Abstract
Over the last decade, TLIF (Transforaminal Lumbar Interbody Fusion) has become a popular technique for achieving segmental interbody fusion. The recent advances in minimal access technology have helped to execute the procedure through a minimally invasive approach and provide adequate decompression with a solid fusion. The minimally invasive technique also helps to avoid many of the disadvantages of the traditional posterior open approach [1, 2]. A study by Schwender et al. [3] reported clinically significant improvements in visual analog scores and Oswestry disability index scores along with a 100% fusion rate in a cohort of patients who underwent a minimally Invasive TLIF (MIS-TLIF) procedure. Visualization is through a smaller and narrower dissection in MIS cases. The presence of complex spine pathologies such as rotated spine in degenerative scoliosis, poor anatomy on fluoroscopy, asymmetric and abnormally shaped pedicles can pose serious challenges in MIS-TLIF, resulting in incorrect placement of pedicle screws and cages [4]. Image-guided navigation during spinal surgery can be of an invaluable assistance to MIS surgeons as it allows for a larger area of visualization of bony and soft tissues through a smaller area of surgical dissection. Pedicle screw placement by freehand techniques is primarily based on anatomical landmarks, and various methods have been described so far based on cadaveric studies. The high variability in the morphology of pedicles makes it more challenging in complex spinal deformities. Fluoroscopy can assist screw placement; however, it increases the operative time and radiation exposure to the surgeon and operating room personnel. Misplacement rates of up to 30% in the lumbar spine and up to 50% in the thoracic spine have been reported with freehand and fluoroscopic guided pedicle screw placement. Mal-positioned screws risk potential damage to the spinal cord, nerve roots, and great vessels and also decrease the stability of the fixation. Medico-legal concerns over patient safety have further reinforced the need for image-guided screw placements to improve accuracy [5].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Over the last decade, TLIF (Transforaminal Lumbar Interbody Fusion) has become a popular technique for achieving segmental interbody fusion. The recent advances in minimal access technology have helped to execute the procedure through a minimally invasive approach and provide adequate decompression with a solid fusion. The minimally invasive technique also helps to avoid many of the disadvantages of the traditional posterior open approach [1, 2]. A study by Schwender et al. [3] reported clinically significant improvements in visual analog scores and Oswestry disability index scores along with a 100% fusion rate in a cohort of patients who underwent a minimally Invasive TLIF (MIS-TLIF) procedure. Visualization is through a smaller and narrower dissection in MIS cases. The presence of complex spine pathologies such as rotated spine in degenerative scoliosis, poor anatomy on fluoroscopy, asymmetric and abnormally shaped pedicles can pose serious challenges in MIS-TLIF, resulting in incorrect placement of pedicle screws and cages [4]. Image-guided navigation during spinal surgery can be of an invaluable assistance to MIS surgeons as it allows for a larger area of visualization of bony and soft tissues through a smaller area of surgical dissection. Pedicle screw placement by freehand techniques is primarily based on anatomical landmarks, and various methods have been described so far based on cadaveric studies. The high variability in the morphology of pedicles makes it more challenging in complex spinal deformities. Fluoroscopy can assist screw placement; however, it increases the operative time and radiation exposure to the surgeon and operating room personnel. Misplacement rates of up to 30% in the lumbar spine and up to 50% in the thoracic spine have been reported with freehand and fluoroscopic guided pedicle screw placement. Mal-positioned screws risk potential damage to the spinal cord, nerve roots, and great vessels and also decrease the stability of the fixation. Medico-legal concerns over patient safety have further reinforced the need for image-guided screw placements to improve accuracy [5].
Computer-assisted spine surgery (CASS) is a discipline that uses novel computer-based technologies, including stereotaxy, navigated surgery, and robotics. Navigation-assisted spine surgery is a group of technologies, which allow the surgeon to access real-time, three-dimensional, and virtual images of the spine in relation to the surgical instruments intra-operatively. This is a combination of image acquisition and processing that is followed by intra-operative navigation. The primary goal of navigation is to optimize the surgical intervention by providing the surgeon with advanced visualization of the operative field and to see the exact position of the handheld instrument in relation to the bony anatomy. The overall benefits include accurate and safe instrumentation, minimal radiation exposure to the surgical team, reduction of surgeon fatigue and surgical duration. Spine navigation was initially used to improve the accuracy of pedicle screw placement. However, over the years, its use has extended into minimally invasive surgical techniques, cervical spine surgery, revision surgery, and spine tumour surgery [5].
2 Components in Spine Navigation Systems [5]
There are numerous navigation systems available commercially now. The basic fundamentals, however, remain the same and include the following.
2.1 Image Acquisition and Processing Unit
The first step in spinal navigation is to acquire high-resolution images of the region of interest, either pre-operatively or intra-operatively, which then allows the surgeon to navigate upon these processed images. Intra-operative imaging is currently being used in most navigated surgeries as it involves the acquisition of images after positioning the patient for surgical intervention, and this reduces the rate of errors in matching and registration. Intra-operative imaging can be done either by fluoroscopy, computerized tomography (CT) scan and of late even magnetic resonance imaging (MRI).
2.2 Referencing System
This includes Dynamic Reference Frame/Array (DRA), Light Emitting Diodes (LEDs), and Tracking system.
2.2.1 Dynamic Reference Array
The dynamic reference array (DRA) is usually attached to fixed anatomical landmarks, such as the spinous process. The accuracy of the navigation depends on the stable fixation of this DRA, and, therefore, it must be left undisturbed throughout the surgery.
2.2.2 Light-Emitting Diodes
DRA has provisions for attaching three or more spheres known as light-emitting diodes (LEDs). These LEDs emit light which is tracked by an electro-optical camera and are known as active arrays. Specialized surgical instruments are used, which also have LEDs attached to them and are called passive arrays as they reflect the infrared rays emitted from the camera and gives the surgeon a real-time tracking of the exact location of these devices over the surgical field. The 3D orientation between these active and passive LEDs thus facilitates navigation.
2.2.3 Tracking System
Various tracking systems are available that include optical, mechanical, acoustic, or electro-magnetic systems. Optical tracking systems are the most frequently used due to superiority in terms of accuracy. They use infrared camera devices to actively track the light emitted or reflected from the LEDs, which are attached to the DRA and surgical instruments which requires the “line of sight” maintenance between the LEDs and cameras at all times.
