CMC hydrogel, and the effect of pH on dye adsorption is observed. As a result of the study, increase of the pH decreases the adsorption capacity of the hydrogel, and it is seen that the adsorption process occurs by the chemical interaction between the bioabsorbent surfaces and the dyestuff [60].
Cai et al. had research on the hydrolysis degree effect of hydrolyzed polyacrylamide grafted carboxymethyl cellulose (CMC-g-HPAM) on dye removal efficiency. In the study, series of CMC-g-HPAM is produced and it is used as flocculants to remove methylene blue dye from aqueous solution. As a result, using FTIR-spectroscopy, characterization of CMC-g-HPAM is done. The flocculation performance of the CMC-g-PAM, CMC-g-HPAM and CMC are compared, and CMC-g-HPAM made higher flocculation performance for removal of methylene blue [61].
Zhang et al. had researched the sorption behavior of carboxymethyl cellulose for methylene blue and its reuse in secondary sorption. In the study, the carboxymethyl cellulose is prepared, and the sorption of methylene blue is done at different pHs. FTIR, SEM, and ‘Y’ potential measurement test methods are used to find that methylene blue removal is pH dependent but temperature independent [52].
Wang et al. studied on the subject of synthesizing carboxymethyl cellulose/organic montmorillonite (CMC/OMMT) nanocomposite at different weight ratios and using the synthesized nanocomposite at the removal of Congo red dye. At the experiment, CMC/OMMT nanocomposite preparation and adsorption experiments are done. FTIR, XRD, TEM, SEM, TG, and UV-vis tests are done to figure out the best absorbency of Congo red dye. As a result, the best absorbency occurred at a weight ratio of CMC to OMMT was 1:1, having a reaction temperature of 60 °C and a reaction time of 6 h [53].
5.3 Chitosan and Its Derivatives
Chitosan is a soluble form of chitin which has the second biggest polymer supply in the world following cellulose. To form chitosan, approximately, 50% of chitin is deacetylated (see Fig. 7). This deacetylation amount that is mentioned at the chitosan definition can change according to polymer origin of chitin polysaccharide. When –NH2 group in d-glucosamine is protonated, chitosan turns into polyelectrolyte forms in an acidic media. It has many usage areas including protein and depollution recovery as flocculent. It is very unique material due to being the only cationic pseudo-natural polymer. Thanks to the solubility property of chitosan, it is applicable in many areas with various forms [62].
Szygula et al. studied the removal of Acid Black 1, Reactive Black 5, and Acid Violet 5 which are sulfonated azo dyes via coagulation with chitosan. At acidic solutions, removing of dyestuff was related to the neutralization of charges. It was obvious that with the use of chitosan, the efficiency of amine groups was increased, while the contact time needed to reach equilibrium was decreased, and the coagulation–flocculation mechanism was affected by initial pH values and the amount of coagulant, while stirring did not make any change [64].
Wang and Wang evaluated the adsorption behavior of Congo red dye which is an anionic dyestuff by using a nanocomposite that includes N, O-carboxymethyl chitosan/montmorillonite with the aim of recovery of anionic dyestuff and effects of molar ratio on recovery. They used FTIR, XRD, and SEM for testing their results. According to SEM results when molar ratio was increased, the adsorption capacity also increased, and maximum adsorption was seen at a 5:1 ratio. Besides, decreasing pH increased the dye adsorption thanks to increasing the attraction between nanocomposite and dyestuff, and when the temperature was increased, adsorption capacity increased because the composite swelled and bigger space was opened for bigger dyestuffs. Finally, it was observed that the adsorption equilibrium of Congo red dyestuff by N, O-CMC-MMT was well fit with Langmuir and pseudo-second-order models [65].
Bhattacharyya and Ray worked on removing synthetic dyes from waters via micro- and nanocomposites of bentonite filled with chitosan and acrylic copolymer. They used FTIR, XRD, SEM, DTA, and TGA for the evaluation of their results. The best swelling ratio in water was yield with a comonomer ratio of acrylic acid and acrylamide 7.5:1, and the highest adsorption for methyl violet and Congo red dye was yield with chitosan which is 12 wt% of total monomer weight in a polymethacrylic acid gel. The highest swelling of clay including hydrogel samples was 2 wt% nano-sized clay (NF2) and 4 wt% micro-sized clay (MF4). When all samples were treated with methyl violet and malachite green dyes with different concentrations, adsorption amount from high to low was, respectively, NF2, MF4, and F0. Furthermore, it was seen that methyl violet adsorption is less than malachite green, and adsorption characteristics were well-fitting with first- and second-order kinetics, and the combined Langmuir–Freundlich model [66].
Bulut and Karaer examined adsorption of a composite including cross-linked chitosan and bentonite for methylene blue which is a cationic dyestuff to remove the dyestuff from aqueous solution. By using FTIR, it was seen that the maximum adsorption capacity of the dyestuff was 95.24 mg/g at 298 °K, and thermodynamic and kinetics of the process were well-fitting with Langmuir model [67].
