Abstract
Gold nanoparticles (GNPs) are widely used for medical purposes, both in diagnostics as well as drug delivery, and hence are prone to release and distribution in the environment. Thus, we have explored the effects of GNPs with two distinct surface capping (citrate and PVP), and three different sizes (16, 27, and 37 nm) at 0.01-, 0.1-, and 1-mg L−1 concentrations on a predominant freshwater alga Scenedesmus obliquus in the sterile freshwater matrix. We have also investigated how an abundant metal ion from freshwater, i.e., Zn2+ ions may modulate the effects of the selected GNPs (40 nm, citrate, and PVP capped). Preliminary toxicity results revealed that gold nanoparticles were highly toxic in comparison to zinc ions alone. A significant modulation in the toxicity of Zn ions was not noticed in the presence of GNPs. In contrast, zinc ions minimized the toxicity produced by GNPs (both CIT-37 and PVP-37), despite its individual toxicity. Approximately, about 42, 33, and 25% toxicity reduction was noted at 0.05-, 0.5-, and 5-mg L−1 Zn ions, respectively, for CIT-37 GNPs, while 31% (0.05 mg L−1), 24% (0.5 mg L−1), and 9% (5 mg L−1) of toxicity reduction were noted for PVP-37 GNPs. Maximum toxicity reduction was seen at 0.05 mg L−1 of Zn ions. Abbott modeling substantiated antagonistic effects offered by Zn2+ ions on GNPs. Stability and sedimentation data revealed that the addition of zinc ions gradually induced the aggregation of NPs and in turn significantly reduced the toxicity of GNPs. Thus, the naturally existing ions like Zn2+ have an ability to modulate the toxicity of GNPs in a real-world environment scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gold NPs (GNPs) have found increasing applications in a multitude of biomedical approaches, such as diagnostics and therapy vectorization (Stuchinskaya et al. 2011; Brown et al. 2010; Ali et al. 2012; Perrault and Chan 2010). The wide variation in the optical (i.e., absorbance and fluorescence) features of GNPs due to its particular surface plasmon resonance made its enormous usage in various fields (Priyadarshini and Pradhan 2017; Saha et al. 2012; Riddhi et al. 2014). Also, the ease of synthesis and surface functionalization with ligands that confer different properties and biocompatibility has made GNPs one of the best-characterized systems (Larguinho et al. 2014). However, the increasing use of GNPs not only for research purposes but also in the industrial sector might be associated with environmental risk due to inappropriate disposal (Larguinho et al. 2014). The occupational and public exposures to nanomaterials are assumed to increase dramatically in anticipated years, and therefore, there is a desperate need for information on toxicity and safety of manufactured nanoparticles (Kasemets et al. 2009). Owing to the inert and insoluble nature of GNPs, they have gained attention as a general model to determine its toxicity and behavior in various systems (Riddhi et al. 2014; Yah 2013). Although gold NPs has been used for several in vivo studies for decades due to its significant advantage of functionalization with any ligands, distinct cytotoxic effects were noted for functionalized nanoparticles based on the moieties attached (Goodman et al. 2004). A definite conclusion has not been made so far from the literature available on the toxicity studies performed in various model organisms concerning the properties of GNPs (Alkilany and Murphy 2010). Hence, the effect of different aspects of gold nanoparticles such as size, shape, surface charge, capping agent, and functionalized ligands needs to be addressed that may interfere with the toxicity of NPs (Yah 2013).
Microalgae are primary producers of the aquatic ecosystem and play a consequent role in the ecological balance of food chain. They are strongly affected by pollutants of organic and metallic origins, as a result of their small sizes and consequently high surface-to-volume ratios (Gilroy et al. 2014). Available information on the toxicity of GNPs to algae is limited to a few studies, each focusing on different algae species and different types of particles with regard to their coating or functionalization and colloidal stability (Behra et al. 2015). The cytotoxicity of GNPs alone on the freshwater algae Scenedesmus sp. has been reported by Renault et al. (2008) where statistically significant GNP toxicity was observed (50% effect at 1 mg L−1). Dedkova et al. (2014) reported an EC50 value of 0.028 and 0.014 mg mL−1 for synthesized GNPs on Desmodesmus subspicatus and Raphidocelis subcapitata, respectively. Whereas PVP-stabilized GNPs showed an EC50 of 0.4823 mg mL−1 in D. subspicatus. In contrast, PVP-stabilized GNPs stimulated the growth of R. subcapitata. Amphiphilic-coated gold NPs with a size range of 4–5 nm exhibited a 72-h EC50 of about 7.5 ± 2.3 mg L−1 for Pseudokirchneriella subcapitata (Van Hoecke et al. 2013).
It has been reported in various studies that size and surface capping has an impact on the toxicity of gold nanoparticles (Pan et al. 2007; Bozich et al. 2014; Kim et al. 2013). The toxic effects of different surface cappings of GNPs were previously explored by our group on Chlorella sp. (Iswarya et al. 2015). Still, current knowledge on dispersion routes of nanomaterials in the environment and consequent hazards is limited (Moore 2006). Nanoparticles entering the aquatic environment react in a highly dynamic manner, which depends on the environmental conditions (Rana and Kalaichelvan 2013; Maurer-Jones et al. 2013).
Various metal ions have been known to impart toxicity on algal cells, with a few studies showing the joint effects of these metal ions. Campbel and Stokes (1985) outlined ten potent heavy metals (Ag, Al, Cd, Co, Cu, Hg, Mn, Ni, Pb, and Zn) associated with acidification and/or speciation thereby causing toxicity to freshwater algae. Harris and Ramelow (1990) have shown the relative binding efficiencies of silver, copper, cadmium, and zinc, where zinc proved to be least effective adhering to the surface of Chlorella and Scenedesmus sp. Contrarily, zinc and cadmium showed maximum adherence at around pH 7, which further decreased with a decrease in pH exhibiting a pH dependent sorption. Franklin et al. (2002) analyzed the impact of metals such as Cu, Cd, and Zn both individually as well as mixtures (binary and ternary) upon the cell division of freshwater alga, Chlorella sp. They showed distinct toxic effects from antagonistic to synergistic based on the combinations of metal mixtures tested. Also, the metallic uptake changed when the metals exist in mixtures, especially in the presence of Cu. Although, with the advent of nanoparticles, the exposure conditions of metals to the environment has considerably changed at a global level. Zou et al. (2013) studied the toxic effect of Fe3O4 NPs in combination with As(V) on Tetrahymena pyriformis where they noticed that Fe3O4 NPs alone were non-toxic. When they were combined with As(V), it promoted significant ROS generation and led to oxidative damage and cell death. Yang et al. (2012) noticed a decrease in the toxicity of Cd2+ ions in the presence of TiO2 NPs towards Chlamydomonas reinhardtii. Adsorption of Cd2+ by TiO2 NPs reduced its bioavailability and its toxicity.