2.3 Registration Process
The process of establishing the synchronization between virtual images and the real anatomy is called registration. Once the image is acquired, the data is transferred to the navigational system, which then performs an automated registration eliminating the need for manual registration.
3 Evolution
The methodology of pedicle screws insertion techniques in spine fusion surgery is the most significant advancement, extending from conventional open procedures to accurately placed percutaneous pedicle screws. Numerous studies in literature have highlighted clinically significant sequelae from inaccurate implant placement. For achieving a safe and ideal screw placement, a number of imaging methods and image guidance systems have been used. The use of stereotactic navigation based intra-operative CT is a promising modality offering the benefits of highly accurate pedicle screw placement, reduced operative radiation exposure, and seamless integration into minimally invasive spine surgery. Recently, extensive minimally invasive spinal systems have surged, almost all based on the principle of using a series of dilators of different lengths and increasing diameters to create a path between muscle fascicles to access the posterior spinal elements [6,7,8]. Initial surgeries using these access portals involved simple decompressive procedures; however, over the last decade, these systems have been expanded to facilitate interbody and posterolateral arthrodesis in addition to the placement of pedicle screws in a less invasive fashion in traumatic to deformity correction cases [9]. Spinal navigation is closely related to intra-operative 3D imaging providing an imaging dataset for navigational use and the opportunity for immediate intra-operative assessment of final screw position giving the option of immediate screw revision if necessary.
4 Generations of Navigation System [5]
The history of spine navigation systems can be considered to have undergone three generations of evolution as shown in Table 4.1.
4.1 First-Generation Spine Navigation
First-generation spine navigation systems employed image acquisition using thin-slice CT scan pre-operatively.
4.2 Second-Generation Spine Navigation
Second-generation spine navigation managed to overcome the shortcomings noted in the first generation. They offered intra-operative reconstruction images of the spinal anatomy using two-dimensional (2D) and three-dimensional (3D) fluoroscopy. The 2D fluoroscopy system provided images in two planes. Axial reformatting was not available. The advantage of this system was that the computer software and image acquisition system could be paired with routinely used fluoroscopy units available in the operating room.
Further improvement was seen in the form of cone-beam CT that used basic multiplane fluoroscopy to reconstruct three-dimensional CT like images. The drawbacks were that limited segments of the spine could only be scanned during the process. This made multiple level fixation spanning long segments difficult as multiple scans needed to be performed for a single procedure, increasing the radiation exposure, and operative time.
4.2.1 3D C-Arm Navigation System
This system depends on the concept of isocentricity. The fluoroscopy unit is coupled with a special reference system and computer software to provide axial, sagittal, and coronal reformatted images. The fluoroscopy unit moves through an arc of 180° while focusing on a solitary point in the spine. The system can be calibrated to a high spatial resolution protocol, which takes multiple fluoroscopy images while the arc moves through the 180° or lower resolution protocol, which may take fewer images during the process. The system allows for automatic reference. The advantage of the system was that it did not require a pre-operative CT scan. Intra-operative image acquisition allowed for a post-operative scan to assess the accuracy of the screw position possible. The 3D C-Arm can be used as a routine fluoroscopy unit and can be paired with image guidance surgery software to work as a navigation system for complex spinal surgery.
However, there are a few disadvantages to this navigation system. It scans patients based on the selected isocentric point. Therefore, all the images obtained are from a segment of the spine in the field of the scan. This limits the scan to 6–7 vertebral segments. Although the images generated by the 3D C-Arm are similar to a reformatted CT scan, the image quality is inferior to conventional pre-operatively performed CT scans.
4.2.2 Cone Beam CT
Plenty of Cone Beam CT (CBCT) devices are available commercially, and again they can be used either pre-operatively or intra-operatively. The image quality is superior to 3D C-Arm, and the time for image acquisition is also shorter. Intra-operative CBCT devices allow automatic registration and have a larger field of scan and, therefore, can screen more vertebral segments in a single scan when compared to the 3D-C Arm system. They can provide both routine fluoroscopy images and reformatted CT images in the axial, sagittal, and coronal sections. The radiation dose of the CBCT devices, however, is lower than a conventional CT scanner, and it may be used to assess the accuracy of placement of screws intra-operatively.
4.2.3 Third-Generation Spine Navigation Systems
Third-generation spine navigation systems are considered the most recent developments in the field. These navigation systems can perform an intra-operative CT scan with subsequent automatic registration. They provide excellent CT images with a scan field that can screen the entire spinal column. It offers an opportunity to use the navigation in conjunction with minimal access surgical procedure. The radiation exposure to the patient with the use of such CT based systems can be much higher than fluoroscopy-based navigation systems. These imaging devices have adjustable radiation density thresholds, which provide good images even when the density is reduced by 25–50% of the maximum dosage.
4.3 Senior Author’s MIS Navigation Surgical Technique
The senior author’s MIS surgical technique is centred around navigation when performing specific portions of his operations. We will outline the operating room setup, data acquisition for tracking, registration of instrumentation/patient, and operative steps while performing navigated MIS-TLIF.
5 Indications
-
1.
Degenerative spondylolisthesis with difficult facet morphology.
-
2.
Grade I-III spondylolytic spondylolisthesis and spondyloptosis with narrow pedicles.
-
3.
Degenerative scoliosis with an indication for selective fusion with rotated pedicles.
-
4.
Revision spine surgery—Adjacent segment disease.
5.1 Operating Room Setup
The senior author (SA) sets up the operating room with the patient prone in the centre of the operating room. The image intensifier comes in from the right side of the room (as seen from the foot of the patient). The monitor with the navigation guide stays above the right side of the patient’s right shoulder. The registration camera is above the head of the bed.
5.2 Anaesthesia
General anaesthesia is used for Navigated TLIF.
5.3 Positioning
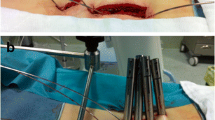
The patient is placed prone on a radiolucent operating table following intubation which allows tilting in all directions and is secured with tapes/belts. The elbows are placed at 90° to decrease traction on the brachial plexus and pads are placed under the ulnar and peroneal nerves. In addition, pillows are placed under the lower extremities (Fig. 4.1). After positioning, the mobility of the Foley catheter is checked, the endotracheal tube is secured, and the fluoroscopic machine is draped into the operative field. Reverse Trendelenburg position is given to make the involved level as vertical as possible to the floor and avoid prolonged abnormal postures with microscope usage.
