Abstract
Currently, engineered nanomaterials are used in a wide range of applications and enter the aquatic environment directly via consumer applications and industrial waste or by unintended discharge. In the coming years, the production rate of nanomaterials is bound to grow, and so are their predicted environmental levels. The toxicological approaches are of significant importance and require noteworthy attention for a sustainable ecosystem. The risk assessment of nanomaterials is, however, a very intricate process. Thus, in this chapter, we aim to provide basic information on the toxic aspects of engineered nanomaterials to freshwater microalgae and fish. The initial section deals with the release of nanomaterials and the principles of their toxicity. The later part of the chapter discusses the toxic impacts of metallic, carbon-based, and metal oxide nanoparticles.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
Nanotechnology in recent years has diversified its applications in various fields of medicine, consumer products, and also the environment. Nanomaterials are defined as materials with the external dimensions in the nanoscale or having an internal or surface structure in the nanoscale (1–100 nm range). They occur in various forms that include nanoparticles (NPs), nanotubes, nanocomposites, nanofibers, and nanowires. The behavior of most nanomaterials in the environment depends on their size, shape, surface reactivity, and degree of agglomeration (Sengul and Asmatulu 2020). The unique physicochemical properties such as extremely small size, large surface area to volume ratio, and size-dependent optical properties are the reasons that make the NPs versatile (Sajid et al. 2015). For instance, titanium dioxide and zinc oxide NPs have been widely used in cosmetic and beauty products as sun-guard to shield the skin against the penetration of harmful ultraviolet rays (Stark et al. 2015). Gold NPs are widely explored in the biomedical industry owing to their easy modification, tunable size, and strong optical properties (Jia et al. 2017). Silver NPs are vastly utilized as antimicrobial agents due to their antibacterial, antifungal, antifilarial, and antiviral properties (Cameron et al. 2018).
Despite their application across various fields (Fig. 6.1), NPs pose a threat of exposure and unfavorable effects on the environment and organisms. With a surge in their production, it is quite certain that the nanomaterials will end up in the aquatic systems (Moore 2006). This is concerning because most of the industrial wastes are washed off into the water bodies (lakes, drainage ditches, rivers, and oceans) despite safety measures. The accidental spillage or the permitted release of the NPs in the form of industrial effluent can result in direct exposure to humans via skin contact, inhalation of aerosols, and direct ingestion of contaminated water or food and vegetables coated with NPs (Liu et al. 2014). Besides, indirect exposure could also result from the ingestion of fish and mollusks contaminated with NPs expelled into the water bodies.
Even though the use of NPs has contributed significantly to the improvement of various fields over recent years, their application raises serious concerns regarding the exposure and adverse effects on the environment and organisms. Recent studies in this area have investigated the toxicological impacts and the various hazards of the exposure of NPs towards the environment but there is still a notable gap of knowledge regarding the toxicities of different NPs and their effects on freshwater organisms.
Release of NPs in the Environment
The discharge of nanomaterials in the aquatic ecosystem can either be accidental or deliberate. They are released into the environment during the different phases of their life cycle, from production to release (Nowack and Bucheli 2007). The run-offs from the nanotechnology-based industries are one of the major sources of nanomaterials in the aquatic environment (Daughton 2004). Once entered the aquatic environment, the fate of the nanomaterials depends upon several factors such as natural organic matter content, pH, and ionic strength. The nanomaterials can easily enter the aquatic organisms via endocytosis and phagocytosis and are passed onto the higher trophic levels via ingestion of those lower-level organisms. Hence, it is of utmost importance to study nanomaterials’ potential toxic effects and health hazards.
Principles of NP Toxicity
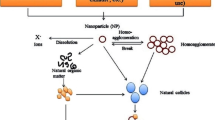
Several factors may alter the toxicity of NPs such as (1) physicochemical properties of NP, (2) functional behavior of NPs, and (3) interaction with other pollutants in the aquatic environment (Turan et al. 2019). NPs tend to show unique and greater toxicity as compared to their bulk counterparts. This can be attributed to their small size and relatively high surface area. The toxicity of NPs generally depends on their size. Particle size affects the cellular uptake of NPs in organisms. A study conducted by Chithrani and Chan (2007) showed that there could be an optimal size for NP uptake. Similarly, the surface charge of the particle plays a crucial role in determining the toxicity of the NPs. Overall, the positively charged NPs are quickly adsorbed to cells as compared to the negatively charged ones due to the net negative charge of the cell surfaces. Therefore, the higher toxicity of the positively charged NPs could be attributed to their higher cellular uptake (Oh et al. 2012). In addition to particle size and surface charge, the shape can also influence the toxicity of the NPs. It determines whether NPs are phagocytosed by the cells. In addition to phagocytosis, NPs can enter the cells by physically piercing or rupturing the cell membranes. Carbon nanotubes (CNTs), for example, have been suggested by several researchers to employ their toxicity by the virtue of their needle-like structure that provides a high aspect ratio to rupture the cell membranes. This can apply to some of the multiwall carbon nanotubes (MWCNTs) with a relatively high diameter and rigidity (Nagai et al. 2011). Since the toxicity of NPs is affected by the material properties, the toxic effects of NPs will be discussed in the next sections by the main classes of nanomaterials based on the composition (Fig. 6.2.).
Toxic Effects of Metallic NPs
Metallic NPs are usually composed of a metal core of an inorganic metal. This is generally covered with a shell consisting of organic or inorganic materials or metal oxide (Khan 2019). Metallic NPs have various applications in day-to-day life. Since the new techniques of NP production have become economically feasible, there has been a surge in the use of metallic NPs in various consumer products like shampoos, creams, footwear, clothing, and also plastic containers (Diegoli et al. 2008).
Even though NPs have proved to be essential in a broad aspect, the fact that they pose various health risks to organisms, as well as the environment, cannot be avoided. In recent years, studies conducted by several researchers using gold and silver NPs have proved this notion. But still, there is a gap of knowledge when it comes to determining the toxic effects of specific metal NPs in freshwater organisms. This is discussed in the next sections.
Gold
Gold NPs (Au-NPs) have been extensively studied for potential use in the biomedical field especially for diagnostics, drug delivery, therapeutics, and cancer treatment (Jia et al. 2017). This is primarily because of the unique characteristics of the Au-NPs. The increased use of Au-NPs has led to its increased diffusion into the environment that comes with an unavoidable risk towards the aquatic organisms. The cytotoxic effects of Au-NPs have been reported in mammalian cells, and also their shape, size, and external coating play a major role in enhancing their toxic effects (Chueh et al. 2014). Although studies on the toxic effects of Au-NPs have been done on mammalian cell lines, research on their toxicity on aquatic organisms is still scarce. Hence it is crucial to investigate the harmful effects of Au-NPs on aquatic organisms to provide a background for ecotoxicological hazard review.