Zinc is an essential metal, inevitable for the plant growth and metabolism. In contrast, it also behaves as a potent toxicant at the higher concentrations beyond its threshold level (Rout and Das 2009). The maximum permissible limit of zinc in the drinking water was determined to be 5 mg L−1, as per the WHO standards (WHO 2003). In the freshwater environment, the concentration of zinc varies from 0.002 to 50 mg/L, with an average of <0.05 mg/L (Roney 2005; Bodar et al. 2005; Luoma and Rainbow 2008; Naito et al. 2010). The toxicity of zinc to green algae in freshwater has been well elucidated by Wong and Chau (1990), where they have shown concentrations as low as 50 μg/L to exhibit toxicity by hampering both cell growth and productivity. A similar report was provided by Kumar et al. (2014), where the toxicity of zinc ions was examined over five different algal species with zinc concentrations ranging from 5 to 1500 μg/L. Moreover, both GNPs and Zn2+ ions find their route to the freshwater majorly through the sewage treatment plants. Since ZnO is one of the most abundant metal oxide contaminants found in stationary aquatic systems (Gottschalk et al. 2009; Sposito et al. 1982), the effects of zinc ions (at different concentrations) on the toxicity of gold nanoparticles should be examined comprehensively.
The current study aims to explore the impact of Zn2+ ions on the toxicity of GNPs over Scenedesmus obliquus, a predominant green algal species. Different-sized (16, 27, and 37 nm) and surface-capped (citrate- and PVP-capped) GNPs were synthesized by the Turkevich method, and their sizes were characterized by both TEM and DLS. To examine the effect of size and surface capping on the toxicity of gold nanoparticles, various-sized (16, 27, and 37 nm) and surface-capped (citrate- and PVP-capped) GNPs were tested on S. obliquus primarily at different concentrations such as 0.01, 0.1, and 1 mg L−1 in a freshwater matrix. Since various metal ions were known to exist in the freshwater, the impact of zinc ions (a major essential metal ion present in the freshwater) on the toxicity of GNPs was also assessed. The minimum concentration of Zn2+ ions tested in the study was kept as 0.05-mg L−1 equivalents to the actual concentration of zinc in source freshwater. Since the study uses sterile freshwater as an experimental matrix, to mimic the real environmental scenario, it provides a better holistic understanding of the combined toxic effects of GNPs (1 mg L−1) and zinc (0.05, 0.5, and 5 mg L−1) on green algae, S. obliquus. Further, the toxicity results have been defended with the particle size assessment and sedimentation analysis.
Materials and methods
Chemicals and reagents
Tetra aurochloric acid (HAuCl4), trisodium citrate, and polyvinyl pyrrolidone (PVP, K-30) were purchased from Sisco Research Laboratories Pvt. Ltd. (Mumbai, India). BG-11 medium was procured from Hi-media Laboratories Pvt. Ltd. (Mumbai, India).
Synthesis of gold nanoparticles
Different-sized (16, 27, and 37 nm) and surface-capped (citrate and PVP) GNPs were synthesized and used further for toxicity assessment. Citrate-capped GNPs of three different sizes such as 16, 27, and 37 nm are hereafter designated as CIT-16, CIT-27, and CIT-37 GNPs, respectively. While PVP-16, PVP-27, and PVP-37 GNPs represent PVP-capped GNPs with a size of 16, 27, and 37 nm, respectively.
Citrate-capped GNPs
Citrate-capped gold nanoparticles (GNPs) were synthesized by the citrate reduction method according to the Turkevich protocol as described by Frens (1973) with slight modifications. One millimolar tetra aurochloric acid (250 μL) was added drop by drop to the 25 mL of de-ionized water and heated at 90 °C with constant stirring. Varying amounts of 1% trisodium citrate (CIT-16: 2.7 mL; CIT-27: 2.3 mL; and CIT-37: 2 mL) was added quickly to the reaction mixture within a minute. The molar ratio of gold (Au):citrate in the solution ranges from 1:367.2 to 1:272. The solution was further heated for about 10 min until a red wine color was obtained indicating the formation of GNPs. The synthesized gold nanoparticles (GNPs) solution was allowed to cool down to room temperature and stored at 4 °C for further use. Besides, the synthesized GNPs were centrifuged at 1000g, 4 °C for about 10 min to remove the excess citrate remained in the suspension.
PVP-capped GNPs
For the synthesis of different-sized PVP-capped GNPs (PVP-16, PVP-27, and PVP-37), a similar procedure was followed as for CIT-16, CIT-27, and CIT-37 GNPs other than the addition of PVP. After the formation of GNPs, i.e., red color, 5% PVP solution was added finally to the solution in less than a minute, followed by a constant stirring for an hour to achieve a uniform capping over the gold nanoparticles. The molar ratio of Au:PVP was 1:5. Solutions were centrifuged at 1000g for 10 min (4 °C), and the supernatant containing PVP-capped GNPs was stored at 4 °C and used for further studies.
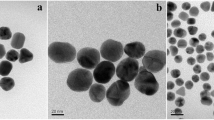
After synthesis, shape and diameters of the GNPs were confirmed with the transmission electron microscopy (TEM, FEI Tecnai T20 S-TWIN TEM). All the GNPs were of spherical in shape (Fig. 1). Sizes of the citrate-capped gold nanoparticles were observed to be 16 ± 2, 27 ± 1, and 37 ± 2 nm for CIT-16, CIT-27, and CIT-37, respectively. Similar sizes were obtained for the PVP-capped gold nanoparticles. Their sizes were noted to be 15 ± 3, 26 ± 2, and 36 ± 2 nm for PVP-16, PVP-27, and PVP-37, respectively.
Characterization of nanoparticles
The hydrodynamic diameters of all the as-synthesized GNPs (both citrate and PVP capped) in the stock suspension were evaluated using dynamic light scattering analysis (90 Plus Particle Size Analyzer, Brookhaven Instruments Corp., USA).
Test organism and experimental setup
S. obliquus, an axenic and green single-celled freshwater microalgae isolated from Vellore Institute of Technology (VIT) Lake, was used as a test organism for the toxicity experiments. BG-11 medium, a specific growth media, was used for growth and maintenance of the culture. A day/light rhythm of 16/8 h was maintained for the growth of algal cultures and illumination was provided by white fluorescent lights (18 W, TL-D Super 80 linear fluorescent tubes, Phillips, India) with an intensity of 3000 Lux. All the toxicity studies were conducted in the freshwater matrix, i.e., sterile freshwater as per OECD guidelines (OECD 2011).
