Abstract
To estimate the associations between vitamin D status and Parkinson’s disease (PD). We searched electronic databases of the human literature in PubMed, EMBASE and the Cochrane Library up to February, 2014 using the following keywords: ‘vitamin D’ or ‘25(OH)D’ and ‘status’ or ‘deficiency’ or ‘insufficiency’ and ‘Parkinson’s disease’. A systematic review and meta-analysis were conducted on observational studies that reported the association between blood vitamin D levels and PD. Seven studies met the inclusion criteria. 1,008 patients and 4,536 controls were included. Results of our meta-analysis show that PD patients had lower mean levels of 25-hydroxyvitamin D [25(OH)D] than healthy controls [weighted mean difference (MD), −16.9, 95 % confidence interval (CI)], −33.5 to −0.2). Patients with vitamin D insufficiency [25(OH)D level <75 nmol/l] had an increased risk of PD (OR 1.5, 95 % CI 1.1–2.0). Patients with vitamin D deficiency [25(OH)D level <50 nmol/l] experienced a twofold increased risk of PD (OR 2.2, 95 % CI 1.5–3.4). Low vitamin D levels are associated with an increased risk of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the very common neurodegenerative disease. It is characterized by degeneration of the dopaminergic neurons in the substantia nigra, resulting in clinical symptoms such as resting tremor, rigidity and bradykinesia [1, 2]. It has been suggested that PD is the result of a complex of nutritional factors, genetic factors, environmental factors and aging [3]; however, the nature of the environmental factors remains largely unclear.
Recently, emerging data suggest that vitamin D may play an important role in the progression of the development of PD. It is well established that the vitamin D endocrine system plays a critical role in calcium homeostasis and bone health; however, in recent decades, the broad range of physiological actions of vitamin D has been increasingly recognized. In addition to its role in proliferation, differentiation and immunomodulation, there is mounting evidence to support an intricate role of vitamin D in brain development and function in health and disease [4]. Optimal balance, muscle strength, and innate immunity require sufficient vitamin D levels, and its deficiency is correlated with increasing risk for a range of adverse health outcomes including cardiovascular diseases [5], stroke [6, 7], multiple sclerosis [8], infectious disease [9, 10] and cancer [11]. Increasing evidence has shown that individuals with PD have lower levels of 25-hydroxyvitamin (25[OH]D) relative to healthy controls and vitamin D deficiency has been proposed to be linked to PD through multiple mechanisms [12]. There is an increasing interest in a range of actions of vitamin D. Low vitamin D status play an important role in the development or pathogenesis of PD [13]. It is reported that the distribution of vitamin D receptors in the substantia nigra is widely known to be affected in PD, and the involvement of this vitamin has been revealed in the regulation of tyrosine hydroxylase gene expression and consequently dopamine biosynthesis [14, 15].
However, there is a lack of systematic reviews and meta-analysis on the evidence regarding the association between vitamin D and PD. Given the high prevalence of low vitamin D status worldwide, we conducted a systematic review to shed light on the relationship between vitamin D levels and the risk of PD. In the present study, we performed a meta-analysis to evaluate comprehensively the vitamin D levels in individuals with PD, which has potential implications for the prevention and treatment of this disease.
Methods
We followed the guidelines for meta-analysis of observational studies in epidemiology (MOOSE) [16].
Data sources
Our electronic literature searches targeted studies on vitamin D status and PD. We searched the human literature in PubMed, EMBASE and the Cochrane Library up to February 2014 for articles on levels of circulating 25(OH)D levels and the risk of PD. The following keywords were used in the search: ‘vitamin D’ or ‘25(OH)D’ and ‘status’ or ‘deficiency’ or ‘insufficiency’. Relevant studies were further sought manually in the reference lists of primary papers and reviews.