Liua et al. used a composite including cross-linked chitosan and bentonite to removing Amino Black 10B(AB10B) which is an anionic azo dye and adsorption behavior. The results of the study were tested with FTIR, SEM, XRD, and TGA. It was observed that removing AB10B decreased when pH and dye concentration of the solution were increased while the removal increased when contact time and dosage of adsorbent were increased. Time for reaching the equilibrium was extended when the initial dye concentration was increased. Moreover, kinetic and equilibrium behavior were well-fitting, respectively, with the Langmuir model and pseudo-second-order kinetic model. Maximum adsorption capacity values were yield at 313 °K with natural pH as 350.9 mg/g and at 293 °K with pH 2 as 323.6 mg/g. As understood from this data, relatively high temperatures and low pH are more suitable for adsorption of the dyestuff [68].
Guo et al. studied for removing of dyestuff from wastewater by a composite including chitosan and CTAB-modified bentonite. 1CTS–10 CTAB-bentonite was prepared and used for weak acid scarlet adsorption which resulted in more than 85% adsorption efficiency; thus, it was a very good adsorbent composite that was reusable for three cycles. For the testing stage, FTIR and XRD are used, and it was seen that the adsorption behavior of the composite was well-fitting with Langmuir and Temkin models [69].
Mahdavinia and Karami synthesized a nanocomposite that is magnetic carboxymethyl chitosan-poly(acrylamide)/laponite RD with improved dye adsorption capacity. They used X-ray diffraction, transmittance electron microscopy, thermogravimetric analysis, vibrating sample magnetometer, and scanning electron microscopy techniques for the testing stage. It was clear that water absorbency is related to magnetic laponite RD content, and when the amount of magnetic clay increased, the swelling capacity and salt sensitivity decreased, while dye removing and adsorption capacity at acidic medium increased. Besides, analyses were done according to Langmuir and Freundlich models, and it was understood that the composite could be used many times with the same dye adsorption capacity [70].
5.4 Activated Biochars and Alternative Biomaterials
Biomass is turned into a carbon-based end product by the pyrolysis process, and as a result, biochar is formed. It has some advantages as being a low-cost product and providing a recycle for the waste of agriculture and forest, so it adds value to waste [71]. Biomass is decomposed in oxygen-free media with very high temperatures as 350–650 °C. Biochar is produced in a charcoal form with volatile gases in two forms. The first one is gas that could turn into bio-oil with condensation, and the second one is gas as CO, CO2, CH4, and H2 that cannot turn into bio-oil. Pyrolysis can be categorized as slow, intermediate, and fast due to its heating rate, maximum temperature, and time, and with differences, characteristics of biochar can be changed. The slow one is more advantages because of being simple and suitable for agricultural products and small production amounts, but other options can be selected according to the needs of the end product and usage area [72]. To enhance the properties of biochar as pore and surface area increasing, functional group adding, and enhancing the capacity, some modification can be done by some additives (see Fig. 8). If this modification is chemical, it is known as chemical activation, and it can enhance the full process as decreasing the steps of the process (carbonization + activation), lowering the needed temperatures for desired properties, and increasing the capacity of catalytic oxidation [71]. Also activation can be done via physical methods such as steam or gas activation. Steam activation is the application of steam after thermal carbonization. If the activation is done by using CO2, N2, NH3, air, O2 gases, or a mixture of these, then it is gas activation. Both of them can enhance the porosity but micro- or mesoporous structures by gas activation [73].
Active biochar/carbon structures that have well-distributed pores, high surface area, and good surface properties use the physisorption methods but oxygen around the surface can enhance the absorption according to adsorbate characteristics.
Radaei et al. produced activated carbon with 572.53 m2/g surface area from the waste of pomegranate by using 37% phosphoric acid acids for the removal of Reactive Blue 19 dyestuffs. Their adsorbent whose amount is 3.5 g/L could adsorb maximum 98.16% dyestuff under conditions with pH 11, 5 min of contact time, and 300 mg/L initial dyestuff concentration [27].
Kahka and Piri used a waste of citrulluscolosynthis which is a kind of watermelon, to yield a bio-adsorbent for reactive red dyestuff. The waste of the plant is modified by using sodium hypochlorite. The best condition for maximum adsorption is with pH 2, 1.75 g/L initial adsorbent amount, 90 mg/L initial dyestuff concentration, and 70 min of contact time, and they could yield 36 mg/L adsorption capacity [28].
Jin et al. convert municipal solid wastes into biochar by using pyrolysis for removing arsenic (As(V)) that is a heavy metal. They examined the effects of potassium hydroxide on the activation of biochar. They observed that activated form has very high adsorption capacity than nonactivated biochar as 30.98 mg/g which is 1.3 times of it because this activation brings functional groups and more surface area to the pure biochar [71].