Freshwater used in this study was collected from VIT Lake located in the VIT University, Vellore, India. It was further filtered with Whatmann No 1 and subsequently with the blotting paper. Besides, they were sterilized to deprive of microbes and used for the toxicity studies. The composition and metal content of the freshwater has been reported in our previous study (Iswarya et al. 2016). It contains about 1730 ± 100 mg L−1 of dissolved solids, 14.6 ± 0.5 mg L−1 of total organic carbon (TOC) with pH of 7.2 ± 0.4, conductance of 2.45 ± 0.23 mS/cm, and temperature of 24 ± 1.4 °C. The metal ion content of the freshwater was Cr6+ 0.002 mg L−1, Zn2+ 0.060 mg L−1; Fe 0.048 mg L−1, Mn2+ 0.011 mg L−1, Ni 0.004 mg L−1, and Ti 0.006 mg L−1.
Algal cells were harvested at the exponential phase from the growth medium by centrifugation (3000g, 10 min, 4 °C) and washed with sterilized freshwater to remove the residues/exudates from the growth medium. Then, the algal cells with an initial cell count of about 5 × 105 cells were prepared in sterile freshwater and used for the toxicity assessment.
Toxicity assessment of gold nanoparticles
Algal cells (5 × 105 cells) prepared in sterilized freshwater were treated with three different-sized (16, 27, and 37 nm) and two differently capped (citrate- and PVP-capped) GNPs at three different concentrations of 0.01, 0.1, and 1 mg L−1. The cells were thereafter kept in the visible light condition (18 W, TL-D Super 80 linear fluorescent tubes, Philips, India) for about 72 h.
After the interaction period, cell viability was assessed by the cell enumeration method using a hemocytometer as detailed in our previous work (Pakrashi et al. 2013). The absolute cell number of viable cells alone was counted to evaluate the cell viability. The cells without any morphological deformity were counted as live/viable cells, whereas the cells with damaged surface and shrinks were considered non-viable. Then, the number of the viable cells obtained after GNP treatment were normalized with respect to control cells and expressed as percentage relative toxicity (%). Similarly, the impact of capping agents alone such as 1% citrate and 5% PVP was studied on freshwater algae as that of GNPs.
Effect of zinc ions on the toxicity of gold nanoparticles
Preparation of zinc solution
Zinc sulfate (ZnSO4.7H2O) was used for the preparation of zinc (Zn2+) solution. Zinc stock solution of about 100 mg L−1 was prepared by dissolving 43.98 mg of ZnSO4.7H2O in 100 mL of de-ionized water. Further, the test concentrations (0.05, 0.5, and 5 mg L−1) were analyzed by atomic absorption spectroscopy (AAanalyst400, PerkinElmer) to determine its actual Zn concentration in a test matrix, i.e., sterile freshwater. Zinc concentration was corrected with blank, i.e., freshwater Zn ions. The actual zinc concentration was found to be 0.061 ± 0.01, 0.519 ± 0.07, and 4.46 ± 0.07 mg L−1 for 0.05, 0.5, and 5 mg L−1 of zinc, respectively.
Toxicity assessment of zinc ions in the absence and presence of gold nanoparticles
From the preliminary toxicity assays with different-sized and surface-capped GNPs, gold nanoparticles (CIT-37 and PVP-37) which showed highest toxicity was selected to evaluate the effect of zinc ions on GNPs. Initial concentration of GNPs was kept constant at 1 mg L−1, while Zn nominal ion concentrations were 0, 0.05, 0.5, and 5 mg L−1. Suspension of algal cells made in sterile freshwater with an initial cell count of 5 × 105 cells was treated with GNPs along with three different concentrations of Zn2+ ions (0, 0.05, 0.5, and 5 mg L−1) for about 72 h under the visible light condition. Experiments were carried out in triplicates per treatment. After the interaction period of 72 h, the viability of treated cells was assessed by the cell enumeration method with the help of a hemocytometer using the phase contrast microscope (Zeiss Axiostar Microscope, USA). Similarly, the toxicity of zinc ions alone, i.e., without GNPs, was also determined. Then, the viability of the treated cells was normalized with control cells and represented as percentage relative toxicity (%).
Abbott modeling
Abbott’s statistical model was widely used to characterize the interactions (synergism, antagonism, or addition) between two different toxicants when they were present as a binary mixture in the system (Teisseire et al. 1999; Chesworth et al. 2004). From the toxicity results, i.e., cell viability (%) results of GNPs and zinc ions alone, expected toxicity (C exp ) of the mixture (GNP + Zn) was calculated with the help of Eq. (1):
where
A represents the percentage toxicity caused by the GNPs alone and
B represents the percentage toxicity caused by the zinc ions alone.
Then, the ratio of inhibition (R I ) was calculated by comparing the observed toxicity with expected toxicity using Eq. (2)
where the observed toxicity is the percentage relative toxicity noted for the mixtures (GNP + Zn) after a 72-h exposure to algae.
By comparing R I value with 1, the effect of the binary mixture was characterized into antagonistic (when R I is less than 1), synergistic (when R I is larger than 1), and additive (when R I value is equal to 1). The calculated R I value was presumed to be statistically different from additivity when the mean R I value obtained from the triplicates was either larger or lesser than its standard deviation from 1. Also, two-way ANOVA (p < 0.01) was performed to confirm the modeling data by analyzing the statistical differences between the observed and expected toxicity using the software, GraphPad Prism, Version 5.
Influence of zinc ions in the stability of gold nanoparticles in the experimental matrix
The hydrodynamic size and sedimentation profile of CIT-37 and PVP-37 GNPs (1 mg L−1) were analyzed in the presence and absence of zinc ions to determine the effect of zinc ions on the stability of GNPs in the sterile freshwater.
Hydrodynamic size analysis by DLS
Hydrodynamic size analysis was performed using dynamic light scattering. This study was done to see the effect of zinc ions on the aggregation and stability of gold nanoparticles (CIT-37 and PVP-37) regarding size (Dalai et al. 2014). Various concentrations of zinc ions (0, 0.05, 0.5, and 5 mg L−1) were added to the sterile and filtered freshwater along with 1 mg L−1 of GNP (CIT-37 and PVP-37). Then the sizes of the GNPs were analyzed at different time intervals (0, 24, 48, and 72 h) using dynamic light scattering analysis (DLS, 90 Plus Particle Size Analyzer, Brookhaven Instruments Corp., USA).
Sedimentation analysis
Sedimentation analysis was done to find out aggregation potential of the gold nanoparticles in the presence and absence of zinc ions (Dalai et al. 2012).The experiment was carried out in the 50-mL centrifuge tube where freshwater matrix, i.e., sterile freshwater, was added. GNPs (CIT-37 and PVP-37) at a concentration of 1 mg L−1, and different concentrations of zinc ions such as 0, 0.05, 0.5, and 5 mg L−1 were added to the sterile freshwater. Then, they were kept under the visible light condition and left undisturbed for about 72 h. At different time intervals, i.e., 0, 24, 48, 72 h, 1 mL of the solution was taken without disturbing the experimental setup and analyzed under UV-vis spectrophotometer (HITACHI, Japan) at the wavelengths 520 and 525 nm for CIT-37 and PVP-37, respectively.