Study selection
Full-length articles of studies evaluating vitamin D status and PD were scrutinized and subsequently selected if they fulfilled the following inclusion criteria: (a) study design was observational study; (b) study population was PD without pre-existing chronic disease; (c) contained relevant data to calculate the effect size; (d) met the predefined methodological quality assessment criteria for non-randomized observational studies (Table 1) [17]. Studies were excluded if: (a) they were reviews, case reports, letters or comments; (b) vitamin D levels were measured using non-blood biological samples such as amniotic fluid or urine; (c) vitamin D level that was measured was the active metabolite 1,25 dihydroxyvitamin D [1,25(OH)2D] only; (d) incomplete or conflicting result data.
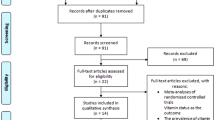
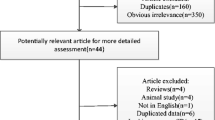
Studies were selected in a two-stage process. Two reviewers (ZL and HPQ) independently scrutinized the electronic literature searches and obtained full-length articles of all citations that met the predefined selection criteria. Final inclusion or exclusion decisions were then made after we read these articles. In cases of duplicate publications, we selected the most complete version. We resolved any disagreements through consensus or arbitration by a third reviewer (SB). We identified 119 articles and after screening the abstracts, we read 56 papers. Seven primary studies met the inclusion criteria (details see Fig. 1).
We evaluated the methodological quality of each study based on the study design, selection of participants, comparability of groups, definition of outcomes, ascertainment of outcomes and sample size, using the assessment criteria for non-randomized observational studies adapted from Duckitt and Harrington [17]. We excluded any study with a score of zero in any of the 6 items or a total score <7 out of 10 maximal points. Quality scores of all included studies are summarized in Table 2.
Tabulation and integration
The following information was extracted from the study reports: the first author’s last name, year of publication, country of origin, study design, sample size, gender, season of blood sampling, assay method, mean age, adjusted odds ratio and the potential confounding variables in the adjustments. Two authors extracted the data independently and in duplicate. Discrepancies were resolved through discussion to achieve a consensus.
Data on dichotomous outcomes were combined using the Mantel–Haenszel method, and measures of effect are presented as odds ratio (OR) with 95 % confidence intervals (CIs). For continuous data, we calculated the sample size weighted mean difference (MD) when outcomes were measured in the same way between studies. We used forest plots to show the point estimate (95 % CIs) for each study, with a diamond at the bottom representing the pooled point estimate (95 % CIs) for each outcome of interest. The presence of significant heterogeneity was examined by the I squared (I 2) statistic. In cases where I 2 exceeded 50 %, we pooled results using the random effects models. Otherwise, fixed effects models were applied. There is no universally accepted definition of vitamin D deficiency.
We used the cut-off point of 50 nmol/l which has been suggested by most experts as the cut-off for vitamin D deficiency. We used the cut-off point of 75 nmol/l which has been suggested by most experts as the cut-off for vitamin D insufficiency [18].
We also conducted sensitivity analysis including only prospective cohort or nested case–control studies. Two-tailed P < 0.05 was considered statistically significant. Funnel plots were applied to evaluate publication bias. Meta-analyses were performed using the Review Manager (RevMan) 5.2 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011).
Results
Seven studies [19–25] (including 1,008 PD and 4,536 controls) met the eligibility criteria. Table 2 shows the quality scores of included studies on vitamin D status and PD of each paper. Table 3 shows the first author, year of publication, the country, number of cases and controls, percentage of male and female patients, assay methods, the mean age of the subjects and adjusted OR (95 % CI) included in each studies. The results of the meta-analysis are shown in Fig. 2. They indicate that patients with PD had lower levels of 25(OH)D relative to healthy controls (weighted mean difference −16.9; 95 % CI, –33.5 to –0.2). There were statistically significant heterogeneity (P < 0.00001; I 2 = 99 %). The significant heterogeneity may be due to the differences in country, ethnicity and age of the participants studied (Table 3). Participants in Abou-Raya et al.’s study were white or black; in Bos et al. and Knekt et al.’s studies were all white; in Ding et al.’s and Evatt et al.’s studies they were white, Asian, black, and Hispanic; those in Sato et al.’s study were all Asian. First, vitamin D levels vary in subjects from different ethnicities. For example, vitamin D insufficiency is more prevalent among black Americans than non-black Americans [26]. Second, international comparison studies have shown that serum 25(OH)D levels vary among countries because of postulated factors, such as variation of sunshine exposure due to latitude of the country, vitamin D supplementation from food, and genetic factors. The different countries from where the subjects resided also may account in part for the high heterogeneity. In addition, as age is an important factor influencing the status of vitamin D, the differences in mean ages of the participants in different studies also may result in significant heterogeneity [27].
