Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disorder of the elderly affecting around 1% of individuals older than 65 years of age. PD poses a major health and economic burden, due to its chronic and progressive nature and the lack of available effective medications to stop or even slow its progression. Majority of PD cases is not linked to known genetic mutations and could be related to environmental factors and an unhealthy lifestyle including deficiency of micronutrients like vitamin D. In the current work, we reviewed the literature to understand the role of vitamin D in the brain and the association between vitamin D insufficiency and deficiency and risk of developing PD. We focus on the molecular mechanisms explaining the increased PD risk concentrating on the nutrigenomic effects of vitamin D. Due to the fact that the prevalence of PD in Egypt is higher than the international one, vitamin D deficiency and some other modifiable risk factors for PD among the Egyptian were discussed. The potential therapeutic role of vitamin D for managing PD was further reviewed. Finally, we could draw the conclusion that vitamin D deficiency may be associated with an increase in the risk and progression of PD. More research is warranted to elucidate the role of vitamin D as preventive and therapeutic options for patients with PD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Epidemiology of Parkinson’s Disease (PD)
Parkinson’s disease (PD) is considered to be the second most common neurodegenerative disorder in the elderly which affects approximately 1% of the population older than 60 years (Hirtz et al. 2007). The prevalence of PD in the USA is expected to increase by 2.25-folds, and in Europe, by 1.83-fold between 2010 and 2050 (Bach et al. 2011). Furthermore, the crude prevalence of PD in the USA is expected to increase from 0.401% in 2005 to 0.535% by 2040, and the number of PD patients will reach 700, 000 individuals (Rossi et al. 2018).
As the disease progresses, both the motor and non-motor manifestations worsen. Until now, there is no effective therapy available to halt or even to slow down the disease progression. Indeed, symptomatic treatment is the only available therapy and is associated with many side effects (Oertel 2017; Toulouse and Sullivan 2008). L-dopa is one of the gold standard treatments for PD. However, it induces many side effects including dyskinesia in approximately 30–80% of patients with PD (Phillips et al. 2016). Additionally, it may even enhance progression of the disease by increasing the fraction of free dopamine induced-oxidative stress (Peritore et al. 2012).
PD exerts a major economic burden due to direct and indirect factors. It was estimated that the annual cost of the PD care is around 20,000 US dollars. Direct cost is mainly driven by healthcare services, medications, deep brain stimulation, and physiotherapy. Indirect cost could be driven by reduction of work performance and hours of patients with PD, absentees from work, and care of patients by family members (Bovolenta et al. 2017; Dowding et al. 2006; Kowal et al. 2013; Martinez-Martin et al. 2019). It has been reported that reduction of PD progression to half could reduce such cost to about third (Johnson et al. 2013).
The lack of specific etiology of PD has led investigators to study the association between PD and plethora of genetic and environmental factors that could play a role in its pathogenesis. One of these factors is vitamin D.
12.2 Association Between Low Level of Vitamin D and Risk of PD
Epidemiological studies indicate a positive association of vitamin D deficiency and risk of PD. A prospective study was conducted in Finland between 1978 and 1980 (Mini-Finland health survey), which included 3173 individuals not suffering from PD. Around 29 years later, 50 individuals developed PD. High level of vitamin D seems to show a protective effect against PD as concluded from the low relative risk (RR) between the highest and lowest quartile (RR: 0.33, 95%CI 0.14–0.8; Knekt et al. 2010). Contrarily, another prospective study contradicted these findings not reporting a protective effect of vitamin D against the risk of developing PD. Levels of vitamin D were assessed among 12,762 individuals, and the samples were collected from 1990 to 1992. At the end of 2008, 67 PD patients were identified. No association was observed between the level of vitamin D and risk of PD, as the hazard ratios (HRs) were (1.05: 95% CI: 0.58–1.90) when vitamin D levels are between 20 and 30 ng/mL and (1.14: 95% CI: 0.59–2.23) when vitamin D levels equal or higher than 30 ng/mL compared to individuals having lower levels than 20 ng/mL (Shrestha et al. 2016). These contradictory results between these studies could be attributed to differences in sample sizes of the individual studies, vitamin D assays, and PD diagnostic validation, dictating the need for more studies.
Systematic review of the literature including human observational studies assessing the relationship between vitamin D and PD risk shows an inverse relationship between vitamin D levels and risk of developing PD. One review included 7 studies where 1008 patients and 4536 controls were assessed. Both vitamin D insufficiency and deficiency increased the risk of PD ((odds ratio (OR): 1.5, 95% CI: 1.1–2.0)) and (OR: 2.2, 95% CI: 1.5–3.4), respectively (Lv et al. 2014). Consistently, another systemic review investigating the relationship between vitamin D and PD included both human and rodents studies. The review also supported the hypothesis that vitamin D can have both protective and symptomatic effects against PD (Rimmelzwaan et al. 2016).
A meta-analysis of 8 studies including 5690 PD patients and 21,251 controls that were published until March 2015 showed an increased risk of PD among individuals with vitamin D insufficiency and deficiency (OR: 1.29, 95% CI: 1.10–1.51) and (OR: 2.08, 95% CI: 1.63–2.65), respectively. A more recent meta-analysis of 20 studies published until January 2018 including 2866 PD patients and 2734 individuals reported that reduced vitamin D levels were associated with an increase in the risk of PD. Vitamin D deficiency and insufficiency were associated with PD risk (OR: 2.08, 95% CI: 1.35–3.19) and (OR: 1.73, 95% CI: 1.48–2.03), respectively. Moreover, reduced vitamin D level increases the severity of PD (r = −0.55, 95% CI −0.73, −0.29; Luo et al. 2018). Notably, the authors reported high heterogeneity depending on the assay methods. Furthermore, vitamin D supplementation reduced PD risk (OR: 0.62, 95% CI: 0.35–0.90: Shen and Ji 2015).
In conclusion, it could be suggested that low vitamin D levels is associated with increased risk of PD. In the next section, we will review potential molecular and non-molecular factors that could explain such association.
12.3 How Could Vitamin D Alter the Risk of Developing PD on the Molecular Level?
12.3.1 Vitamin D Synthesis and Its Role in the Brain
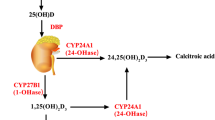
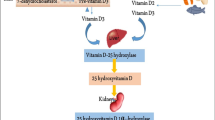
Vitamin D can be found in food, or it can be synthesized by the human skin when exposed to sunlight. Our skin first synthesizes the precursor for vitamin D, 25-(OH)D. This is further hydroxylated in the liver and then in the kidney to form 1,25 dihydroxyvitamin D (1,25-(OH)2D3), or calcitriol, which is the bioactive form of vitamin D. These hydroxylations are carried out by the enzyme 1a-hydroxylase. Calcitriol activates vitamin D receptor (VDR) and regulates gene transcription (Rimmelzwaan et al. 2016). While VDR is a nuclear receptor, 1a-hydroxylase is found in the cytoplasm (Lương and Nguyễn 2012). Both vitamin D receptor and 1a-hydroxylase are found in neurons and glial cells. The active form of vitamin D, 1,25-(OH)2D, induces its neuroprotective action in several ways. First, it inhibits nitric oxide synthase which produces the free radical, nitric oxide. It also induces the activity of γ-glutamyl transpeptidase, which synthesizes glutathione. Glutathione is an important antioxidant and free radical neutralizer. In addition to its neuroprotective actions, 1,25-(OH)2D induces the production of growth factors like nerve growth factor (NGF), glial derived neurotrophic factor (GDNF), and neurotrophin 3 (NT3) (Rimmelzwaan et al. 2016).