Statistical analysis
All the toxicity assays were carried out as three independent experiments with triplicates for each treatment. Data were represented as mean ± SD. Statistical analysis was performed with software, GraphPad Prism v.5. Two-way ANOVA was followed to compare the statistical differences among toxic effects of the GNPs and zinc ions. The level of significance was kept at 1%. One-way ANOVA was followed to compare the statistical differences among the control and GNPs at p < 0.05.
Results and discussion
Characterization of gold nanoparticles
Through the dynamic light scattering method, the hydrodynamic diameters of citrate-capped GNPs were found to be 15, 33, and 39 nm for CIT-16, CIT-27, and CIT-37, respectively (Fig. 2). Whereas the hydrodynamic diameters of the PVP-capped GNPs were doubled than that of the citrate-capped GNPs and found to be 33, 63, and 72 nm for PVP-16, PVP-27, and PVP-37, respectively (Fig. 2). From the TEM and DLS results, it has been inferred that the size of the PVP-capped GNPs was double than its TEM size. Whereas the sizes obtained from TEM and DLS were similar for citrate-capped GNPs. The size variation obtained for PVP-capped GNPs were due to a long chain polymer, PVP, used as a surface capping agent for the synthesis of PVP-capped GNPs (Tejamaya et al. 2012). As illustrated by Nur (2013), the variation among the sizes of GNPs measured by TEM and DLS is higher for PVP-capped GNPs than citrate-capped GNPs.
Effective hydrodynamic diameter of initially 37-nm-sized citrate (CIT-37) and PVP-capped (PVP-37) GNPs under different exposure concentrations of zinc ions over a period of 0–72 h in freshwater. The statistical significance with respect to 0 h of suspension is shown. Asterisks represent the statistical significance in the following order: ***p < 0.001, **p < 0.1, and *p < 0.5. No asterisk denotes insignificant difference
Citrate-capped GNPs showed higher aggregation with respect to time which indicates its unstable nature in the test matrix tested, i.e., sterile freshwater (Fig. 2). At 1 mg L−1 of GNPs, the hydrodynamic diameter of CIT-37 was found to be increased from 214 nm (0 h) to 561, 654, and 1004 nm at 24, 48, and 72 h, respectively. The differences between the sizes were found to be statistically significant at each time interval except 24 to 48 h. When PVP-37 GNPs (1 mg L−1) were present alone in the sterile freshwater, sizes of GNPs were found to be consistent throughout the experimental period in the size range of 43–53 nm.
Toxicity assessment of gold nanoparticles
The cytotoxicity of various-sized (16, 27, and 37 nm) citrate- and PVP-capped GNPs was evaluated on Scenedesmus sp. by cell enumeration method and expressed as percentage relative toxicity in Fig. 3. As the concentration of GNPs was increased, the relative toxicity of algal cells was also found to increase for all the types of GNPs tested in the study. They were found to be statistically significant with respect to control. Among the different-sized citrate-capped GNPs, the highest toxicity was observed at 1 mg L−1 of CIT-27 (53 ± 2.6%) and CIT-37 (54 ± 1.2%) GNPs. Smaller-sized citrate-capped GNPs (CIT-16) showed significantly less toxicity in comparison with CIT-37 and CIT-27 GNPs, larger-sized GNPs, whereas significant differences were not observed between CIT-27 and CIT-37 at the concentrations 0.1 and 1 mg L−1. Size-dependent toxicity of citrate-capped GNPs was obtained till CIT-27. Smaller-sized citrate-capped GNPs react more rapidly with the substances in the solution and induce higher aggregation than larger-sized GNPs (Zeng et al. 2012). Probably, due to the more facile aggregation of the smaller-sized citrate-capped GNPs, they were less toxic than the larger-sized citrate-coated GNPs.
The cytotoxic effects of 16-, 27-, and 37-nm citrate- and PVP-capped GNPs (0.01, 0.1, and 1 mg L−1) were evaluated on Scenedesmus sp. after 72 h. The relative toxicity (%) was calculated by normalizing the number of viable cells with respect to control cells (no exposure of GNPs). After normalization, the relative toxicity (%) for control group was considered as zero. Asterisks (***) representing the statistical significance of GNP treatment with respect to control at p < 0.001
Among the PVP-capped GNPs, highest toxicity of about 42% was observed at 1 mg L−1 of PVP-16 and PVP-37. PVP-16 and PVP-37 showed similar toxicity at all the concentrations tested. PVP-27 showed significantly less toxicity than other PVP-capped GNPs only at 1 mg L−1. PVP-capped GNPs did not show any size-dependent toxicity on S. obliquus. Distinctive size-dependent toxicity results obtained for PVP-capped GNPs were contradictory with the reports (Pan et al. 2007; Coradeghini et al. 2013) studied so far, i.e., as the size of NPs decreases, the toxicity of nanoparticles increases. Zhang et al. (2011) studied the in vivo toxicity of different-sized PEG-coated GNPs (5, 10, 30, and 60 nm) using a mice model system. They found that the GNPs toxicity did not vary with relevance to size, i.e., independent of size.
Comparing citrate- and PVP-capped GNPs, citrate-capped GNPs showed higher toxicity than PVP-capped GNPs at all the concentrations, irrelevant of size. These differences in the toxicity of GNPs were found to be statistically significant with each other. It could be due to the primary difference in the capping agent on the surface of GNPs. Citrate-capped GNPs have citrate weakly bound to the surface of GNPs, which interacts rapidly with the substances in the suspension (Tolaymat et al. 2010). While PVP-capped GNPs has PVP, a large polymer strongly constrained to the GNPs and thereby less prone to the substantial interaction with other substances (Romer et al. 2011; Cumberland and Lead 2009). Wang et al. (2014) reported a similar, less toxic effect of PVP-capped Ag NPs than citrate-capped NPs. It has been previously described that the protective coating of GNPs helped in decreasing its cytotoxicity (Sathishkumar et al. 2014).
The effect of capping agents such as 1% citrate and 5% PVP on the toxicity of GNPs towards S. obliquus was also studied. Citrate and PVP showed 76 ± 8 and 55 ± 5% relative toxicity on S. obliquus, which clearly signifies that PVP was less interactive with the algal cells in comparison to citrate. Thus, PVP-capped GNPs were of less toxic than citrate-capped GNPs.
Influence of zinc ions on toxic effects of gold nanoparticles
As zinc ions were predominant in the freshwater matrix used in the study among other metal ions, the effect of zinc ions on the GNPs (CIT-37 and PVP-37) toxicity towards S. obliquus were checked. Toxicity exhibited by zinc ions and their effects on the toxicity of GNPs are illustrated in Fig. 4.
