Abstract
Over-exploitation of groundwater causes a drop in water levels and, as a result, the intrusion of saltwater along the coastal region, which is a natural occurrence. A groundwater quality assessment was carried out to investigate such phenomena along the coast of Visakhapatnam, Andhra Pradesh. The groundwater samples were collected from sixteen different locations (from Bheemunipatnam to Burujupeta). The available groundwater quality data set is based on physicochemical analysis of groundwater samples collected from the coastal aquifer. The characteristics of the groundwater samples were statistically evaluated using correlation to investigate interrelationships between various groundwater parameters and to assess the current stage of contamination and the occurrence of seawater intrusion. The analysis of groundwater samples collected from the coastal aquifer for determination of specific chemical characteristics such as Ca2+/Mg2+, Cl−/(CO32− + HCO3−), and the ratio of Total Alkalinity (TA) to Total Hardness (TH) revealed that coastal groundwater appeared to be slightly contaminated by seawater intrusion. The Gibbs diagram demonstrated that the controlling mechanism of groundwater samples was governed by rock weathering.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Water is essential to people's lives and livelihoods. It causes the demand for clean water to rise in lockstep with global population growth. Without groundwater, the largest and most reliable of all freshwater resources, it would be impossible to maintain secure water supplies for drinking, industry, and agriculture. Groundwater, unlike other natural resources or raw materials, is found all over the world. Because of rainfall patterns and aquifer distribution, the possibilities for its extraction vary greatly from place to place (rocks, sand layers, and so on, in whose pore spaces the groundwater sits). Because of the hydraulic gradient of groundwater above mean sea level, freshwater is also widespread in the coastal aquifer [1, 2]. The mass transport of saline waters into previously occupied zones by fresher waters is known as saltwater intrusion. The movement of saltwater with high total dissolved solids (TDS) content into freshwaters is characterised as saltwater intrusion. Coastal aquifers are important sources of freshwater supply in many countries around the world, particularly in arid and semi-arid zones [3]. Many coastal areas in India are heavily urbanised, exacerbating the need for freshwater. Aquifers along the coast are vulnerable to disturbances. Inadequate management of a coastal aquifer may result in its demise as a source of freshwater much sooner than other aquifers not connected to the sea. The threat of seawater intrusion is the reason [4, 5].
The chemical composition of groundwater is determined primarily by three processes: atmospheric inputs, water interaction with soil and rock, and anthropogenic activities. Precipitation, climate change, and natural hazards all contribute to atmospheric inputs, while water interaction with soil and rock causes weathering and erosion of crustal materials. Over-exploitation of groundwater and the location of industries above sensitive zones have a significant impact on groundwater quality [6]. The presence of highly brackish waters in certain localised pockets of the coastal aquifer, such as Kaniti-Paravada near Visakhapatnam, is caused by hydrogeological processes in the area, not by sea influence [7]. The purpose of this paper is to investigate the controlling mechanism of seawater intrusion in Visakhapatnam's coastal aquifers.
-
MeghadriGedda Reservoir
-
Raiwada Link Canal
-
Yeleru Left Bank Canal
2 Materials and Methods
2.1 Study Area
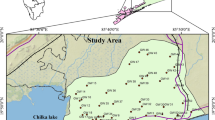
Greater Visakhapatnam, once a small fishing village, has grown into a major port city over the years. It also serves as the administrative centre for the Visakhapatnam district. Visakhapatnam is located on India's east coast, at 17o42’ North latitude and 82o02’ East range of hills. The city and its surroundings can be divided into four categories based on topographical conditions: Hilly region, Upland tracks, Rolling plains, and Plains.