Patients with vitamin D insufficiency [25(OH)D level <75 nmol/l] had an increased risk of PD (OR 1.5, 95 % CI 1.1–2.0) (see Fig. 3). Patients with vitamin D deficiency [25(OH)D level <50 nmol/l] experienced a twofold increased risk of PD (OR 2.2, 95 % CI 1.5–3.4) (see Fig. 4).
No obvious publication biases were observed in Funnel plots (see Fig. 5). Similar results were observed if the meta-analyses were restricted to prospective cohort and nested case–control studies (data available upon request).
Discussion
The main findings in this systematic review and meta-analysis are that PD patients had lower mean 25(OH)D levels. Patients with vitamin D insufficiency [25(OH)D level <75 nmol/l] had an increased risk of PD (OR 1.5, 95 % CI 1.1–2.0) (see Fig. 3). Patients with vitamin D deficiency [25(OH)D level <50 nmol/l] experienced a twofold increased risk of PD (OR 2.2, 95 % CI 1.5–3.4) (see Fig. 4).
Previously, Zhao et al. [28] conducted meta-analysis of vitamin D levels in Alzheimer’s and Parkinson’s disease, the results of this meta-analysis also showed that the PD patients had lower levels of 25(OH)D than healthy controls. However, our study included more recent observational studies, and our systematic review also includes category variables such as 25(OH)D <75 or 50 nmol/l.
Vitamin D deficiency is an important condition in the elderly. Prevalence of neurodegenerative disease is also higher in these patients. Vitamin D is produced in body in skin on exposure to UV-B radiation and is found in limited food sources [29].
Some involved factors in vitamin D deficiency are advanced age, avoidance of sun exposure, residence in northerly latitudes, and darker skin. Serum 25(OH)D is the most useful indicator of vitamin D level of body.
In recent years, the terms vitamin D insufficiency and deficiency have been employed to characterize the suboptimal serum levels of 25(OH)D. However, presently it is difficult to give a clear value of 25(OH)D concentrations for the vitamin D insufficiency and deficiency. Thus, an alternative useful gradual scale has been proposed as follows: vitamin D insufficiency is defined as the 25(OH)D level <30 ng/ml (75 nmol/l) and vitamin D deficiency as the 25(OH)D level <20 ng/ml (50 nmol/l) [18]. As shown in Fig. 1, according to the mean 25(OH)D levels individuals with PD in six studies are vitamin D deficient, whereas the vitamin D status in the control group is much better.
The quality of systematic reviews is dependent upon the quality of studies included. We scrutinized the selected studies and excluded studies of poor methodological quality using strict quality assessment criteria. Methodological issues that may affect the study quality such as cohort being selected an unrepresentative group, unspecified or unacceptable definition of outcomes, or study design not described or poorly designed, were not applicable to the studies reviewed.
