Abstract
Nowadays, respiratory viruses are the most common cause of diseases in humans, with a substantial effect on the morbidity and mortality around the world. In addition, the emergence of SARS-CoV-2 in recent years threats the public health. Till now, there is no effective therapy for COVID-19, and many techniques are being tried. The current anti-respiratory viral drugs destroy not only the respiratory viruses, but also the host’s metabolic processes.
Nanotechnology has transformed the world by providing advanced solutions to a wide range of challenges in healthcare nowadays. Current advancements in the strategy and manufacturing of nanomedicines have provided a variety of benefits over traditional techniques of respiratory viral infection therapy. Upcoming research can be aimed on functionalized nanomaterials in order to enable site-specific, concomitant delivery of several medicines in order to treat a wide range of diseases. Designing nanomaterials and the issue of long-term toxicity should be prioritized. However, with rapid advances nanomaterials, there is hope that the overall treatment of respiratory viral infections can be more efficient. This review displays viruses’ classification according to the genomic materials, respiratory viruses’ threat, the antiviral efficiency of nanodrugs, and nanomaterials against respiratory viruses, and the possible antiviral mechanism of nanomaterials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Respiratory viruses
- SARS-CoV-2
- Antiviral drugs
- Toxicity
- Nanomaterials
- Lactoferrin nanoparticles
- Silica nanocarriers
- Antiviral mechanism
1 Introduction
Globally, respiratory diseases are the most public diseases. These diseases are generally restricted to the upper air routes and are self-limiting. In some case, the infection can proceed to he lower respiratory tract, such as bronchitis and pneumonia. Youngsters and the old people are particularly vulnerable, particularly in underdeveloped nations. Respiratory viruses have a considerably larger role in the infection of the respiratory tract and bronchitis in children, but bacteria are the leading cause of pneumonia, particularly in adults and the clinical symptoms are very overlapping, and there is a growing evidence of bacterial infections followed by the viral diseases (Van Doorn and Yu 2020). There are various respiratory viruses that regularly circulate across all ages and are identified as suited for the effective transmission of individuals to individuals. Furthermore, in recent years, the risks posed to public health are SARS Coronavirus (SARS-CoV), COVID-19 (SARS-CoV-2), and H5N1 avian influenza. However, H5N1 avian influenza virus has caused a few outbreaks of human illnesses. Despite the fact that respiratory viruses cause a large number of illnesses, there is now just a few preventative or therapeutic interventions available (Boncristiani et al. 2009). The most significant task persisting in the progress of active antiviral agents is the viruses’ replication in the host cell. The host immunity in this state is weakened. Furthermore, due to the intricacies of viruses, cure is mostly accompanied with symptoms, and complete treatment of viruses might be impossible. Treatments are regarded as a red sign by the researcher, and innovative tools have been investigated in order to conquer the restrictions of current therapies (Zhu et al. 2015).
Because of the nanotechnology efficacy in treating the viral diseases, it has appeared as one of the most hopeful breakthroughs, overcoming the restrictions of conventional antiviral medications. It not only allowed us to conquer difficulties with drug solubility, bioavailability, bio-distribution, and toxicity, but it also offered medicines distinctive characteristics, which improved their potency and selectivity toward viral cells over host cells (Milovanovic et al. 2017a, b). The use of nanotechnology to combat SARS-CoV-2 may involve processes that affect the viruses’ entrance into the host cell, where blocking of the proteins of viruses’ surface may render the virus inactive. Also, specific targeting nanoparticles to certain viral protein may stop the internalization of the virus (Kerry et al. 2019). More research is needed to understand the interaction between nanoparticles and SARS-CoV-2 in order to plan coherent targeted curatives (Mainardes and Diedrich 2020). The current review compiles the threat of respiratory viral infections, the toxicity of conventional antiviral medicine, and the recent advancements of nanomedine that opens up innovative ways for advance research for the management of respiratory viral diseases.
2 Viruses Classification According to the Genetic Materials
Genetic materials of viruses are the main key for survivability and replication for viruses. They store the genetic database and information necessary for virus evolution and revolution (Brister et al. 2014). Viruses can be classified into different types according to the type of genetic material into two types: DNA virus and RNA virus. The DNA virus may be either double strand DNA (DSDNA) as iridoviridae and herpesviridae, or single strand DNA (ss-DNA) as anellovirus and parvoviridae (Wolf et al. 2018). RNA virus also have two types, double strand RNA (ds-RNA) as birnaviridae and reoviridae, and single strand RNA (ss-RNA) in which there are two sub-categories of it: positive sense RNA (+RAN) as coronaviridae, and negative sense RNA (−RNA) as orthomyxoviridae (Fig. 6.1). Additionally, according to the presence and albescence of envelope virus, they are categorized into: enveloped and non-enveloped virus. Hepadna viruses are an example of enveloped DNA, and corona virus, hepatitis D, and retroviruses are enveloped RNA viruses. However, examples of non-enveloped viruses are adenoviruses (DNA viruses) and hepatitis A and E viruses (RNA viruses).
Virus replication and mechanism of action in host cell vary according to type of genetic material, and number of strands (ss or ds) for each genetic material. In the double stranded DNA (ds-DNA) viruses, viruses invade the cell nucleus firstly, and then it begins to replicate using the polymerase enzyme inside the host cell to replicate itself as in herpesviridae family. The ss-DNA viruses have a circular genome, the replication process occurs inside the nucleus with a mechanism called rolling cycle, the single stranded genome is converted into ds-DNA intermediates then transcription to mRNA is occurred as in parvoviridae family (Wolf et al. 2018; Kaufman et al. 2015; Malathi and Renuka 2019; Modrow et al. 2013). However, the double stranded (ds-RNA) virus enters the host cell and uses the core capsid to replicate in the cell cytoplasm using the host cell polymerase enzyme for replication. The ds-RNA then splits and one strand acts as a template for mRNA generation as in rheoviridae family (Malathi and Renuka 2019; Modrow et al. 2013). Additionally, the replication processes in the ssRNA (+RNA) viruses occur in the cytoplasm where poly proteins are translated from the genomic RNA and polymerase enzyme synthesizing the complementary strand (negative polarity) for the genomic RNA strand (positive sense RNA template). The new produced complementary strand acts as a template for production of new viral genome and sub-genomic mRNA as in corona virus. Another type of RNA virus is the positive sense ssRNA reverse transcriptase virus. This type of virus contains two copies of (ssRNA), which use the reverse transcriptase enzyme to be converted to ds-DNA. The newly produced transcribed DNA is then transported to the host cell genome and start transcription and translation to mRNA using integrase enzyme as retrovirus. Moreover, there is a ds-DNA reverse transcriptase virus, which contains partial class ds-DNA genome, and produces the ssRNA intermediate (act as mRNA). The produced mRNA can be reverse transcribed to ds-DNA using reverse transcriptase enzyme to reproduction (Fig. 6.1) (Matamoros et al. 2011).
3 The Threat of Respiratory Viral Infections
Globally, viral diseases provide a substantial threat to both public health and global economy. Current infectious diseases, such as influenza, coronavirus, ebola, and dengue that disseminated directly or indirectly from individual to another, have emphasized the urgent need for new antiviral therapeutics (Lozano et al. 2012).
Respiratory viruses are the utmost common cause of infection in humans, having a considerable influence on morbidity and death globally, particularly in children. Severe respiratory infections are responsible for around 20% of all pediatric fatalities globally, particularly among poor people in tropical climates, where the percentage of cases of severe respiratory infections per fatality can be considerably higher than in temperate countries. Several respiratory viruses infected human of all ages, and well-adapted to cause transmission from person to other (Table 6.1) (Boncristiani et al. 2009).
The mucosa of human respiratory system is the greatest noteworthy portion and the initial locate of the entry of several viruses infection. These infectious pathogens primarily invade the upper respiratory tract and then influence the lower respiratory tract, leading to morality. The diseases of lower respiratory tract clarify a principal cause of the spread human disease and mortality with ~3 million deaths annually worldwide (WHO 2016).
The respiratory viruses often come into the host via airborne spreads, and then disseminate through direct adherent or droplets/aerosols, efficiently propagate in the respiratory tract, and generally origin of clinical indications, specifically fever, dyspnea, cough, bronchiolitis, and/or pneumonia as in Table 6.1 (Kutter et al. 2018). For instance, the pandemic corona viruses, which are a large family of RNA viruses, pass on a disease to the upper respiratory and gastrointestinal tract of vertebrates. The key symptoms in human comprise cough, fever, and, in more critical cases, troubles in breathing have been described with potential death from SARS-CoV, MERS-CoV, and SARS-CoV-2 (Gurunathan et al. 2020a, b).