5.4 3D Navigation Registration
Following standard skin preparation and sterile draping, navigation reference frame is docked on the adjacent spinous process (usually one level above). The 3D C-arm is triggered to spin around the patient and the procured images get formatted into images in all planes (sagittal, coronal, and axial). These images are then transferred to the Stealth monitor. The Stealth™ camera can detect and track anatomy using infrared rays to whichever part/instrument the tracker is attached and registered. At the time of spinning the 3D C-arm, operating team are off the operating room to avoid radiation. The total time taken from draping to registering patients data to 3D navigation takes approximately around 45 min. Authors noticed that anchoring reference frame, static position of patient, and temporary suspension of ventilation to sidestep respiratory movements (generally for a minute) at the time of image capture by the C-arm play a key role to minimize anatomical (registration) errors [10, 11]. Literature suggests that error margins were positive in <1 mm translation and 5° rotation of the patient reference array in all regions of spine [12].
As a first step following verification, navigated Jamshedi needle is registered and tracked to the optical system following which pedicle cannulation is performed using real-time visualization in all the three planes. Percutaneous guide wires are then passed into the pedicles through the Jamshedi needle (11 G) (the authors prefer to place the pedicle guide wires first followed by interbody cage and finally pedicle screws with interconnecting rods. This is because of the change in the real anatomy as a result of disc space preparation and insertion of the cage v/s the virtual anatomy that was captured earlier). Once the placement of the navigated Jamshedi needle within the vertebral body at an appropriate orientation is confirmed, a blunt-tipped threaded guidewire is passed through the cannulated centre of the entry needle. Care should be taken not to advance the guide wire to within 10 mm from the anterior wall of the vertebral body. Following confirmation by lateral view from navigated images, tip of the guidewire from the navigated Jamshedi needle is withdrawn. The steps are repeated for rest of the pedicles and all the guide wires are bent away from the operative field securing them to the draping without introducing sharp bends into them (Fig. 4.2).
5.5 Decompression
Using the Wiltse’s approach, with 3D navigation, successive serial dilators of increasing diameters till 22 mm are inserted. The tubular retractor of appropriate length (5/6/7 cm) is placed over the dilator and accurately docked on the lamina–facet complex (Fig. 4.3). After removal of dilators, the final retractor system can be a fixed rigid tube (METRx), or a split blade tubular retractor (QUADRANT, MARS 3 retractor, etc.) that can be expanded. The surgical microscope is then moved into the field and decompression and interbody fusion are performed through the tubular retractor with variations in the operative steps as per the demands of the indication. The soft tissue over the facet is removed with a long monopolar cautery and Kerrison rongeur. The facet–lamina junction is delineated using navigated curette. Using an angled curette, the space between the lamina and the ligamentum flavum is defined after thinning out the lamina with a high-speed navigated burr. Using the Kerrison rongeur, the lamina–facet junction is removed. If there is no stenosis, then a small laminotomy can be done to allow the visualization of the neural elements in close proximity to the facet joint. If the patient has stenosis on the ipsilateral side, a complete laminectomy should be performed. In cases of bilateral stenosis, the spinous process is undercut and a contralateral laminectomy and medial-facetectomy accomplished by tilting the tube. If stenosis is severe or there is a significant foraminal component on the contralateral side, we suggest decompressing the lateral recess down to the exit zone by wanding the tube caudally [13]. For confirming adequate decompression, navigated probe is checked into spinal canal and foramina in both ipsilateral and contralateral sides (Fig. 4.4). A navigated burr may be used to drill the lamina and the facets, but this decreases the quantity of bone graft, since the surgeon relies on locally excised bone for fusion.
5.6 Disc Space Preparation
The next step is identifying the disc space. In general, the traversing root is medial to the pedicle and only minimal retraction is justified. The exiting nerve root hugs the superior pedicle as it exits the neural foramen and is generally cephalad to the level of the disc in the foramen. Although we do not necessarily dissect out the exiting root, it may be protected by placing a patty directed towards the cephalad pedicle in the foramen. Discectomy and disc space preparation are performed with the help of disc forceps, Kerrison rongeurs, bayonetted curettes, and rotating end plate shavers. The completeness of excision of the intervertebral disc is evaluated by introducing the navigation array probe in all directions: contralateral-posterior, anterior and ipsilateral-anterior, posterior quadrants of disc space (Fig. 4.5) [14]. Once disc space is cleared of the remnant disc, superior and inferior cartilaginous endplates are curetted till superficial bleeding appears on the bed of endplates to promote fusion. In certain complex situations such as high-grade spondylolisthesis, conditions with collapsed disc spaces, etc., identification of the posterior annulus and intervertebral disc may be difficult and the navigation probe has a role in identifying the precise anatomy.
The appropriate size trial interbody cage is then placed into the disc space. After confirming proper placement on navigated screen, the trial is removed and any fragment of bone and cartilage is removed. Autologous bone graft is then packed into the anterior disc space using a funnel and checked with navigated probe for equal distribution of graft. The interbody structural device (cage filled with bone graft) is then advanced into the disc space. The size and position of the cage to be placed was calculated using calibration applications on the Stealth monitor. Interbody fusions are performed using either titanium/PEEK cage and autograft, the cage being precisely positioned and verified with navigation assistance.
5.7 Percutaneous Pedicle Screw and Rod Fixation
The skin and underlying fascia are dilated by means of sequential dilators to create a pathway for the pedicle screws over the initially placed guide wires. The largest dilator is left in place to protect surrounding soft tissue. Using navigation assistance tracker attached to the handles of cannulated tap, advanced over the guidewire down to the pedicle. Depth and diameter of pedicle can be calculated using navigated measurement software at the end of tapping. Care should be taken to prevent the guidewire from advancing or backing-out. Once the pedicle is tapped, the tap and tissue dilator sleeve are withdrawn while the screwdriver and tower assembly are placed over the guidewire. The pedicle screw is advanced with the navigated assistance polyaxial screwdriver avoiding cranio-facet joint violation until the appropriate depth is achieved (Fig. 4.6). Coronal, axial, sagittal images are checked intra-operatively to confirm the screw’s placement within the pedicle, orientation, and overall depth. Care should be taken to avoid advancing the screw head to bone, which would limit the ability to seat the rod. The guidewire is withdrawn as the screw enters the pedicle in order to avoid it getting bent ahead of screw tip and trapped. The screwdriver is withdrawn from the tower assembly. Subsequent pedicle screws are placed with this same technique. It is important to note that all screw tower assemblies should line up in the same orientation and height before the next step of the procedure (Fig. 4.6).