The main surface sources of water for the city of Visakhapatnam are
-
Mudasarlova Reservoir
-
GambheeramGedda Reservoir
-
Gosthani River
-
Thatipudi Reservoir
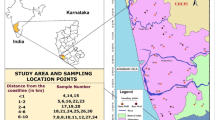
Ground water samples (from open wells, bore wells, and hand pumps) were collected from sixteen locations along Visakhapatnam's coastline (from Burujupeta to Bheemunipatnam). The sampling sites are located 5–8 kms from the coast, and the details of the sampling sites are provided in Table 1. Water samples were collected in 2-L polythene bottles (soaked overnight in 2% nitric acid and washed thoroughly in distilled water) from each location and analysed for pH, Conductance, Total Dissolved Solids, Total Hardness, Calcium Hardness, Magnesium Hardness, Alkalinity, Chloride, Sulphate, Nitrate, Phosphate, Iron, Sodium, and Potassium as per standard methods [8].
3 Results and Discussion
Table 2 shows the results of the various quality parameters for all samples. A brief description of the essential physicochemical properties of groundwater is provided below.
3.1 pH
The pH in the study areas ranged from 8.21 at (S-16) in Burujupeta to 7.02 at (S-12) PeddaWaltair. All of the pH values in the samples are well within the ranges for various water uses, including drinking and other domestic supplies [8, 9].
3.2 Conductance
The conductance ranged from a maximum of 2.36 mmhos/cm at (S-8) Appugarh to a minimum of 0.31 mmhos/cm at (S-3). Due to the high concentration of Ionic constituents present in the water bodies under study, almost all of the water samples under study exceeded the permissible limit (0.3 mmhos/cm), reflecting the contributions from salinity intrusion and pollution by domestic wastes. The dissolved ions such as bicarbonates, chlorides, sodium, potassium, magnesium, and sulphate are primarily responsible for electrical conductivity [11].
3.3 Total Dissolved Solids (TDS)
The values of Total Dissolved Solids (TDS) in groundwater in the current study range from 170.5 to 1298 mg/l, indicating high mineralization in the study area. Furthermore, organic substances commonly found in polluted water may contribute to the Dissolved Solids. Water with more than 500 mg/l TDS is not considered desirable for drinking water supplies, though more highly mineralized water is used in places where better water is unavailable. As a result, for drinking water, 500 mg/l has been proposed as the desirable limit and 2000 mg/l as the maximum permissible limit [8, 9]. TDS levels greater than 500 mg/l in water cause gastrointestinal irritation [8, 9].
3.4 Chlorides
Ranged from 24 to 199 mg/l in the study areas. Chloride limits have been established primarily based on taste considerations. A desirable limit of 250 mg/l chlorides has been recommended for drinking water [8, 9]. However, no adverse health effects on humans have been reported from drinking water with a higher chloride content [10, 11].
3.5 Alkalinity
Alkalinity in the natural system is primarily composed of carbonates, bicarbonates, and hydroxides. These constituents are formed as a result of mineral dissolution in the soil and atmosphere [12]. Carbonate and bicarbonates can also be produced by microbial decomposition of organic matter. The alkalinity ranged from 440 mg/l at S-8 in Appugarh to 110 mg/l at S-3 in Mangamaripeta. The alkalinity of almost all of the areas studied was found to be higher than the allowable limit (120 mg/l). This could be due to the percolation of the area's domestic sewage [13].
3.6 Total Hardness
The carbonates, sulphates, and chlorides of calcium and magnesium, as well as their carbonates, sulphates, and chlorides, harden the water. Total Hardness ranged from a maximum of 380.0 mg/l at S-8, Appugarh, to a minimum of 120.0 mg/l at S-3, Mangamaripeta. For drinking water, a desirable limit of 300 mg/l and a permissible limit of 600 mg/l have been recommended [8]. Hardness has no known negative effects on health; however, some evidence suggests that it may play a role in heart disease [14].
3.7 Calcium Hardness
Calcium concentrations in the study area ranged from 70 mg/l in Bheemili to a maximum of 220 mg/l in Appugarh. Calcium in drinking water has a maximum desirable limit of 75 mg/l and a maximum permissible limit of 200 mg/l. The calcium ions in the majority of the study samples exceeded the desirable limit, but remained within allowable limits. Calcium is a necessary component of the human body. Rickets and tooth decay can be caused by low calcium levels in drinking water. It is required for nervous system function, cardiac function, and blood coagulation [15].