When activated, microglia, the immune cells of the nervous system, transform into phagocytes, identical to macrophages. Microglia are implicated in the pathogenesis of PD. Activated microglia lead to perpetuation of inflammation and neuronal cell death which progresses to dopaminergic neurodegeneration in PD. The substantia nigra has the highest number of microglia, rendering it the neuronal organ most vulnerable to inflammation and neurodegeneration. The substantia nigra contains mostly dopaminergic neurons and is hypothesized to be the location of pathology for PDMicroglial activation negatively affects the structure and function of dopaminergic neurons. Dopaminergic neurons in the substantia nigra also possess lower levels of glutathione, a powerful antioxidant, which reduces their antioxidant activity and therefore significantly increase susceptibility to microglia even more (Yan et al. 2014).
The activation of microglia can be mediated by interferon gamma (IFN-γ), inducible nitric oxide synthase (iNOS), interleukin 1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α).
1a-hydroxylase is found in the substantia nigra neurons as well as glial cells and neurons of the hypothalamus (Lương and Nguyễn 2012). Vitamin D deficiency is associated with tyrosine hydroxylase gene suppression. Tyrosine hydroxylase is an essential enzyme needed for dopamine synthesis and neurotrophic factors (Zhou et al. 2019).
In PD, iNOS expression is increased, which aggravates dopaminergic neuronal death. The chronic inflammatory process, stimulated by the activation of pro-inflammatory cytokines which activate microglia, is thought to be leading pathology to the progression of PD (Yan et al. 2014). iNOS expression was attenuated in cortical neurons when exposed to vitamin D treatment. The INOS gene has VDR response element on it. As VDR is one of the receptors for vitamin D, this highlights the potential direct action of vitamin D on iNOS (Dursun et al. 2014).
12.3.2 Association of VDR Polymorphism and PD
Observational studies comparing VDR genetic variation rs10735810 (FokI) C allele in PD patients and controls have shown mixed results. While some studies showed an increased frequency of the C allele of VDR among patients with PD, others noticed an association of the CC allele and mild forms of PD in Japanese patients, which could be explained population variation. Another study showed that the VDR BsmI genotype in Koreans was associated with PD, while the VDR gene increased the susceptibility to PD in Caucasians (Lương and Nguyễn 2012). A study on Hungarian patients showed an association between an increase in the FokI C allele and PD as compared to controls, and other genetic variants did not show any associations (Török et al. 2013). In contrast, a study of Taiwanese population showed no association of genetic variants of the VDR with PD (Lin et al. 2014). Another study in Han Chinese population showed an association between VDR FokI T/C polymorphism and sporadic PD (Han et al. 2012). Other polymorphisms of the VDR gene include ApaI, BsmI, and TaqI. A meta-analysis found no association of these polymorphisms and PD susceptibility, while FokI polymorphism showed an increased risk in the Asian populations specifically (Gao et al. 2010). TNF-a production is induced by microglial activation (Kuno et al. 2005). Calcitriol was found to inhibit the expression of TNF in microglial cells in two ways: increasing IL-10 expression and forming a complex of LITAF (a transcription factor of TNF) and VDR, which prevents the LITAF from binding to the promoter of the TNF gene (He et al. 2017). VDR is known to affect many diseases by regulating gene transcription (Butler et al. 2011). Vitamin D is known for its anti-inflammatory effect in neurodegenerative diseases. Its effect on calcium homeostasis is thought to play a major protective role in the pathogenesis of PD. This could be explained by the fact that dopaminergic neurons in the substantia nigra are vulnerable to the effect of sustained opening of L-type calcium channels (Butler et al. 2011). More studies are needed to confirm the relationship between VDR polymorphism and risk of PD (Rimmelzwaan et al. 2016).
12.3.3 Molecular Pathways of the Association Between Vitamin D and PD
12.3.3.1 MHCII Complex
Major histocompatibility complex (MHC) is an important locus in human DNA which contains many polymorphic genes. They encode for cell surface proteins that are important for the adaptive immune system. Some genes in this region confer to an increased risk of PD. Specifically, increased levels of MHC class II expression were found in monocytes of PD patients.
Astrocytes increase the levels of glial fibrillary acidic protein (GFAP) and glutathione peroxidase to protect dopaminergic neurons. In PD, astrocytes increase the expression of GFAP. Astrocytes also develop type 2 helper T-cell immune responses that suppress IL-12 expression and increase the expression of MHC and stimulatory cytokines. All of which leads to an inflammatory response (Yan et al. 2014). In response to toxic factors, astrocytes release toxic factor which damage the dopaminergic cell bodies which later lead to degeneration of neurons in the striatum (Yan et al. 2014).
Interestingly, 1,25-(OH)2 D3 (calcitriol) is known to suppress MHC class II antigen expression in monocytes and macrophages (Lương and Nguyễn 2012). This inhibitory effect of vitamin D on MHC expression could partly explain the potential protective effect of vitamin D in PD.
12.3.3.2 Cytochrome P450
Cytochrome P450 is a group of enzymes responsible for the metabolism of endogenous as well as exogenous compounds. They can also be found in the brain and are known to be polymorphic and therefore may contribute to diseases, either by exhibiting increased or decreased activity. CYP2D6*4 allele was found to be more prevalent among patients with PD as compared to controls. Interestingly, CYP2D6 can also act as 25-hydroxylase, activating vitamin D3 (formed by the skin) conversion to 25-(OH)D. Deficiency of the 25-hydroxylase enzymes is associated with vitamin D deficiency (Lương and Nguyễn 2012).
Moreover, CYP2D6 polymorphism is also associated with PD. According to ethnicities, CYP2D6 was found to be absent in less than 1% of the Asian population, and in up to 10% of Caucasians. Finding a significant association between this polymorphism and PD would require a large study group, and stratification according to ethnicities.
Of significance is that CYP2D and PD loci are located on chromosome 22. Deletion of chromosome 22q11 resulted in lower serum calcium, bone mineral density, and parathyroid hormone levels (Lương and Nguyễn 2012). CYP2D6 is a potential 25-hydroxylase, converting vitamin D3 to 25OHD. The lack of this enzyme will result in vitamin D deficiency (Lương and Nguyễn 2012).
12.3.3.3 Heme Oxygenase-1 (HO-1)
HO-1 is an inducible cytoprotective enzyme which is expressed in response to oxidative stress. It is responsible for the catabolism of heme to biliverdin and subsequently bilirubin alongside the production of carbon monoxide (CO) (Gozzelino et al. 2010). Low dose of CO has been found to exert anti-inflammatory effects in animal models of inflammatory bowel disease (Hegazi et al. 2005).
While in normal brains, HO-1 is found in low levels. HO-1 was overexpressed in astrocytes of PD especially in substantia nigra and Lewy bodies in dopaminergic neurons. 1,25-(OH) D3/calcitriol could exert its protective effects in PD via HO-1 dependent mechanism. Consistently, calcitriol has been shown to delay HO-1 immunoreactivity after cerebral ischemia (Lương and Nguyễn 2012).
12.3.3.4 Poly(ADP-Ribose) Polymerase-1 (PARP-1)
PARP-1 is a protein acting on the nucleus of the cell, and in response to stress, it can either induce neuronal death or survival (Lương and Nguyễn 2012). The role of PARP-1 in stress response is explained in detail in a review by (Xin Luo and Lee Kraus 2012). MPTP is a well-known neurotoxin that causes parkinsonian symptoms. PARP gene lacking mice have shown to be spared from the effects of MPTP. PARP-1 was also overexpressed in the dopaminergic neurons in the substantia nigra of PD patients. High levels of vitamin D suppress PARP-1 expression, in a dose-dependent manner. Vitamin D is hypothesized to induce this anti-inflammatory effect by inhibiting microglial activation.