A rod measurement guide is placed to facilitate measurement of the rod size. The rod is passed percutaneously through a separate stab incision (SEXTANT) or placed freehand in other designs leaving adequate lengths at both ends. Once the rod is seated, a cap inserter is placed in the tower assembly. Subsequent screw caps are now placed. Compression can be achieved by system specific methods. Final tightening of the construct is performed with an anti-torque stabilizer and torque-limited driver. The screw tower assemblies are loosened and removed. Final radiograph is obtained to confirm proper positioning of screws, cage, and rod (Fig. 4.7). Dorso-lumbar fascia is approximated with absorbable No. 2-0 Vicryl and subcuticular running closure with Monocryl 3-0 done.
5.8 Post Operative Care
Ambulation usually begins on post-operative day 1. The average hospital stay is 2 days to longer for patients who have additional medical comorbidities with most patients being discharged on POD 4 with assisted ambulation. The scar at 6 weeks follow-up is cosmetic (Fig. 4.8).
5.9 Advantages of MIS
The conventional open posterior approach contributes to wide soft tissue dissection and leads to localized denervation of muscles, extensive blood loss, fibrous tissue (dead space), persistent back pain, and muscle spasm after the procedure [15,16,17]. Kawaguchi et al. [18] demonstrated that the duration of muscle retraction during spine surgery, pressure of the retractors, and the number of levels exposed directly correlate with the post-operative elevation of serum creatinine phosphokinase isoenzymes, a marker of muscle injury. The MIS-TLIF procedure has overt advantages over open TLIF in reducing blood loss (intra-operative and post-operative) thus abolishing need for transfusion, reduced infection rates [19, 20]. These specific advantages can be attributed to fall back of the dilated muscles in the tracts thus collapsing the dead space, which in turn helps to hasten post-operative recovery and early rehabilitation in MIS-TLIF.
6 Advantages of Navigation-Assisted Surgery
Although MIS-TLIF with fluoroscopy causes lesser damage to the patients, the intra-operative challenges faced by surgeons in inserting percutaneous pedicle screw are spinal alignment, quality/quantity of multifidus muscle, and depth of screw entry point. Furthermore, the pedicle dimensions, facet joint arthritis, screw location (ipsilateral and contralateral), screw length, screw diameter, cortical encroachment, frank penetration, and screw trajectory angle are all uncertainty screw-related variable [4].
6.1 Accuracy
Navigation-assisted screw positioning has reported lower misplacement rate compared to the freehand placement. Rajasekaran et al. in a recent article have analyzed pedicles and documented an accuracy rate of 96.2% using intra-operative CT based navigation [21]. In addition to pedicle screw placement, navigation helps to classify these non-negotiable pedicles and prevents the surgeon from attempting to instrument it. Navigation has resulted in pedicle perforation rates as low as 1–5%. The accuracy of 3D navigation system is considered to be superior to virtual fluoroscopy and 2D navigation [22]. A meta-analysis of 9019 thoracic pedicle screws established the superiority of CT navigated instrumentation over fluoroscopic guidance [23]. Castro et al. noted a 40% pedicle breach following freehand pedicle screw placement in fluoroscopy-assisted surgery in spite of anatomic visualization of entry points [24]. MISS is likely to have much higher misplacement rates. Navigated spine surgery has the potential to create phantom screw trajectories and helps the surgeon to apply stab incision at the appropriate level through which screws can be placed with ease in correlation with these phantom images. Baaj et al. used intra-operative navigation to apply percutaneous pedicle screws in short constructs in degenerative spine [25]. Kim et al. observed an accuracy rate of 96.6% in MISS using computer aided navigation and intra-operative CT [26].
6.2 Radiation Safety
It has been noted that for the spine surgeons, radiation exposures are up to 10–12 times greater than in other orthopaedic procedures and may approach or exceed guidelines for cumulative exposure [27]. Minimally invasive spine surgeries (MISS) involve notoriously high amount of radiations to the surgeon and other operating room staff due to the non-visualization of anatomical landmarks for freehand placement of screws. In such a scenario, navigation-assisted surgery reduces the radiation exposure for the operative team, as all members are protected during the scanning procedure. They also found 87% less exposure time to radiation while using intra-operative CT in comparison to fluoroscopy used in MIS procedures [28]. From the patient’s perspective, the radiation exposure for CT based navigation systems is significantly higher when compared to fluoroscopy-based systems, yet they fall within permissible limits.
6.3 Surgical Site Infection
A review of MIS-TLIF studies suggest an infection rate of 0–10% [26]. Similar experience has been highlighted by the author’s team [20]. O’Toole et al. found that the incidence of surgical wound infection was significantly lower after MIS–TLIF (0.6%) than after open TLIF (4.0%) [29]. To reduce the rate of infection with MIS-TLIF, it is recommended to avoid placing fingers into the surgical wound, which may increase the risk of surgical wound infection if there are microscopic breaks in the surgeons gloves. Nassr A also concluded that MIS-TLIF is associated with lower incidence of surgical site infection than open TLIF [30].
6.4 Facet Joint Preservation
There is also a high chance of facet joint violation in MISS which in turn results in adjacent segment degeneration. The real advantage of navigated MIS-TLIF lies in the fact that precise facet joint sparing entry can be taken and optimal trajectory in axial plane can be made with maximal screw length to achieve a near perfect and extremely safe pedicle screw with maximum possible pull-out strength (Fig. 4.9). Lau et al. observed lesser facet joint violations in MISS while using intra-operative navigation [31].
6.5 In Obese/Osteoporotic Patients
Instrumentation using MISS in obese patients and frail osteoporotic patients is challenging as manual tactile feel of the pedicles would not be possible, and spinal navigation comes to the rescue in such scenarios.