The toxicity imparted by zinc ions at concentrations of 0.05, 0.5, and 5 mg L−1 and its effect on CIT-37 and PVP-37 GNPs (1 mg L−1) induced toxicity on Scenedesmus sp. at the end of 72 h. Asterisks, ***p < 0.001, represents the statistical significance with respect to toxicity exhibited by either CIT-37 or PVP-37 to that of zinc effected GNPs toxicity
Zinc ions alone shown 12, 25, and 31% toxicity at the concentration 0.05, 0.5, and 5 mg L−1, respectively, and were statistically significant with respect to control. A concentration-dependent increase in the toxicity was observed when algal cells were treated with zinc ions alone. Zinc ions were found to be significantly less toxic than GNPs. In a study by Omar (2002), Zinc ion was found to accelerate the growth of S. obliquus and S. quadricauda at its low concentrations (0.5–1.5 mg L−1) and gradually decreased the growth at higher concentrations (4.5–8 mg L−1). They also reported a growth inhibition of about 24 and 33% for S. obliquus and S. quadricauda, respectively, at the concentration, 8 mg L−1. Monteiro et al. (2011) reported that S. obliquus has a potential to tolerate higher concentrations of zinc.
A sharp reduction in the GNPs (both CIT-37 and PVP-37) toxicity was observed when zinc ions were added to it. The decrease in the toxicity of GNPs by Zn ions was found to be statistically significant with respect to the toxicity of GNPs alone. When the concentration of zinc ions was low, i.e., 0.05 mg L−1, the toxicity of GNPs was found to be reduced drastically from 54 to 12% for CIT-37 and 42 to 11% in case of PVP-37. As the concentration of zinc ions was increased to 0.5 and 5 mg L−1, the effective toxicity of GNPs + Zn increased to 21 and 29% for CIT-37-capped GNPs and 18 and 33% for PVP-capped GNPs, respectively. This increase in toxicity was due to the toxic effect of Zn ions at higher concentrations tested. This reduction reveals an antagonistic effect of Zn ions over the GNPs toxicity. Although a significant difference in the toxicity of GNPs alone with respect to GNPs + Zn was observed, an insignificant difference was noted while comparing the toxic effect of GNPs + Zn with Zn ions alone for both CIT-37 and PVP-37. In other words, the presence of GNPs did not reduce the toxicity of Zn ions in this study. A similar insignificant difference in the toxicity of As(V) and Cu was noted by Kim et al. (2016) upon the addition of citrate-capped Ag NPs towards Daphnia magna. Hence, the toxicity imparted by GNPs was entirely masked in presence of Zn ions, and therefore, the effective toxicity was exclusively that of Zn alone.
Antagonistic effect of Zn ions over GNPs toxicity was confirmed with a statistical model, Abbott modeling (Table 1). From the Abbott modeling, ratio of inhibition (R I ) was calculated and found to be in the range of 0.2 to 0.5 for all the combinations of GNP + Zn, even for both CIT-37 and PVP-37. Since the R I values were less than 1, the type of interaction was found to be antagonistic and statistically significant from 1 (Abbott et al. 1986). Hence, it was confirmed once again that the antagonistic action will be produced when GNPs and zinc ions were present together.
From the reports available, it has been known that toxicity of heavy metals was found to be reduced in the presence of various nanoparticles such as TiO2 and Fe2O3. (Dalai et al. 2014; Zou et al. 2013). Our previous work explored the effects of TiO2 and Al2O3 NPs on the toxic effects of Cr (VI) on the same Scenedesmus sp., showing the antagonistic effects between these metal species (Dalai et al. 2014). At other instances, a synergistic effect was observed on addition of nanoparticles such as As(V) + TiO2 NPs (Wang et al. 2011). At non-toxic concentrations of Fe2O3 (200 μgmL−1) and Al2O3 (150 μg mL−1), the toxicity of As(V) was increased (Hu et al. 2012).
As an essential nutrient, zinc plays a significant role in the defense mechanisms of plants being antioxidants, a key factor of various vital enzymes and proteins, structural stabilizers, etc. (Hafeez et al. 2013; Bray and Bettger 1990). A recent study by Tsuji et al. (2002) demonstrated that the zinc pretreatment on cells reduced the toxic effects of heavy metals (Cd, Hg, Cu, Pb, and arsenate) on the marine alga, Dunaliella tertiolecta. Higher production of phytochelatins by zinc pretreatment helped in the elimination of toxicity as well as oxidative stress produced by the heavy metals. Aravind and Prasad (2005) stated that zinc supplementation attenuated the oxidative stress induced by cadmium upon Ceratophyllum demersum by modulating the redox pathway. Thus, the potent antioxidant property of zinc ions might have imparted the toxicity of GNPs and thereby induced the antagonistic effect.
Moreover, the physicochemical processes such as agglomeration, surface adsorption, dissolution, and bioavailability of NPs in the test matrix during the exposure period also reflect the toxicity of nanoparticles (Auffan et al. 2009, 2010; Kahru et al. 2008). Adsorption of heavy metals/other contaminants onto to the surface of nanoparticles may either decrease its bioavailability through entrapment or increase its uptake through Trojan horse effect (Von Moos and Slaveykova 2014; Auffan et al. 2010). Thus, in this case, adsorption of zinc over the gold NPs surface might have resulted in a larger agglomeration of the NPs (as detailed in section 3.4) which would have reduced the toxicity of GNPs. Adsorption of various heavy metals such as Hg, Cd, and Cr onto the surface of GNPs and subsequent NPs aggregation have been reported earlier in various sensing studies (Sugunan et al. 2005; Shellaiah et al. 2016; Guo et al. 2014). This property has been widely utilized for the development of NPs sensors to determine the concentration of heavy metals (Priyadarshini and Pradhan 2017). Henceforth, one or more complex processes were involved in modulating the toxicity of GNPs.
Influence of zinc ions on the stability of gold nanoparticles in the experimental matrix
Hydrodynamic size analysis by DLS
Stability of gold nanoparticles (CIT-37 and PVP-37) in the presence and absence of zinc ions were investigated at different time intervals (0, 24, 48, and 72 h) using DLS method. Sizes of GNPs in the sterile freshwater were expressed as hydrodynamic diameter and shown in Fig. 2, for CIT-37 and PVP-37 GNPs, respectively. When CIT-37 GNPs were exposed to various concentrations of Zn ions, a similar size pattern was noted as of GNPs alone. The sizes of GNPs were rapidly increased with increase in the concentration of Zn ions. At 5 mg L−1 of Zn ions, the hydrodynamic diameter of CIT-37 GNPs was found to be 138.33, 943, 1121.33, and 1379 nm at 0, 24, 48, and 72 h, respectively. This aggregation was more intense than the aggregation of gold nanoparticles observed, i.e., without any zinc ions. Differences between GNPs and GNPs +Zn were found to be statistically significant only at the concentration, 5 mg L−1 of Zn ions. Interaction of GNPs with the natural colloids and ions exists in the freshwater might have destabilized the GNPs and thereby induced the aggregation of nanoparticles (Stankus et al. 2011). The presence of zinc exacerbated the aggregation of GNPs which might be due to its sorption over the surface of NPs.