The ecotoxic effects of two polymer-coated Au-NPs were observed by Hoecke et al. on the freshwater microalgae Pseudokirchneriella subcapitata (Van Hoecke et al. 2013). In aquatic environments, agglomeration/aggregation of Au-NPs is common and does not necessarily decrease NP toxicity but could facilitate ingestion. Gilroy et al. (2014) established the potential transfer of Au-NPs in the food webs and stated that most NPs that remained in the digestive tract did not affect reproduction and were eliminated with ejection. Iswarya et al. (2017) explored the impact of Zn2+ present in the freshwater environment at an average concentration of <0.05 mg/L, on the toxicity of Au-NPs with different sizes and surface capping (citrate and PVP) to the green alga Scenedesmus obliquus. They found that as the concentration of Au-NPs increased, the relative toxicity of all the types of Au-NPs tested increased. Citrate-capped Au-NPs were found to be more toxic than PVP-capped Au-NPs, and the toxicity depended also on NP size. It was confirmed that Zn ions showed an antagonistic ability to interfere with the toxicity caused by Au-NPs on green algae Scenedemus sp. independently from the surface capping.
Zebrafish (Danio rerio) are increasingly being employed as an in vivo model to assess Au-NP toxicity. The impact of Au-NP (12 and 50 nm) exposure in the food of zebrafish showed that exposure even in the low doses can result in various cellular dysfunctions and cause genomic alterations (Geffroy et al. 2012). Smaller malpigmented eyes were seen after zebrafish embryos were exposed to 1.3 nm Au-NPs functionalized with a cationic ligand, N,N,N-trimethylammoniumethanethiol (TMAT-Au-NPs) (Kim et al. 2013). This was related to the increase in cell death in the eyes caused by the overexpression of genes p53 and bax. The effects of contaminated sediment containing Au-NPs were investigated during a 20-day exposure study of D. rerio. The chronic exposure resulted in a series of detrimental effects on the tissues of the organism including increased expression of genes involved in oxidative stress, mitochondrial metabolism, and modifications in the genome (Dedeh et al. 2015). A study comparing the different terminal modifications of Au-NPs on zebrafish using peptide-capped Au-NPs revealed that the terminal alteration was essential with terminal histidines causing higher toxicity than terminal tryptophans, and methionine causing the least toxicity (Harper et al. 2014). The biodistribution of differently shaped Au-NPs (nanospheres, nanorods, nano-urchins, and nanobipyramids) on the toxicity of zebrafish revealed shape-dependent biodistribution patterns after exposure to different-shaped gold particles. The differently shaped particles were found to be distributed in different ratios in the digestive organs such as the gall bladder, liver, and pancreas. The biodistribution patterns suggested that long-term exposure could cause shape-dependent sublethal consequences (van Pomeren et al. 2019).
Silver
Studies exposing cultures of algae and zooplankton to engineered Ag-NPs have revealed several factors that may alter the toxicity of Ag-NPs (Zhao and Wang 2010; Das et al. 2013). The trophic transfer of Ag-NPs may be altered by the nature of the exposure (waterborne or diet borne) (Zhao and Wang 2011). Significant effort has been made to study the contribution of silver ions on Ag-NP toxicity to algae and zooplankton (Navarro et al. 2008b; Das et al. 2013). It has been shown that the presence of algae may trigger the release of silver ions from Ag-NPs and consequently alter their toxicity (Navarro et al. 2008b). At the organism level, physiological and functional characteristics may also alter the toxicity of Ag-NPs (Oukarroum et al. 2012; Pokhrel et al. 2013). For instance, Chlorella vulgaris could efficiently detoxify Ag-NP induced ROS species via the induction of antioxidant enzymes, allowing photosynthesis to continue even at high Ag-NPs concentrations (Qian et al. 2016). The toxicity of Ag-NPs was higher in cultures at the early phases of growth. Finally, Ag-NP toxicity was different depending on the examined species according to species sensitivity distributions (SSDs) (Coll et al. 2016). This was also observed in the present literature review where vulnerability to Ag-NP toxicity was higher for D. magna compared to other daphnids (Völker et al. 2013), for D. galeata compared to D. magna and Bosmina longirostris (Sakamoto et al. 2015), for Dunaliella tertiolecta compared to C. vulgaris (Oukarroum et al. 2012), and for Microcystis aeruginosa (prokaryotic) compared to C. vulgaris (eukaryotic) (Qian et al. 2016).
In a size-dependent (30–72 nm) in vivo study conducted on zebrafish, it was observed that Ag-NPs were able to diffuse into the embryos via Brownian motion through chorionic pores, thereby generating toxicity (Lee et al. 2012). Bar-Ilan et al. (2009), on the other hand, synthesized Ag-NPs of various sizes (3, 10, 50, and 200 nm) and applied them to zebrafish embryos in a rearing container. They discovered size-independent mortality rates after 120 h post-fertilization (Bar-Ilan et al. 2009). In another investigation, Ag-NPs were discovered to have a size-dependent effect on the neural development of zebrafish embryos. Four-nm Ag-NPs were taken up more efficiently than 10-nm Ag-NPs in this circumstance, with the exposed zebrafish embryos’ heads accumulating more Ag-NPs than the trunks (Xin et al. 2015). As a result, the size-dependent toxicity profile of Ag-NPs remains a point of contention. In both fish cell lines and zebrafish embryos, George et al. (2012) confirmed the surface defect-driven toxicity of Ag-NPs. Surface reactivity caused by crystal defects increased the toxicity of Ag nanoplates compared to other Ag-NPs. According to another study, Ag-peptide NPs were substantially more biocompatible than citrate-coated Ag-NPs (Lee et al. 2013). The study showed that the combination of numerous physicochemical properties of the NPs defined their harmful effects on embryonic development, emphasizing the significance of investigating their effects one factor at a time. Another investigation revealed that exposing Ag-NPs to simulated sunlight increased their embryonic toxicity (George et al. 2014). Zebrafish exposed to Ag-NPs during their early development experienced a variety of side effects, including a decrease in heart rate, damage to neuromast hair cells, and smaller but statistically significant increases in mortality and teratogenicity (Yoo et al. 2016). After chronic exposure to Ag-NPs, a recent study looked at the reproductive toxicity and associated probable adverse outcome pathway (AOP) in zebrafish. Adult zebrafish (three months old) were treated with varying concentrations (0, 10, 33, and 100 µg/L) of Ag-NPs for five weeks and the results were observed. Female zebrafish fertility was dramatically reduced after exposure to 33 and 100 µg/L Ag-NPs, which was accompanied by an increase in apoptotic cells in the ovarian and testicular tissue (Ma et al. 2018). The size-related effects of chronic Ag-NPs exposure on intestinal Na/K-ATPase and SOD activities in adult zebrafish were suggested in a recent study. The study also revealed Ag-NPs had higher toxicity in the intestine than in the liver, showing that Ag-NPs have organ-specific toxicity. It was also demonstrated in this study that the response of zebrafish to Ag-NPs was sex-dependent since the males showed more susceptibility as compared to the females (Bao et al. 2020).