7 Concerns with Spine Navigation
7.1 Operative Time
The older generation of navigation systems employing manual point matching registration did lead to increased operative times. This drawback has been overcome with newer generation navigation systems that allow for automatic registration and a larger field of scan (BRAINLAB) extending to multiple vertebral segments. Improvement in quality of virtual images, reduction in acquisition time, and automatic registration process have contributed to the reduction in the duration of a surgery over the years. The overall duration is set to improve steadily as the experience of the surgeon and operating room personnel rises resulting in a systematic workflow in the long run.
7.2 Wobbling and Motion Related Artefacts
Whilst the entry points and trajectories of instrumentation are clearly defined by image-guided surgery, the wobble created by manually tapping or inserting screws across the trajectories involved might result in inaccuracies due to the maximal radial movement from its centre of axis [10]. This is best avoided by postponing the screw insertion process after creating trajectories of all planned screws. Nowadays, powered pedicle screw drive systems are available which enhance surgeon experience with faster, accurate screw insertions. In lean and poorly built patients, ventilation related movement of the thoracic spine may hinder the accuracy of navigation. It is better to acquire images in a non-ventilation mode and reduce the tidal volume in such scenarios to reduce motion-related artefacts. More important, all the nursing staff and assisting surgeons who are involved in the handling of instruments around the surgical field must be aware of the fact that the slightest deflection of the fixed reference array might result in severe inaccuracy. In doubtful scenarios, the surgeon needs to re-verify the accuracy. If the tip of the pointer appears to be either underneath the lamina or hanging above in space, one can be sure that there has been a disturbance of the array, and the entire navigation needs to be repeated. Sometimes in spite of placing the surgical instruments and camera in the “line of sight,” navigation might be troublesome. It might be due to bloodstain or debris covering the spherical diodes. Care should be taken to gently clear it to avoid disturbing the position of reference array.
7.3 Distance from Reference Array
The accuracy of instrumentation is directly proportional to the distance of the level of interest from the reference array. Even though the current systems are capable of imaging the whole spine, the accuracy is questionable at the farthest point from the reference array. This can be solved in two ways. Firstly, when the surgeon requires imaging of the entire spine in case of complex deformity and surgery involves more than 12 segments, it would be appropriate to affix the reference array midway between the ends of the surgical incision. On the other hand, where the surgeon is not able to get an adequate fixation point as in paediatric cervical spine, considering the far distance of iliac crest from the area of instrumentation, it would be better to place the reference array on immobile regions such as Mayfield clamp. Whenever instrumentation is attempted at distal levels, it is better to re-verify the accuracy manually.
7.4 Cost-Effectiveness
The uptake of navigation technology has been limited by start-up, acquisition, and maintenance costs. The opponents of spinal navigation cite this as one of the major drawbacks. The economical evaluations have recognized limitations and challenges as the cost-effectiveness depends on multiple factors such as the number of surgeries performed, the intricateness of surgical procedures undertaken, complications, and the cost of revision surgeries. But a study also concluded that it would actually be a cost-saving surgery for a spine unit that does more than 254 spinal instrumentations yearly [32]. Al-Khouja et al.in his systematic review states that the biggest advantage of image-guided surgery is the prevention of reoperation and four out of seven studies had a zero reoperation rate [33].
7.5 Learning Curve
As with any new technology and its user experience, navigated spine surgery does have a learning curve. However, here, it requires well-organized operating room personnel to function as a single unit, and the success depends on the learning curve of the entire team. Each of the team needs to understand and execute their roles efficiently to reduce the nuances of surgical duration and technical flaws. Bai et al. in his prospective study analyzed the learning curve of surgeons using image-guided navigation spinal surgery and noticed a steep incline in operating time and screw perforation rate by 6 months and reached a plateau by 12 months [34]. Sasso et al. in his retrospective analysis of 4-year data, noted an average reduction of 40 min in operative time for lumbar fusion using navigation and image-guided surgery [35]. Ryang et al. in his prospective analysis of the learning curve using 3D fluoroscopy found a learning curve of 4 months in placing lumbar and thoracic pedicle screws [36].
8 Senior Authors Experience
The authors ventured to assess the impact of 3D navigation in MI-TLIF in evaluating
-
1.
Navigation setting time
-
2.
Radiation exposure
-
3.
Disc space preparation
-
4.
Cage placement
-
5.
Accuracy of pedicle screw placement
-
6.
Cranial facet violation, and
-
7.
Evaluation of canal decompression
8.1 Results
3D Navigation Setting Time
Total time taken for setting up of navigation including pre- surgical time, i.e. scrubbing of the parts, draping, initializing the 3D C-arm and the navigation workstation, mounting reference array on the patient, acquiring scans, and transferring the same onto the navigation workstation was 46.65 ± 9.45 min. As displayed in results, the navigation setting up time progressively reduced with increasing experience. Our setting time values were in consensus with a study conducted by Balling et al. Balling [37] recorded an O-arm guided 3D navigation setting time of 46.2 ± 10.1 min in a prospective study of 306 posterior instrumentations. In our study, we experienced navigation error in one case probably due to translation of the reference array while operating. And this caused a medial breach in one patient which was rectified immediately. Rampersaud et al. suggested that error margins were positive in <1 mm translation and 5° rotation of the patient reference array in all regions of spine [38]. Furthermore, a study by Rahmathullah et al., with his experience of 1500 cases in navigation commented that turning on the warmers during registration can cause image artefacts leading to error [39]. Again, while registration and setting up of navigation take additional time, the total operating time may get shorter in patients with complex anatomy, as compared to fluoroscopy-assisted MI-TLIF. To minimize anatomical errors that could be secondary to respiratory movements, the authors temporarily suspend ventilation (generally for a minute) at the time of image capture by the C-arm [40].
Radiation Exposure
In author’s experience, 117 patients were treated with single-level 3D navigated MI-TLIF and 15 have lost to follow-up. A total of 408 pedicle screws were implanted, the mean time for fluoroscopy usage was 97.6 ± 11.67, and mean amount of radiation from fluoroscopy was 4.43 ± 0.87 which was similar to those found by Mendelsohn et al. who reported that radiation exposure to patients using O arm navigation was 2.77 times more when compared to non-navigated surgeries. However, the dose of 5.69 mSv was much lower than a conventional CT (7.5 mSv) and amounts to one-quarter of the total occupational exposure allowed per year. They also found 87% less exposure time to radiation while using intra-operative CT in comparison to fluoroscopy used in MIS procedures. From the patient’s perspective, the radiation exposure for CT based navigation systems is significantly higher when compared to fluoroscopy-based systems, yet they fall within permissible limits [28]. Kim et al. have also concluded that the use of navigation-assisted fluoroscopy is feasible and safe for minimally invasive spine surgery. Radiation exposure is decreased to the patient as well as the surgical team [41].