3.1 The SARS-CoV-2 Threat
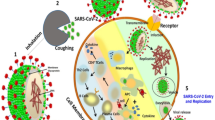
Coronaviruses belong to the subfamily Coronavirinae (order: Nidovirales, family: Coronaviridae), which are enclosed rounded viruses with the ssRNA genome (Dhama et al. 2014; Schoeman and Fielding 2019). The beta-coronavirus, which is responsible for COVID-19, was discovered for the first time in Wuhan, in the end of 2019. SARS-CoV-2 is a zoonotic virus, or animal origin virus, that be adherent to coronaviridae viruses family, has challenged the world due to its vigorously spreading, about 85 million confirmed cases and 2 million death up till now have been confirmed with COVID-19 with (in 218 countries) (WHO 2022). This virus is thought to be originated in bats hosts then jump from other animals to infect human then spread between infected people via droplets of sneezing and coughing either in air or surfaces (Das et al. 2020; Ahmed et al. 2020a, b; Wu et al. 2020a, b). Unfortunately countries cannot lockdown completely due to the economic issues thus early detection of infected individuals besides monitoring and treatment is required as quick as possible. Infected hosts display various clinical types, extending from asymptomatic to serious symptoms in their reproductive organs, circulatory, respiratory, and digestive systems (Gurunathan et al. 2020a, b). Thus, the viral infection can fight with by controlling the chain of transmission especially till now no available approved drug specific for this virus and all available vaccines are still under trials.
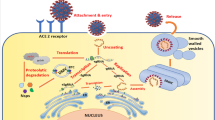
SARS-CoV-2 is about 50–500 nm ssRNA virus composed of four proteins N, S, M, and E proteins which refer, respectively, to nucleocapsid that hold the viral RNA, spike, membrane for attachment of virus with host cells, while M for membrane and E for envelope proteins both of them lie exterior to complete the viral structure (Yan et al. 2020). The S protein can attach to the angiotensin-converting enzyme 2 receptors (ACE2Rs) at the membrane surface of the host cell that help the virus to enter the host (e.g., human) cells via attachment to this cell receptor. ACE2Rs are focused at the lung, intestine, and kidney of human body proposing that corona virus can actually target these organs (Mehranfar and Izadyar 2020). Gene sequence analysis of SARS-CoV-2 suggested a great similarity to the RNA sequence of SARS and MERS which are two coronaviruses emerged in 2003 and 2012, respectively, and were epidemic which are reason for the deadly acute respiratory diseases in humans and flu-like diseases (Badgujar et al. 2020). Moreover, Zhou et al. (2020) reported the genome sequence of the novel SARS-CoV-2 is similar to the genome of bat coronavirus by 96.2%. However, the comparison between SARS-CoV and SARS-CoV-2 in human to human transmission is in the interest of the last one, that can spread much faster, which has already WHO declared it as a global pandemic on 11 March 2020 (Wan et al. 2020). However, this homology can be useful in two points; the first is that antiviral drugs used for these viruses can be used temporarily for COVID-19 infection (Zhou et al. 2020). Drugs such as chloroquine, acyclovir, hydroxychloroquine, ganciclovir, remdesivir, ganciclovir, ribavirin, lopinavir, and ritonavir are applied for COVID-19 treatment, but no drug is permitted by the FDA for the COVID-19 treatment, these drugs display better interactions with the active site of SARS-CoV-2 because of greater electrostatic and dispersion interfaces (Badgujar et al. 2020; Wang 2020). The second important point of homology is the available steps of vaccine designing for SARS/MERS in literature data that can decrease the steps of designing rapid vaccines for COVID19 that can take 2–3 years (Lu et al. 2020). Recent studies have revealed that novel SARS-CoV-2 and SARS-CoV infect host cells by using the same receptor (angiotensin-converting enzyme 2, ACE2), and the adhering of SARS-CoV-2 to the surface receptors of host cells is facilitated by the S proteins (Wu et al. 2020a, b; Wan et al. 2020; Hofmann et al. 2020). Moreover, it was detected that cells that possess ACE2, and not possess the enzymes aminopeptidase N and human dipeptidyl peptidase-4, were more liable for SARS-CoV-2 infection (Wrapp et al. 2020).
3.2 Toxicity of Conventional Antiviral Drugs
Chemical antiviral drugs such as Emtricitabine, Lamivudine, Aciclovir, Nevirapine, and others are currently in use. Most of those chemical drugs, on the other hand, may cause side effects or dose-limiting toxicity (Guo et al. 2019). One of the most difficult aspects of treating viral infections is getting enough drugs to reach pathogens inside their intracellular compartments (Li and Armstead 2011). Furthermore, antiviral drug may have short half-life, necessitating repeated and outsized doses to produce a therapeutic effectiveness, resulting in high costs, poor patient compliance, and serious side effects. Moreover, drug resistance can arise when patients do not adhere to their treatment protocols perfectly (Goossens 2009) or when infections are exposed to suboptimal drug dosages for a prolonged period of time (Sandegren and Andersson 2009).
Amantadine drugs prevent replication by inhibiting the action of the M2 protein. Amantadine is only effect on influenza A, not influenza B, since influenza B lacks an M2 protein and instead uses a replacement protein known as NB, which is unaffected by amantadine (Betakova et al. 1996). However, amantadine was related with central nervous system (CNS) toxic effects such as irritability, anxiety, insomnia, agitation, concentration disorder, ataxia, lisping, depression, and hallucinations (Keyser et al. 2000). Furthermore, lower extremity oedema and involuntary myoclonic jerks were also reported after treatment with amantadine (Yarnall and Burn 2012).
Similarly, oseltamivir is an oseltamivir carboxylate medication (Ro 64-0802; GS4071), which is a powerful and specific neuraminidase inhibitor of the glycoprotein that is crucial to influenza A and B viruses’ propagation (McClellan and Perry 2001). However, severe toxic effects of oseltamivir, such as hepatitis, an increase in liver enzymes, and allergic reactions that lead to anaphylaxis, are less common. It also has the potential to cause Stevens-Johnson syndrome (Simón-Talero et al. 2012). In recent years, toxic epidermal necrolysis, cardiac arrhythmia, convulsions, elevated diabetes, and hemorrhagic colitis have all been recorded (Chen and Lai 2013). Neurological effects of oseltamivir included abnormal behaviors and hallucinations (Guisado-Macías et al. 2012). Its safety is not clear whether in pregnant women or in pediatrics (Kiso et al. 2004). Unfortunately, as previously mentioned, current antiviral medications also damage not only the viral infection but also the host’s metabolic processes. There are plenty of other challenges to overcome in the development of effective antiviral therapies (Sumbria et al. 2021). Current advances in nanotechnology can help to resolve these hurdles, opening up new possibilities for the development of innovative broad-spectrum nanotherapeutic platforms to fight viral infections which presents a very promising approach (Fang et al. 2018). Antiviral drugs may be incorporated into nanoparticles to improve bioavailability, thus decreasing systemic toxicity, improving effectiveness, and keeping the therapeutic window for longer time (Stephen et al. 2020; Sharmin et al. 2021).
Any nanomaterials have an intrinsic toxicity that helps them to destroy viruses directly (Zhou et al. 2021). As nanoparticles invade the human body, they can pass through numerous cell barriers to influence the most sensitive organs, such as the lungs, liver, and kidney, causing mitochondrial impairment, DNA mutations, and ultimately cell apoptosis or death (Gulati et al. 2018).
4 Nanodrugs and Their Efficacy in Killing Viruses
A particle’s antibacterial and antiviral activity is totally related with chemicals that kill bacteria and viruses or decrease their rate of growth without being very hazardous to neighboring tissues. The most recently found antibacterial agents are natural substances that have been chemically changed (von Nussbaum et al. 2006). Nanotechnology has emerged as one of the most promising developments, overcoming the shortcomings of conventional antiviral medicines, because of its efficiency to deal with viral diseases. Not only did it allow us to solve snags associated with the solubility, bioavailability, bio-distribution, and drugs toxicity, but it also gave drugs distinctive properties, which consequently improved their effectiveness and selectivity in the direction of viral cells against the host cells. Nanoformulations can act as antiviral agents through different mechanisms as well. One of the most influential properties of nanoparticles is having immunochemically inert surfaces that minimize their enzymatic degradation and uptake by phagocytes of the reticuloendothelial system and give them high in vivo retention in turn. Also, nanoparticles have improved deposition to the diseased sites and high efficacy, and this is attributed to the enhanced permeability phenomenon that causes vasculatures to be compromised. Several nanoparticles have been proposed over the years as carriers for antiviral agents (Milovanovic et al. 2017a, b). Nanoparticles have been known with their ability to interfere with the cycle of viral infections in an efficient manner. Since the contact of viruses with the host cells is mediated by multivalent interactions and given that nanostructure has multivalent character that allows for their attachment to several ligands, nanostructures are capable of interfering with viral attachment and blocking viral entry into host cells (Łoczechin et al. 2019).