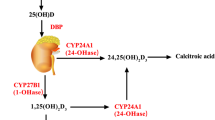
A number of biologically plausible mechanisms may explain the associations between vitamin D status and PD. Vitamin D has been shown to exhibit neuroprotective effects through antioxidative mechanisms, neuronal calcium regulation, immunomodulation, enhanced nerve conduction, and detoxification mechanisms. The vitamin D receptors and an enzyme responsible for the formation of the active form 1,25-hydroxyvitamin D have been found in high levels in the substantia nigra, the region of the brain affected most by Parkinson disease [30, 31]. This raises the possibility that chronic inadequacy of vitamin D leads to the loss of dopaminergic neurons in the substantia nigra region and further Parkinson disease. Vitamin D receptor (VDR) is widely expressed in human brains and is responsible for the formation of the highly active vitamin D metabolite. Animal studies have investigated the effects of VDR gene transcription in neuronal cells, and have shown that VDRs and vitamin D are key molecules to brain development, the prevention of anxiety, the induction of glial-derived neurotrophic factor, and the induction of nerve growth factor synthesis [32].
Some limitations of this review should be acknowledged. We need to interpret the results cautiously. Low vitamin D levels are found in PD, however, in the lack of large multi-center double-blinded randomized controlled clinical trials of vitamin D supplementation for the PD, this could be an epiphenomenon. We cannot exclude the drug’s influence, since we had no information for most cases of treatment with anti-parkinsonian drugs, which may occur years before the date of first hospitalization. Second, there were different assay techniques used to measure circulating 25(OH)D level. Third, since there is no information regarding sunlight exposure or ethnicity-specific results in most studies, we could not pool the findings by sunlight exposure or ethnicity. However, sunlight exposure and ethnicity (a partly surrogate measure for sunlight exposure and typical intake or supplementation level in a population) are upstream factors affecting vitamin D status; their effects should have already been reflected in blood vitamin D levels––the basis of the meta-analyses. In addition, we do not have uniformly collected measures of PD severity (Hoehn and Yahr stage, Unified Parkinson Disease Rating Scale scores). Therefore, we cannot assess exactly what effect, if any, differences in age and race or the time of plasma samples drawn might have on these findings. Finally, we reviewed only published studies in English.
In summary, patients with lower vitamin D status were associated with high risk of PD. Further confirmation of these findings in a large cohort is needed. As well, further research is required to better understand the role of vitamin D in PD associated pathologies. Large multi-center double-blinded randomized controlled clinical trials of vitamin D supplementation for the prevention of PD are needed to be conducted to determine the risks and benefits. Such an intervention, if proven safe and effective, could have substantial public health importance.
Highlights
-
1.
The levels of 25(OH)D were lower in Parkinson’s disease (PD) than in controls.
-
2.
Patients with circulating 25(OH)D levels <75 nmol/l had an increased risk of PD.
-
3.
Patients with 25(OH)D levels <50 nmol/l experienced a twofold increased risk of PD.
References
Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Counsell C, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Söderkvist P, Felice A, Geoparkinson study group (2007) Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med 64(10):666–672
Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, Scott BL, Vance JM, Scott WK (2008) Pesticide exposure and risk of Parkinson’s disease: a family-based case–control study. BMC Neurol 8(3):6
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4(11):600–609
DeLuca GC, Kimball SM, Kolasinski J, Ramagopalan SV, Ebers GC (2013) Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol 39(5):458–484
Zittermann A, Schleithoff SS, Koerfer R (2006) Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev 11(1):25–33
Chaudhuri JR, Mridula KR, Alladi S, Anamika A, Umamahesh M, Balaraju B, Swath A, Bandaru VS (2014) Serum 25-hydroxyvitamin D deficiency in ischemic stroke and subtypes in Indian patients. J Stroke 16(1):44–50
Brøndum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M (2013) 25-hydroxyvitamin D and symptomatic ischemic stroke: an original study and meta-analysis. Ann Neurol 73(1):38–47