Volume of Disc Excised
Adequate disc space preparation is extremely vital for optimum fusion. In our study, the amount of disc removed was 75% in the ipsilateral-anterior, 81% in ipsilateral-posterior, 63% in contralateral-anterior, and 43% in contralateral-posterior quadrants. Following discectomy, Hurly et al. [42] compared the area of empty disc space between two techniques; cone beam navigation and open technique using a navigation probe. Disc removed using cone beam navigation was ipsilateral-anterior = 75%, ipsilateral-posterior = 81%, contralateral-anterior = 63%, and contralateral-posterior = 43%. Rhin et al. showed in his randomized study of 40 lumbar TLIF that the percent disc removed by volume (80% versus 77%, p = 0.41), percent disc removed by mass (77% versus 75%, p = 0.55), and percent total disc removed by area (73% versus 71%, p = 0.63) between the open and MIS approaches were nearly same. The posterior contralateral quadrant was associated with the lowest percent of disc removed compared with the other three quadrants in both open and MIS groups (50% and 60%, respectively). Thus, concluding that navigation can help guide adequate disc space preparation intra-operatively and the surgeon should be generous during discectomy from the posterior contralateral corner to minimize the likelihood of pseudoarthrosis [43].
Cage Placement
Transforaminal lumbar interbody fusion entails packing the anterior one-thirds of disc space with bone graft and navigation allows assessment of the thickness of this mantle of bone graft using the navigation probe. While the guidelines for exact placement of the cage have not been published, numerous papers show encouraging results with anterior and central placement within the intervertebral disc space [44]. In our study, the cage position was central in 87 patients, contralateral antero-central in six patients, and ipsilateral postero-central in eight patients. The Cohen’s kappa statistic test for interobserver co-relation was 0.92 for the two examiners with regard to cage placement. Progressive posterior cage migration was noticed in a patient with initial postero-lateral placement of the cage and this was revised. Schupper et al. had employed navigation in his revision L3L4 case, as an adjunct, to help localize the interspace for cage deployment through minimal exposure. The TLIF cage was able to be appropriately placed in the collapsed disc space, as well as the pedicle screws, which allowed for improvement of lumbar lordosis. Similarly, Lian et al. in his 33 cases had determined the size and orientation of the cage by the navigation and after the cage insertion, a second scan was made to verify the accuracy of all the implants. Navigation also allows the surgeons to place and impact the cage in the desired spot and also most importantly avoid mishaps such as accidental penetration of anterior longitudinal ligament (ALL) and retroperitoneal positioning of the cage [45].
Blood Loss
The mean intra-operative blood loss was 89.65 ± 23.67 mL which is lower as compared to Xu YF et al. [46] and Foley et al. [47].
Accuracy of Pedicle Screw Placement
Regarding accuracy 95.6% showed grade 0 and 4.4% had grade 1 pedicle breach. In one case a grade 3 pedicle screw breach occurred; this was suspected intra-operatively on the C-arm images and confirmed by spinning the 3D C-arm again and extracting images before extubating the patient. The Cohen’s kappa statistic test with regard to pedicle screw breach was 0.889 which demonstrated high reproducible accuracy. Freehand screw misplacement rates in spine is much higher than other spinal segments, and it becomes much more challenging in dysmorphic pedicles as seen in deformities and in areas where there is distortion of normal anatomical landmarks such as trauma, revision surgeries, and ankylosed spine. Navigation has resulted in pedicle perforation rates as low as 1–5%. The accuracy of 3D navigation system is considered to be superior to virtual fluoroscopy and 2D navigation [22]. A meta-analysis of 9019 pedicle screws established the superiority of CT navigated instrumentation over fluoroscopic guidance [22, 23]. Similarly 94.6% had grade 0 and 5.4% demonstrated grade 1 cranial facet violation as was observed by Lau et al. [31]. Thus, 3D-navigation makes sure that the pedicle screw is implanted in the most precise trajectory in all the 3 planes with added benefit of protection against radiation.
Cranio-Facet Violation
The facet joint cranial to the level of fixation is a critical anatomic structure and protection of this joint is vital in avoiding adjacent segment disease [48, 49]. In the current study, only 25 out of 408 pedicle screws (6.1%) violated the cranial facet joint, with 94.6% and 5.4% of pedicle screws demonstrated grade 0 and grade 1 cranial facet violation, respectively, reinforcing the advantages of navigation-assisted insertion of pedicle screws. Again, the degree of violation in these 6.1% of screws appears relatively inconsequential (grade 1), based on the classification of Babu et al. [50]. The Cohen’s kappa statistic test with regard to cranial facet violation was 0.878 which demonstrated high reproducible accuracy. Ohba et al. [51] reviewed 194 pedicle screws in 28 consecutive patients and found that 87.5% and 94% of screws inserted using conventional fluoroscopy and 3D navigation group, respectively, did not violate the facet joint. Park et al. [48] reported a high rate of cranial-facet joint violation in fluoroscopic MISS surgery when compared to open surgeries (31.5% vs. 15.2% of all screws, p < 0.001).
Evaluation of Canal Decompression
In our study, the navigation array probe was utilized to verify the adequacy of decompression and to confirm the anatomical landmarks as and when necessary. In their study on 28 patients undergoing MIS-TLIF, Lee et al. [52] found that the Mean spinal canal cross section area at disc spaces have increased significantly at 12 months post-operatively from 157.5 mm2 to 294.3 mm2, (p = 0.012) leading to a good clinical outcome, which could easily be evaluated intra-operatively using the navigation like in our study [42].
Reduced Surgical Site Infection
In the present study of 117 patients, no surgical site infection was seen. In our another study of 1043 patients treated with MIS techniques, 763 underwent non-instrumented surgeries and 280 underwent instrumented fusion. The overall infection rate after MISS was 0.29%, 0% in non-instrumented cases and 1.07% [3 out of 280 cases] in instrumented cases. Nassr A also concluded that MIS-TLIF is associated with lower incidence of surgical site infection than open TLIF [30].