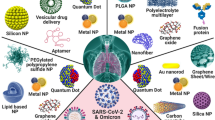
Nanoparticles (NPs) in the range of 1–100 nm size were applied as a tool for drug delivery, identification, and cure of various infectious diseases (Aderibigbe 2017; Maduray and Parboosing 2020; Prasad et al. 2018). Multiple nanomaterials and nanocarriers can act as viral activity inhibitor, they utilized in many new pharmaceutical applications due to its high accuracy in delivering of drugs to the target sites devoid of the healthy cells, detecting the viral infections in early stages, delivering nanotherapeutic molecules or nano-vaccines to certain specific organs or cells. Several nanomedicines are undergoing investigation for the treatment of viral infections. For instances, silver nanoparticles (AgNPs), gold nanoparticles (AuNPs), organic nanoparticles, graphene oxides, zinc oxide, liposomes, quantum dots nanoparticles (Lei et al. 2008; Gurunathan et al. 2020b; Rafiei et al. 2016; Michalet et al. 2005). These nanoparticles may display a brilliant perspective in the future of virus therapy especially in the pandemic Coronaviruses (CoVs). There are many studies providing a deep view about these particles and the mechanism of their action as antiviral in the cell. This short review represents some of these studies about part of these nanoparticles and how they work in the cell.
4.1 Nanomedicine Weapon Against SARS-CoV-2 Threat
Nanomedicine has an influence on all sectors of medicine and is regarded as a significant tool for innovative diagnostics, imaging therapy, nanomedicine treatments, vaccinations, and the development of biological materials for regeneration human cells, and organs (Fluhmann et al. 2018). Polymeric nanoparticles, liposomal and protein nanoparticles have been utilized in nanodrugs, particularly for drug delivery. A basis for their usage in various medical purposes is the amount of interactions between nanoparticles and biologically active molecules (Patra et al. 2018). Nanodrugs have been produced for years, and numerous are now being tested in experimental trials for treating several diseases such as infectious, cancer, cardiovascular, and any inflammation. However, only a handful has been authorized for the human practice (Kupferschmidt and Cohen 2020). Furthermore, nanoparticles can enhance particular medication targeting and regulated the rate of drug release, consequently influencing therapy effectiveness and safety. Also, metallic nanoparticles have also been used in nanodrugs, owing to their antimicrobial properties (Kupferschmidt and Cohen 2020).
The use of nanoparticles to battle SARS-CoV-2 might entail processes that affect the virus’s entrance into the cell of the host until it is inactivated. Because blocking viral surface proteins may inactivate the virus, tailored nanoparticles specific to virus produced proteins may limit the internalization of the virus (Kerry et al. 2019). Metallic nanoparticles have been demonstrated to impede viral adhesion to the surface of the cell, hence inhibiting internalization of the virus and reducing the replication of virus. Titanium, silver, gold, and zinc nanoparticles have already demonstrated their antiviral activity against HIV, influenza virus, respiratory syncytial virus, and others (Kupferschmidt and Cohen 2020). The nanoparticles bind to the viral envelope or protein, affecting the adherence with the host cell, according to the mode of action. The treatment’s efficacy is related to the dimensions, shape, and the charge of nanoparticles’ surface; however, precautions must be taken concerning the nanoparticles’ concentration to bypass their cytotoxicity (Singh et al. 2017).
Organic nanoparticles have been utilized to administer antiviral drugs such as zidovudine, dapivirine, acyclovir, and others with the goal of increasing the bioavailability of drug, promoting effective drug delivery, and promoting targeted antiviral action. The fundamental restriction of antiviral drugs is the absence of particular targeting, which causes the cytotoxic effect on the host cell. This issue can be solved by the organic nanoparticles because of their adaptability; nanoparticles can be used as adjustable vectors for viral targeting and selective medication delivery (Milovanovic et al. 2017a, b). Antiviral drugs such as chloroquine, lopinavir, ribavirin, ritonavir, and remdesivir have shown encouraging efficacy against SARS-CoV-2 (Li et al. 2020). Nanoencapsulation of antiviral medications may aid in the progress of safer therapeutics for SARS-CoV-2 and other viral infections. Although it is well recognized that nanotechnology-based drug delivery approach improves current therapies in medicine, it remains under investigation as shown in the SARS-CoV-2 pandemic (Uskokovic 2020). In conclusion, nanoparticles may play a crucial role in multiple phases of SARS-CoV-2 pathogenesis, given their ability to prevent viral adherence and their fusion with membrane during the entrance of virus. Furthermore, nanoencapsulated medications may be more effective in triggering intracellular pathways that produce permanent virus damage and limit transcription, translation, and replication of the virus (Mainardes and Diedrich 2020).
4.2 Biogenic and Non-biogenic Metallic Nanoparticles and Its Antiviral Efficiency
There are extensive groups of biological, chemical, and physical approaches to produce NPs (Khandel et al. 2018). Chemical routine production of metallic nanoparticles includes the bottom-up approach with procedures such as the polyol synthesis, sol-gel method, microemulsion, and hydrothermal synthesis. A well-defined size nanoparticles are produced by chemical approaches (Yu et al. 2008). The synthesis mechanism requires reducing metal salt ions by reducing agents or decay of metal salts with further energy in the existence of a stabilizer (Khandel et al. 2018). Despite the benefits of chemical synthesis, there may be significant drawbacks, such as the usage of hazardous and non-biodegradable compounds and NP unsuitability for biological purposes (Khandel et al. 2018; Patel et al. 2015).
The physical routine for the NPs production comprises techniques such as ultraviolet (UV) radiation, sonochemical, microwave irradiation, thermal decomposition, laser ablation, photochemical, and radical induction (Khandel et al. 2018; Dhand et al. 2015; Maduray and Parboosing 2020). In appropriately, the rich waste produced by physical approaches for NP production seems to be economically unfavorable (Dhand et al. 2015). Biosynthesis of metallic nanoparticles depends on the bottom-up method that enrolls unicellular and multicellular living organisms (e.g., actinomycetes, bacteria, viruses, fungus, yeast, algae, and plants) (Khandel et al. 2018; Pantidos 2014; Ingale and Chaudhari 2013). Biogenic nanoparticles are environmentally friendly, rapidly formed in large amounts, biocompatible, and of definite size and shape (Khandel et al. 2018; Shah et al. 2015).
Biological nanoparticles can attack drug-resistant viruses, and assisting in the progress of antiviral drugs. Recent literature by El-Sheekh et al. (2020) demonstrates the inhibitory efficiency of cyanobacterial synthesized Ag2O|AgO-NPs and gold-NPs for the replication of the Herpes Simplex (HSV-1) virus. The results revealed a 90% decrease in cytopathic effect of Herpes Simplex virus using Ag2O|AgO nanoparticles and gold nanoparticles at 31.25 μL, with a higher decrease rate (49.23%) using Ag2O|AgO-nanoparticles than gold nanoparticles (42.75%). Additionally, the antiviral activity of biosynthesized Ag nanoparticles was demonstrated against Herpes Simplex Virus (HSV-1), Hepatitis virus-10, and Coxsackie B4 virus. The antiviral mode of action of biosynthesized silver nanoparticles has not been detected, but most probably the antiviral activity of them is attributed to the blocking virus’s entrance into host cells by binding the viral envelope glycoproteins. SNPs also may exhibit their antiviral activity through interaction with viral genetic material or via interfering with viral replication pathways (Haggag et al. 2019).
The metallic nanoparticles’ antiviral action is based on competitive interaction with cell receptors and viral envelope rupture (Rai et al. 2016). The viral infection is dependent on the virus’s entrance and adherence to host cells via the interaction of virus’s surface constituents through ligands and proteins of the cellular membrane. The most effective technique for generating novel antiviral medications is to hamper the contacts between virus’s ligand and cellular membrane, hence preventing virus adherence and entrance into cells. By analyzing the mode of action of metallic nanoparticles in microorganisms, silver nanoparticles have emerged as one of the most promising antiviral agents (Salleh et al. 2020). Figure 6.2 showed the antiviral mechanism of silver nanoparticles.