12.3.3.5 Neurotrophic Factors (NTFs)
NTFs are important proteins for the survival of neurons (Brockmann et al. 2016). NTFs can promote neuronal regeneration or protect them from insult. NTFs include brain-derived neurotrophic factors (BDNF), glial-derived neurotrophic factor (GDNF), mesencephalic-astrocyte-derived neurotrophic factor (MANF), and cerebral dopamine neurotropic factor (CDNF). Their respective receptors are found in the striatum and the substantia nigra. In PD, expression of NTFs is reduced. In Koreans, CDNF single-nucleotide polymorphism (rs7094179) increased the susceptibility to PD. In the Chinese Han population, an allele of BDNF was a risk factor for PD. Calcitriol acts on the expression of neurotrophic receptors and expression of GDNF. By increasing GDNF and restoring tyrosine hydroxylase expression in the substantia nigra and striatum, calcitriol protects against dopamine loss (Lương and Nguyễn 2012). GDNF is an important neuroprotective agent for dopamine neurons in the midbrain. GDNF administration alleviated symptoms in PD patients and primate models. In vitro, calcitriol increased glutathione, and its precursor γ-glutamyl transpeptidase and neurotrophin-3 as well (Smith et al. 2006). Decreased levels of neurotrophins in the nigrostriatal region of postmortem brain of PD patients were evident in previous studies. These neurotrophins included BDNF. A suggested mechanism for that is microglial activation which subsequently resulted in the death of dopaminergic neurons (Nagatsu and Sawada 2005). Activated monocytes secrete bioactive BDNF during inflammatory processes to aid in neuronal survival (Brockmann et al. 2016). Increased BDNF serum levels were associated with disease duration and severe motor impairment in PD patients. BDNF also correlated positively with inflammatory markers of neurodegenerative diseases (Brockmann et al. 2016).
12.3.3.6 Sp1 Transcription Factor
Sp1 transcription factor is a DNA-binding protein. In response to oxidative stress in neurons, it gets acetylated. Sp1 family is important in the expression of dopamine transporter gene (Lương and Nguyễn 2012). Sp1 sites act synergistically with vitamin D-responsive elements to induce CYP24 (25-OH-D 24-hydroxylase) production which is important for the metabolism of 1,25OH-D (Lương and Nguyễn 2012). A previous study showed that Sp1 inhibition was seen to reduce monoamine oxidase B activity which resulted in neuroprotective effects (Yao et al. 2018).
12.3.4 Nonmolecular Mechanism of the Association Between Vitamin D and PD
Vitamin D, as one of the fat-soluble vitamins, is stored in adipose tissue, and it alters the inflammatory response of adipocytes (Stevens 2021). Hypertrophy of the adipose tissues associated with obesity results in insufficient blood supply, hypoxia, macrophages infiltration, release of pro-inflammatory cytokines (IL6 & 8, MCP1, TNF-alpha and resistin), and altering adipokines secretion. These changes are associated with insulin resistance (de Luca and Olefsky 2008; Heilbronn and Campbell 2008). It was consistently shown in recent literature that DM increases the risk of PD and its clinical progression. This could be attributed to mitochondrial dysfunction, oxidative stress, neuroinflammation, impaired protein hemostasis. Insulin resistance and poor glycemic control worse PD progress (Hassan et al. 2020). The potential beneficial effect of vitamin D in PD could be explained by its anti-inflammatory effects and inhibition of insulin resistance.
Vitamin D decreases insulin resistance by affecting the release of adipokines as it increases adiponectin and inhibits leptin release. Furthermore, vitamin D plays a critical physiological role as a powerful anti-inflammatory molecule by inhibiting P38MAP kinase and NF-kB signaling pathways, reducing the expression of pro-inflammatory factor genes (IL1-beta, IL-8, and TNF-alpha), (Koszowska et al. 2014; Szymczak-Pajor and Śliwińska 2019). It’s totally accepted among the scientific community that insulin resistance is observed during developing diabetes mellitus (DM: Reusch 2002; Taylor et al. 1994) and vitamin D deficiency increases the risk of developing DM through losing the protective roles, as reviewed in details by Berridge (2017). The prevalence of vitamin D deficiency among Kenyan patients with DM was 38.4% and was associated with poor glycemic control (Karau et al. 2019). Haidari et al. (2016) reported similar prevalence of vitamin D deficiency among the Persian DM patients (35.72%). Vitamin D has protective effects through antagonizing the inflammatory response observed early during the pre-DM by reducing release of cytokines, chemokines, and reducing the monocytes chemotaxis (Bartels et al. 2010; Calton et al. 2015). It is also important for preserving the mitochondrial functions including maintaining the energetic one through preserving the function of the electron transport chain (ECT; Ashcroft et al. 2021). ECT is responsible for producing the majority of the energy needed by the cells; hence, mitochondria are called the powerhouse of the cells (Siekevitz 1957). Vitamin D antagonizes apoptosis (Moz et al. 2020; Riachy et al. 2002) and plays a pivotal role in controlling calcium hemostasis by increasing the expression of plasma calcium ATPase (Kip and Strehler 2004), NCX1, and calbindin (Ko et al. 2009; Pu et al. 2016) and decreasing expression of L-type calcium channels (Brewer et al. 2001). Together with its antioxidant effects (Wu et al. 2021), it regulates histone demethylase genes which control hypermethylation of promoter regions of many genes (Pereira et al. 2012; Yu et al. 2018).
Exposure to environmental contaminants, like pesticides, has hazardous effects. Organochlorine pesticides were introduced in the market and were extensively used around 80 years ago and were banned in the 1970s in many countries after many reports about their toxic effects (Blus 2002). A negative correlation existed between the serum concentration of organochlorine pesticides and vitamin D levels in a cross-sectional study that included 1275 participants (Yang et al. 2012). This toxic effect might be attributed to the endocrinal disruptor effects of these pesticides (Lee et al. 2010). Furthermore, the organochlorine pesticides interfere with vitamin D activity, its medicated intestinal absorption, intestinal alkaline phosphatase activity, and bone resorption (Nowicki et al. 1972).
Later, organophosphate pesticides were introduced in the market and as replacers of the banned organochloride ones (Costa 1987). In spite of the fact that many of scientific publications show their neurotoxic actions, they represent one of the most commonly used pesticides worldwide. Their neurotoxic actions could be attributed to inhibition of cholinesterase enzyme, mitochondrial dysfunction, oxidative stress, and inducing neuroinflammation (Jokanović 2018; Sakata 2005; Salama et al. 2014). To the extent of our knowledge, limited data exist regarding the effects of organophosphate pesticides on vitamin D. Chlorpyrifos is one of the most commonly used organophosphate pesticides. It increases the expression of VDR at the level of the skin which could indicate interference with vitamin D metabolism (Sawicki et al. 2019). Furthermore, vitamin D antagonized the chlorpyrifos-induced toxicity at retinal and renal levels of Wistar rats (El-Hossary et al. 2009).
12.4 Potential Association of Environmental Factors and PD, the Egyptian Experience
The occurrence of PD in some agricultural countries such as Egypt is higher than industrialized countries. Among 100,000 individuals, around 2748 individuals aged 50 years old or older and 7263 individuals aged between 70 and 79 years old are diagnosed with PD in Egypt (Khedr et al. 2012).
Only 5–15% of PD could be attributed to well-identified genetic mutation, while majority of PD patients are classified as idiopathic cases, postulated to be associated with exposure to environmental contaminants (e.g., pesticides, unhealthy lifestyle, and nutritional habits leading to micronutrients deficiency and central obesity) (Ball et al. 2019; Chen et al. 2004; Guo et al. 2019; Sherzai et al. 2016). These modifiable risk factors among the Egyptian population will be discussed in more details in the next section.