Martinelli V, Dalla Costa G, Colombo B, Dalla Libera D, Rubinacci A, Filippi M, Furlan R, Comi G (2014) Vitamin D levels and risk of multiple sclerosis in patients with clinically isolated syndromes. Mult Scler 20(2):147–155
Guzmán-Fulgencio M, García-Álvarez M, Berenguer J, Jiménez-Sousa MÁ, Cosín J, Pineda-Tenor D, Carrero A, Aldámiz T, Alvarez E, López JC, Resino S (2014) Vitamin D deficiency is associated with severity of liver disease in HIV/HCV coinfected patients. J Infect 68(2):176–184
Camargo CA Jr, Manson JE (2014) Vitamin D supplementation and risk of infectious disease: no easy answers. Am J Clin Nutr 99(1):3–4
Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF (2007) Optimal vitamin D status for colorectal cancer prevention: a quantitative meta-analysis. Am J Prev Med 32(3):210–216
Evatt ML (2010) Beyond vitamin status: is there a role for vitamin D in Parkinson disease? Arch Neurol 67(7):795–797
Newmark HL, Newmark J (2007) Vitamin D and Parkinson’s disease––a hypothesis. Mov Disord 22(4):461–468
Evatt ML, DeLong MR, Kumari M, Auinger P, McDermott MP, Tangpricha V, Parkinson Study Group DATATOP Investigators (2011) High prevalence of hypovitaminosis D status in patients with early Parkinson disease. Arch Neurol 68(3):314–319
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29(1):21–30
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta- analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Duckitt K, Harrington D (2005) Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 330(7491):565
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281
Abou-Raya S, Helmii M, Abou-Raya A (2009) Bone and mineral metabolism in older adults with Parkinson’s disease. Age Ageing 38(6):675–680
van den Bos F, Speelman AD, van Nimwegen M, van der Schouw YT, Backx FJ, Bloem BR, Munneke M, Verhaar HJ (2013) Bone mineral density and vitamin D status in Parkinson’s disease patients. J Neurol 260(3):754–760
Ding H, Dhima K, Lockhart KC, Locascio JJ, Hoesing AN, Duong K, Trisini-Lipsanopoulos A, Hayes MT, Sohur US, Wills AM, Mollenhauer B, Flaherty AW, Hung AY, Mejia N, Khurana V, Gomperts SN, Selkoe DJ, Schwarzschild MA, Schlossmacher MG, Hyman BT, Sudarsky LR, Growdon JH, Scherzer CR (2013) Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology 81(17):1531–1537
Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V (2008) Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol 65(10):1348–1352
Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M (2010) Serum vitamin D and the risk of Parkinson disease. Arch Neurol 67(7):808–811
Sato Y, Honda Y, Kaji M, Asoh T, Hosokawa K, Kondo I, Satoh K (2002) Amelioration of osteoporosis by menatetrenone in elderly female Parkinson’s disease patients with vitamin D deficiency. Bone 31(1):114–118
Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K (2005) Abnormal bone and calcium metabolism in immobilized Parkinson’s disease patients. Mov Disord 20(12):1598–1603
Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ (2008) Vitamin D insufficiency among African–Americans in the southeastern United States: implications for cancer disparities (United States). Cancer Causes Control 19(5):527–535
Tsiaras WG, Weinstock MA (2011) Factors influencing vitamin D status. Acta Derm Venereol 91(2):115–124
Zhao Y, Sun Y, Ji HF, Shen L (2013) Vitamin D levels in Alzheimer’s and Parkinson’s diseases: a meta-analysis. Nutrition 29(6):828–832
Lawson DE, Paul AA, Black AE, Cole TJ, Mandal AR, Davie M (1979) Relative contributions of diet and sunlight to vitamin D state in the elderly. Br Med J 2(6185):303–305
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29(1):21–30
Buell JS, Dawson-Hughes B (2008) Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med 29(6):415–422
Han X, Xue L, Li Y, Chen B, Xie A (2012) Vitamin D receptor gene polymorphism and its association with Parkinson’s disease in Chinese Han population. Neurosci Lett 525(1):29–33
Acknowledgments
This work was supported by the Harbin Special Funds for Research of Scientific and Technological Innovative Talents (No. 2011RFQYS092) and Innovative Foundation of the Harbin Medical University (HCXS2010014).
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Z. Lv and H. Qi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lv, Z., Qi, H., Wang, L. et al. Vitamin D status and Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci 35, 1723–1730 (2014). https://doi.org/10.1007/s10072-014-1821-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1821-6