The intricacy of viral structures may lead to a lack of understanding of the mode of nanoparticles’ actions toward pathogenic viruses. Silver nanoparticles interact with the viruses in two ways: (1) Silver nanoparticles bind to the virus’s outer coat, preventing viral adherence to the receptors of the cell, and (2) the silver nanoparticles attach to the virus’s genetic material, preventing the virus from replicating or propagating within the host cells (Salleh et al. 2020). Table 6.2 demonstrates the antiviral activity of biogenic and non-biogenic silver nanoparticles against some respiratory viral infections. Also there is a hypothesis that silver nanoparticles inhibit SARS-CoV-2 binding to the cell receptor through binding to its spike glycoprotein and the releasing of silver decreases pH resulting in virus denaturation (Salleh et al. 2020).
Furthermore, the antiviral efficiency of zinc oxide nanoparticles and polyethylene glycol coated zinc oxide nanoparticles was evaluated against HINI influenza virus. Ghaffari et al. (2019) found that polyethylene glycol coating enhances the potential antiviral activity of the zinc oxide nanoparticles compared to non-coated zinc oxide nanoparticles. Polyethylene glycol (PEG) covered zinc oxide nanoparticles with 200 g/mL concentration hinders at the percentage of around 92% in replicates of the DNA genomic of HSV-1 and it lessens virus titer as well (Tavakoli et al. 2018). The hydroxyl group-ZnNPs, oleic acid modified-ZnNPs, and chitosan-ZnNPs have antiviral action against herpes simplex virus type-1 (Farouk and Shebl 2018). Also, it was known that intracellular Zn2+ concentration inhibited the replication of Nidovirus and many platforms of RNA viruses (Ishida 2019).
In recent study, Kumar et al. (2019) evaluated the antiviral efficacy of iron oxide nanoparticles against the PR8-H1N1 strain, and they recommended it as a powerful influenza virus inhibitor with an eightfold decline in the viral RNA. Also, Lin et al. (2017) examined the antiviral characteristics of selenium nanoparticles and zanamivir coated selenium nanoparticles against H1N1 influenza virus, and their findings showed that the zanamivir coating had greater antiviral activity than non-coated selenium nanoparticles.
Similarly, Li et al. (2017) investigated the higher antiviral properties of oseltamivir coated selenium nanoparticles against the H1N1 influenza. With oseltamivir, selenium nanoparticles were modified to form more stable and compact globular nanocomposites.
Inhibitory activity of oseltamivir-coated selenium nanoparticles is attributed to suppressing the activity of hemagglutinin (HA) glycoprotein, which is found on the surface of the virus and responsible for combining receptors containing sialic acid on the host cells, and neuraminidase (NA) glycoprotein, which assists the linkage between sialic acid and HA to be cleaved to let the virus enter the host cells. Therefore, oseltamivir-coated selenium nanoparticles prevent the viral fusion and entry into host cells. The underlying molecular mechanisms verified that oseltamivir-coated selenium nanoparticles significantly suppressed the expression levels of PARP, caspase 3, p53 and increased the level of AKt. This indicates that oseltamivir-coated selenium nanoparticles depressed H1N1-induced host cells apoptosis. Oseltamivir-coated selenium nanoparticles markedly decreased ROS generation compared to oseltamivir and selenium nanoparticles as well. Therefore, additional antiviral properties against multidrug resistance may be provided by the oseltamivir-coated selenium nano system to prospective selenium species (Li et al. 2017).
Gold nano-rods (AuNRs) have been widely used in biomedical applications as they can greatly improve therapeutic efficacy of drugs through delivering them to target tissues in an efficient way. Their surface can be easily modified with biocompatible materials as well which makes them ideal drug delivery systems. Additionally, AuNRs have tunable surface plasmon and photo thermal properties that provide them with worthy photo acoustic and photo thermal properties. They also have been used as nanocarriers for chemotherapeutic agents giving effective combined chemo-photo thermal therapy. Recently, AuNRs should be recognized as prospective biocompatible target-specific antiviral-drug carriers. For instance, AuNRs were developed for delivery of ssRNA immune activator for inhibition of seasonal and pandemic flu viral replication (Chakravarthy et al. 2010). The AuNRs have been used in developing antiviral therapy for combatting Middle East respiratory syndrome corona virus (MERS-CoV) by having the HR1 peptide inhibitor called pregnancy induced hypertension (PIH) immobilized in gold nano-rods. As the fusion between MERS-COV envelope and host cell membrane is mediated by S2 subunit of Spike protein of the viral envelope and since the process of drawing host cell membrane and viral envelope is initiated by the 6-helix bundle (6-HB) that is formed after binding of HR1 and HR2, which are two of three major domains of S2 subunit, takes place, PIH α-helix peptide was recognized as HR1 peptide inhibitor using the docking based virtual screening based on HR2 sequence. It was found that PIH mimics the HR2 conformation which means it can be used to inhibit HR1 and block the formation of 6-HB. Therefore, fusion between viral envelope and host cell membrane won’t take place and viral genome won’t be released into the host cells.
In spite of PIH alone showed effective inhibitory activity, it suffered major drawbacks like other peptides. These drawbacks include poor metabolic stability and bioavailability. PIH-modified gold nano-rods (PIH-AuNRs) exhibited more efficient inhibitory activity besides improving bio-stability and biocompatibility that leaded to improved physical and pharmaceutical profiles than those of PIH alone (Huang et al. 2019).
As per the previous research, nanomaterials developed with various forms and structures show unique benefits for practice in antiviral therapy, notably; nanometric diameter that allows drug delivery across resistant barriers, high surface area to volume ratios for the inclusion of large drug loads and enhanced efficiency, surface functionalization availability that facilitates passage across cellular membranes and improves stability as well as bioavailability, antiviral activity against several viruses due to bio-mimetic properties, high specificity, enhanced drug delivery and controlled drug release to target tissues, reduced drug resistance, possibility for personalized therapy and last but not least low incidence of drug adverse effects upon using nan-based therapeutics (Cojocaru et al. 2020).
Moreover, bio-functionalization of gold nanoparticles with seaweed Sargassum wightii extract was applied to achieve more efficient drug delivery to target cells. Antiviral activity of seaweed-gold nanoparticles against Herpes Simplex Virus (HSV) was assessed by the lessening of cytopathic effect (CPE) caused by HSV in a dose-dependent mode. It was found that 10 and 25 μL of seaweed-gold nanoparticles decreased HSV-1 and HSV-2 CPE by 70%. According to literature, functionalized gold nanoparticles have size-dependent interface and the capability to inhibit the adhering and virus’s entrance, and this was the accountable for their antiviral efficiency (Dhanasezhian et al. 2019).
Recently, it was known that copper (Cu) abolishes the propagation tendency of SARS-CoV, influenza, and other respiratory viruses, having high prospective decontamination in hospitals, communities, and households (Cortes and Zuñiga 2020; Raha et al. 2020). Copper oxide nanoparticles (CuO-NPs) have antiviral activity against HCV by directing the adhering of HCV to hepatic cells and the entrance of the virus. Thus, it can be used as a nontoxic drug for HCV infected patients (Hang et al. 2015). In addition CuO-NPs are related with a noteworthy antiviral effectiveness against HSV-1 at nontoxic concentration to host cells causing 83% inhibition in virus titer (Tavakoli and Hashemzadeh 2019). Moreover Cu surfaces scored less time of stable infective SARS-CoV and SARS-CoV-2 as compared with the surfaces of plastic and steel (Van Doremalen et al. 2020) and thus, Cunano coating can decrease viral transmission (Pemmada et al. 2020). Cu-NPs were used with other materials for synthesizing a face mask that may protect from COVID-19 infection (Ahmed et al. 2020a, b).