Rajapaksha et al. formed biochar with steam activation from an invasive plant (Sicyos angulatus L.) for sulfamethazine (SMT) in water. They observed that the most effective parameter is process pH for biochar properties. The best adsorption which is 37.7 mg/g is yielded at activated one with pH 3, and this capacity referred to 55% growth compared to pure biochar [74].
Li et al. investigate the formation of biochar from Enteromorpha prolifera (EPAC) in one step with oily sludge addition for methylene blue dyestuff. With this production, they yielded better surface area, pore, and adsorption capacity as 910 mg/g and prove the importance of pH levels [75].
Zhang and Lu studied on biochar formation from coconut shell at 450 degree for 2 h with wet impregnation and calcination for Reactive Brilliant Blue KN-R remove. Their biochar is modified with TiO2, and its crystals are dispersed suitably and removal ability enhanced than untreated [76].
Ramie bars are used by Cai et al. for removing Safranine T. Their adsorbent is prepared by pyrolysis of ramie at 500 degree for 20 h after titanium butoxide is treated. With this modification, pore volume, surface area, and adsorption increase than pristine biochar thanks to homogenous TiO2 particles [77].
Chicken feathers are a source of biochar that belongs to Li et al. for Rhodamine B removal. They used pyrolysis for 1 h at 450 degrees with tetrabutyl titanate treatment. This method increased surface area and degradation rate due to the TiO2 particle surface of biochar [78].
5.5 Other Polymers
Other polymers, especially biopolymers for the natural cycle, are mostly used in the form of hydrogels, bead pellets, etc., for being an adsorbent, and they have very advantageous usage in wastewater refinement processes of the textile industry. Some studies are summarized below:
Ekici and Guntekin assert that low-temperature dyestuff removal can be done with their hydrogel. They could yield 111–122 mg dyestuff adsorption per gram of adsorbent by using polyampholytes (PAHs) hydrogels in simultaneous Remazol-type dyestuff removal studies at 35 °C and 20 °C. They had dyestuff adsorption ability from 94 to 98% [79].
Inal et al. studied on methylene blue dyestuff removal by using hydrogels that are acrylamide–crotonic acid. When hydrogels which consist of only acrylamide, adsorption can be increased by increasing pH. Maximum adsorption was yielded with pH 9 for 300-min treatment. For acrylamide–crotonic acid hydrogels, pH 8 causes a decreased adsorption, while pH 7, 9, and 10 bring similar adsorption. When crotonic acid amount in hydrogel structure increased, adsorption amount increased. Desorption can be done by treating colored hydrogels at pH 2 HCl-KCl buffer solution for one hour. Also, their hydrogel did not lose adsorption capacity when it is used 20 times repeatedly [80].
Sudarsan et al. suggested using reusable hydrogels which consist of sodium alginate for methylene blue removal procedure. Condensation of ethylene glycol and acrylic acid and then free-radical polymerization were steps for introducing ionic pendant functionalities on sodium alginate, and pH-tunable hydrogels were prepared. They observed higher swelling at higher pH. Removal amount of tried hydrogels is between 80 and 98%. Also, up to 90% desorption of hydrogels could be done at 0.1 N HCl solution, and they could be reused [81].
Ma and Zhang investigated the adsorption of alizarin red S(ARS) by Fe/Al–alginate composite hydrogel electrode electrocoagulation (EC). They added 2.0% (w/v) sodium alginate solution with scrap iron to 5.0% (w/v) Ca2+(CaCl2 · 2H2O) which is hardening solution at 60 °C for 2 h and then storing at 3% (w/v) Al3+ (AlCl3) for the dye degradation study. Initial pH 3 with O2 is suitable for the optimal degradation of ARS. COD is removed by the electrode application up to 90% efficiency. Maximum color removal is 99%, and 30 min is the optimum time for electrolysis. These electrodes have better results compared to conventional ones, and they suggest using the ultrasonic application because it accelerates electrocoagulation [82].
Ilgın and Ozay emphasized that they produced hydrogels from poly(acrylamide-co-methacrylamido-4-(2-aminoethyl) morpholine) and poly(Aam-c-MAEM) with four different molar ratios (nAam/nMAEM: 100/0, 95/5, 90/10, 80/20) by using single-step free-radical aqueous polymerization. The produced hydrogel is used for reactive orange (RO) removal which is anionic dyestuff. They used 0.1 mol% MBA as a cross-linker. When the comonomer ratio and initial adsorbent amount increased, the removal ability increased; while temperature and pH of solution increased, the removal percent decreased. Also, maximum removal was done by distilled water. Salt amount affects removal amount adversely such that Al ion presence in solution has the worst removal performance [83].