Toxic Effects of Carbon-based Nanomaterials
Carbon nanomaterials (CNMs) can be described as the allotropes of carbon that have at least one of their dimensions in the range of 1- 100 nm. The major classes of CNMs are fullerenes, CNTs, graphene, and carbon black. These materials can originate in diverse ways, some of these could be liberated into the environment naturally (in consequence of forest fires or volcanic eruptions), whereas others could be produced by anthropogenic combustions or could also be manufactured in industries (Freixa et al. 2018). Due to their unique physicochemical, mechanical, and electrical properties, they have been used in various fields such as engineering, sports equipment, optics, automotive industry, cosmetic and medical applications (Navarro et al. 2008a). The increased usage of CNMs has increased the exposure risk of the aquatic environment to CNMs. Hence it is evident that methodologies are devised to study the effects of CNMs on the organisms to get cumulative knowledge on their toxic effects and potential bioaccumulation.
Algae are one of the most sensitive organisms to CNMs. It is reported that the toxicity of CNMs to algal cells can be both directly related to their exposure as well as to the indirect effects such as shading effects by the nanomaterials (resulting in reduced light absorption and photosynthesis) and to the nutrient depletion caused by the absorption of nutrients on CNMs (Schwab et al. 2011; Long et al. 2012; Zhao et al. 2017). For instance, Zhao et al. (2017) studied the toxicity of graphene nanomaterials to freshwater algae (Chlorella pyrenoidosa) showing that graphene significantly decreased the membrane integrity of algal cells. Sensitivity to CNM exposure differs between species. For instance, the growth rate of C. vulgaris was more strongly affected at lower CNT concentrations (EC50 = 1.8 mg/L) than that the growth rate of P. subcapitata (EC50 = 20 mg/L) (Schwab et al. 2011). Similar toxicity of CNTs for P. subcapitata (EC50 = 17.95 mg/L) was also reported in another study (Lukhele et al. 2015). Moreover, differences in toxicity to CNMs observed between planktonic and biofilm communities have been attributed to the presence of extracellular polymeric substances (EPS) matrix (Luongo and Zhang 2010; Rodrigues and Elimelech 2010), where planktonic communities were more severely affected by CNM exposure. The protective role of EPS to pollutants has been widely studied (Flemming and Wingender 2010). Other studies have observed fast overproduction of EPS in algae after exposure to high concentrations of CNMs, which was interpreted as a natural defensive mechanism against double-walled CNTs (Verneuil et al. 2015b), MWCNT (Verneuil et al. 2015a), or graphene (Garacci et al. 2017).
The bioaccumulation and distribution of multiwalled CNTs were recently investigated using a zebrafish model, which revealed a bioaccumulation factor of 16 L/kg fish wet weight (Maes et al. 2014). Li et al. (2015) discovered CNT-induced biochemical changes in zebrafish. They showed that CNT exposure can activate the brain and cause gonadal changes. In another study, the toxicity of functionalized CNTs of various lengths was assessed in zebrafish embryos, with the conclusion that the length of CNTs has a significant impact on their toxicity profile in vivo (Cheng and Cheng 2012). Another study found that single-wall (SW)CNTs functionalized with polyethylene glycol increased mortality, delayed hatching, and decreased overall larval length only at the highest dosage examined (1 mg/L), with no evidence of genotoxicity or nanotube uptake by tissues (Girardi et al. 2017). A study using oxidized-MWCNT along with Cd showed that oxidized-MWCNT promoted apoptosis and necrosis in ZFL (zebrafish liver cell lines) cells and increased Cd toxicity at low concentrations, most likely through a “Trojan horse” and/or synergistic action (Morozesk et al. 2018). In another study, Ren et al. (2021) explored the effect of MWCNTs on the enantioselectivity of bioaccumulation of a chiral insecticide indoxacarb in zebrafish and found that MWCNTs did not affect the preferential bioaccumulation pattern of R-(-)-indoxacarb. However, the amount of R-(-)-indoxacarb that accumulated in zebrafish was 65% higher when co-exposed with MWCNTs compared to single exposure (Ren et al. 2021).
Toxic Effects of Metal Oxide NPs
Metal oxide NPs (MOx NPs) include both synthesized and naturally found particles that are in the nanoscale range. MOx NPs, especially the engineered ones, have gained popularity in recent years owing to their diversity in the crystal structure, intriguing magnetic and electronic properties, and the existence of metal–oxygen bonding (Amde et al. 2017). They are used in almost all fields, which include medicine, biomedical applications, material chemistry, agriculture, environmental remediation, and catalysis (Chavali and Nikolova 2019). The endless applications have paved the way for their deliberate and accidental release into the aquatic environment. However, in the aquatic matrix, MOx NPs undergo various physicochemical transformations that alter their pristine nature (Garner et al. 2017) and subsequently their toxic impact on aquatic species. Thus, the following sections address the toxic effects of different MOx NPs and their mechanisms of toxic action on aquatic species.
Titanium Dioxide NPs
TiO2 NPs are the most frequently used metal oxide NPs in multiple commercial sections such as topical sunscreens, light-emitting diodes, surface coatings, and disinfectant sprays (Saxena and Harish 2018). Such large-scale use of TiO2 NPs has prompted several researchers to study their impacts on the aquatic environment. Toxic effects associated with TiO2 NPs on freshwater microalgae include the shading effect (Zhang et al. 2020b), oxidative stress generation (Gao et al. 2020), cellular membrane damage (Roy et al. 2020), and a decrease in photosynthetic efficiency (Middepogu et al. 2018). These toxic effects vary with differences in particle size, crystalline form (Chen et al. 2019b), and illumination conditions (Iswarya et al. 2018). Smaller-sized particles have a larger specific surface area to volume ratio that increases the likelihood of interaction with the algal surface. Moreover, the different crystalline forms of TiO2 NPs display dissimilar toxic effects due to the differences in semiconductor bandgap and surface chemistry. Since TiO2 NPs are photocatalysts, light source plays a vital role in imparting toxic effects. The activation of TiO2 NPs in the presence of UV illuminations generated reactive oxygen species (ROS) in the medium that augmented the toxic effects (Sendra et al. 2017; Roy et al. 2020). Until now, ROS generation has been described as the early stress response and the basic mechanism of TiO2 NPs toxicity in freshwater microalgae. However, Middepogu et al. (2018) supported a paradigm shift in the toxic mechanism of TiO2 NPs from oxidative stress to metabolic disruptions involved in photosynthesis. Besides all these particle-associated and experimental factors, various environmental parameters such as pH and temperature also alter the properties and the toxicological effects of TiO2 NPs on microalgae (Zhang et al. 2020b).
In freshwater fishes such as D. rerio and Carassius gibelio, TiO2 NPs stimulated the immune system with ROS generation, lysosomal membrane destruction, lipid peroxidation, protein carbonylation, DNA damage, and lastly apoptosis (Bobori et al. 2020). Dietary uptake of TiO2 NPs caused morphological alterations in the kidney, intestine, and liver and biochemical changes in the liver of D. rerio (Cunha and de Brito-Gitirana 2020). Besides, TiO2 NPs altered the gene expression associated with the development of the dorsoventral axis and neural network of D. rerio embryos (Kansara et al. 2020). Mechanistic investigation using shotgun proteomics revealed that the chronic exposure of TiO2 NPs altered the insulin-responsive compartment of D. rerio offspring (Chen et al. 2019a). Overall, the evaluation of the toxic effects of TiO2 NPs on microalgae and fishes has taken a shift from using conventional toxicity endpoints (such as mortality and oxidative stress determination) to a more specific mechanistic approach.