4.3 The Antiviral Activity of Organic Nanoparticles
4.3.1 Polymeric Nanoparticles
Polymeric nanoparticles were created after liposomes in order to increase their stability and medicinal payload. They are compact colloidal nanoparticles with a size of less than 500 nm that are formed of a biocompatible polymeric medium comprised of artificial or natural origin polymers (Zazo et al. 2016). Polymeric nanoparticles may be more stable than liposomes in biological liquids and under storage circumstances due to their configuration. Polymeric nanoparticles can be made via a variety of techniques, including solvent evaporation, spontaneous emulsification, solvent diffusion, and polymerization (Lembo et al. 2018). They could be loaded with lipophilic and hydrophilic medicines, and several chemical methods, such as covalent chemistry and hydrophobic interactions, have been recommended. Above the threshold micelle concentration and temperature, the polymers unexpectedly form micellar configurations, forming hydrophobic aggregate polymeric chains (Zazo et al. 2016). Polymers, however, converted insoluble in liquids and behave like inactive ingredients below the above-mentioned precarious limits. They have piqued the interest of researchers as nanosized drug delivery systems, not only because of their several advantages (Cagel et al. 2017).
In the viral therapy, although, the conventional treatments against influenza virus infections were considered to target the proteins of the virus, the development of viral variants carrying drug-resistant transformations could hinder the progress of pathogen-targeting antivirals. For this reason, growing efforts have been directed towards developing host-targeted antiviral agents which act by controlling host aspects involved in viral replication. Since targeted antiviral approaches do not employ a choosy pressure on the target pathogen, they may be less liable to strain variations and mutations. Vacuolar ATPases (V-ATPases), which are abundant proton pumps found in the endo-membrane structure of all eukaryotic cells, have been identified as target for blocking virus entry into host cells due to their critical role in allowing viral entry by V-ATPase-mediated endosomal acidification. A lot of V-ATPase inhibitors have been developed such as plecomacrolide bafilomycin and diphyllin. However, their clinical application is limited by toxicity concerns and delivery challenges associated with their poor water solubility. Therefore, poly(ethylene glycol)-block-poly(lactide-coglycolide) (PEG-PLGA-)-based polymeric nanoparticle system was developed to encapsulate bafilomycin and diphyllin in its hydrophobic polymeric core. The drug-loaded nanoparticles achieved sustained drug release kinetics over 3 days and were proficiently taken up by various types of cell lines. The nanoparticulate V-ATPase inhibitors exhibited diminished cytotoxicity, enhanced antiviral activity as well as increased therapeutic index in comparison with free diphyllin and bafilomycin drugs. Treatment with diphyllin nanoparticles in a mouse model of sublethal influenza challenge exhibited good tolerability and achieved reduced body weight loss and viral load in the lungs. Additionally, diphyllin nanoparticle treatment offered a significant survival advantage following a lethal influenza viral challenge. Moreover, host-targeted treatment by diphyllin-loaded nanoparticles can be applied to multiple strains of influenza viruses as a broad-spectrum antiviral (Hu et al. 2018).
4.3.2 Carbon-Based Nanomaterials as Antivirals
It has been reported that carbon-based nanomaterials have potent antiviral properties. Carbon quantum dots (CQDs) can be synthesized using several simple and inexpensive methods with a very small diameter and efficient water dispersion to be used in several therapeutic applications. Furthermore, they have excellent optical properties that facilitate in vivo tracking, and they are known for lacking signs of toxicity in animals as well. CQDs resulting from citric acid/ethylene diamine then conjugated with boronic acid functions showed highly effective anti-HCoV-229E coronavirus behavior by interaction with the S protein of human coronavirus and therefore blocking its entry into host cells (Łoczechin et al. 2019).
Antiviral cationic carbon dots based on curcumin have been developed as multi-site inhibitors for enteric coronavirus. Although curcumin (CCM) has been reported to have antiviral activity against several viruses, it could not be widely applied in its pure form due to poor solubility and bioavailability. Encapsulating CCM in inorganic-based carriers has been widely used to overcome these two problems. This method could overcome the poor solubility and bioavailability of CCM without causing significant improvement on its antiviral activity. It was relatively tedious and time-consuming as well. Therefore, another method was developed to improve solubility, bioavailability, and antiviral activity of curcumin.
Curcumin was applied as a precursor to prepare cationic carbon dots (CCM-CDs) with antiviral properties using one-step method. The effectiveness of (CCM-CDs) was studied using porcine epidemic diarrhea virus (PEDV) as corona virus typical. As-prepared CCM-CDs treatment was found to effectively hinder PEDV proliferation compared to corporate CDs (EDA-CDs). The CCM-CDs modify the configuration of viral surface protein which leads to blocking the viral entry. They also suppress the production of negative-strand RNA in virus, budding of the virus and the accumulation of reactive oxygen species (ROS) by PEDV. Moreover, CCM-CDs inhibit viral propagation by activating the generation of interferon-stimulating genes (ISGs) and pro-inflammatory cytokines (Ting et al. 2018).
4.3.3 Lactoferrin Loaded Nanoparticles as Antivirals
As long as the recommended first-line highly active antiretroviral therapy (HAART) for HIV/AIDS has been known to be a mixture of one non-nucleoside reverse transcriptase, and two nucleoside/nucleotide reverse transcriptase inhibitors, a mixture of Zidovudine (AZT), Efavirenz (EFV), and Lamivudine (3TC) is one of the commonly used main line treatment. Patient needs to take this regimen in a fixed schedule which may cause several adverse effects and health complications as the prolonged use of these drugs has been reported to cause different toxicities like cardio-toxicity and erythrocyte toxicity. From this perspective, a formulation of lactoferrin nanoparticles loaded with triple drug combination of zidovudine, efavirenz, and lamivudine has been developed with enhanced bioavailability, improved pharmacokinetic profile and minimal drug-associated toxicity over the free drugs. Lactoferrin is a pleiotropic particle with wide-ranging practical activities including anti-HIV activity. Lactoferrin nanoparticles exhibit high drug loading capacity and provide the loaded drugs with the advantage of bypassing first pass metabolism which leads to reduced drug dose and therefore reduced drug-associated toxicity (Kumar et al. 2016).
Lactoferrin nanoparticles were prepared using sol-oil protocol. In this protocol, an equal amount (3.33 mg) of drugs was dissolved separately in 100 μL of dimethyl sulfoxide solvent, and then different concentrations of lactoferrin were solvated in 500 μL of phosphate buffer (1×) saline (pH 7.4) separately. The incubation of drugs and protein solutions were on ice for 1 h and then mixed with 25 mL of olive oil. Further, they were sonicated for 15 min at 4 °C using an ultrasonic homogenizer. Samples were then instantly transferred into liquid nitrogen for 15 min and incubated on ice for 4 h. Centrifugation of the formed particles at 6000 rpm for half an hour was taken place. The oil containing supernatant was thrown away and the ice-cold di-ethyl ether washed pellet suspended in phosphate buffer for the next experiment (Kumar et al. 2016).
First-line antiretroviral therapy nanoparticles (FLART-NP) enter the cells slowly and reach the maximum level at 4 h, then become maintained at a constant level for long period until a significant decline takes place over a period of 8 h. This suggests that lactoferrin NPs undergo exocytosis after release of its payload making no burden on the cells by the delivery vehicle. The maintenance period also delivers longer period for the drugs to perform against HIV existing inside macrophages leading to enhanced antiviral activity. Lactoferrin NPs show pH dependent drug release with maximum drug release in the endosomal pH (pH 5.0) and minimal release in the physiological pH (pH 7.4) which indicates that there is no drug release in extracellular condition, thus targeted drug release and reduced drug-related toxicity have been achieved. This is also supported by the microscopic analysis that shows characteristic surface projections/depressions that maintain lactoferrin’s structural features which may be involved in recognition and receptor binding on the target tissue. With FLART-NP, liver and kidney damage has been completely abolished which confirms the advantage of targeted drug delivery (Kumar et al. 2016).
4.3.4 Silica Nanocarriers as Antivirals
Mesoporous Si-NP of 2–50 nm size are frequently used in drug delivery systems against viruses these particles can protect drug till reach the specific site besides improving its solubility and stability and enhancing drug circulation time and controlled release (LaBauve et al. 2018). Si-NP is a stable biocompatible that can carry and pass RNA/DNA molecules through the cells and protect them from degradation via nuclear enzymes which is an important step for development viral vaccine (Tarn et al. 2013). Moreover these particles can pass into the cells without damaging the cells membrane compared to lipid based delivery systems also do not produce inflammation at the site of injection or systemic side effects (Mehta et al. 2020). Also, De Souza et al. (2016) showed that functionalized Si-NP can be used as antiviral drug against HIV that can interact specifically with viral envelope at nontoxic concentrations to mammalian cells and prevent virus to enter the cells. The Si-NP associated with didodecyldimethylammonium bromide has virucidal activity against H1N1 (Capeletti et al. 2018).