Rahimdokht et al. synthesized a photocatalyst as TiO2/gum tragacanth hydrogel for the methylene blue dyestuff removal from polluted solutions. The hydrogel was produced by sonication of TiO2 nanoparticles into gum tragacanth, and they added glutaraldehyde as a cross-linking agent. They yielded maximum efficiency as 88.86% with a pH of 9.02 a 124.34 min removal time [84].
Patel and Patel assert that their Cationic Poly [acrylamide/N-vinyl pyrrolidone/N,N-diallylpyrrolidinium bromide] [AAm/NVP/DAPB] hydrogels are produced by free-radical solution polymerization with a cross-linker (N,N-methylene bisacrylamide), initiator (2.2′-azobis (2-methylpropionamidine) dihydrochloride). They used the hydrogels for removal of acid dyestuffs as Acid Yellow, Orange-II and Reactive Golden Yellow. The best swelling was observed at YH5 with higher DAPB amount, and dyestuff removing performance is seen as Acid Yellow < Orange-II < Reactive Golden Yellow [85].
Dey et al. studied on semi-IPN and ester-based hydrogels production for wastewater refining treatments as an adsorbent. They produced their comonomer cross-linker that was poly(ethylene glycol) di-itaconate by functionalizing poly(ethylene glycol) (PEG 1500) with itaconic acid using melt esterification. Then, copolymerization was done by using acrylamide and methylene bisacrylamide. For storage modulus, dyestuff adsorption ability, and the anti-fungal, better results were yielded with ester-based gel than semi-IPN-type gels. Regardless of medium pH, swelling of hydrogel decreased when the PEG amount was increased at semi-IPN gels; thus, dyestuff adsorption decreased [86].
5.6 Comparison of All Adsorbent Materials
Clay is easily findable because its source is very high in nature, and it is ready to adsorb thanks to high surface area, but it is very good to modify and produce a composite with many polymer sources. Cellulose and chitosan derivatives are a good example of composite production, especially with clay. They have also very wide sources, but cellulose derivatives have more sources than chitosan derivatives in nature. Chitosan is more likely to form cationic adsorbents, while cellulose derivative is close to slightly anionic characteristics. For further usages of cellulose derivate-based adsorbents, usage conditions, especially temperature, are very important because they are easily flammable, while chitosan derivatives provide flame-retardancy effect. Chitosan and cellulose derivatives need attention for solubility, and they must be turned into suitable forms. Also, chitosan has some viscosity problems because of forming a gel-like solution even with water; thus, it needs extra control in processes. However, it has a very valuable advantage as an antibacterial behavior for further reuse applications of an adsorbent. Biochars are also natural sources, but it needs extra processes to turn them into suitable forms of adsorbent materials and activation of the adsorbent. These processes must need attention not to lose the quality of the adsorbent. Biopolymers are also good alternatives for using them as hydrogel form or in a composite structure, but adsorption characteristics must be enhanced. Also, biopolymers are the best option for further usages because they are ready to form or reshape in different conditions, but their adsorption is limited.
With the lights of all information, Table 3 is produced for comparison of all adsorbent materials. All of them are valuable and have many different advantages with prior behaviors specific to different usages. However, it is better to combine them in a composite according to characteristics of the waste that would be refined to yield better adsorption and more refining. All of them have reusage option with different conditions that can serve a better cycle for the environment.
6 An Alternative Composite Structure Study
In the present composite structure study, firstly, chitosan modification is done to turn it into carboxymethyl chitosan. 50 mL NaOH solution (20 wt%) is used to swell 5 g chitosan for 12 h and filtration applied to the mixture. Filtered chitosan is taken into a volumetric flask, and 50 mL ethyl alcohol is added to stir for 30 min. 4 g butanetetracarboxylic acid addition is done, and the mixture is stirred for half an hour 30 min more. After that, cooling is done to make the temperature of the mixture 20 °C, and the mixture is kept at 20 °C for 1 h. Filtration is applied to the treated sample, and distilled water is used for dissolving filtered sample. Further, acetic acid is added to set pH as 7.0. The mixture is poured into ethyl alcohol to yield foam gel precipitation, and pouring is applied to take the white precipitation into Petri dishes. Ethanol with a ratio of 70% is used to wash the precipitate for three times by using, and ethyl alcohol is used for one time. Drying of the CMCTS is done by putting Petri dishes into the oven at 80 °C. Finally, CMCTS is turned into dust form by scraping off from the Petri dishes [30].
In the case of a clay and modified chitosan combination, firstly, 1 g clay is swelled at the 100 mL distilled water. Secondly, the CMCTS solution is prepared with the distilled water which refers to a 5:1 weight ratio of CMCTS-clay at nanocomposite. Further, the CMCTS solution and clay suspension is mixed slowly, and stirring is done at 60 °C for 6 h. Then, washing of nanocomposites is done until the supernatant pH becomes 7.0 by using distilled water. After pH setting, oven at 60 °C is used for drying of composite for 12 h. Finally, the CMCTS-clay nanocomposite is turned into dust form by scraping off [30].