3.8 Magnesium Hardness
Magnesium concentrations in the current study area ranged from 30 mg/l in Mangamaripeta to a maximum of 180 mg/l in Burujupeta. Magnesium in drinking water has a maximum desirable limit of 30 mg/l and a maximum permissible limit of 100 mg/l. Magnesium ions exceeded the allowable limit in 90 per cent of the study samples. Magnesium concentration in water is lower than calcium concentration, possibly due to the lower occurrence of Magnesium Minerals. Magnesium is a moderately toxic element when present in high concentrations in drinking water [15].
3.9 Phosphates
Phosphate concentrations in the study area are low in all locations. Phosphorus is a necessary plant nutrient that is widely used in fertilisers. In acidic soils, phosphate is adsorbed or fixed as aluminium or iron phosphate, while in alkaline or neutral soils, it is fixed as calcium phosphate. As a result, phosphate concentrations in groundwater are typically low, but phosphate mobility in subsoil and groundwater may be induced by various chemical processes in soil strata.
3.10 Sulphates
The soluble salts of calcium, magnesium, and sodium make up the majority of the sulphate content in groundwater. The sulphate content of rainfall infiltration and groundwater recharge, primarily from stagnant water pools and surface runoff water collected in low-lying areas, changes significantly over time. Sulphate levels in the study area ranged from 3.5 mg/l in Maharanipeta to 33.5 mg/l in Babujinagar. Sulphate levels are within the prescribed standard (200 mg/l) at all sampling stations. Sulphate is poorly absorbed from the human intestine and slowly penetrates into the cellular membranes of mammals before being rapidly eliminated through the kidneys. In humans, sodium and magnesium sulphates have a cathartic effect. It has also been linked to respiratory diseases [16].
3.11 Nitrates
The presence of nitrates in drinking water is thought to be necessary for its negative health effects. The presence of high nitrate levels in groundwater is a major issue in many parts of the country. The Nitrate content in the study area is low in all sampling locations. Nitrate is a plant nutrient that is both effective and moderately toxic. [8, 9] have set a limit of 45 mg/l for drinking water supplies. Its concentrations above 45 mg/l may be hazardous to human health. At higher concentrations, nitrate can cause Methaemoglobinaemia (blue baby syndrome) [17], a disease that primarily affects bottle-fed infants. Ingestion of high doses of nitrates on a regular basis may also result in carcinogenic diseases.
3.12 Iron
The highest iron content was found in the current study areas at Chepaluppada, at 0.22 mg/l. Iron content was found to be low in the other regions, and in Sagarnagar colony, Jodugullapalem, Appugarh, MVP Colony, and EastPoint Colony, iron content was found to be below the detectable level of 0.3 mg/l, as prescribed by BIS [9] for drinking water supplies. Iron concentrations above 0.3 mg/l stain clothing and utensils. The water is also unsuitable for processing food, beverages, ice, dyeing, bleaching, and other similar processes. The limit for iron in water is determined by aesthetic and taste considerations rather than physiological effects [18].
3.13 Sodium and Potassium
In the study area, sodium concentrations range from 32 mg/l in Mangamaripeta to 200 mg/l in Appugarh. Sodium has not been included in drinking water standards set by the Bureau of Indian Standards. The high sodium levels could be due to base-exchange phenomena. The potassium concentration in groundwater in the study area ranged from 1.8 to 68.4 mg/l. Potassium is an essential element for humans, plants, and animals, and it is derived primarily from vegetation and soil in the food chain. Rainwater, potash silicate minerals, the use of potash fertilisers, and surface water for irrigation are the primary sources of potassium in groundwater. It is more common in sedimentary rocks and is found in feldspar, mica, and other clay minerals. Potassium has not been included in drinking water standards set by the Bureau of Indian Standards. However, the European Economic Community [19] has set a guideline level of potassium in drinking water at 10 mg/l. Potassium levels were found to be high in Sagarnagar Colony (25.8 mg/l) and Burujupeta (68.4 mg/l).