Zinc Oxide NPs
ZnO NPs have a wurtzite structure and are used in paints, pigments, lubricants, ceramic glass, fire retardants, and batteries because of their optoelectronic, catalytic, and antimicrobial properties (Saxena and Harish 2018). Exposure to a very low or environmentally relevant concentration of ZnO NPs is known to affect the growth and lipid content, induce plasmolysis, destruct the membrane, and disrupt thylakoids in the chloroplast of Scenedesmus sp. (Meng et al. 2018; Aravantinou et al. 2020). As discussed earlier, NP transformation in the water matrix can alter their toxic effects. The presence of bovine serum albumin reduced the toxic effects of ZnO NPs on C. pyrenoidosa by forming a protein corona on the surface of ZnO NPs (Janani et al. 2020) whereas the presence of phosphate in water transformed ZnO NPs into zinc phosphate and hopeite resulting in their reduced toxic effects to Chlorella sorokiniana (Zhang et al. 2020a). Unlike TiO2 NPs, ZnO NPs can undergo dissociation and the released ions are internalized by the algal cells that impart toxic effects (Ye et al. 2018). Moreover, it is essential to infer the toxic effects through feedback between microalgae and the aquatic ecosystem. Tang et al. (2018) investigated such environmental feedback caused by the release of algal organic matter to the aquatic environment using standard analytical parameters, such as excitation-emission matrices, molecular weight distribution, hydrophilic and hydrophobic properties, and microcystin-LR.
The toxicity of ZnO NPs in fish has been assessed based on behavior (Campos et al. 2019), physiological markers (Chupani et al. 2018), and molecular biological approaches (Hou et al. 2019). With the gastrointestinal route being the most important exposure pathway in aquatic species, toxicity studies have been conducted incorporating ZnO NPs in the feed. Chronic exposure of Cyprinus carpio to dietary ZnO NPs (50 and 500 mg/kg of feed) did not affect the blood biochemistry, hematology, lipid peroxidation, and Zn accumulation levels but affected liver and kidney function (Chupani et al. 2018). Likewise, Dekani et al. (2019) revealed that the dietary exposure of C. carpio to ZnO NPs resulted in greater accumulation in the target organs and caused higher toxicity than the dietary exposure to organic and inorganic forms of Zn. Using the observational assessment of fish behavior, Campos et al. (2019) found that ZnO NPs can induce food demotivation and alter the anti-predatory defensive behavior of Oreochromis niloticus, which suggests possible neurotoxicity. Besides, ZnO NPs were toxic to developing vascular and nervous systems of D. rerio and its succeeding generations (Kteeba et al. 2018). However, these toxic effects were reversed in the presence of dissolved organic matter. At the molecular level, ZnO NPs inhibited the growth and development of D. rerio by affecting the cell cycle processes (Hou et al. 2019).
Cerium Oxide NPs
CeO2 NPs are extremely versatile owing to their unique surface area and redox activity, and high stability that makes them a potential candidate in the manufacture of biosensors, catalysis, corrosion-resistant coatings, therapeutic agents, drug delivery vectors, and anti-parasitic ointments (Nadeem et al. 2020). Very few studies have reported the toxic effects of CeO2 NPs on microalgae. Pulido-Reyes et al. (2019) demonstrated that the surface coating of CeO2 NPs completely modifies the interaction of NPs with algal cells and also influences the mechanism of toxic effects. Pristine CeO2 NPs damaged the cell membrane and reduced the metabolic activity while the PVP-coated CeO2 NPs induced toxicity (ROS generation) without damage to the cell membrane. These conflicting effects of CeO2 NPs will be very useful during the manufacturing of safer-by-design NPs. CeO2 NPs are insoluble under environmental pH > 7.5. However, the small fraction of dissolved Ce3+ can produce harmful effects (Röhder et al. 2014). In contrast, Kosak née Röhder et al. (2018) reported a very high median effective concentration of CeO2 NPs and Ce3+ probably due to the negligible uptake of CeO2 NPs in the wild type Chlamydomonas reinhardtii and relatively slow uptake of Ce3+ in both the wild type and cell wall free mutant of C. reinhardtii. Similarly, Xiong et al. (2020) also reported a negligible effect of CeO2 NPs on the growth and pigments of Scenedesmus obliquus. Recently, Hund-Rinke et al. (2020) studied the attachment behavior of three sub-types of CeO2 NPs to algae and found a correlation between growth inhibition (Raphidocelis subcapitata) and attachment efficiency of CeO2 NPs. To sum up, the toxic effects of CeO2 NPs majorly depend on the dissociation of Ce ions in the experimental matrix and subsides in the presence of a surface coating.
Copper Oxide NPs
Similar to TiO2 NPs, CuO also has a strong light absorption reaction that makes them an essential photocatalyst. They are used in products such as catalysts, sensors, surfactants, and antimicrobials (Wu et al. 2020). Most studies described the toxic effects related to CuO NPs by the release of Cu2+ from the NPs as the ionic form is highly toxic (Joonas et al. 2019; Wu et al. 2020). To decipher biological processes, it is important to consider the metabolomes such as lipids, glycans, and the array of small molecules along with the genome, the transcriptome, and the proteome, as the metabolomes function as a substrate and product of biochemical reactions and aid in biological regulation (Doerr 2016). Wang et al. (2020) used such an approach (global metabolomics) and found similar metabolic responses (lipid bilayer remodeling, perturbation of glutathione metabolism, and accumulation of osmoregulants and chlorophyll intermediates) in C. vulgaris after treatment with CuO NPs, CuO microparticles (1 and 10 mg/L), and Cu ions (0.08 and 0.8 mg/L), and also confirmed dissolution as a major driving factor resulting in the metabolic reprogramming of algae. Alho et al. (2020) also proposed the shedding of Cu2+ from CuO NPs, which was the cause of toxic effects, as both NPs and ions had similar toxicity targets and responses in R. subcapitata.
NP toxicity assessments in the matrix representing the natural environment are understated. The correlation of study settings with real exposure conditions is bound to enhance the ecotoxicological results of NPs. In support of this, Joonas et al. (2019) studied the behavior and toxic effects of CuO NPs and their ions in nutrient-adjusted natural water. A decrease in the toxicity of CuO NPs and Cu ions was observed due to the differences in bioavailability arising from the binding of Cu ions to natural organic matter. Similarly, Yin et al. (2020) reported that the presence of C. reinhardtii significantly affected the fate (reduced colloidal stability, adsorption, and assimilation) and toxic effects of CuO NPs.