The composite was tried with cationic dyestuff [30, 31] previously, and very high adsorption values are yielded. In this study, anionic dyestuff is used to observe the anionic adsorption and prove the amphoteric characteristic of the composite thanks to the anionic characteristic of the carboxymethyl end group and cationic characteristic of chitosan. Dye bath simulating solution is prepared by using 200 mL pure water and 0.5 g dyestuff which is Nyloset Brilliant Red from Setaş Color Center and anionic dyestuff for nylon dyeing. Water is used to observe the real usage of dyestuff under natural pH instead of observing the full performance of composite by using other solvents. 2 g composite is added to the simulating dye bath, and the bath is mixed with a magnetic stirrer for 12 h. Then, UV-visible spectrophotometer analysis is done for dye bathes before and after the adsorbent addition. Adsorption graph is presented in Fig. 9.
Removal amount is calculated by using Formula 1, and R refers to removal percent, while c0 attributes to the initial concentration of solution and cf attributes to the final concentration of the solution. As a result, anionic dyestuff removal is 42% as given in Table 4. This result shows that in addition to adsorbing cationic dyestuff, the composite can adsorb anionic dyestuff thanks to its amphoteric characteristic. This also proves that the modification chitosan is applied well enough to hold anionic dyestuff.
7 Conclusion
The textile industry produces very harmful wastewater with very high amounts. These must be refined before discharging; even the reuse can make a profit for both economies an environment. For the refinement, there are many conventional methods as physical, chemical, and biological treatments, but adsorbent usage is a very promising alternative because of low cost, ease of applicability, and reusability in further cases as dyestuff or filler, etc. These adsorbents can be clay, cellulose and chitosan derivatives, biochars, and other polymers with various forms according to the needs of wastewater characteristics. For better, easier, and faster refining, adsorbents in composite forms are useful. In this chapter, also a study related to a composite adsorbent which is based on modified chitosan and clay for removal of dyestuff and enhancements is evaluated. It is shown that the novel nanocomposite can adsorb both cationic and anionic dyestuffs thanks to its amphoteric structure.
References
White SB, Biernat JF, Duffy K, Kavalar MH, Kort WE, Naumes JS, Slezak MR, Stoffel CR (2010) Water markets of United States and the world: a strategic analysis for Milwaukee Water Council, Wisconsin
Agrawal R, Sharan M (2015) Municipal textile waste and its management. Res J Fam Community Consum Serv 3(1):4–9
Kumar PS, Saravanan A (2017) Sustainable wastewater treatments in textile sector. In: Sustainable fibers and textiles
Lemeneh DY, Tesema AF (2019) Valuation of polyvinyl alcohol and maize starch as sizing agent for textile processing. Int J Mod Sci Technol 4(11):286–293
Fu S, Farrell MJ, Ankeny MA, Turner ET, Rizk V (2019) Hydrogen peroxide bleaching of cationized cotton fabric. AATCC J Res 6(5):21–29
Hossain T, Hossain A, Saha PK, Alam Z (2019) Effect of scouring and bleaching agents on whiteness index and bursting strength of cotton knitted fabric. Global J Res Eng 19(1):23–28
Patil S, Mahapatra A, Gotmare VD, Patil PG, Bharimalla AK, Arputharaj A (2019) Effect of different mercerization techniques on tactile comfort of cotton fabric. Indian J Fibre Text Res 44:217–222
Chemchame Y, El Mouden M, Mansar A (2017) Comparison between mordant and alkaline dyeing of wool fiber with dyes from Rubia tinctorum plant. Am J Phys Chem 6(1):1–5
Moniruzzaman M, Mondal MS, Hossain N (2018) The influence of mordant and mordanting techniques on ecofriendly dyeing of cotton fabric by extracted used tea. J Eng Sci 9(1):111–117
El-Sayed WA, El-Ola SMA, AElsayed NA, Tawila DMN (2017) Multi-functional finishing of woolen fabric. J Sci Res Sci 34(1):1–18
Moga IC, Iordănescu M, Prıcop F, Scarlat R, Dorogan A (2013) Quality monitoring for wastewater generated by the textile finishing. Ind Textila 6(4):222–228