The health effects of exposure to pesticides among the Egyptian population have been fully reviewed by Mansour (2004 and 2008). While pesticides play a crucial role in increasing crop productivity, exposure to many pesticides can be associated with many health hazards, including metabolic and neurodegenerative disorders. Egypt was ranked as the fifth highest consumer of pesticides in Africa. Even though organochlorine pesticides like dichlorodiphenyltrichloroethane (DDT) have been banned in the 1970s, they are still detected in water and food. Furthermore, organophosphate pesticides, like chlorpyrifos and malathion, are one of the most widely used pesticides in Egypt. The problem of exposure to pesticides in Egypt is not only related to the amount of used pesticides but also to the lack of using protective equipment during mixing and application. We previously reported association between exposure to pesticides and risk of PD among the Egyptian population (OR: 7.09, 95% CI: 1.12–44.01). This risk is altered by polymorphism of the butyrylcholinesterase gene, which is responsible for metabolizing the organophosphate pesticides (Rösler et al. 2018).
DM increases the risk of developing PD, as was discussed in the previous section. Egypt is located in the red zone of DM, where more than half of diabetic patients reside (Al-Rubeaan 2010). The International Diabetes Federation reported that around 15.56% of Egyptian aged between 20 and 79 in 2011 were diabetic. In 2007, around 4.4 million Egyptian were diabetic, and the number increased to 7.5 and 8.9 million in 2013 and 2019, respectively. Furthermore, the number of diabetic patients in Egypt is expected to reach 11.9 and 16.9 million by 2030 and 2045, respectively (Aguiree et al. 2013; Karuranga et al. 2019; Whiting et al. 2011). We discussed the potential risk factors in our previous work, and this high prevalence of DM among the Egyptian population could be explained by unhealthy nutritional habits and lack of physical exercise which are one of the leading factors of the high prevalence of overweight and obesity among the Egyptian (Hegazi et al. 2015), as 50% and (65–80%) of Egyptian men and women, respectively, are overweight and obese (El-Zanaty and Way 2009). Furthermore, Sharara et al. (2018) show in their meta-analysis that the prevalence of physical inactivity among the Egyptian population is between 32% and 91%. Different types of exercise could offer neuroprotective effect and reduce PD progression at motor and non-motor levels. These protective effects could be attributed to the fact that exercise improves the mitochondrial function, antagonizes oxidative stress, enhances growth factor formation, positively affects neurogenesis and plasticity, and decreases the other modifiable risk factors like DM and cardiovascular diseases, as reviewed in detail by Feng et al. (2020) and Xu et al. (2019). Other potential risk factors for DM in Egypt are exposure to pesticides and the high prevalence of hepatitis C infection (Hegazi et al. 2015).
Regarding the status of vitamin D in Egypt, some of trails were conducted to assess the degrees of vitamin D insufficiency and deficiency among the Egyptian populations; however, small sample size was obvious among the majority of these studies. The prevalence of vitamin D insufficiency and deficiency among 200 Egyptian school students aged between 9 and 11 years old was 15 and 11.5%, respectively. Obesity and lack of adequate milk intake, sun exposure, and physical activity are linked to low vitamin D levels (Abu Shady et al. 2016). In one study, 24 and 21.3% of 75 adolescent Egyptian girls aged between 14 and 17 years old have insufficient and deficient vitamin D, respectively. This was associated with lack of sun exposure (Amr et al. 2012). Furthermore, El Badawy et al. (2015) showed that 18.5 and 5.3% of 500 Egyptian students between 13 and 18 years of age have insufficient and deficient vitamin D. Botros et al. (2015) reported in their cross-sectional study which included 404 Egyptian females that 77.2, 72.6, 72, and 39.5% of the geriatric, nursing, childbearing, and elderly females are suffering from vitamin D deficiency. Gerges et al. (2021) reported that 13 and 43% of 100 Egyptian females in the childbearing period have insufficient and deficient vitamin D levels. Another cross-sectional study assessed vitamin D levels among 135 pregnant Egyptian women and their neonates. Maternal levels of vitamin D were correlated to the levels in their neonates, as vitamin D insufficiency and deficiency were reported among 28.9 and 40% of the pregnant women and 32.6 and 60% of their neonates. Increased BMI and lack of fish intake and sun exposure were associated with low maternal vitamin D levels (El Rifai et al. 2014). As it was discussed in the previous section, vitamin D deficiency increases the risk of insulin resistance and in turn DM. The prevalence of vitamin D deficiency among 60 Egyptian patients with DM was reported by Abdelsadek et al. (2018) as 73.3% compared to 35% among 30 control individuals. Consistently, 450 obese Egyptian women aged between 25 and 35 years old were categorized according to the existence of vitamin D deficiency into two groups. Metabolic changes in the form of higher blood pressure, dyslipidemia, and insulin resistance with evidence of higher fasting blood glucose, and higher levels of inflammatory markers, C reactive protein, and interleukin 6, were obvious among vitamin D-deficient group. VDR gene polymorphisms alter the body response, by which women having ApaI (Aa, aa), FokI (Ff, ff), and for TaqI (Tt, tt) alleles have lower vitamin D levels, higher inflammatory markers, and more insulin resistance than those having common homozygous alleles ApaI (AA), FokI (FF), and TaqI (TT; Zaki et al. 2017).
12.5 Vitamin D as a Potential Therapy for PD
12.5.1 Experimental Studies
Experimental Parkinson’s disease is modeled by different regimens of administration of toxins like 6-OHDA, MPTP, and rotenone, as reviewed recently by El-Gamal et al. (2021). Previous in vitro and in vivo studies reported protective effects of vitamin D against these toxins. Jang et al. (2014) show a neuroprotectant effect of vitamin D against rotenone-induced toxicity (reduced cell viability and oxidative stress) in SH-SY5Y cells, as in vitro model of PD, through activating the autophagy degradation pathway. Furthermore, Wang et al. (2001) reported the neuroprotective effect of vitamin D at both in vitro and in vivo levels. Daily administration of vitamin D to Sprague-Dawley rats for 8 days before stereotactic injection of 6-OHDA into the medial forebrain bundle improves the locomotor impairment and increase the dopamine and its metabolites in the substantia nigra. Coherently, vitamin D antagonized 6-OHDA-induced cell death of primary ventral mesencephalic culture. The protective effect of vitamin D in the 6-OHDA mouse model was further confirmed by Kim et al. (2020), as vitamin D administration antagonizes 6-OHDA dopaminergic neurodegeneration and neuroinflammation in substantia nigra. This beneficial effect could be explained that vitamin D reversed the 6-OHDA-induced reduction in the brain endothelial P-glycoprotein level and expression of VDR and its target genes MDR1a and CYP24. Similar results obtained among the 6-OHDA rat model of PD, as vitamin D, improves the locomotor impairment and dopamine content in the corpus striatum by antagonizing the inflammatory and oxidative stress processes (Lima et al. 2018). Together with that, vitamin D exerted a neuroprotective effect against MPTP-induced nigrostriatal neurodegeneration through reducing microglial activation, TLR4 receptor expression, pro-inflammatory cytokines expression, and increase expression of anti-inflammatory cytokines (IL-4, IL-10, and TGF-β) and CD (163, 204, and 206; Calvello et al. 2017).