In contrast to the shedding of Cu ions from CuO NPs that induced toxic effects in microalgae, CuO NPs as a whole were responsible for inducing ROS in gills and increasing the number of cells in the early apoptotic and necrotic phases of Hyphessobrycon eques (Mansano et al. 2018). Although the presence of clay particles and humic acid-induced heteroagglomeration with CuO NPs and decreased the bioavailability of CuO NPs, altered levels of developmental gene expression and abnormalities were observed in the embryos of D. rerio (Kansara et al. 2019). Canli et al. (2018) reported that the exposure to CuO NPs (0, 1, 5, and 25 mg/L) altered the serum biomarkers levels in freshwater fish Oreochromis niloticus. Boyle et al. (2020) investigated the effects of pH and intermittent pulse on the toxicity of CuO NPs to D. rerio and found that CuO NPs were more toxic in pulse exposure and acidic conditions. In the study by Braz-Mota et al. (2018), species-specific metabolic stress responses to CuO NPs were reported which were possibly caused by different osmoregulatory strategies between two Amazon fish Apistogramma agassizii and Paracheirodon axelrodi.
Conclusions
The growing use of nanomaterials, especially in applications from which they are discharged directly, will lead to increased exposure to aquatic organisms. The authors believe that this chapter will provide basic and substantial information on the toxicity of nanomaterials to freshwater microalgae and fish. The nanomaterials as a whole and their dissolved ions contribute to the toxicity. Advances in the toxicity assessment of nanomaterials on microalgae and fish facilitate the understanding of their mode of action. However, a shift from a toxicological approach (stimulating toxicity with extreme concentrations) to an ecotoxicological perspective (assays under environmentally relevant conditions) is required.
References
Alho L de OG, Souza JP, Rocha GS et al (2020) Photosynthetic, morphological and biochemical biomarkers as tools to investigate copper oxide nanoparticle toxicity to a freshwater chlorophyceae. Environ Pollut 265:114856. https://doi.org/10.1016/j.envpol.2020.114856
Amde M, Liu J, Tan ZQ, Bekana D (2017) Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A Review. Environ Pollut 230:250–267
Aravantinou AF, Andreou F, Manariotis ID (2020) Long-term toxicity of ZnO nanoparticles on Scenedesmus rubescens cultivated in semi-batch mode. Nanomaterials 10(11):2262. https://doi.org/10.3390/nano10112262
Bao S, Tang W, Fang T (2020) Sex-dependent and organ-specific toxicity of silver nanoparticles in livers and intestines of adult zebrafish. Chemosphere 249(7). https://doi.org/10.1016/j.chemosphere.2020.126172
Bar-Ilan O, Albrecht RM, Fako VE et al (2009) Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 5(16):1897–1910. https://doi.org/10.1002/smll.200801716
Bobori D, Dimitriadi A, Karasiali S et al (2020) Common mechanisms activated in the tissues of aquatic and terrestrial animal models after TiO2 nanoparticles exposure. Environ Int 138:105611. https://doi.org/10.1016/j.envint.2020.105611
Boyle D, Clark NJ, Handy RD (2020) Toxicities of copper oxide nanomaterial and copper 2sulphate in early life stage zebrafish: Effects of pH and intermittent pulse exposure. Ecotoxicol Environ Saf 190:109985. https://doi.org/10.1016/j.ecoenv.2019.109985
Braz-Mota S, Campos DF, MacCormack TJ et al (2018) Mechanisms of toxic action of copper and copper nanoparticles in two Amazon fish species: Dwarf cichlid (Apistogramma agassizii) and cardinal tetra (Paracheirodon axelrodi). Sci Total Environ 630:1168–1180. https://doi.org/10.1016/j.scitotenv.2018.02.216
Cameron SJ, Hosseinian F, Willmore WG (2018) A current overview of the biological and cellular effects of nanosilver. Int J Mol Sci 19(7):1–40. https://doi.org/10.3390/ijms19072030
Canli EG, Dogan A, Canli M (2018) Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environ Toxicol Pharmacol 62:181–187. https://doi.org/10.1016/j.etap.2018.07.009
Chavali MS, Nikolova MP (2019) Metal oxide nanoparticles and their applications in nanotechnology. SN Appl Sci 1(6):607. https://doi.org/10.1007/s42452-019-0592-3
Chen L, Hu C, Guo Y et al (2019a) TiO2 nanoparticles and BPA are combined to impair the development of offspring zebrafish after parental coexposure. Chemosphere 217:732–741. https://doi.org/10.1016/j.chemosphere.2018.11.052
Chen X, Zhu Y, Yang K et al (2019b) Nanoparticle TiO2 size and rutile content impact bioconcentration and biomagnification from algae to daphnia. Environ Pollut 247:421–430. https://doi.org/10.1016/j.envpol.2019.01.022
Cheng J, Cheng SH (2012) Influence of carbon nanotube length on toxicity to zebrafish embryos. Int J Nanomedicine 7:3731–3739. https://doi.org/10.2147/IJN.S30459
Chithrani BD, Chan WCW (2007) Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett 7(6):1542–1550. https://doi.org/10.1021/nl070363y
Chueh PJ, Liang RY, Lee YH et al (2014) Differential cytotoxic effects of gold nanoparticles in different mammalian cell lines. J Hazard Mater 264(2014):303–312. https://doi.org/10.1016/j.jhazmat.2013.11.031
Chupani L, Niksirat H, Velíšek J et al (2018) Chronic dietary toxicity of zinc oxide nanoparticles in common carp (Cyprinus carpio L.): Tissue accumulation and physiological responses. Ecotoxicol Environ Saf 147:110–116. https://doi.org/10.1016/j.ecoenv.2017.08.024
Coll C, Notter D, Gottschalk F et al (2016) Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes). Nanotoxicology 10(4):436–444. https://doi.org/10.3109/17435390.2015.1073812
Cunha RLD da, de Brito-Gitirana L (2020) Effects of titanium dioxide nanoparticles on the intestine, liver, and kidney of Danio rerio. Ecotoxicol Environ Saf 203:111032. https://doi.org/10.1016/j.ecoenv.2020.111032
Das P, Xenopoulos MA, Metcalfe CD (2013) Toxicity of silver and titanium dioxide nanoparticle suspensions to the aquatic invertebrate, Daphnia magna. Bull Environ Contam Toxicol 91(1):76–82. https://doi.org/10.1007/s00128-013-1015-6