Raman AAA, Asghar A, Buthiyappan A, Daud WMAW (2017) Treatment of recalcitrant waste. In: Current developments in biotechnology and bioengineering: biological treatment of industrial effluents, pp 409–442
Holkar CR, Jadhav AJ, Pinjari DV, Mahamuni NM, Pandit AB (2016) A critical review on textile wastewater treatments: possible approaches. J Environ Manage 182:351–366
Siddique K, Rizwan M, Shahid MJ, Ali S, Ahmad R, Rizvi H (2017) Textile wastewater treatment options: a critical review. In: Enhancing cleanup of environmental pollutants
Akarsu C (2014) Treatment of industrial wastewater by electrocoagulation process. In: ISITES
Hakulinen R (1988) The use of enzymes for wastewater treatment in pulp and paper industry—a new possibility. Water Science Technol 20(1):251–262
Anijiofor Sandra C, Jamil NAM, Jabbar S, Sakyat S, Gomes C (2017) Aerobic and anaerobic sewage biodegradable processes: the gap analysis. Int J Res Environ Sci 3(3):9–19
Sarayu K, Sandhya S (2012) Current technologies for biological treatment of textile wastewater—a review. Appl Biochem Biotechnol 167:645–661
Mashall H (2018) Natural nanomaterials. In: Environmental nanotechnology, p 133
Crini G, Lichtfouse E, Wilson L, Morin-Crini N (2019) Conventional and non-conventional adsorbents for wastewater treatment. Environ Chem Lett 17(1):195–213
De Gisi S, Lofrano G, Grassi M, Michele N (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40
Burakov AE, Galunin EV, Burakova IV, Kucherova AE, Agarwal S, Tkachev AG, Gupta VK (2018) Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol Environ Saf 148:702–712
Sadegh H, Ali GAM (2018) Potential applications of nanomaterials in wastewater treatment: nanoadsorbents performance. In: Advanced treatment techniques for industrial wastewater
Liu J, Boo WJ, Clearfield A, Sue HJ (2006) Intercalation and exfoliation: a review on morphology of polymer nanocomposites reinforced by inorganic layer structures. Mater Manuf Process 21(2):143–151
Şen F, Palancıoğlu H, Aldaş K (2010) Polymeric nanocomposites and usage areas. Electron J Mach Technol 7(1):11–18
Chen S, Zhou M, Wang HF, Wang T, Wang XS, Hou HB, Song BY (2018) Adsorption of reactive brilliant red X-3B in aqueous solutions on clay–biochar composites from bagasse and natural attapulgite. Water 10(703)
Radaei E, Alavi Moghadam SMR, Arami M (2014) The study of the adsorption of reactive blue 19 dye by activated carbon from pomegranate residue. Water and Wastewater Vol. 25, 4(92):27–34
Kahkha MR, Piri J (2016) Adsorption of reactive red dye from wastewater using modified citrulluscolosynthis ash. Water and Wastewater 27(3):32–37
Şen F, Demirbaş Ö, Çalımlı MH, Aygün A, Alma MH, Nas MS (2018) The dye removal from aqueous solution using polymer composite films. Appl Water Sci 8(206)
Emekdar E, Şahin UK (2018) Design of a zwitter-ionic nanocomposite for dyestuff removal. IOP Conf Ser Mater Sci Eng 460(012039)
Emekdar E, Şahin UK (2019) Katyonik Atık Suların Arıtılması İçin Tekstil Atıklarından Çevreci Kompozit Üretimi. In: UÇTEK Ulusal Çukurova Tekstil Kongresi, Adana, Turkey
Hussin F, Aroua MK, Daud WMAW (2011) Textural characteristics, surface chemistry and activation of bleaching earth: a review. Chem Eng J 170(1):90–106
Bergaya F, Lagaly G (2006) General Introduction: clay, clay minerals and clay science. In: Hand book of clay science, pp 1–18
De Paiva LB, Morales AR, Díaz FRV (2008) Organoclays: properties, preparation and applications. Appl Clay Sci 42:8–24
Yılmazbayhan A (2006) Maleik Anhidritle Graftlanmış Oligomerlerin Ve İPP/Silikat Nanokompozitlerin Tepkimeli Ekstrüzyon Yöntemiyle Sentezi Ve Karakterizasyonu. Hacettepe Üniversitesi Fen Bilimleri Enstitüsü, Ankara
Heller-Kallai L, Yariv S (1981) Swelling of montmorillonite containing coordination complexes of amines with transition metal cations. J Colloid Interface Sci 79(2):479–485
Yariv S (1976) Organophilic pores as proposed primary migration media for hydrocarbons in argillaceous rocks. Clay Sci 5(1):19–29
Sivathasan J (2007) Preparation of clay-dye pigment and its dispersion in polymers. Thesis dissertation masters by research, RMIT University School of Engineering, MMS ID 9921861231801341
Turan D (2012) Organokil esaslı nanokompozit endüstriyel liflerin üretilmesi ve karakterizasyonu. Kocaeli Üniversitesi. Fen Bilimleri Enstitüsü, Kocaeli