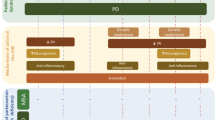
Other potential mechanisms for the beneficial effects of vitamin D can be due to the fact that vitamin D plays a crucial role in proliferation and differentiation of neural stem cells (Shirazi et al. 2015) and increases the expression of tyrosine hydroxylase, the rate-limiting enzyme for DA synthesis (Cui et al. 2015). Furthermore, its deficiency is associated with impairment on the ontogeny of DA neurons in the developing brain (Cui et al. 2010). The potential molecular and environmental factors that could explain association of vitamin D and PD were illustrated in Fig. 12.1.
12.5.2 Clinical Studies
Furthermore, few clinical trials were conducted to assess the potential therapeutic role of vitamin D. Daily administration of vitamin D (10 000 IU) for 16 weeks in PD patients did not improve balance. However, a significant effect of vitamin D was reported in younger PD patients (aged 52 to 66 years old) but not the older PD patients (Hiller et al. 2018). Daily vitamin D supplementation (1200 IU) to PD patients for 1 year seems to be helpful in reducing the progression of the disease. However, this beneficial effect is variable according to different VDR Fokl genotype, as this effect was significant with Fokl CT and TT but not CC genotype (Suzuki et al. 2013). Zhou et al. (2019) demonstrated in their recent systematic review and meta-analysis that included eight studies that both vitamin D insufficiency and deficiency significantly increase the risk of PD (OR, 1.77; 95% CI, 1.29–2.43) and (OR, 2.55; 95% CI, 1.98–3.27), respectively. Exposure to sun for at least 15 minutes per week significantly reduces PD risk (OR, 0.02; 95% CI, 0.00–0.10). In spite of the fact that both vitamin D insufficiency and deficiency significantly increase the PD risk, vitamin D supplement did not significantly reduce motor impairment associated with PD.
With the declaration of the COVID-19 pandemic in the first half of 2020, it was suggested that COVID-19 can induce neurodegeneration and worsen PD manifestations. It has also been postulated that vitamin D administration could have a beneficial therapeutic effect (de Barros Viana et al. 2021). Hribar et al. (2020) concluded in their recent review that vitamin D supplements could have beneficial effects on reducing PD progression and both the risk and severity of COVID-19 among PD patients. Interestingly, a recent review of literature suggested that the association of low level of vitamin D among PD patients and severity of the PD motor symptoms could be attributed to limited mobility and lack of exposure to the sun as the disease progresses (Fullard and Duda 2020).
12.6 Conclusion
Like other noncommunicable chronic diseases, PD has complex etiology and risk factors. The current review suggests that vitamin D deficiency is one of these risk factors. This interesting association could be related to the pleiotropic effects of vitamin D especially its anti-inflammatory and antioxidant properties. Further research is warranted to better understand this association and may open the door for potential utilizing vitamin D as one of therapeutic modalities and preventive strategies of PD.
References
Abdelsadek SE, El Saghier EO, Abdel Raheem SI (2018) Serum 25(OH) vitamin D level and its relation to diabetic peripheral neuropathy in Egyptian patients with type 2 diabetes mellitus. Egypt J Neurol Psychiatry Neurosurg 54(1):36. https://doi.org/10.1186/s41983-018-0036-9
Abu Shady MM, Youssef MM, Salah El-Din EM, Abdel Samie OM, Megahed HS, Salem SME, Mohsen MA, Abdel Aziz A, El-Toukhy S (2016) Predictors of serum 25-Hydroxyvitamin D concentrations among a sample of Egyptian schoolchildren. Sci World J 2016. https://doi.org/10.1155/2016/8175768
Aguiree F, Brown A, Cho NH, Dahlquist G, Dodd S, Dunning T, Hirst M, Hwang C, Magliano D, Patterson C, Scott C, Shaw J, Soltesz G, Usher-Smith J, Whiting D (2013) IDF diabetes atlas, 6th edn
Al-Rubeaan K (2010) Type 2 diabetes mellitus red zone. Int J Diabetes Mellitus 2(1):1–2. https://doi.org/10.1016/j.ijdm.2009.12.009
Amr N, Hamid A, Sheta M, Elsedfy H (2012) Vitamin D status in healthy Egyptian adolescent girls. Georgian Med News 210:65–71. https://europepmc.org/article/med/23045423
Ashcroft SP, Fletcher G, Philp AM, Jenkinson C, Das S, Hansbro PM, Atherton PJ, Philp A (2021) Diet-induced vitamin D deficiency reduces skeletal muscle mitochondrial respiration. J Endocrinol 249(2):113–124. https://doi.org/10.1530/JOE-20-0233
Bach J-P, Ziegler U, Deuschl G, Dodel R, Doblhammer-Reiter G (2011) Projected numbers of people with movement disorders in the years 2030 and 2050. Mov Disord 26(12):2286–2290. https://doi.org/10.1002/mds.23878
Ball N, Teo W-P, Chandra S, Chapman J (2019) Parkinson’s disease and the environment. Front Neurol 10:218. https://doi.org/10.3389/fneur.2019.00218
Bartels LE, Hvas CL, Agnholt J, Dahlerup JF, Agger R (2010) Human dendritic cell antigen presentation and chemotaxis are inhibited by intrinsic 25-hydroxy vitamin D activation. Int Immunopharmacol 10(8):922–928. https://doi.org/10.1016/j.intimp.2010.05.003
Berridge MJ (2017) Vitamin D deficiency and diabetes. Biochem J 474(8):1321–1332. https://doi.org/10.1042/BCJ20170042
Blus LJ (2002) Organochlorine pesticides. In: Handbook of ecotoxicology, 2nd edn. CRC Press, pp 313–339. https://doi.org/10.1201/9781351069656-1
Botros RM, Sabry IM, Abdelbaky RS, Eid YM, Nasr MS, Hendawy LM (2015) Vitamin D deficiency among healthy Egyptian females. Endocrinol Nutr 62(7):314–321. https://doi.org/10.1016/j.endonu.2015.03.010
Bovolenta TM, De Azevedo Silva SMC, Arb Saba R, Borges V, Ferraz HB, Felicio AC (2017) Systematic review and critical analysis of cost studies associated with Parkinson’s disease. Parkinson’s Dis 2017. https://doi.org/10.1155/2017/3410946
Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM (2001) Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 21(1):98–108. https://doi.org/10.1523/jneurosci.21-01-00098.2001
Brockmann K, Apel A, Schulte C, Schneiderhan-Marra N, Pont-Sunyer C, Vilas D, Ruiz-Martinez J, Langkamp M, Corvol JC, Cormier F, Knorpp T, Joos TO, Gasser T, Schüle B, Aasly JO, Foroud T, Marti-Masso JF, Brice A, Tolosa E et al (2016) Inflammatory profile in LRRK2-associated prodromal and clinical PD. J Neuroinflammation 13(1). https://doi.org/10.1186/s12974-016-0588-5
Butler MW, Burt A, Edwards TL, Zuchner S, Scott WK, Martin ER, Vance JM, Wang L (2011) Vitamin D receptor gene as a candidate gene for Parkinson disease. Ann Hum Genet 75(2):201–210. https://doi.org/10.1111/j.1469-1809.2010.00631.x
Calton EK, Keane KN, Newsholme P, Soares MJ (2015) The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One 10(11):e0141770. https://doi.org/10.1371/journal.pone.0141770