Example 1
Figure 4.10 demonstrates the use of navigation in L4L5 MI-TLIF in a patient with adult degenerative scoliosis in which only selective fusion of L4 L5 is indicated.
-
(a)
Accurate placement of screws across rotated pedicles with malformed anatomy due to advanced degenerative arthritis is seen.
-
(b)
The cage can be placed optimally using navigation.
-
(c)
Post-operative X-ray and healed scar of MI-TLIF.
Example 2
Figure 4.11 demonstrates the use of 3D navigation in ill-defined anatomy at L4L5 in advanced degenerative arthritis
-
(a)
Poorly defined anatomy on 2D fluoroscopy images.
-
(b)
Pedicle screw insertion using 3D navigation.
-
(c)
Post operative X-ray of MI-TLIF.
9 Conclusions
At author’s institution, almost all cases requiring fusion are operated with Minimally Invasive Transforaminal Lumbar Interbody Fusion [MIS-TLIF] technique with fluoroscopy and 3D navigation. With vast experience in minimally invasive techniques, we find MIS to be associated with less post-operative infection rates as compared to open techniques. With 3D navigation, MIS becomes safer and highly accurate. MIS-TLIF with 3D navigation has satisfactory clinical outcomes and fusion rates with the additional benefits of less initial post-operative pain, less blood loss, earlier rehabilitation, and shorter hospitalization. MIS-TLIF with 3D navigation is a more cost-effective treatment than MIS-TLIF with fluoroscopy.
References
Eliyas JK, Karahalios D. Surgery for degenerative lumbar spine disease. Dis Mon. 2011;57:592–606.
Vaccaro AR, Bono CM. Minimally invasive spine surgery. CRC Press; 2007.
Phillips FM, Lieberman IH, Polly DW Jr, Wang MY. Minimally invasive spine surgery: surgical techniques and disease management. Springer Nature; 2020.
Kim M-C, Chung H-T, Cho J-L, Kim D-J, Chung N-S. Factors affecting the accurate placement of percutaneous pedicle screws during minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2011;20:1635–43.
Rajasekaran S, Shetty AP. Section 11, Chapter 14: Navigation in spine surgery. In: Lumbar spine online textbook. DT Internet publisher - ISSLS, Wheeless Online; 2020.
Jahng T-A, Fu T-S, Cunningham BW, Dmitriev AE, Kim DH. Endoscopic instrumented posterolateral lumbar fusion with Healos and recombinant human growth/differentiation factor-5. Neurosurgery. 2004;54:171–81.
Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine. 2003;28:S26–35.
Kambin P. Letters. Spine. 2004;29:598–9.
Kim DH, Jaikumar S, Kam AC. Minimally invasive spine instrumentation. Neurosurgery. 2002;51:S15–25.
Rahmathulla G, Nottmeier EW, Pirris SM, Deen HG, Pichelmann MA. Intraoperative image-guided spinal navigation: technical pitfalls and their avoidance. Neurosurg Focus. 2014;36:E3.
Guha D, Jakubovic R, Gupta S, Fehlings MG, Mainprize TG, Yee A, Yang VXD. Intraoperative error propagation in 3-dimensional spinal navigation from nonsegmental registration: a prospective cadaveric and clinical study. Glob Spine J. 2019;9:512–20.
Rampersaud YR, Simon DA, Foley KT. Accuracy requirements for image-guided spinal pedicle screw placement. Spine. 2001;26:352–9.
Kulkarni AG, Sagane SS, Kunder TS. Management of spondylolisthesis using MIS techniques: recent advances. J Clin Orthop Trauma. 2020;11(5):839–47. https://doi.org/10.1016/j.jcot.2020.07.015.
Hurley RK Jr, Anderson ER 3rd, Lawson BK, Hobbs JK, Aden JK, Jorgensen AY. Comparing lumbar disc space preparation with fluoroscopy versus cone beam-computed tomography and navigation: a cadaveric study. Spine. 2018;43:959–64.
Sihvonen T, Herno A, Paljärvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575–81.
Styf JR, Willén J. The effects of external compression by three different retractors on pressure in the erector spine muscles during and after posterior lumbar spine surgery in humans. Spine. 1998;23:354–8.
Gejo R, Matsui H, Kawaguchi Y, Ishihara H, Tsuji H. Serial changes in trunk muscle performance after posterior lumbar surgery. Spine. 1999;24:1023–8.
Kawaguchi Y, Matsui H, Tsuji H. Changes in serum creatine phosphokinase MM isoenzyme after lumbar spine surgery. Spine. 1997;22:1018–23.
Kulkarni AG, Patel RS. Is closed-suction drainage essential after minimally invasive lumbar fusion surgery? A retrospective review of 381 cases. J Minim Invasive Spine Surg Tech. 2017;2:27–31.
Kulkarni AG, Patel RS, Dutta S. Does minimally invasive spine surgery minimize surgical site infections? Asian Spine J. 2016;10:1000–6.
Rajasekaran S, Bhushan M, Aiyer S, Kanna R, Shetty AP. Accuracy of pedicle screw insertion by AIRO intraoperative CT in complex spinal deformity assessed by a new classification based on technical complexity of screw insertion. Eur Spine J. 2018;27:2339–47.
Silbermann J, Riese F, Allam Y, Reichert T, Koeppert H, Gutberlet M. Computer tomography assessment of pedicle screw placement in lumbar and sacral spine: comparison between free-hand and O-arm based navigation techniques. Eur Spine J. 2011;20:875–81.
Meng X-T, Guan X-F, Zhang H-L, He S-S. Computer navigation versus fluoroscopy-guided navigation for thoracic pedicle screw placement: a meta-analysis. Neurosurg Rev. 2016;39:385–91.
Castro WHM, Halm H, Jerosch J, Malms J, Steinbeck J, Blasius S. Accuracy of pedicle screw placement in lumbar vertebrae. Spine. 1996;21:1320–4.
Baaj AA, Beckman J, Smith DA. O-Arm-based image guidance in minimally invasive spine surgery: technical note. Clin Neurol Neurosurg. 2013;115:342–5.
Kim TT, Drazin D, Shweikeh F, Pashman R, Johnson JP. Clinical and radiographic outcomes of minimally invasive percutaneous pedicle screw placement with intraoperative CT (O-arm) image guidance navigation. Neurosurg Focus. 2014;36:E1.