Maes A, Van Leemput L, Cremers A, Uytterhoeven J (1980) Electron density distribution as a parameter in understanding organic cation exchange in montmorillonite. J Colloid Interface Sci 77(1):14–20
İpekoğlu B, Kurşun İ, Bilge Y, Barut A (1997) Türkiye bentonit potansiyeline genel bir bakış. In: 2. Endüstriyel Hammaddeler Sempozyumu
Demir E (2008) Isıl İşlemin Bir Bentonitin Katyon Değiştirme Kapasitesi, Adsorpsiyon, Gözenekliliği, Yüzey Alanı Ve Yüzey Asitliği Gibi Bazı Fizikokimyasal Özelliklerine Etkisi. Ankara Üniversitesi. Fen Bilimleri Enstitüsü, Ankara
Kayır YZ (2007) Bentonit nedir. In: Endüstriyel Fırınlar ve Refrakter Sempozyumu
Wentworth SA (1969) Illite. Clay Sci 3(6):140–155
Karim AB, Mounir B, Hachkar M, Bakasse M, Yaacoubi A (2009) Removal of basic red 46 dye from aqueous solution by adsorption onto Moroccan clay. J Hazard Mater 168(1):304–309
Vimonses V, Jin B, Chow CW, Saint C (2009) Enhancing removal efficiency of anionic dye by combination and calcination of clay materials and calcium hydroxide. J Hazard Mater 171:941–947
Li Z, Chang PH, Jiang WT, Jean JS, Hong H (2011) Mechanism of methylene blue removal from water by swelling clays. Chem Eng J 168(3):1193–1200
Teng MY, Lin SH (2006) Removal of methyl orange dye from water onto raw and acidactivated montmorillonite in fixed beds. Desalination 201:71–81
Karagozoglu B, Tasdemir M, Demirbas E, Kobya M (2007) The adsorption of basic dye (Astrazon Blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell activated carbon: kinetic and equilibrium studies. J Hazard Mater 147:217–228
Gürses A, Doğar Ç, Yalçın M, Açıkyıldız M, Bayrak R, Karaca S (2006) The adsorption kinetics of the cationic dye, methylene blue, onto clay. J Hazard Mater 131:217–228
Weng CH, Pan YF (2007) Adsorption of a cationic dye (methylene blue) onto spent activated clay. J Hazard Mater 144:355–362
Yan H, Zhang W, Kan X, Dong L, Jiang Z, Li H, Cheng R (2011) Sorption of methylene blue by carboxymethyl cellulose and reuse process in a secondary sorption. Colloids Surf A 380:140–153
Wang MM, Wang L (2013) Synthesis and characterization of carboxymethyl cellulose/organic montmorillonite nanocomposites and its adsorption behavior for Congo red dye. Water Sci Eng 6(3):272–282
Bilgen M (2006) Wrinkle recovery for cellulosic fabric by means of ionic crosslinking. Thesis dissertation MSc.Textile Chemistry, NCSU, USA
Gordon S, Hsieh YL (eds) (2006) Cotton: science and technology. Woodhead Publishing Series in Textiles, Woodhead Publishing Limited, Cambridge, England, ISBN-13: 978-1845690267
Borsa J, Ravichandran V, Obendorf SK (1999) Distribution of carboxyl groups in carboxymethylated cotton fibers. J Appl Polym Sci 72(2):203–207
Rácz I, Borsa J, Obendorf SK (1998) Carboxymethylated cotton fabric for pesticide-protective work clothing. Text Res J 68(1):69–74
Parikh DV, Sachinvala ND, Calamari TA, Negulescu I (2003) Carboxymethylated cotton for moist wound healing. AATCC Rev 3(6)
Begum HA, Mahbub MKB (2003) Effectiveness of carboxymethyl cellulose for the removal of methylene blue from aqueous solution. Dhaka Univ J Sci 61(2):193–198
Salama A, Shukry N, El-Sakhawy M (2015) Carboxymethyl cellulose-g-poly (2-(dimethylamino) ethyl methacrylate) hydrogel as adsorbent for dye removal. Int J Biol Macromol 73:72–75
Cai T, Yang Z, Li H, Yang H, Li A, Cheng R (2013) Effect of hydrolysis degree of hydrolyzed polyacrylamide grafted carboxymethyl cellulose on dye removal efficiency. Cellulose 20(5):2605–2614
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31(7):603–632
Cheaburu-Yilmaz CN, Yilmaz O, Vasile C (2015) Eco-friendly chitosan-based nanocomposites: chemistry and applications. In: Eco-friendly polymer nanocomposites, New Delhi, Springer, pp 341–386
Szyguła A, Guibal E, Ruiz M, Sastre AM (2008) The removal of sulphonated azo-dyes by coagulation with chitosan. Colloids Surf A 330:219–226
Wang L, Wang A (2008) Adsorption behaviors of Congo red on the N, O-carboxymethyl-chitosan/montmorillonite nanocomposite. Chem Eng J 143:43–50
Bhattacharyya R, Ray SK (2014) Micro-and nano-sized bentonite filled composite superabsorbents of chitosan and acrylic copolymer for removal of synthetic dyes from water. Appl Clay Sci 101:510–520
Bulut Y, Karaer H (2015) Adsorption of methylene blue from aqueous solution by crosslinked chitosan/bentonite composite. J Dispersion Sci Technol 36(1):61–67