Calvello R, Cianciulli A, Nicolardi G, De Nuccio F, Giannotti L, Salvatore R, Porro C, Trotta T, Panaro MA, Lofrumento DD (2017) Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, shifting M1 to M2 microglia responses. J Neuroimmune Pharmacol 12(2):327–339. https://doi.org/10.1007/s11481-016-9720-7
Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Willett WC, Ascherio A (2004) Obesity and the risk of Parkinson’s disease. Am J Epidemiol 159(6):547–555. https://doi.org/10.1093/aje/kwh059
Costa LG (1987) Toxicology of pesticides: a brief history. In: Costa LG, Galli CL, Murphy SD (eds) Toxicology of pesticides. Springer, Berlin Heidelberg, pp 1–10
Cui X, Pertile R, Liu P, Eyles DW (2015) Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience 304:90–100. https://doi.org/10.1016/j.neuroscience.2015.07.048
Cui X, Pelekanos M, Burne THJ, McGrath JJ, Eyles DW (2010) Maternal vitamin D deficiency alters the expression of genes involved in dopamine specification in the developing rat mesencephalon. Neurosci Lett 486(3):220–223. https://doi.org/10.1016/j.neulet.2010.09.057
de Barros Viana M, Rosário BDA, de Fátima Santana de Nazaré M, Estadella D, Ribeiro DA, Socorro de Barros Viana G (2021) COVID-19 in age-related neurodegenerative diseases: is there a role for vitamin D3 as a possible therapeutic strategy? Rev Neurosci 32(2):235–247. https://doi.org/10.1515/revneuro-2020-0074
de Luca C, Olefsky JM (2008) Inflammation and insulin resistance. FEBS Lett 582(1):97–105. https://doi.org/10.1016/j.febslet.2007.11.057
Dowding CH, Shenton CL, Salek SS (2006) A review of the health-related quality of life and economic impact of Parkinson’s disease. Drugs Aging 23(9):693–721. https://doi.org/10.2165/00002512-200623090-00001
Dursun E, Gezen-AK D, Yilmazer S (2014) Vitamin D Uygulamasının Primer Hippokampal Nöronlardaki İndüklenebilir Nitrik Oksit Sentaz (İNOS) Anlatımı Üzerine Etkileri. Nöro Psikiyatri Arşivi 51(2):163–168. https://doi.org/10.4274/npa.y7089
El-Gamal M, Salama M, Collins-Praino LE, Baetu I, Fathalla AM, Soliman AM, Mohamed W, Moustafa AA (2021) Neurotoxin-induced rodent models of Parkinson’s disease: benefits and drawbacks. Neurotox Res:0123456789. https://doi.org/10.1007/s12640-021-00356-8
El-Hossary GG, El-Gohary AA, Ahmed NS, Mohamed AS, Mansour SM (2009) Amelioration of chlorpyrifos induced retinal and renal toxicity by vitamin D3. Aust J Basic Appl Sci 3(3):2304–2314. https://www.researchgate.net/publication/288452421_Amelioration_of_Chlorpyrifos_Induced_Retinal_and_Renal_Toxicity_by_Vitamin_D
El-Zanaty F, Way A (2009) Egypt demographic and health survey 2008. https://www.unicef.org/egypt/reports/egypt-demographic-and-health-survey-2014
El Badawy AA, Aboserea MM, El Seifi OS, Mortada EM, Bakry HM, Waly EH, Raafat N, Etewa RL, El Badawy SA (2015) Vitamin D, parathormone and associated minerals among students in Zagazig district, Sharkia governorate, Egypt. Int J Vitam Nutr Res 84(3–4):173–182. https://doi.org/10.1024/0300-9831/a000204
El Rifai NM, Abdel Moety GAF, Gaafar HM, Hamed DA (2014) Vitamin D deficiency in Egyptian mothers and their neonates and possible related factors. J Matern Fetal Neonatal Med 27(10):1064–1068. https://doi.org/10.3109/14767058.2013.849240
Feng YS, Yang SD, Tan ZX, Wang MM, Xing Y, Dong F, Zhang F (2020) The benefits and mechanisms of exercise training for Parkinson’s disease. In: Life sciences, vol 245. Elsevier. https://doi.org/10.1016/j.lfs.2020.117345
Fullard ME, Duda JE (2020) A review of the relationship between vitamin D and Parkinson disease symptoms. In: Frontiers in neurology, vol 11. Frontiers Media S.A., p 454. https://doi.org/10.3389/fneur.2020.00454
Gao L, Tao Y, Zhang L, Jin Q (2010) Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis 14(1):15–23
Gerges MES, Amin GEA, Andraous F, Abdel Hamid DM, Allam MF (2021) Vitamin D level in a sample of Egyptian females of childbearing age attending a family medicine center. Int J Clin Pract 75(4):e13738. https://doi.org/10.1111/ijcp.13738
Gozzelino R, Jeney V, Soares MP (2010) Mechanisms of cell protection by heme Oxygenase-1. In: Annual review of pharmacology and toxicology, vol 50, pp 323–354. https://doi.org/10.1146/annurev.pharmtox.010909.105600
Guo Y, Xu W, Liu FT, Li JQ, Cao XP, Tan L, Wang J, Yu JT (2019) Modifiable risk factors for cognitive impairment in Parkinson’s disease: a systematic review and meta-analysis of prospective cohort studies. Mov Disord 34(6):876–883. https://doi.org/10.1002/mds.27665
Haidari F, Zakerkish M, Karandish M, Saki A, Pooraziz S (2016) Association between serum vitamin D level and glycemic and inflammatory markers in non-obese patients with type 2 diabetes. Iran J Med Sci 41(5):367–373
Han X, Xue L, Li Y, Chen B, Xie A (2012) Vitamin D receptor gene polymorphism and its association with Parkinson’s disease in Chinese Han population. Neurosci Lett 525(1):29–33. https://doi.org/10.1016/j.neulet.2012.07.033
Hassan A, Sharma Kandel R, Mishra R, Gautam J, Alaref A, Jahan N (2020) Diabetes mellitus and Parkinson’s disease: shared pathophysiological links and possible therapeutic implications. Cureus 12(8). https://doi.org/10.7759/cureus.9853
He J, Guo X, Liu ZQ, Yang PC, Yang S (2017) Vitamin D inhibits the staphylococcal enterotoxin B-induced expression of tumor necrosis factor in microglial cells. Immunol Res 65(4):913–919. https://doi.org/10.1007/s12026-017-8930-2
Hegazi RAF, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE (2005) Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med 202(12). https://doi.org/10.1084/jem.20051047
Hegazi R, El-Gamal M, Abdel-Hady N, Hamdy O (2015) Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann Glob Health 81(6):814–820. https://doi.org/10.1016/j.aogh.2015.12.011
Heilbronn L, Campbell L (2008) Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des 14(12):1225–1230. https://doi.org/10.2174/138161208784246153
Hiller AL, Murchison CF, Lobb BM, O’Connor S, O’Connor M, Quinn JF (2018) A randomized, controlled pilot study of the effects of vitamin D supplementation on balance in Parkinson’s disease: does age matter? PLoS One 13(9). https://doi.org/10.1371/journal.pone.0203637
Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R (2007) How common are the & quot; common& quot; neurologic disorders? Neurology 68(5):326–337. https://doi.org/10.1212/01.wnl.0000252807.38124.a3
Hribar CA, Cobbold PH, Church FC (2020) Potential role of vitamin d in the elderly to resist covid-19 and to slow progression of Parkinson’s disease. Brain Sci 10(5):6–13. https://doi.org/10.3390/brainsci10050284