Recently AbouAitah et al. (2020) showed that Si-NP-(NH2)-(shikimic acid)-(quercetin) displayed both an antiviral against H5N1 and anti-inflammatory effect by inhibition of cytokines (TNF-α, IL-1β) and nitric oxide production in rat model. As the biggest challenge facing COVID-19 vaccine development is confirming that the host cell receives the introduced genetic material this can be achieved using viral vector or nanotailored delivery system to promote the synthesis of spike protein. Thus functionalized Si-NP can propose a possibly nontoxic and active delivery structure for DNA/RNA vaccines and may be suitable in the pursuit for a COVID-19 vaccine. Recently a new Si-NP based delivery system for COVID19 is synthesized and is under trial called Nuvec® (Theobald 2020).
Finally, nanoparticles prevent the viral propagation or blocking the virus’ entrance in to the host cell through their several interfaces with glycoprotein receptor and/or viral coat, these can hinder the viral propagation in the host cell. Nanoparticles are novel antimicrobial agents owing to unique chemical and physical features with their high surface area. The viral replication and assembly in the intracellular compartment of an infected cell require host cellular and viral factors for progeny virion production. The mechanisms of interaction between nanoparticles with these aspects are the crucial to an efficient viral propagation inhibition (Dos Santos et al. 2014).
The mechanism of viral infection comprises attachment, penteration, replication, and budding. Blocking or suppressing any of these steps is the antiviral functional nanoparticles. There are many antiviral functional nanoparticles mechanisms. We will mention these mechanisms, as promising therapeutic strategies (Chen and Liang 2020). The early steps of virus entry are sites of action of the inhibitor, because of its accessibility and extracellular location making it attractive therapeutic strategy (Dos Santos et al. 2014). Consequently, the well-designed nanoparticles can be used as a wide-ranging antiviral agent by suppressing the attachment of the virus. The highly conserved target of viral attachment ligands (VALs) heparan sulfate proteoglycans is mimicked by a series of antiviral nanoparticles with long and flexible linkers which designed by Stellacci’s group. In vitro nanomolar irreversible activity of these nanoparticles on papilloma virus, herpes simplex virus, dengue, respiratory syncytial virus, and lenti virus achieved efficient prevention of viral attachment (Cagno et al. 2018). The second way of viral suppression is hindering their dissemination and host cells entrance by varying the cell surface membrane and protein structures. These can be achieved by interaction of nanostructure with viruses and changing their capsid protein structure to reduce its virulence and entry into the host cell (Chen and Liang 2020). Haag and his collaborators prevent the glycoprotein coat of the vesicular stomatitis virus and the interaction of baby hamster kidney cells (Donskyi et al. 2018). Therefore, blocking between viruses and host cells is efficient therapeutic strategy to conquer viral infections. The third strategy to prevent viral infection is inhibiting the viral replication. By suppressing the expression of certain enzymes require for the viral DNA or RNA replication, these destroy the viral replication inside the host cell. As known, the offspring of a mother virus is more virulent. Therefore, the inhibition of virus budding and its excretion from host cells are another antiviral functional nanoparticle mechanism. This through prevent viral binding and reduce the number of viral offspring resulting in decreasing its virulence (Chen and Liang 2020).
5 Conclusion
Because of the virus’s unusual behavior and particular viral metabolic activities, developing an effective management plan is difficult. If SARS-CoV-2 undergoes a genetic change, like the influenza virus did, this might be a limiting step in controlling viral transmission. Finally, nanomaterials offer numerous tools for use as nanotherapeutics against respiratory viral infections. The antiviral efficiency of metallic nanoparticles is a significant and potentially solution to the present SARS-CoV-2 pandemic; nanoparticles are capable of interfacing with virus particles. As a result, additional research in the chemistry and biology of nanotechnology is needed to design multifunctional nanoparticles that may be used as drug nanocarriers.
References
AbouAitah K et al (2020) Virucidal action against avian influenza H5N1 virus and immunomodulatory effects of nanoformulations consisting of mesoporous silica nanoparticles loaded with natural prodrugs. Int J Nanomedicine 15:5181
Aderibigbe BA (2017) Metal-based nanoparticles for the treatment of infectious diseases. Molecules 22(8):1370
Ahmed MK, Afifi M, Uskoković V (2020a) Protecting healthcare workers during COVID-19 pandemic with nanotechnology: a protocol for a new device from Egypt. J Infect Public Health 13(9):1243–1246. https://doi.org/10.1016/j.jiph.2020.07.015. Epub 2020 Aug 3
Ahmed MK, Afifi M et al (2020b) Protecting healthcare workers during COVID-19 pandemic with nanotechnology: a protocol for a new device from Egypt. J Infect Public Health 13(9):1243–1246
Badgujar CK, Ram AH, Zanzany R et al (2020) Remdesivir for COVID-19: a review of pharmacology, mechanism of action, in-vitro activity and clinical use based on available case studies. J Drug Deliv Therap 10(4-s):264–270
Betakova T, Nermut MV, Hay AJ (1996) The NB protein is an integral component of the membrane of influenza B virus. J Gen Virol 77(Pt 11):2689–2694. https://doi.org/10.1099/0022-1317-77-11-2689
Boncristiani HF, Criado MF, Arruda E (2009) Respiratory viruses. In: Encyclopedia of microbiology, pp 500–518
Branche AR, Falsey AR (2016) Parainfluenza virus infection. Semin Respir Crit Care Med 37(4):538–554. https://doi.org/10.1055/s-0036-1584798
Brister JR, Ako-Adjei D, Bao Y et al (2014) NCBI viral genomes resource. Nucleic Acids Res 2014:1. https://doi.org/10.1093/nar/gku1207
Cagel M, Tesan FC, Bernabeu E et al (2017) Polymeric mixed micelles as nanomedicines: achievements and perspectives. Eur J Pharm Biopharm 113:211–228. pii: S0939-6411(16)30694-4
Cagno V, Androzzi PD, Alicarnasso M et al (2018) Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat Mater 17(2):195–203
Capeletti LB et al (2018) Silica nanoparticle applications in the biomedical field. In: Smart nanoparticles for biomedicine. Elsevier, Amsterdam, pp 115–129
Chakravarthy KV et al (2010) Gold nanorod delivery of an SsRNA immune activator inhibits pandemic H1N1 influenza viral replication. Proc Natl Acad Sci U S A 107(22):10172–10177. https://doi.org/10.1073/pnas.0914561107
Chen YH, Lai HJ (2013) Acute hemorrhagic colitis after oral administration of oseltamivir for influenza. Gastrointest Endosc 77(6):976. https://doi.org/10.1016/j.gie.2013.02.005
Chen L, Liang J (2020) An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater Sci Eng C 112:110924
Chen N, Zheng Y, Yin J et al (2013) Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J Virol Methods 193:470–477
Cojocaru F-D et al (2020) Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics 12(2):171. https://doi.org/10.3390/pharmaceutics12020171
Cortes AA, Zuñiga JM (2020) The use of copper to help prevent transmission of SARS-coronavirus and influenza viruses. A general review. Diagn Microbiol Infect Dis 98:115176
Das C et al (2020) Silver-based nanomaterials as therapeutic agents against coronaviruses: a review. Int J Nanomedicine 15:9301
De Souza E, Silva JM et al (2016) Viral inhibition mechanism mediated by surface-modified silica nanoparticles. ACS Appl Mater Interfaces 8(26):16564
Dhama K, Pawaiya RVS, Chakrabort S et al (2014) Coronavirus infection in equines: a review. Asian J Anim Vet Adv 9:164–176
Dhanasezhian A, Srivani S, Govindaraju K et al (2019) Anti-Herpes Simplex Virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Indian J Geo Mar Sci 48:1252–1257
Dhand C, Dwivedi N, Loh XJ et al (2015) Methods and strategies for the synthesis of diverse nanoparticles and their applications: a comprehensive overview. RSC Adv 5(127):105003–105037
Donskyi I, Druke M, Silberreis K et al (2018) Interactions of fullerene-polyglycerol sulfates at viral and cellular interfaces. Small 14(17):1800189