3.14 Correlation Analysis
The correlation analysis of 13 physicochemical parameters of groundwater from 16 different sites in Visakhapatnam city was performed, and the correlation coefficients (r) are shown in Table 3. Between EC and TDS (r = 1), Sulphates and Nitrates (r = 0.94), Total Hardness and Calcium Hardness (r = 0.91), Total Hardness and Magnesium Hardness (r = 0.9), TDS and Chlorides (r = 0.90), and TDS and Alkalinity (r = 0.90), there is a strong correlation (r = 0.9). A very high positive correlation also exist between TDS and Sodium (r = 0.86), EC and Total Hardness (r = 0.84), EC and Magnesium Hardness (r = 0.80), Chlorides and Nitrates (r = 0.78), Alkalinity and Sodium (r = 0.78), Chlorides and Sulphates (r = 0.77), TDS and Sulphates (r = 0.77), EC and Nitrates (r = 0.73), pH and Alkalinity (r = 0.72), EC and Calcium Hardness (r = 0.72), Chlorides and Sodium (r = 0.71), pH and Total Hardness (r = 0.70). It can thus be assumed that the concurrent increase/decrease in ion composition in these waters is primarily due to dissolution/precipitation reactions and concentration effects.
3.15 Sea Water Contamination Using Chemical Ratio
The ratio of specific chemical parameters such as Ca2+/Mg2+, Cl/(CO3 + HCO3), and TA/TH can be used to assess saltwater intrusion into coastal aquifers [20]. Because magnesium is much more abundant in seawater than calcium, the Ca/Mg ratio is recommended as a parameter for determining saltwater contamination. A low Ca/Mg ratio could also indicate saltwater contamination [21].
. If the ratio is less than one, the area is thought to be heavily impacted by saltwater intrusion. Similarly, one of the criteria used to assess the presence of seawater intrusion is the Cl/(CO3 + HCO3) ratio. Table 4 shows the Cl/(CO3 + HCO3), TA/TH, and Ca/Mg ratios.
A low Ca/Mg ratio may also indicate saltwater contamination. If the ratio is less than one, the area is considered to be heavily impacted by saltwater intrusion. Similarly, the Cl/(CO3 + HCO3) ratio is one of the criteria used to determine the presence of seawater intrusion. Table 4 shows the ratios of Cl/(CO3 + HCO3), TA/TH, and Ca/Mg.
3.16 GIBB’S Diagram
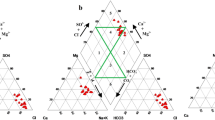
The Gibb's ratio for the ions (Na/Na + Ca and Cl/(Cl + HCO3) of groundwater samples was plotted against the TDS values. The plot shows that all of the groundwater samples belong to the rock dominant category. The interaction between aquifer rocks and groundwater causes rock dominance in the samples (Fig. 1).
4 Summary and Conclusion
Water samples were collected and analysed to assess the quality (in terms of physical and chemical content) of Visakhapatnam City's coastal aquifers. The study revealed the following findings. Groundwater in the study area is moderately complex and slightly alkaline. According to the current study, seawater intrusion is the cause of increased ion concentrations in the coastal aquifer. According to the findings of this study, the coastal aquifer is slightly contaminated by seawater intrusion, which is most likely due to increased over-exploitation of groundwater sources. More research on seawater intrusion into the coastal aquifer and geological weathering is needed to accurately quantify groundwater contamination.
5 Suggestions
-
To protect coastal ground water, the sources of saline water intrusion and mobility mechanisms must be identified and investigated for sustainable groundwater resource development.
-
Data collected during this study should be expanded to include all seasons of the year, as changes in water quality that occur as a result of normal seasonal variations are not reflected in this report due to the limited period of performance.
-
The 2002 National Water Policy emphasises that “overexploitation of groundwater should be avoided, particularly near the coast, to prevent ingress of seawater into sweet water aquifers”. As a result, determining the extent of seawater intrusion is critical for planning and managing groundwater resource protection in coastal aquifers.