Rampersaud YR, Raja Rampersaud Y, Foley KT, Shen AC, Williams S, Solomito M. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine. 2000;25:2637–45.
Mendelsohn D, Strelzow J, Dea N, et al. Patient and surgeon radiation exposure during spinal instrumentation using intraoperative computed tomography-based navigation. Spine J. 2016;16:343–54.
O’Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine. 2009;11:471–6.
Nassr A. CORR insights®: does minimally invasive surgery have a lower risk of surgical site infections compared with open spinal surgery? Clin Orthop Relat Res. 2014;472:1725–6.
Lau D, Terman SW, Patel R, La Marca F, Park P. Incidence of and risk factors for superior facet violation in minimally invasive versus open pedicle screw placement during transforaminal lumbar interbody fusion: a comparative analysis. J Neurosurg Spine. 2013;18:356–61.
Dea N, Fisher CG, Batke J, Strelzow J, Mendelsohn D, Paquette SJ, Kwon BK, Boyd MD, Dvorak MFS, Street JT. Economic evaluation comparing intraoperative cone beam CT-based navigation and conventional fluoroscopy for the placement of spinal pedicle screws: a patient-level data cost-effectiveness analysis. Spine J. 2016;16:23–31.
Drazin D, Al-Khouja L, Shweikeh F, Pashman R, Johnson J, Kim T. Economics of image guidance and navigation in spine surgery. Surg Neurol Int. 2015;6:323.
Wang D, Zhang K, Qiang M, Jia X, Chen Y. Computer-assisted preoperative planning improves the learning curve of PFNA-II in the treatment of intertrochanteric femoral fractures. BMC Musculoskelet Disord. 2020; https://doi.org/10.1186/s12891-020-3048-4.
Sasso RC, Garrido BJ. Computer-assisted spinal navigation versus serial radiography and operative time for posterior spinal fusion at L5-S1. J Spinal Disord Tech. 2007;20:118–22.
Ryang Y-M, Villard J, Obermüller T, Friedrich B, Wolf P, Gempt J, Ringel F, Meyer B. Learning curve of 3D fluoroscopy image–guided pedicle screw placement in the thoracolumbar spine. Spine J. 2015;15:467–76.
Balling H. Time demand and radiation dose in 3D-fluoroscopy-based navigation- assisted 3D-fluoroscopy-controlled pedicle screw instrumentations. Spine (Phila Pa 1976). 2018;43(9):E512–9.
Rampersaud YR, Simon DA, Foley KT. Accuracy requirements for image-guided spinal pedicle screw placement. Spine (Phila Pa 1976). 2001;26(4):352–9.
Rahmathulla G, Nottmeier EW, Pirris SM, et al. Intraoperative image-guided spinal navigation: technical pitfalls and their avoidance. Neurosurg Focus. 2014;36(3):E3.
Guha D, Jakubovic R, Gupta S, et al. Intraoperative error propagation in 3-dimensional spinal navigation from nonsegmental registration: a prospective cadaveric and clinical study. Glob Spine J. 2019;9(5):512–20.
Kim CW, Lee Y-P, Taylor W, Oygar A, Kim WK. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J. 2008;8:584–90.
Hurley RK, Anderson ER, Lawson BK, et al. Comparing lumbar disc space preparation with fluoroscopy versus cone beam-computed tomography and navigation. Spine (Phila Pa 1976). 2018;43(14):959–64.
Rihn JA, Gandhi SD, Sheehan P, Vaccaro AR, Hilibrand AS, Albert TJ, Anderson DG. Disc space preparation in transforaminal lumbar interbody fusion: a comparison of minimally invasive and open approaches. Clin Orthop Relat Res. 2014;472:1800–5.
Castellvi AD, Thampi SK, Cook DJ, et al. Effect of TLIF cage placement on in vivo kinematics. Int J Spine Surg. 2015;9:38.
Lian X, Navarro-Ramirez R, Berlin C, Jada A, Moriguchi Y, Zhang Q, Härtl R. Total 3D Airo® navigation for minimally invasive transforaminal lumbar interbody fusion. Biomed Res Int. 2016;2016:5027340.
Xu Y-F, Le X-F, Tian W, et al. Computer-assisted, minimally invasive transforaminal lumbar interbody fusion. Medicine. 2018;97:e11423.
Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl):S1–6.
Park Y, Ha JW, Lee YT, et al. Cranial facet joint violations by percutaneously placed pedicle screws adjacent to a minimally invasive lumbar spinal fusion. Spine J. 2011;11(4):295–302.
Chen Z, Zhao ÆJ, Xu ÆH, et al. Technical factors related to the incidence of adjacent superior segment facet joint violation after transpedicular instrumentation in the lumbar spine. Eur Spine J. 2008;17(11):1476–80.
Babu R, Park JG, Mehta AI, et al. Comparison of superior-level facet joint violations during open and percutaneous pedicle screw placement. Neurosurgery. 2012;71(5):962–70.
Ohba T, Ebata S, Fujita K, et al. Percutaneous pedicle screw placements: accuracy and rates of cranial facet joint violation using conventional fluoroscopy compared with intraoperative three-dimensional computed tomography computer navigation. Eur Spine J. 2016;25(6):1775–80.
Lee CK, Park JY, Zhang HY. Minimally invasive transforaminal lumbar interbody fusion using a single interbody cage and a tubular retraction system: technical tips, and perioperative, radiologic and clinical outcomes. J Korean Neurosurg Soc. 2010;48:219–22.
Conflict of Interest
The authors have no conflicts of interests to disclose.
Ethical Review Committee Statement
The study is approved by institutional review board (approval vide - SaHo7840), Saifee Hospital, Mumbai.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kulkarni, A.G., Rathi, P., Rajamani, P.A. (2022). MI-TLIF with 3D Navigation. In: Kim, JS., Härtl, R., Wang, M.Y., Elmi-Terander, A. (eds) Technical Advances in Minimally Invasive Spine Surgery. Springer, Singapore. https://doi.org/10.1007/978-981-19-0175-1_4
Download citation
DOI: https://doi.org/10.1007/978-981-19-0175-1_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0174-4
Online ISBN: 978-981-19-0175-1
eBook Packages: MedicineMedicine (R0)