Dos Santos CA, Seckler MM, Ingle AP et al (2014) Silver nanoparticles: therapeutical uses toxicity, and safety issues. J Pharm Sci 103(7):1931–1944
El-Sheekh MM, Shabaan MT, Hassan L et al (2020) Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int J Environ Health Res. https://doi.org/10.1080/09603123.2020.1789946
Fang RH, Kroll AV, Gao W et al (2018) Cell membrane coating nanotechnology. Adv Mater 30. https://doi.org/10.1002/adma.201706759
Farouk F, Shebl RI (2018) Comparing surface chemical modifications of zinc oxide nanoparticles for modulating their antiviral activity against herpes simplex virus type-1. Int J Nanopart Nanotechnol 4(021):1–14
Fluhmann B, Ntai I, Borchard G et al (2018) Nanomedicines: the magic bullets reaching their target? Eur J Pharm Sci 128:73–80
Gaikwad S, Ingle A, Gade A, Incoronato N, Russo L, Galdiero S, Galdiero M (2013) Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine 8(1):4303–4314. https://doi.org/10.2147/IJN.S50070
Ghaffari H, Tavakoli A, Moradi A et al (2019) Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci 26:70. https://doi.org/10.1186/s12929-019-0563-4
Goossens H (2009) Antibiotic consumption and link to resistance. Clin Microbiol Infect 15(3):12–15. https://doi.org/10.1111/j.1469-0691.2009.02725.x
Guisado-Macías JA, Rodríguez FF, Méndez-Sánchez F et al (2012) Oseltamivir-related psychiatric manifestations. Actas Espanolas de Psiquiatria 40(1):46–48. https://europepmc.org/article/med/22344496
Gulati S, Sachdeva M, Bhasin KK (2018) Emerging applications of nanoparticles: biomedical and environmental. AIP Conf Proc 1953:030213. https://doi.org/10.1063/1.5032548
Guo H, Wan X, Niu F et al (2019) Evaluation of antiviral effect and toxicity of total flavonoids extracted from Robinia pseudoacacia cv. idaho. Biomed Pharmacother 118:109809. https://doi.org/10.1016/j.biopha.2019.109335
Gurunathan S, Qasim M, Choi Y et al (2020a) Antiviral potential of nanoparticles-can nanoparticles fight against coronaviruses? Nanomaterials (Basel, Switzerland) 10(9):1645. https://doi.org/10.3390/nano10091645
Gurunathan S et al (2020b) Antiviral potential of nanoparticles—can nanoparticles fight against coronaviruses? Nanomaterials 10(9):1645
Haas LE, Thijsen SF, van Elden L et al (2013) Human metapneumovirus in adults. Viruses 5(1):87–110. https://doi.org/10.3390/v5010087
Haggag EG, Elshamy AM, Rabeh MA et al (2019) Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int J Nanomedicine 14:6217–6229. https://doi.org/10.2147/IJN.S214171
Hang X, Peng H, Song H et al (2015) Antiviral activity of cuprous oxide nanoparticles against hepatitis C virus in vitro. J Virol Methods 222:150–157
Hofmann M, Kleine-Weber H, Krüger N et al (2020) The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. Mol Biol. https://doi.org/10.1101/2020.01.31.929042
Hu C-MJ et al (2018) Antiviral efficacy of nanoparticulate vacuolar ATPase inhibitors against influenza virus infection. Int J Nanomedicine 13:8579–8593. https://doi.org/10.2147/ijn.s185806
Huang X et al (2019) Novel gold nanorod-based HR1 peptide inhibitor for Middle East respiratory syndrome coronavirus. ACS Appl Mater Interfaces 11(22):19799–19807. https://doi.org/10.1021/acsami.9b04240
Ingale AG, Chaudhari AN (2013) Biogenic synthesis of nanoparticles and potential applications: an eco-friendly approach. J Nanomed Nanotechol 4(165):1–7
Ishida T (2019) Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell-virus growth inhibition. Am J Biomed Sci Res 2:28–37
Jacobs SE, Lamson DM, St George K (2013) Human rhinoviruses. Clin Microbiol Rev 26(1):135–162. https://doi.org/10.1128/CMR.00077-12
Kaufman HL, Kohlhapp FJ, Zloza A (2015) Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 14:642–664
Kerry RG, Malik S, Redda YT (2019) Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomedicine 18:196–220
Keyser LA, Karl M, Nafziger AN et al (2000) Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch Intern Med 160(10):1485–1488. https://doi.org/10.1001/archinte.160.10.1485
Khandel P, Yadaw RK, Soni DK, Kanwar L, Shahi SK (2018) Biogenesis of metal nanoparticles and their pharmacological applications: present status and application prospects. J Nanostruct Chem 8(3):217–254
Kiso M, Mitamura K, Sakai-Tagawa Y et al (2004) Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364(9436):759–765. https://doi.org/10.1016/S0140-6736(04)16934-1
Körner RW, Söderlund-Venermo M, van Koningsbruggen-Rietschel S et al (2011) Severe human bocavirus infection, Germany. Emerg Infect Dis 17(12):2303–2305. https://doi.org/10.3201/eid1712.110574
Kumar P et al (2016) Triple drug combination of zidovudine, efavirenz and lamivudine loaded lactoferrin nanoparticles: an effective nano first-line regimen for HIV therapy. Pharm Res 34(2):257–268. https://doi.org/10.1007/s11095-016-2048-4
Kumar R, Nayak M, Sahoo GC et al (2019) Iron oxide nanoparticles based antiviral activity of H1N1 influenza a virus. J Infect Chemother 25(5):325–329
Kupferschmidt K, Cohen J (2020) Race to find COVID-19 treatments accelerates. Science 367(6485):1412–1413
Kutter JS, Spronken MI, Fraaij PL et al (2018) Transmission routes of respiratory viruses among humans. Curr Opin Virol 28:142–151
LaBauve AE et al (2018) Lipid-coated mesoporous silica nanoparticles for the delivery of the ML336 antiviral to inhibit encephalitic alphavirus infection. Sci Rep 8(1):1–13
Lei L, Sun RWY, Chen R et al (2008) Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther 13:252–262
Lembo D, Donalisio M, Civra A et al (2018) Nanomedicine formulations for the delivery of antiviral drugs: a promising solution for the treatment of viral infections. Expert Opin Drug Deliv 15(1):93–114. https://doi.org/10.1080/17425247.2017.1360863
Li B, Armstead AL (2011) Nanomedicine as an emerging approach against intracellular pathogens. Int J Nanomedicine 6:3281. https://doi.org/10.2147/ijn.s27285
Li Y, Lin Z, Guo M et al (2017) Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int J Nanomedicine 12:5733–5743. https://doi.org/10.2147/ijn.s140939
Li H, Liu S, Yu X, Tang S et al (2020) Coronavirus disease 2019 (COVID-19): current status and future perspective. Int J Antimicrob Agents 55:105951
Lin Z, Li Y, Guo M et al (2017) Inhibition of H1N1 influenza virus by selenium nanoparticles loaded with zanamivir through p38 and JNK signaling pathways. RSC Adv 7(56):35290–35296
Łoczechin A et al (2019) Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl Mater Interfaces 46:42964–42974. https://doi.org/10.1021/acsami.9b15032
Lozano R, Naghavi M, Foreman K et al (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128
Lu R, Zhao X, Li J et al (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224):565–574
Maduray K, Parboosing R (2020) Metal nanoparticles: a promising treatment for viral and Arboviral infections. Biol Trace Elem Res 199(8):3159–3176. https://doi.org/10.1007/s12011-020-02414-2
Mainardes RM, Diedrich C (2020) The potential role of nanomedicine on COVID-19 therapeutics. Ther Deliv 11(7):411–414. https://doi.org/10.4155/tde-2020-0069
Malathi VG, Renuka DP (2019) ssDNA viruses: key players in global virome. Virusdisease. https://doi.org/10.1007/s13337-019-00519-4
Matamoros T, Álvarez M, Barrioluengo V et al (2011) Reverse transcriptase and retroviral replication. IntechOpen, Rijeka, Croatia. https://doi.org/10.5772/21660
McClellan K, Perry CM (2001) Oseltamivir: a review of its use in influenza. Drugs 61(2):263–283. https://doi.org/10.2165/00003495-200161020-00011. Erratum in: Drugs 61(6):775
Mehranfar A, Izadyar M (2020) Theoretical design of functionalized gold nanoparticles as antiviral agents against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). J Phys Chem Lett 11:10284–10289
Mehta M et al (2020) Advanced drug delivery systems can assist in targeting coronavirus disease (COVID-19): a hypothesis. Med Hypotheses 144:110254
Michalet X, Pinaud FF, Bentolila LA et al (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538–544
Milovanovic M, Arsenijevic A, Milovanovic J et al (2017a) Nanoparticles in antiviral therapy. In: Antimicrobial nanoarchitectonics: from synthesis to applications, 1st edn. Elsevier Inc., Amsterdam, pp 383–410
Milovanovic M, Arsenijevic A, Milovanovic J et al (2017b) Nanoparticles in antiviral therapy. In: Antimicrobial nanoarchitectonics. Elsevier, Amsterdam, pp 383–410. https://doi.org/10.1016/B978-0-323-52733-0.00014-8