Liu Q, Yang B, Zhang L, Huang R (2015) Adsorption of an anionic azo dye by cross-linked chitosan/bentonite composite. Int J Biol Macromol 72:1129–1135
Guo J, Chen S, Liu L, Li B, Yang P, Zhang L, Feng Y (2012) Adsorption of dye from wastewater using chitosan–CTAB modified bentonites. J Colloid Interface Sci 382(1):61–66
Mahdavinia GR, Karami S (2015) Synthesis of magnetic carboxymethyl chitosan-g-poly (acrylamide)/laponite RD nanocomposites with enhanced dye adsorption capacity. Polym Bull 72(9):2241–2262
Jin H, Capareda S, Chang Z, Gao J, Xu Y, Zhang J (2014) Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: adsorption property and its improvement with KOH activation. Biores Technol 169:622–629
Manyàa JJ, Azuara M, Manso JA (2018) Biochar production through slow pyrolysis of different biomass materials: seeking the best operating conditions. Biomass Bioenerg 117:115–123
Tan X-F, Liu S-B, Liu Y-G, Gu Y-L, Zeng G-M, Hua X-J, Wang X, Liu S-H, Jiang L-H (2017) Biochar as potential sustainable precursors for activated carbon production: multiple applications in environmental protection and energy storage. Biores Technol 227:359–372
Rajapaksha AU, Vithanage M, Ahmad M, Seod D-C, Cho J-S, Lee S-E, Lee SS, Ok YS (2015) Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J Hazard Mater 290:43–50
Li X-Y, Han D, Xie J-F, Wang Z-B, Gong Z-Q, Li B (2018) Hierarchical porous activated biochar derived from marine macroalgae wastes (Enteromorpha prolifera): facile synthesis and its application on methylene blue removal. Roy Soc Chem Adv 8:29237–29247
Zhang S, Lu X (2018) Treatment of wastewater containing reactive brilliant blue KN-R using TiO2/BC composite as heterogeneous photocatalyst and adsorbent. Chemosphere 206:777–783
Cai X, Li J, Liu Y, Yan Z, Tan X, Liu S, Zeng G, Gu Y, Hu X, Jiang L (2018) Titanium dioxide-coated biochar composites as adsorptive and photocatalytic degradation materials for the removal of aqueous organic pollutants. J Chem Technol Biotechnol 93:783–791
Li H, Hu J, Zhou X, Li X, Wang X (2018) An investigation of the biochar-based visible-light photocatalyst via a self-assembly strategy. J Environ Manag 217:175–182
Ekici S, Guntekin G (2018) Utilization of polyampholyte hydrogels for simultaneous removal of textile dyes from aqueous solutions. Sep Sci Technol 53(11):1777–1790
Inal M, ÇAĞDAŞ TUNALI BE, Yiğitoğlu M (2017) Akrilamid-Krotonik Asit İç İçe Geçmiş Ağ Yapılı Hidrojellerinin Metilen Mavisinin Adsorpsiyonunda Kullanımı. Uluslararası Mühendislik Araştırma ve Geliştirme Dergisi 9(2)
Sudarsan S, Franklin DS, Sakthivel M, Chitra G, Sridharan TB, Guhanathan S (2018) Ecofriendly pH-tunable hydrogels for removal of perilous thiazine dye. J Polym Environ 26:3773–3784
Ma SS, Zhang YG (2016) Electrolytic removal of alizarin red S by Fe/Al composite hydrogel electrode for electrocoagulation toward a new wastewater treatment. Environ Sci Pollut Res 23:22771–22782
Ilgin P, Ozay O (2017) Novel stimuli-responsive hydrogels derived from morpholine: synthesis, characterization and absorption uptake of textile azo dye. Iran Polym J 26:391–404
Rahimdokht M, Pajootan E, Ranjbar‐Mohammadi M (2018) Titania/gum tragacanth nanohydrogel for methylene blue dye removal from textile wastewater using response surface methodology, Polym Int 68(1):134–140. 10. https://doi.org/1002/pi.5706
Patel YN, Patel MP (2012) Novel cationic poly[AAm/NVP/DAPB] hydrogels for removal of some textile anionic dyes from aqueous solution. J Macromol Sci Part A Pure Appl Chem 49:490–501
Dey A, Bera R, Chakrabarty D (2017) Synthesis of poly(ethylene glycol) di-itaconate and investigation of its influence on acrylamide based hydrogels meant for water treatment. Polymer 116:178–190
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Emekdar, E., Şahin, U.K. (2021). Dyestuff Adsorbing Natural Composites for Wastewater Treatments. In: Muthu, S.S. (eds) Advances in Textile Waste Water Treatments. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-16-0065-4_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-0065-4_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0064-7
Online ISBN: 978-981-16-0065-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)