Jang W, Kim HJ, Li H, Jo KD, Lee MK, Song SH, Yang HO (2014) 1, 25-Dyhydroxyvitamin D3 attenuates rotenone-induced neurotoxicity in SH-SY5Y cells through induction of autophagy. Biochem Biophys Res Commun 451(1):142–147. https://doi.org/10.1016/j.bbrc.2014.07.081
Johnson SJ, Diener MD, Kaltenboeck A, Birnbaum HG, Siderowf AD (2013) An economic model of Parkinson’s disease: implications for slowing progression in the United States. Mov Disord 28(3):319–326. https://doi.org/10.1002/mds.25328
Jokanović M (2018) Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: a review. Toxicology 410(December):125–131. https://doi.org/10.1016/j.tox.2018.09.009
Karau PB, Kirna B, Amayo E, Joshi M, Ngare S, Muriira G (2019) The prevalence of vitamin D deficiency among patients with type 2 diabetes seen at a referral hospital in Kenya. Pan Afr Med J 34:1–11. https://doi.org/10.11604/pamj.2019.34.38.18936
Karuranga S, Malanda B, Saeedi P, Salpea P (2019) IDF atlas 9th edition and other resources. In: International diabetes federation, 9th edn https://www.diabetesatlas.org/en/resources/
Khedr EM, Al Attar GS, Kandil MR, Kamel NF, Abo Elfetoh N, Ahmed MA (2012) Epidemiological study and clinical profile of Parkinson’s disease in the Assiut governorate, Egypt: a community-based study. Neuroepidemiology 38(3):154–163. https://doi.org/10.1159/000335701
Kim H, Shin JY, Lee YS, Yun SP, Maeng HJ, Lee Y (2020) Brain endothelial p-glycoprotein level is reduced in Parkinson’s disease via a vitamin d receptor-dependent pathway. Int J Mol Sci 21(22):1–15. https://doi.org/10.3390/ijms21228538
Kip SN, Strehler EE (2004) Vitamin D3 upregulates plasma membrane Ca2+-ATPase expression and potentiates apico-basal Ca2+ flux in MDCK cells. Am J Physiol Renal Physiol 286(2):363–369. https://doi.org/10.1152/ajprenal.00076.2003
Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M (2010) Serum vitamin D and the risk of Parkinson disease. Arch Neurol 67(7):808–811. https://doi.org/10.1001/archneurol.2010.120
Ko S-H, Lee G-S, Vo TTB, Jung E-M, Choi K-C, Cheung K-W, Kim JW, Park J-G, Oh GT, Jeung E-B (2009) Dietary calcium and 1, 25-dihydroxyvitamin D3 regulate transcription of calcium transporter genes in Calbindin-D9k knockout mice. J Reprod Dev 55(2):137–142. https://doi.org/10.1262/jrd.20139
Koszowska AU, Nowak J, Dittfeld A, Brończyk-Puzoń A, Kulpok A, Zubelewicz-Szkodzińska B (2014) Obesity, adipose tissue function and the role of vitamin D. Central Eur J Immunol 39(2):260–264. https://doi.org/10.5114/ceji.2014.43732
Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A (2013) The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord 28(3):311–318. https://doi.org/10.1002/mds.25292
Kuno R, Wang J, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A (2005) Autocrine activation of microglia by tumor necrosis factor-α. J Neuroimmunol 162(1–2):89–96. https://doi.org/10.1016/j.jneuroim.2005.01.015
Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR (2010) Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect 118(9):1235–1242. https://doi.org/10.1289/ehp.0901480
Lima LAR, Lopes MJP, Costa RO, Lima FAV, Neves KRT, Calou IBF, Andrade GM, Viana GSB (2018) Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J Neuroinflammation 15(1):1–11. https://doi.org/10.1186/s12974-018-1266-6
Lin CH, Chen KH, Chen ML, Lin HI, Wu RM (2014) Vitamin D receptor genetic variants and Parkinson’s disease in a Taiwanese population. Neurobiol Aging 35(5):1212.e11–1212.e13. https://doi.org/10.1016/j.neurobiolaging.2013.10.094
Luo X, Ou R, Dutta R, Tian Y, Xiong H, Shang H (2018) Association between serum Vitamin D levels and Parkinson’s disease: a systematic review and meta-analysis. Front Neurol 9(Nov):1–10. https://doi.org/10.3389/fneur.2018.00909
Luo X, Lee Kraus W (2012) On par with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev 26(5):417–432. https://doi.org/10.1101/gad.183509.111
Lương K, Nguyễn L (2012) Role of vitamin D in Parkinson’s disease. ISRN Neurol 2012:1–11. https://doi.org/10.5402/2012/134289
Lv Z, Qi H, Wang L, Fan X, Han F, Wang H, Bi S (2014) Vitamin D status and Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci 35(11):1723–1730. https://doi.org/10.1007/s10072-014-1821-6
Mansour SA (2008) Environmental impact of pesticides in Egypt. Rev Environ Contam Toxicol 196:1–51. https://doi.org/10.1007/978-0-387-78444-1_1
Mansour S a (2004) Pesticide exposure--Egyptian scene. Toxicology 198(1–3):91–115. https://doi.org/10.1016/j.tox.2004.01.036
Martinez-Martin P, Macaulay D, Jalundhwala YJ, Mu F, Ohashi E, Marshall T, Sail K (2019) The long-term direct and indirect economic burden among Parkinson’s disease caregivers in the United States. Mov Disord 34(2):236–245. https://doi.org/10.1002/mds.27579
Moz S, Contran N, Facco M, Trimarco V, Plebani M, Basso D (2020) Vitamin D prevents pancreatic cancer-induced apoptosis signaling of inflammatory cells. Biomol Ther 10(7):1–16. https://doi.org/10.3390/biom10071055
Nagatsu T, Sawada M (2005) Inflammatory process in Parkinsons disease: role for cytokines. Curr Pharm Des 11(8):999–1016. https://doi.org/10.2174/1381612053381620
Nowicki HG, Wong RG, Myrtle JF, Norman AW (1972) Inhibition of biological activity of cholecalciferol (vitamin D3) by o, p’-DDT or, p, p’-DDT in rachitic cockerel. J Agric Food Chem 20(2):376–380. https://doi.org/10.1021/jf60180a058
Oertel WH (2017) Recent advances in treating Parkinson’s disease. F1000Research 6:260. https://doi.org/10.12688/f1000research.10100.1
Pereira F, Barbáchano A, Singh PK, Campbell MJ, Muñoz A, Larriba MJ (2012) Vitamin D has wide regulatory effects on histone demethylase genes. Cell Cycle 11(6):1081–1089. https://doi.org/10.4161/cc.11.6.19508
Peritore CS, Ho A, Yamamoto BK, Schaus SE (2012) Resveratrol attenuates L-DOPA-induced hydrogen peroxide toxicity in neuronal cells. Neuro Report 23(17):989–994. https://doi.org/10.1097/WNR.0b013e32835a4ea4
Phillips JR, Eissa AM, Hewedi DH, Jahanshahi M, El-Gamal M, Keri S, Moustafa AA (2016) Neural substrates and potential treatments for levodopa-induced dyskinesias in Parkinson’s disease. Rev Neurosci. https://doi.org/10.1515/revneuro-2016-0009
Pu F, Chen N, Xue S (2016) Calcium intake, calcium homeostasis and health. Food Sci Human Wellness 5(1):8–16. https://doi.org/10.1016/j.fshw.2016.01.001
Reusch JEB (2002) Current concepts in insulin resistance, type 2 diabetes mellitus, and the metabolic syndrome. Am J Cardiol 90(5 SUPPL):19–26. https://doi.org/10.1016/S0002-9149(02)02555-9
Riachy R, Vandewalle B, Conte JK, Moerman E, Sacchetti P, Lukowiak B, Gmyr V, Bouckenooghe T, Dubois M, Pattou F (2002) 1, 25-dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: implication of the antiapoptotic protein A20. Endocrinology 143(12):4809–4819. https://doi.org/10.1210/en.2002-220449