In the presence of Zn ions, PVP-37 GNPs were found to be aggregated immediately and with respect to time. Though the size of PVP-37 GNPs was increased, they were considered to be statistically insignificant at all the concentrations tested, except 5 mg L−1 of Zn ions. The hydrodynamic diameter of PVP-37 GNPs in the sterile freshwater was increased from 76.67 nm (0 h) to 159, 187, and 627 nm at 24, 48, and 72 h, respectively upon addition of 5 mg L−1 of Zn ions.
Comparing the PVP-37 and CIT-37 GNPs alone, CIT-37 showed higher aggregation in the sterile freshwater. Their size differences were considered to be statistically significant between them. Nur (2013) illustrated similar aggregation results in the environmentally relevant conditions. Higher stability of PVP-capped GNPs was due to the surface capping agent, PVP, which are less prone to the environmental changes such as ionic strength and pH than citrate-capped GNPs, readily reactive and unstable (Zhang and Zhang 2014; Hitchman et al. 2013; Badawy et al. 2010). Natural organic matter (NOM), a component of natural colloids in the freshwater, may accelerate the rate of aggregation. Stankus et al. (2011) evaluated the consequences of humic acid (from Suwannee River) on the stability of various-coated GNPs. They observed that the citrate-capped GNPs showed higher aggregation than other GNPs which has different coatings.
Similar aggregation profile was found for both CIT-37 and PVP-37 GNPs even after the addition of Zn ions. Citrate-capped GNPs aggregated rapidly in each time intervals in the presence of Zn ions. Dalai et al. (2014) reported a similar increase in the aggregation of TiO2 NPs when exposed to 0.05 mg L−1 of Cr (VI) as a mixture in the freshwater system. They also stated that aggregation of NPs resulted due to the sorption of Cr (VI) on the surface of NPs. In contrast, PVP-capped GNPs does not show any intense aggregation when Zn ions were added to the solution. It remained stable at every time interval, except 5 mg L−1 of Zn at 72 h. Due to the steric hindrance of PVP, coated on the GNPs, significant change in the hydrodynamic diameter was not observed.
The reduced toxicity observed upon addition of zinc could owe to the aggregation of GNPs. Although, we do not see a strict size-dependent toxicity, still, zinc concentration-based effect on toxicity could be clearly seen. PVP-capped GNPs exhibited maximum toxicity in the presence of zinc, possibly due to lesser aggregation and smaller size of the GNPs.
Sedimentation analysis
Sedimentation analysis gives the supporting data to prove the size data of GNPs in the presence of different concentrations of zinc ions. From the Fig. 5, it has been observed that the absorbance of citrate-capped GNPs was found to decline significantly with respect to time both in the presence and absence of Zn ions. Even though a decrease in the absorbance was increased upon addition of Zn ions, they were statistically insignificant when compared with sedimentation rate observed for CIT-37 GNPs at all the concentrations tested, except 5 mg L−1 of Zn ions. As similar to stability data, sedimentation profile for PVP-37 GNPs (Fig. 5) revealed that the concentration of GNPs was found to be stable with the increase in time. Their absorbance ratios were noted in the range of 0.97–0.79, even Zn ions are added to it. Only at the 5 mg L−1 of Zn ions, a slight change in the absorbance ratio was observed which was statistically insignificant with the increase in time. Citrate-capped GNPs showed a higher sedimentation rate than PVP-capped GNPs both in the presence and absence of zinc ions. Gliga et al. (2014) reported a similar higher sedimentation rate for citrate-capped Ag NPs in comparison with PVP-capped Ag NPs.
From the stability results (DLS and sedimentation profile), it has been observed that the agglomeration of GNPs occurred upon addition of Zn ions, especially at 5 mg L−1. Aggregation of GNPs reduces the bioavailability of NPs and in turn affects their uptake and internalization. Albanese and Chan (2011) demonstrated that aggregation of citrate-capped GNPs influences their bioavailability in the system and in turn its interaction with cells. Subsequently, it may affect the uptake of GNPs and makes it more complicated in the toxicity assessments (Alkilany and Murphy 2010). Zn uptake by algal cells decreased, although not significantly, when it was added to GNPs in a freshwater matrix (Fig. S1, detailed in the supplementary information). Thus, aggregation of CIT-37 and PVP-37 GNPs reduced the toxicity of NPs towards algae when GNPs and Zn ions were co-exposed together.
Conclusion
GNPs exhibited surface capping related variability in the toxicity on S. obliquus. Citrate-capped GNPs were more toxic and showed a size dependency, optimum 27 nm, while PVP-capped GNPs did not give a distinctive difference with respect to size, 27 nm being least effective. This study confirms that the zinc ions, present as a predominant metallic species in freshwater, have shown the antagonistic ability to interfere with the toxicity caused by GNPs on green algae Scenedesmus sp. The ability of zinc ions to mask the toxicity of GNPs was observed to be independent of the surface capping of GNPs, which suggests an interaction of the nanoparticle with the cell was completely blocked and hence the toxicity due to GNPs were compromised in total. Zinc ions along with other metal ions present in freshwater should therefore be considered for any agonistic/antagonistic effects over other nanoparticle toxicity. Future studies on the mechanism of combined toxic effect of GNPs with zinc are required to understand the route of inhibition and the cellular dysfunction causing toxicity. Also, this study brings our attention and demands future toxicity studies to consider the presence of other metal ions in the in vivo environment before attributing the effective toxicity exhibited by a pollutant.