Modrow S, Falke D, Truyen U et al (2013) Viruses with single-stranded, positive-sense RNA genomes. In: Molecular virology. Springer, Berlin. https://doi.org/10.1007/978-3-642-20718-1_14
Mori Y, Ono T, Miyahira Y et al (2013) Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res Lett 8:93
Morris D, Ansar M, Speshock J (2019) Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses 11:732
National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases (2021). https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
Pantidos N (2014) Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J Nanomed Nanotechnol 5(5):1–10
Patel P et al (2015) Plant-based synthesis of silver nanoparticles and their characterization. In: Nanotechnology and plant science. Springer, New York, pp 271–288
Patra JK, Das G, Fraceto LF et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16:1–33
Pemmada R et al (2020) Science-based strategies of antiviral coatings with viricidal properties for the COVID-19 like pandemics. Materials 13(18):4041
Prasad M, Lambe UP, Brar B et al (2018) Nanotherapeutics: an insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother 97:1521–1537
Rafiei S, Rezatofighi SE, Ardakani MR et al (2016) Gold nanoparticles impair foot-and-mouthdisease virus replication. IEEE Trans Nanobiosci 15:34–40
Raha S et al (2020) Is copper beneficial for COVID-19 patients? Med Hypotheses 2020:109814
Rai M, Deshmukh SD, Ingle AP et al (2016) Metal nanoparticles: the protective nanoshield against virus infection. Crit Rev Microbiol 42:46–56
Salleh A, Naomi R, Utami ND et al (2020) The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials 10(8):1566. https://doi.org/10.3390/nano10081566
Sandegren L, Andersson DI (2009) Bacterial gene amplification: implications for the evolution of antibiotic resistance. Nat Rev Microbiol 7:578–588. https://doi.org/10.1038/nrmicro2174
Schoeman D, Fielding BC (2019) Coronavirus envelope protein: current knowledge. Virol J 16:69
Shafagati N, Williams J (2018) Human metapneumovirus - what we know now. F1000Res 7:135. https://doi.org/10.12688/f1000research.12625.1
Shah M, Fawcett D, Sharma S et al (2015) Green synthesis of metallic nanoparticles via biological entities. Materials 8(11):7278–7308
Sharmin S, Rahaman MM, Sarkar C et al (2021) Nanoparticles as antimicrobial and antiviral agents: a literature-based perspective study. Heliyon 7:e06456. https://doi.org/10.1016/j.heliyon.2021.e06456
Simón-Talero M, Buti M, Esteban R (2012) Severe anaemia related to oseltamivir during treatment of chronic hepatitis C: a new drug interaction? J Viral Hepat 19:14–17. https://doi.org/10.1111/j.1365-2893.2011.01521.x
Singh L, Kruger HG, Maguire GEM (2017) The role of nanotechnology in the treatment of viral infections. Ther Adv Infect Dis 4(4):105–131
Stephen BJ, Suchanti S, Mishra R et al (2020) Cancer nanotechnology in medicine: a promising approach for cancer detection and diagnosis. Crit Rev Ther Drug Carrier Syst 37(4):375–405. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2020032634
Sumbria D, Berber E, Mathayan M et al (2021) Virus infections and host metabolism—can we manage the interactions? Front Immunol 11. https://doi.org/10.3389/fimmu.2020.594963
Tarn D et al (2013) Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc Chem Res 46(3):792–801
Tavakoli A, Hashemzadeh MS (2019) Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J Virol Methods 275:113688. https://doi.org/10.1016/j.jviromet.2019.113688. Epub 2019 Jul 2
Tavakoli A, Ataei-Pirkooh A, Sadeghi GM et al (2018) Polyethylene glycol-coated zinc oxide nanoparticle: an efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine 13:2675–2690
Theobald N (2020) Emerging vaccine delivery systems for COVID-19: functionalised silica nanoparticles offer a potentially safe and effective alternative delivery system for DNA/RNA vaccines and may be useful in the hunt for a COVID-19 vaccine. Drug Discov Today 25(9):1556–1558
Ting D et al (2018) Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl Nano Mater 10:5451–5459. https://doi.org/10.1021/acsanm.8b00779
Uskokovic V (2020) Why have nanotechnologies been underutilized in the global uprising against the coronavirus pandemic? Nanomedicine (Lond). https://doi.org/10.2217/nnm-2020-0163. Epub ahead of print
Van Doorn HR, Yu H (2020) Viral respiratory infections. In: Hunter’s tropical medicine and emerging infectious diseases. Elsevier, Amsterdam, pp 284–288. https://doi.org/10.1016/B978-0-323-55512-8.00033-8
Van Doremalen N et al (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382(16):1564–1567
Von Nussbaum F, Brands M, Hinzen B et al (2006) Antibacterial natural products in medicinal chemistrydexodus or revival? Angew Chem Int Ed 45(31):5072e129
Walsh EE, Peterson DR, Falsey AR (2008) Human metapneumovirus infections in adults: another piece of the puzzle. Ann Intern Med 168:2489–2496. https://doi.org/10.1001/archinte.168.22.2489
Wan Y, Shang J, Graham R et al (2020) Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94:e00127–e00120
Wang J (2020) Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J Chem Inf Model 60(6):3277–3286
WHO (2022) Coronavirus Update, 83,527,714 confirmed cases and 1,819,905 deaths from COVID-19 virus outbreak. WHO, Geneva. https://covid19.who.int/. Accessed 5 Jan 2021
Wolf Y, Kazlauskas D, Iranzo J (2018) Origins and evolution of the global RNA virome. mBio 9:e02329–e02318. https://doi.org/10.1128/mBio.02329-18
World Health Organization (2003) Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). World Health Organization, Geneva. hdl:10665/70863
World Health Organization (WHO). World Health Statistics (2016) Monitoring health for the SDGs sustainable development goals. World Health Organization, Geneva, Switzerland
Wrapp D, Wang N, Corbett KS et al (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367:1260–1263
Wu F, Zhao S, Yu B et al (2020a) A new coronavirus associated with human respiratory disease in China. Nature 579:265–269
Wu Y, Ho W, Huang Y et al (2020b) SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet 395(10228):949–950
Yan R et al (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367(6485):1444–1448
Yarnall AJ, Burn DJ (2012) Amantadine-induced myoclonus in a patient with progressive supranuclear palsy. Age Ageing 41(5):695–696. https://doi.org/10.1093/ageing/afs043
Yu C-H, Tam K, Tsang ES (2008) Chemical methods for preparation of nanoparticles in solution. Handb Metal Phys 5:113–141
Zazo H, Colino CI, Lanao JM (2016) Current applications of nanoparticles in infectious diseases. J Control Release 224:86–102
Zhou P, Yang X-L, Wang X-G et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273
Zhou B, Thao TTN, Hoffmann D et al (2021) SARS-CoV-2 spike D614G change enhances replication and transmission. Nature 592:122–127. https://doi.org/10.1038/s41586-021-03361-1
Zhu J-D, Meng W, Wang X-J et al (2015) Broad-spectrum antiviral agents. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.00517/abstract
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
No funding.
Availability of Data
The datasets generated or analyzed during the review are available from the corresponding author on reasonable request.
Competing Interests
“The authors declare that they have no competing interests”.
Author Contribution
Heba S. Abbas was responsible for sharing the idea, determining the points and objectives, writing introduction abstract, conclusion, sharing in nanoparticles parts, reviewing, rearrange for whole review and draw Fig. 6.2. Hosam Saleh wrote the classification of viruses and draw Fig. 6.1. Esraa M. M. Mohammad and Ebthal F. M. Elzayat wrote respiratory viruses part. Also, Hala A. Abdelgaid wrote SARS-CoV2 threat, and nanoparticles, Amira S. H. Mohamed shared in writing nanoparticles, Amany Y. El-Sayed shared in writing the antiviral mechanism of nanoparticles, Noha M. Gamil wrote the toxicity of antiviral drugs, and Salma E. S. Ismail wrote organic nanoparticles.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Abbas, H.S. et al. (2022). The Future Therapy of Nanomedicine Against Respiratory Viral Infections. In: Hameed, S., Rehman, S. (eds) Nanotechnology for Infectious Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-16-9190-4_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-9190-4_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9189-8
Online ISBN: 978-981-16-9190-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)