Daughton CG (2004) Non-regulated water contaminants: emerging research. Environ Impact Assess Rev 24(7–8):711–732. https://doi.org/10.1016/j.eiar.2004.06.003
de Campos RP, Chagas TQ, da Silva Alvarez TG et al (2019) Analysis of ZnO nanoparticle-induced changes in Oreochromis niloticus behavior as toxicity endpoint. Sci Total Environ 682:561–571. https://doi.org/10.1016/j.scitotenv.2019.05.183
Dedeh A, Ciutat A, Treguer-Delapierre M et al (2015) Impact of gold nanoparticles on zebrafish exposed to a spiked sediment. Nanotoxicology 9(1):71–80. https://doi.org/10.3109/17435390.2014.889238
Dekani L, Johari SA, Joo HS (2019) Comparative toxicity of organic, inorganic and nanoparticulate zinc following dietary exposure to common carp (Cyprinus carpio). Sci Total Environ 656:1191–1198. https://doi.org/10.1016/j.scitotenv.2018.11.474
Diegoli S, Manciulea AL, Begum S et al (2008) Interaction between manufactured gold nanoparticles and naturally occurring organic macromolecules. Sci Total Environ 402(1):51–61. https://doi.org/10.1016/j.scitotenv.2008.04.023
Doerr A (2016) Global metabolomics. Nat Methods 14:32
Dominguez GA, Lohse SE, Torelli MD et al (2015) Effects of charge and surface ligand properties of nanoparticles on oxidative stress and gene expression within the gut of Daphnia magna. Aquat Toxicol 162:1–9. https://doi.org/10.1016/j.aquatox.2015.02.015
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633. https://doi.org/10.1038/nrmicro2415
Freixa A, Acuña V, Sanchís J et al (2018) Ecotoxicological effects of carbon based nanomaterials in aquatic organisms. Sci Total Environ 619–620:328–337. https://doi.org/10.1016/j.scitotenv.2017.11.095
Gao X, Deng R, Lin D (2020) Insights into the regulation mechanisms of algal extracellular polymeric substances secretion upon the exposures to anatase and rutile TiO2 nanoparticles. Environ Pollut 263:114608. https://doi.org/10.1016/j.envpol.2020.114608
Garacci M, Barret M, Mouchet F et al (2017) Few Layer Graphene sticking by biofilm of freshwater diatom Nitzschia palea as a mitigation to its ecotoxicity. Carbon N Y 113:139–150. https://doi.org/10.1016/j.carbon.2016.11.033
Garner KL, Suh S, Keller AA (2017) Assessing the risk of engineered nanomaterials in the environment: development and application of the nanoFate model. Environ Sci Technol 51(10):5541–5551. https://doi.org/10.1021/acs.est.6b05279
Geffroy B, Ladhar C, Cambier S et al (2012) Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: the role of size, concentration and exposure time. Nanotoxicology 6(2):144–160. https://doi.org/10.3109/17435390.2011.562328
George S, Gardner H, Seng EK et al (2014) Differential effect of solar light in increasing the toxicity of silver and titanium dioxide nanoparticles to a fish cell line and zebrafish embryos. Environ Sci Technol 48(11):6374–6382. https://doi.org/10.1021/es405768n
George S, Lin S, Ji Z et al (2012) Surface defects on plate-shaped silver nanoparticles contribute to its hazard potential in a fish gill cell line and zebrafish embryos. ACS Nano 6(5):3745–3759. https://doi.org/10.1021/nn204671v
Gilroy KD, Hughes RA, Neretina S (2014) Kinetically controlled nucleation of silver on surfactant-free gold seeds. J Am Chem Soc 136(43):15337–15345. https://doi.org/10.1021/ja5081635
Girardi FA, Bruch GE, Peixoto CS et al (2017) Toxicity of single-wall carbon nanotubes functionalized with polyethylene glycol in zebrafish (Danio rerio) embryos. J Appl Toxicol 37(2):214–221. https://doi.org/10.1002/jat.3346
Harper B, Sinche F, Ho WuR et al (2014) The impact of surface ligands and synthesis method on the toxicity of glutathione-coated gold nanoparticles. Nanomaterials 4(2):355–371. https://doi.org/10.3390/nano4020355
Hou J, Liu H, Zhang S et al (2019) Mechanism of toxic effects of Nano-ZnO on cell cycle of zebrafish (Danio rerio). Chemosphere 229:206–213. https://doi.org/10.1016/j.chemosphere.2019.04.217
Hund-Rinke K, Sinram T, Schlich K et al (2020) Attachment efficiency of nanomaterials to algae as an important criterion for ecotoxicity and grouping. Nanomaterials 10(6):1021. https://doi.org/10.3390/nano10061021
Iswarya V, Bhuvaneshwari M, Chandrasekaran N et al (2018) Trophic transfer potential of two different crystalline phases of TiO2 NPs from Chlorella sp. to Ceriodaphnia dubia. Aquat Toxicol 197:89–97. https://doi.org/10.1016/j.aquatox.2018.02.003
Iswarya V, Johnson JB, Parashar A et al (2017) Modulatory effects of Zn2+ ions on the toxicity of citrate- and PVP-capped gold nanoparticles towards freshwater algae, Scenedesmus obliquus. Environ Sci Pollut Res 24(4):3790–3801. https://doi.org/10.1007/s11356-016-8131-x
Janani B, Raju LL, Thomas AM et al (2020) Impact of bovine serum albumin – A protein corona on toxicity of ZnO NPs in environmental model systems of plant, bacteria, algae and crustaceans. Chemosphere :128629. https://doi.org/10.1016/j.chemosphere.2020.128629
Jia YP, Ma BY, Wei XW et al (2017) The in vitro and in vivo toxicity of gold nanoparticles. Chinese Chem Lett 28(4):691–702. https://doi.org/10.1016/j.cclet.2017.01.021
Joonas E, Aruoja V, Olli K et al (2019) Environmental safety data on CuO and TiO2 nanoparticles for multiple algal species in natural water: Filling the data gaps for risk assessment. Sci Total Environ 647:973–980. https://doi.org/10.1016/j.scitotenv.2018.07.446
Kansara K, Kumar A, Karakoti AS (2020) Combination of humic acid and clay reduce the ecotoxic effect of TiO2 NPs: a combined physico-chemical and genetic study using zebrafish embryo. Sci Total Environ 698:134133. https://doi.org/10.1016/j.scitotenv.2019.134133
Kansara K, Paruthi A, Misra SK et al (2019) Montmorillonite clay and humic acid modulate the behavior of copper oxide nanoparticles in aqueous environment and induces developmental defects in zebrafish embryo. Environ Pollut 255:113313. https://doi.org/10.1016/j.envpol.2019.113313
Khan SA (2019) Metal nanoparticles toxicity: Role of physicochemical aspects. Elsevier Inc.