References
Abbott MB, Bathurst JC, Cunge JA, O'Connell PE, Rasmussen J (1986) An introduction to the European hydrological system—Systeme Hydrologique Europeen, “SHE”, 1: history and philosophy of a physically-based, distributed modelling system. J Hydrol 87(1):45–59
Albanese A, Chan WC (2011) Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 5(7):5478–5489
Ali M, Hashim U, Mustafa S, Man Y, Islam KN (2012) Gold nanoparticle sensor for the visual detection of pork adulteration in meatball formulation. J Nanomater 2012:1
Alkilany AM, Murphy CJ (2010) Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanopart Res 12(7):2313–2333
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate–glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43(2):107–116
Auffan M, Bottero JY, Chaneac C, Rose J (2010) Inorganic manufactured nanoparticles: how their physicochemical properties influence their biological effects in aqueous environments. Nanomedicine 5(6):999–1007
Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR (2009) Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol 4(10):634–641
Badawy AME, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM (2010) Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ Sci Technol 44(4):1260–1266
Behra R, Wagner B, Sgier L, Kistler D (2015) Colloidal stability and toxicity of gold nanoparticles and gold chloride on Chlamydomonas reinhardtii. Aqaut Geochem 21(2):331–342
Bodar CW, Pronk ME, Sijm DT (2005) The European Union risk assessment on zinc and zinc compounds: the process and the facts. Integr Environ Assess Manag 1:301–319
Bray TM, Bettger WJ (1990) The physiological role of zinc as an antioxidant. Free Radic Biol Med 8(3):281–291
Bozich JS, Lohse SE, Torelli MD, Murphy CJ, Hamers RJ, Klaper RD (2014) Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna. Environmental Science: Nano 1(3):260–270
Brown SD, Nativo P, Smith J-A, Stirling D, Edwards PR, Venugopal B, Flint DJ, Plumb JA, Graham D, Wheate NJ (2010) Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J Am Chem Soc 132(13):4678–4684
Campbel PGC, Stokes PM (1985) Acidification and toxicity of metals to aquatic biota. Can J Fish Aquat Sci 42(12):2034–2049
Chesworth J, Donkin M, Brown M (2004) The interactive effects of the antifouling herbicides irgarol 1051 and diuron on the seagrass Zostera marina (L.). Aquat Toxicol 66(3):293–305
Coradeghini R, Gioria S, García CP, Nativo P, Franchini F, Gilliland D, Ponti J, Rossi F (2013) Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol Lett 217(3):205–216
Cumberland SA, Lead JR (2009) Particle size distribution of silver nanoparticles at environmentally relevant conditions. J Chromatogr A 1216:7
Dalai S, Pakrashi S, Kumar RS, Chandrasekaran N, Mukherjee A (2012) A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations. Toxicol Res 1(2):116–130
Dalai S, Pakrashi S, Bhuvaneshwari M, Iswarya V, Chandrasekaran N, Mukherjee A (2014) Toxic effect of Cr(VI) in presence of n-TiO2 and n-Al2O3 particles towards freshwater microalgae. Aquat Toxicol 146:28–37
Dedkova K, Bures Z, Palarcík J, Vlcek M, Kukutschova J (2014) Acute toxicity of gold nanoparticles to freshwater green algae. In: In: conference NanoCon, Nov 5the7th. Czech Republic, Brno
Franklin NM, Stauber JL, Lim RP, Petocz P (2002) Toxicity of metal mixtures to a tropical freshwater alga (Chlorella sp.): the effect of interactions between copper, cadmium, and zinc on metal cell binding and uptake. Environ Toxicol Chem 21(11):2412–2422
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 241(105):20–22
Gilroy KD, Neretina S, Sanders RW (2014) Behavior of gold nanoparticles in an experimental algal–zooplankton food chain. J Nanopart Res 16(5):1–8
Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL (2014) Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol 11(1):1
Goodman CM, McCusker CD, Yilmaz T, Rotello VM (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem 15(4):897–900
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol 43(24):9216–9222
Guo Y, Zhang Y, Shao H, Wang Z, Wang X, Jiang X (2014) Label-free colorimetric detection of cadmium ions in rice samples using gold nanoparticles. Anal Chem 86(17):8530–8534
Hafeez B, Khanif YM, Saleem M (2013) Role of zinc in plant nutrition—a review. American Journal of Experimental Agriculture 3(2):374
Harris PO, Ramelow GJ (1990) Binding of metal ions by particulate biomass derived from Chlorella vulgaris and Scenedesmus quadricauda. Environ Sci Technol 24(2):220–228
Hitchman A, Smith GHS, Ju-Nam Y, Sterling M, Lead JR (2013) The effect of environmentally relevant conditions on PVP stabilised gold nanoparticles. Chemosphere 90(2):410–416
Hu J, Wang D, Forthaus BE, Wang J (2012) Quantifying the effect of nanoparticles on as (V) ecotoxicity exemplified by nano-Fe2O3 (magnetic) and nano-Al2O3. Environ Toxicol Chem 31(12):2870–2876
Iswarya V, Manivannan J, De A, Paul S, Roy R, Johnson J, Kundu R, Chandrasekaran N, Mukherjee A, Mukherjee A (2015) Surface capping and size-dependent toxicity of gold nanoparticles on different trophic levels. Environ Sci Pollut Res:1–15
Iswarya V, Bhuvaneshwari M, Chandrasekaran N, Mukherjee A (2016) Individual and binary toxicity of anatase and rutile nanoparticles towards Ceriodaphnia dubia. Aquat Toxicol 178:209–221
Kahru A, Dubourguier HC, Blinova I, Ivask A, Kasemets K (2008) Biotests and biosensors for ecotoxicology of metal oxide nanoparticles: a minireview. Sensors 8(8):5153–5170
Kasemets K, Ivask A, Dubourguier H-C, Kahru A (2009) Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol in Vitro 23(6):1116–1122
Kim I, Lee BT, Kim HA, Kim KW, Kim SD, Hwang YS (2016) Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna. Chemosphere 143:99–105
Kim K-T, Zaikova T, Hutchison JE, Tanguay RL (2013) Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol Sci 133(2):275–288
Kumar D, Santhanam P, Ananth S, Devi AS, Nandakumar R, Prasath BB, Jeyanthi S, Jayalakshmi T, Ananthi P (2014) Effect of different dosages of zinc on the growth and biomass in five marine microalgae. Int J Fish Aquac 6(1):1–8
Larguinho M, Correia D, Diniz MS, Baptista PV (2014) Evidence of one-way flow bioaccumulation of gold nanoparticles across two trophic levels. J Nanopart Res 16(8):1–11
Luoma SN, Rainbow PS (2008) Metal contamination in aquatic environments: science and lateral management. Cambridge University Press, New York
Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL (2013) Toxicity of engineered nanoparticles in the environment. Anal Chem 85(6):3036–3049
Monteiro CM, Fonseca SC, Castro PM, Malcata FX (2011) Toxicity of cadmium and zinc on two microalgae, Scenedesmus obliquus and Desmodesmus pleiomorphus, from northern Portugal. J Appl Phycol 23(1):97–103
Moore MN (2006) Do nanoparticles present ecotoxicologiocal risks for the health of the aquatic environment? Environ Int 32:967–976