Rimmelzwaan LM, Van Schoor NM, Lips P, Berendse HW, Eekhoff EMW (2016) Systematic review of the relationship between vitamin D and Parkinson’s disease. J Parkinsons Dis 6(1):29–37). IOS press. https://doi.org/10.3233/JPD-150615
Rösler TW, Salama M, Shalash AS, Khedr EM, El-Tantawy A, Fawi G, El-Motayam A, El-Seidy E, El-Sherif M, El-Gamal M, Moharram M, El-Kattan M, Abdel-Naby M, Ashour S, Müller U, Dempfle A, Kuhlenbäumer G, Höglinger GU (2018) K-variant BCHE and pesticide exposure: gene-environment interactions in a case–control study of Parkinson’s disease in Egypt. Sci Rep 8(1):16525. https://doi.org/10.1038/s41598-018-35003-4
Rossi A, Berger K, Chen H, Leslie D, Mailman RB, Huang X (2018) Projection of the prevalence of Parkinson’s disease in the coming decades: revisited. Mov Disord 33(1):156–159. https://doi.org/10.1002/mds.27063
Sakata M (2005) II.7.2 Organophosphorus pesticides, pp 534–544. http://eknygos.lsmuni.lt/springer/124/535-544.pdf
Salama M, El-Morsy D, El-Gamal M, Shabka O, Mohamed WMY (2014) Mitochondrial complex I inhibition as a possible mechanism of chlorpyrifos induced neurotoxicity. Ann Neurosci 21(3):85–89. https://doi.org/10.5214/ans.0972.7531.210303
Sawicki K, Czajka M, Matysiak-Kucharek M, Kruszewski M, Skawiński W, Brzóska K, Kapka-Skrzypczak L (2019) Chlorpyrifos stimulates expression of vitamin D 3 receptor in skin cells irradiated with UVB. Pestic Biochem Physiol 154:17–22. https://doi.org/10.1016/j.pestbp.2018.12.003
Sharara E, Akik C, Ghattas H, Makhlouf Obermeyer C (2018) Physical inactivity, gender and culture in Arab countries: a systematic assessment of the literature. BMC Public Health 18(1):1–19. https://doi.org/10.1186/s12889-018-5472-z
Shen L, Ji HF (2015) Associations between vitamin D status, supplementation, outdoor work and risk of Parkinson’s disease: a meta-analysis assessment. Nutrients 7(6):4817–4827. https://doi.org/10.3390/nu7064817
Sherzai AZ, Tagliati M, Park K, Pezeshkian S, Sherzai D (2016) Micronutrients and risk of Parkinson’s disease. Gerontol Geriatr Med 2. https://doi.org/10.1177/2333721416644286
Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang G-X (2015) 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol 98(2):240–245. https://doi.org/10.1016/j.yexmp.2015.02.004
Shrestha S, Lutsey PL, Alonso A, Huang X, Mosley TH, Chen H (2016) Serum 25-hydroxyvitamin D concentrations in mid-adulthood and Parkinson’s disease risk. Mov Disord 31(7):972–978. https://doi.org/10.1002/mds.26573
Siekevitz P (1957) Powerhouse of the cell. Sci Am 197(1):131–144. https://doi.org/10.1038/scientificamerican0757-131
Smith MP, Fletcher-Turner A, Yurek DM, Cass WA (2006) Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res 31(4):533–539. https://doi.org/10.1007/s11064-006-9048-4
Stevens SL (2021) Fat-soluble vitamins. Nurs Clin N Am 56(1):33–45. https://doi.org/10.1016/j.cnur.2020.10.003
Suzuki M, Yoshioka M, Hashimoto M, Murakami M, Noya M, Takahashi D (2013) Randomized, double-blind, placebo-controlled trial of vitamin D. Am J Clin Nutr 97(3):1004–1013. https://doi.org/10.1007/s11481-016-9720-7
Szymczak-Pajor I, Śliwińska A (2019) Analysis of association between vitamin d deficiency and insulin resistance. Nutrients 11(4):794. https://doi.org/10.3390/nu11040794
Taylor SI, Accili D, Imai Y (1994) Insulin resistance or insulin deficiency: which is the primary cause of NIDDM? Diabetes 43(6):735–740. https://doi.org/10.2337/diab.43.6.735
Török R, Török N, Szalardy L, Plangar I, Szolnoki Z, Somogyvari F, Vecsei L, Klivenyi P (2013) Association of vitamin D receptor gene polymorphisms and Parkinson’s disease in Hungarians. Neurosci Lett 551:70–74. https://doi.org/10.1016/j.neulet.2013.07.014
Toulouse A, Sullivan AM (2008) Progress in Parkinson’s disease—where do we stand? Prog Neurobiol 85(4):376–392. https://doi.org/10.1016/J.PNEUROBIO.2008.05.003
Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV, Wang Y (2001) Vitamin D3 attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res 904(1):67–75. https://doi.org/10.1016/S0006-8993(01)02450-7
Whiting DR, Guariguata L, Weil C, Shaw J (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94(3):311–321. https://doi.org/10.1016/j.diabres.2011.10.029
Wu M, Wu Y, Xu K, Lin L (2021) Protective effects of 1,25 dihydroxyvitamin D3 against high-glucose-induced damage in human umbilical vein endothelial cells involve activation of Nrf2 antioxidant signaling. J Vasc Res:1–10. https://doi.org/10.1159/000515512
Xu X, Fu Z, Le W (2019) Exercise and Parkinson’s disease. Int Rev Neurobiol 147:45–74. https://doi.org/10.1016/bs.irn.2019.06.003
Yan J, Fu Q, Cheng L, Zhai M, Wu W, Huang L, Du G (2014) Inflammatory response in Parkinson’s disease (review). Mol Med Rep 10(5):2223–2233. https://doi.org/10.3892/mmr.2014.2563
Yang JH, Lee YM, Bae SG, Jacobs DR, Lee DH (2012) Associations between organochlorine pesticides and vitamin d deficiency in the U.S. population. PLoS One 7(1):e30093. https://doi.org/10.1371/journal.pone.0030093
Yao L, Dai X, Sun Y, Wang Y, Yang Q, Chen X, Liu Y, Zhang L, Xie W, Liu J (2018) Inhibition of transcription factor SP1 produces neuroprotective effects through decreasing MAO B activity in MPTP/MPP+ Parkinson’s disease models. J Neurosci Res 96(10):1663–1676. https://doi.org/10.1002/jnr.24266
Yu S, Wang Y, Li X, Mao Z, Yu F, Wang L, Ba Y, Wang C, Li W (2018) Methylation in 3′ near region of GC gene and its association with the level of vitamin D binding protein and type 2 diabetes mellitus. Nutr Res 54:52–59. https://doi.org/10.1016/j.nutres.2018.03.016
Zaki M, Kamal S, Basha WA, Youness E, Ezzat W, El-Bassyouni H, Amr K (2017) Association of vitamin D receptor gene polymorphism (VDR) with vitamin D deficiency, metabolic and inflammatory markers in Egyptian obese women. Genes Dis 4(3):176–182. https://doi.org/10.1016/j.gendis.2017.07.002
Zhou Z, Zhou R, Zhang Z, Li K (2019) The association between vitamin D status, vitamin D supplementation, sunlight exposure, and parkinson’s disease: a systematic review and meta-analysis. Med Sci Monit 25:666–674. https://doi.org/10.12659/MSM.912840
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
El-Gamal, M., Azar, J., Hegazi, R. (2022). The Emerging Role of Vitamin D Deficiency as a Risk Factor of Parkinson’s Disease. In: Salama, M. (eds) Nutrigenomics and the Brain. Nutritional Neurosciences. Springer, Singapore. https://doi.org/10.1007/978-981-16-9205-5_12
Download citation
DOI: https://doi.org/10.1007/978-981-16-9205-5_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9204-8
Online ISBN: 978-981-16-9205-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)