Kim KT, Zaikova T, Hutchison JE et al (2013) Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol Sci 133(2):275–288. https://doi.org/10.1093/toxsci/kft081
Kosak née Röhder LA, Brandt T, Sigg L et al (2018) Uptake and effects of cerium(III) and cerium oxide nanoparticles to Chlamydomonas reinhardtii. Aquat Toxicol 197:41–46. https://doi.org/10.1016/j.aquatox.2018.02.004
Kteeba SM, El-Ghobashy AE, El-Adawi HI et al (2018) Exposure to ZnO nanoparticles alters neuronal and vascular development in zebrafish: Acute and transgenerational effects mitigated with dissolved organic matter. Environ Pollut 242:433–448. https://doi.org/10.1016/j.envpol.2018.06.030
Lee KJ, Browning LM, Nallathamby PD et al (2012) In vivo quantitative study of sized-dependent transport and toxicity of single silver nanoparticles using zebrafish embryos. Chem Res Toxicol 25(5):1029–1046. https://doi.org/10.1021/tx300021u
Lee KJ, Browning LM, Nallathamby PD et al (2013) Study of charge-dependent transport and toxicity of peptide-functionalized silver nanoparticles using zebrafish embryos and single nanoparticle plasmonic spectroscopy. Chem Res Toxicol 26(6):904–917. https://doi.org/10.1021/tx400087d
Li J, Ying GG, Jones KC et al (2015) Real-world carbon nanoparticle exposures induce brain and gonadal alterations in zebrafish (Danio rerio) as determined by biospectroscopy techniques. Analyst 140(8):2687–2695. https://doi.org/10.1039/c4an02227k
Liu Y, Tourbin M, Lachaize S et al (2014) Nanoparticles in wastewaters: hazards, fate and remediation. Powder Technol 255:149–156. https://doi.org/10.1016/j.powtec.2013.08.025
Long Z, Ji J, Yang K et al (2012) Systematic and quantitative investigation of the mechanism of carbon nanotubes’ toxicity toward Algae. Environ Sci Technol 46(15):8458–8466. https://doi.org/10.1021/es301802g
Lukhele LP, Mamba BB, Musee N et al (2015) Acute toxicity of double-walled carbon nanotubes to three aquatic organisms. J Nanomater 2015. https://doi.org/10.1155/2015/219074
Luongo LA, Zhang X (Jackie) (2010) Toxicity of carbon nanotubes to the activated sludge process. J Hazard Mater 178(1–3):356–362. https://doi.org/10.1016/j.jhazmat.2010.01.087
Ma YB, Lu CJ, Junaid M et al (2018) Potential adverse outcome pathway (AOP) of silver nanoparticles mediated reproductive toxicity in zebrafish. Chemosphere 207:320–328. https://doi.org/10.1016/j.chemosphere.2018.05.019
Maes HM, Stibany F, Giefers S et al (2014) Accumulation and distribution of multiwalled carbon nanotubes in zebrafish (Danio rerio). Environ Sci Technol 48(20):12256–12264. https://doi.org/10.1021/es503006v
Mansano AS, Souza JP, Cancino-Bernardi J et al (2018) Toxicity of copper oxide nanoparticles to Neotropical species Ceriodaphnia silvestrii and Hyphessobrycon eques. Environ Pollut 243:723–733. https://doi.org/10.1016/j.envpol.2018.09.020
Meng Y, Wang S, Wang Z et al (2018) Algal toxicity of binary mixtures of zinc oxide nanoparticles and tetrabromobisphenol A: roles of dissolved organic matters. Environ Toxicol Pharmacol 64:78–85. https://doi.org/10.1016/j.etap.2018.09.010
Middepogu A, Hou J, Gao X et al (2018) Effect and mechanism of TiO 2 nanoparticles on the photosynthesis of Chlorella pyrenoidosa. Ecotoxicol Environ Saf 161:497–506. https://doi.org/10.1016/j.ecoenv.2018.06.027
Moore MN (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int 32(8):967–976. https://doi.org/10.1016/j.envint.2006.06.014
Morozesk M, Franqui LS, Mansano AS et al (2018) Interactions of oxidized multiwalled carbon nanotube with cadmium on zebrafish cell line: the influence of two co-exposure protocols on in vitro toxicity tests. Aquat Toxicol 200(January):136–147. https://doi.org/10.1016/j.aquatox.2018.05.002
Nadeem M, Khan R, Afridi K et al (2020) Green synthesis of cerium oxide nanoparticles (CeO2 NPs) and their antimicrobial applications: a review. Int J Nanomedicine 15:5951–5961. https://doi.org/10.2147/IJN.S255784
Nagai H, Okazaki Y, Chew SH et al (2011) Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci U S A 108(49). https://doi.org/10.1073/pnas.1110013108
Navarro E, Baun A, Behra R et al (2008a) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17(5):372–386. https://doi.org/10.1007/s10646-008-0214-0
Navarro E, Piccapietra F, Wagner B et al (2008b) Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42(23):8959–8964. https://doi.org/10.1021/es801785m
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150(1):5–22. https://doi.org/10.1016/j.envpol.2007.06.006
Oh WK, Jeong YS, Kim S et al (2012) Fluorescent polymer nanoparticle for selective sensing of intracellular hydrogen peroxide. ACS Nano 6(10):8516–8524. https://doi.org/10.1021/nn204899m
Oukarroum A, Polchtchikov S, Perreault F et al (2012) Temperature influence on silver nanoparticles inhibitory effect on photosystem II photochemistry in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Environ Sci Pollut Res 19(5):1755–1762. https://doi.org/10.1007/s11356-011-0689-8
Pokhrel LR, Dubey B, Scheuerman PR (2013) Impacts of select organic ligands on the colloidal stability, dissolution dynamics, and toxicity of silver nanoparticles. Environ Sci Technol 47(22):12877–12885. https://doi.org/10.1021/es403462j
Pulido-Reyes G, Briffa SM, Hurtado-Gallego J et al (2019) Internalization and toxicological mechanisms of uncoated and PVP-coated cerium oxide nanoparticles in the freshwater alga: Chlamydomonas reinhardtii. Environ Sci Nano 6(6):1959–1972. https://doi.org/10.1039/c9en00363k
Qian H, Zhu K, Lu H et al (2016) Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: new insights from proteomic and physiological analyses. Sci Total Environ 572:1213–1221. https://doi.org/10.1016/j.scitotenv.2016.08.039
Ren B, Jia B, Zhang X et al (2021) Influence of multi-walled carbon nanotubes on enantioselective bioaccumulation and oxidative stress toxicity of indoxacarb in zebrafish (Danio rerio). Chemosphere 267(235):128872. https://doi.org/10.1016/j.chemosphere.2020.128872
Rodrigues DF, Elimelech M (2010) Toxic effects of single-walled carbon nanotubes in the development of E Coli Biofilm. Environ Sci Technol 44(12):4583–4589. https://doi.org/10.1021/es1005785
Röhder LA, Brandt T, Sigg L et al (2014) Influence of agglomeration of cerium oxide nanoparticles and speciation of cerium(III) on short term effects to the green algae Chlamydomonas reinhardtii. Aquat Toxicol 152:121–130. https://doi.org/10.1016/j.aquatox.2014.03.027
oy B, Suresh PK, Chandrasekaran N et al (2020) Antibiotic tetracycline enhanced the toxic potential of photo catalytically active P25 titanium dioxide nanoparticles towards freshwater algae Scenedesmus obliquus. Chemosphere 267:128923. https://doi.org/10.1016/j.chemosphere.2020.128923