Naito W, Kamo M, Tsushima K, Iwasaki Y (2010) Exposure and risk assessment of zinc in Japanese surface waters. Sci Total Environ 408:4271–4284
Nur Y (2013) Gold nanoparticles: synthesis, characterisation and their effect on Pseudomonas flourescens. University of Birmingham
OECD (2011) Test no 201: freshwater alga and cyanobacteria, growth inhibition test. OECD guidelines for the testing of chemicals. OECD Publishing Paris
Omar H (2002) Bioremoval of zinc ions by Scenedesmus obliquus and Scenedesmus quadricauda and its effect on growth and metabolism. Int Biodeterior Biodegrad 50(2):95–100
Pakrashi S, Dalai S, Prathna T, Trivedi S, Myneni R, Raichur AM, Chandrasekaran N, Mukherjee A (2013) Cytotoxicity of aluminium oxide nanoparticles towards fresh water algal isolate at low exposure concentrations. Aquat Toxicol 132:34–45
Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Jahnen-Dechent W (2007) Size-dependent cytotoxicity of gold nanoparticles. Small 3(11):1941–1949
Perrault SD, Chan WCW (2010) In vivo assembly of nanoparticle components to improve targeted cancer imaging. Proc Natl Acad Sci 107(25):11194–11199
Priyadarshini E, Pradhan N (2017) Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: a review. Sens Actuators B Chem 238:888–902
Rana S, Kalaichelvan PT (2013) Ecotoxicity of nanoparticles. ISRN toxicology 2013
Renault S, Baudrimont M, Mesmer-Dudons N, Gonzalez P, Mornet S, Brisson A (2008) Impacts of gold nanoparticle exposure on two freshwater species: a phytoplanktonic alga (Scenedesmus subspicatus) and a benthic bivalve (Corbicula fluminea). Gold Bull 41(2):116–126
Riddhi P, Om B, Kuldeep R, Bindiya P. (2014) An incongruent upshot of gold nano particles in middle of cancer treatment with poles apart appliances. Int J Drug Dev Res
Romer I, White TA, Baalousha M, Chipman K, Viant MR, Lead JR (2011) Aggregation and dispersion of silver nanoparticles in exposure media for aquatic toxicity tests. J Chromatogr A 1218(27):4226–4233
Roney N. (2005). Toxicological profile for zinc. Agency for Toxic Substances and Disease Registry
Rout GR, Das P (2009) Effect of metal toxicity on plant growth and metabolism: I. Zinc. In Sustainable Agriculture. Springer Netherlands. pp. 873–884
Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112(5):2739–2779
Sathishkumar M, Pavagadhi S, Mahadevan A, Balasubramanian R (2014) Biosynthesis of gold nanoparticles and related cytotoxicity evaluation using A549 cells. Ecotoxicol Environ Saf 114:232–240
Shellaiah M, Simon T, Sun KW, Ko FH (2016) Simple bare gold nanoparticles for rapid colorimetric detection of Cr3+ ions in aqueous medium with real sample applications. Sens Actuators B Chem 226:44–51
Sposito G, Lund LJ, Chang AC (1982) Trace metal chemistry in arid-zone field soils amended with sewage sludge: I. Fractionation of Ni, Cu, Zn, Cd, and Pb in solid phases1. Soil Sci Soc Am J 46(2):260–264
Stankus DP, Lohse SE, Hutchison JE, Nason JA (2011) Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ Sci Technol 45(8):3238–3244
Stuchinskaya T, Moreno M, Cook MJ, Edwards DR, Russell DA (2011) Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates. Photochemical & Photobiological Sciences 10(5):822–831
Sugunan A, Thanachayanont C, Dutta J, Hilborn JG (2005) Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Sci Technol Adv Mater 6(3):335–340
Teisseire H, Couderchet M, Vernet G (1999) Phytotoxicity of diuron alone and in combination with copper or folpet on duckweed (Lemna minor). Environ Pollut 106(1):39–45
Tejamaya M, Römer I, Merrifield RC, Lead JR (2012) Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol 46(13):7011–7017
Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M (2010) An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer reviewed scientific papers. Sci Total Environ 408(5):999–1006
Tsuji N, Hirayanagi N, Okada M, Miyasaka H, Hirata K, Zenk MH, Miyamoto K (2002) Enhancement of tolerance to heavy metals and oxidative stress in Dunaliella tertiolecta by Zn-induced phytochelatin synthesis. Biochem Biophys Res Commun 293(1):653–659
Van Hoecke K, De Schamphelaere KAC, Ali Z, Zhang F, Elsaesser A, Rivera-Gil P, Parak WJ, Smagghe G, Howard CV, Janssen CR (2013) Ecotoxicity and uptake of plymer coated gold nanoparticles. Nanotoxicology 1:37–47
Von Moos N, Slaveykova VI (2014) Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae–state of the art and knowledge gaps. Nanotoxicology 8(6):605–630
Wang D, Hu J, Irons DR, Wang J (2011) Synergistic toxic effect of nano-TiO2 and As (V) on Ceriodaphnia dubia. Sci Total Environ 409(7):1351–1356
Wang X, Ji Z, Chang CH, Zhang H, Wang M, Liao YP, Lin S, Meng H, Li R, Sun B (2014) Use of coated silver nanoparticles to understand the relationship of particle dissolution and bioavailability to cell and lung toxicological potential. Small 10(2):385–398
Wong PTS, Chau YK (1990) Zinc toxicity to freshwater algae. Toxic Assess 5(2):167–177
WHO (2003) Zinc in drinking-water. Background document for preparation of WHO Guidelines for drinking-water quality. World Health Organization 2003. Geneva. (WHO/SDE/WSH/03.04/17). Available from: www.who.int/water_sanitation_health/dwq/chemicals/zincsum.pdf
Yah CS (2013) The toxicity of gold nanoparticles in relation to their physiochemical properties. Biomed Res 24(3):400–413
Yang WW, Miao A-J, Yang L-Y (2012) Cd2+ toxicity to a green alga Chlamydomonas reinhardtii as influenced by its adsorption on TiO2 engineered nanoparticles. PLoS One 7(3):e32300
Zhang H, Zhang C (2014) Transport of silver nanoparticles capped with different stabilizers in water saturated porous media. Journal of Materials and Environmental Science 5(1):231–236
Zhang X-D, Wu D, Shen X, Liu P-X, Yang N, Zhao B, Zhang H, Sun Y-M, Zhang L-A, Fan F-Y (2011) Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomedicine 6:2071–2081
Zeng S, Cai M, Liang H, Hao J (2012) Size-dependent colorimetric visual detection of melamine in milk at 10 ppb level by citrate-stabilized Au nanoparticles. Anal Methods 4:2499–2505
Zou X-Y, Xu B, Yu C-P, Zhang H-W (2013) Combined toxicity of ferroferric oxide nanoparticles and arsenic to the ciliated protozoa Tetrahymena pyriformis. Aquat Toxicol 134:66–73
Acknowledgements
We sincerely thank PSG-IAS, Coimbatore, TN, India, for the TEM analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Thomas D. Bucheli
Electronic supplementary material
ESM 1
(DOCX 68 kb)
Rights and permissions
About this article
Cite this article
Iswarya, V., Johnson, J., Parashar, A. et al. Modulatory effects of Zn2+ ions on the toxicity of citrate- and PVP-capped gold nanoparticles towards freshwater algae, Scenedesmus obliquus . Environ Sci Pollut Res 24, 3790–3801 (2017). https://doi.org/10.1007/s11356-016-8131-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8131-x