Sajid M, Ilyas M, Basheer C et al (2015) Impact of nanoparticles on human and environment: review of toxicity factors, exposures, control strategies, and future prospects. Environ Sci Pollut Res 22(6):4122–4143. https://doi.org/10.1007/s11356-014-3994-1
Sakamoto M, Ha JY, Yoneshima S et al (2015) Free silver ion as the main cause of acute and chronic toxicity of silver nanoparticles to cladocerans. Arch Environ Contam Toxicol 68(3):500–509. https://doi.org/10.1007/s00244-014-0091-x
Sangabathuni S, Murthy RV, Chaudhary PM et al (2017) Mapping the glyco-gold nanoparticles of different shapes toxicity, biodistribution and sequestration in adult zebrafish. Sci Rep 7(1):1–7. https://doi.org/10.1038/s41598-017-03350-3
Saxena P, Harish (2018) Nanoecotoxicological reports of engineered metal oxide nanoparticles on Algae. Curr Pollut Reports 4:128–142
Schwab F, Bucheli TD, Lukhele LP et al (2011) Are carbon nanotube effects on green algae caused by shading and agglomeration? Environ Sci Technol 45(14):6136–6144. https://doi.org/10.1021/es200506b
Sendra M, Sánchez-Quiles D, Blasco J et al (2017) Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: ultraviolet radiation is key variable for toxicity assessment. Environ Int 98:62–68. https://doi.org/10.1016/j.envint.2016.09.024
Sengul AB, Asmatulu E (2020) Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett 18(5):1659–1683. https://doi.org/10.1007/s10311-020-01033-6
Skjolding LM, Kern K, Hjorth R et al (2014) Uptake and depuration of gold nanoparticles in Daphnia magna. Ecotoxicology 23(7):1172–1183. https://doi.org/10.1007/s10646-014-1259-x
Stark WJ, Stoessel PR, Wohlleben W et al (2015) Industrial applications of nanoparticles. Chem Soc Rev 44(16):5793–5805. https://doi.org/10.1039/c4cs00362d
Tang Y, Xin H, Yang S et al (2018) Environmental risks of ZnO nanoparticle exposure on Microcystis aeruginosa: toxic effects and environmental feedback. Aquat Toxicol 204:19–26. https://doi.org/10.1016/j.aquatox.2018.08.010
Tedesco S, Doyle H, Blasco J et al (2010a) Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis. Aquat Toxicol 100(2):178–186. https://doi.org/10.1016/j.aquatox.2010.03.001
Tedesco S, Doyle H, Blasco J et al (2010b) Exposure of the blue mussel, Mytilus edulis, to gold nanoparticles and the pro-oxidant menadione. Comp Biochem Physiol - C Toxicol Pharmacol 151(2):167–174. https://doi.org/10.1016/j.cbpc.2009.10.002
Turan NB, Erkan HS, Engin GO et al (2019) Nanoparticles in the aquatic environment: usage, properties, transformation and toxicity—A review. Process Saf Environ Prot 130:238–249
Van Hoecke K, De Schamphelaere KAC, Ali Z et al (2013) Ecotoxicity and uptake of polymer coated gold nanoparticles. Nanotoxicology 7(1):37–47. https://doi.org/10.3109/17435390.2011.626566
van Pomeren M, Peijnenburg WJGM, Vlieg RC et al (2019) The biodistribution and immuno-responses of differently shaped non-modified gold particles in zebrafish embryos. Nanotoxicology 13(4):558–571. https://doi.org/10.1080/17435390.2018.1564079
Verneuil L, Silvestre J, Mouchet F et al (2015a) Multi-walled carbon nanotubes, natural organic matter, and the benthic diatom Nitzschia palea: “A sticky story.” Nanotoxicology 9(2):219–229. https://doi.org/10.3109/17435390.2014.918202
Verneuil L, Silvestre J, Randrianjatovo I et al (2015b) Double walled carbon nanotubes promote the overproduction of extracellular protein-like polymers in Nitzschia palea: An adhesive response for an adaptive issue. Carbon 88:113–125. https://doi.org/10.1016/j.carbon.2015.02.053
Völker C, Boedicker C, Daubenthaler J et al (2013) Comparative toxicity assessment of nanosilver on three Daphnia species in acute, chronic and multi-generation experiments. PLoS One 8(10). https://doi.org/10.1371/journal.pone.0075026
Wang L, Huang X, Sun W et al (2020) A global metabolomic insight into the oxidative stress and membrane damage of copper oxide nanoparticles and microparticles on microalga Chlorella vulgaris. Environ Pollut 258:113647. https://doi.org/10.1016/j.envpol.2019.113647
Wu F, Harper BJ, Crandon LE et al (2020) Assessment of Cu and CuO nanoparticle ecological responses using laboratory small-scale microcosms. Environ Sci Nano 7(1):105–115. https://doi.org/10.1039/c9en01026b
Xin Q, Rotchell JM, Cheng J et al (2015) Silver nanoparticles affect the neural development of zebrafish embryos. J Appl Toxicol 35(12):1481–1492. https://doi.org/10.1002/jat.3164
Xiong JQ, Ru S, Zhang Q et al (2020) Insights into the effect of cerium oxide nanoparticle on microalgal degradation of sulfonamides. Bioresour Technol 309:123452. https://doi.org/10.1016/j.biortech.2020.123452
Ye N, Wang Z, Wang S et al (2018) Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: particles outperform dissolved ions. Nanotoxicology 12(5):423–438. https://doi.org/10.1080/17435390.2018.1458342
Yin E, Zhao Z, Chi Z et al (2020) Effect of Chlamydomonas reinhardtii on the fate of CuO nanoparticles in aquatic environment. Chemosphere 247:125935. https://doi.org/10.1016/j.chemosphere.2020.125935
Yoo MH, Rah YC, Choi J et al (2016) Embryotoxicity and hair cell toxicity of silver nanoparticles in zebrafish embryos. Int J Pediatr Otorhinolaryngol 83:168–174. https://doi.org/10.1016/j.ijporl.2016.02.013
Zhang H, Chen Z, Huang Q (2020a) Study of the toxicity of ZnO nanoparticles to Chlorella sorokiniana under the influence of phosphate: spectroscopic quantification, photosynthetic efficiency and gene expression analysis. Environ Sci Nano 7(5):1431–1443. https://doi.org/10.1039/C9EN01464K
Zhang J, Jiang L, Wu D et al (2020b) Effects of environmental factors on the growth and microcystin production of Microcystis aeruginosa under TiO2 nanoparticles stress. Sci Total Environ 734:139443. https://doi.org/10.1016/j.scitotenv.2020.139443
Zhao CM, Wang WX (2010) Biokinetic uptake and efflux of silver nanoparticles in Daphnia magna. Environ Sci Technol 44(19):7699–7704. https://doi.org/10.1021/es101484s
Zhao CM, Wang WX (2011) Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem 30(4):885–892. https://doi.org/10.1002/etc.451
Zhao J, Cao X, Wang Z et al (2017) Mechanistic understanding toward the toxicity of graphene-family materials to freshwater algae. Water Res 111:18–27. https://doi.org/10.1016/j.watres.2016.12.037
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Giri, S., Thiagarajan, V., Chandrasekaran, N., Mukherjee, A. (2022). Ecotoxicity of Nanomaterials to Freshwater Microalgae and Fish. In: Guo, LH., Mortimer, M. (eds) Advances in Toxicology and Risk Assessment of Nanomaterials and Emerging Contaminants. Springer, Singapore. https://doi.org/10.1007/978-981-16-9116-4_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-9116-4_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-9115-7
Online ISBN: 978-981-16-9116-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)