Abbreviations
- APHA:
-
American Public Health Association
- BIS:
-
Bureau of Indian Standards
- Ca:
-
Calcium
- CaH:
-
Calcium Hardness
- Cl:
-
Chlorides
- EC:
-
Electrical Conductivity
- EEC:
-
European Economic Community
- MgH:
-
Magnesium Hardness
- Na:
-
Sodium
- TA:
-
Total Alkalinity
- TDS:
-
Total Dissolved Solids
- TH:
-
Total Hardness
- WHO:
-
World Health Organisation
References
Ghyben, B. W. (1889). Nota in verband met de voorgenomen put boring put boring nabij, Amsterdam Kononkl. Inst. Ing. Tijdschr., 1888–1889, 8–22.
Herzberg, B. (1901). Die Wasserversorgung einiger Nordeebader. Jour Gasbeleuchtung Wasserversorg, 44, 815–844.
Behera, A. K., Chakrapani, G. J., Kumar, S., & Rai, N. (2019). Identification of sea water intrusion signature through geochemical evolution of groundwater. A case study based on coastal region of the Mahanadi delta, Bay of Bengal, India. Natural Hazards, 97, 1209–1230.
Davood, M., & Mohammad, K. (2019). Seawater intrusion and aquifer under flooding events. Journal of Hydrology, 568, 1118–1130.
Korrai, S., Gangu, K. K., Rao, P. P., & Jonnalagadda, S. B. A study on assemment of vulnerability of seawater intrusion to groundwater in coastal areas of Visakhapatnam, India. Environment, Development and Sustainability, 23, 5937–5955.
Kannan, N. (2010). A study on the growth of Indian sector. International Journal of Management, 1(1), 17–22.
Jagannadha , V. V., & Krishnaiah, N. (1979). Quality of groundwater in the costal aquifer near Visakhapatnam. India Ground water, 14(5), 290–295.
APHA. (2005). Standard methods for the examination of water and waste water (21st ed.). American Public Health Association.
Bureau of Indian Standards (BIS). (2012). Amendment no.1 June 2015 to IS 10500: 2012 Drinking water-specification. Bureau of Indian Standards (BIS), Manak Bhavan,9 Bahadur Shah Zafar Marg, New Delhi.
World Health Organization (WHO). (1996). Water quality monitoring—A practical guide to the design and implementation of freshwater quality studies and monitoring programmers. In J. Bartram & R. Balance (Eds.), Published on behalf of United Nation Environment Programme and the World Health Organization (UNEP).
Peavy, H. S., Rowe, D. R., & Tchobanoglous, G. (1985). Donnan dialysis removal of nitrate from water: Effects of process parameters. Environmental Engineering (p. 696). McGraw-Hill, Book Company.
Mittal, S. K., & Verma, N. (1997). Critical analysis of ground water quality parameters. Indian Journal of Environmental Protection, 17(6), 426–429.
Naik, S. (2005). Studies on pollution status of Bondamunda area of Rourkela industrial complex, Doctor of Philosophy, Department of Chemistry, National Institute of Technology, Rourkela, Orissa.
Sarala, C., & Ravi Babu, P. (2012). Assessment of ground water quality parameters in and around Jawaharnagar, Hyderabad. International Journal of Scientific and Research Publications, 2(10), 2250–3153.
Dwivedi, A., Dwivedi, H. P., Tiwari, S. (2017). Quality analysis of Hydrosphear water of Deosar Block in District Singrauli, Madhya Pradesh, India. International Journal of Advanced Research in Chemicals Science (IJARCS), $(1), 22–26.
Sarda, P., & Sadgir, P. (2015). Assessment of multi parameters of water quality in surface water bodies—A review. International Journal for Research in Applied Sciences & Engineering Technology, 3(VIII), 2321–9653.
Soni, N., & Bhatia, A. (2015). Analysis of quality of drinking water of private bore well and piped water supply in Jaipur city, Rajasthan, India. Research Journal pf Recent Science, 4, 313–316.
Kumari, K. (2014). Statistical and Correlation studies of ground water parameters of Kamleshwarpur area at Mainpat, Chhattisgarh. IOSR Journal of Engineering, 04(I), 2278–8719.
European Economic Community (EEC). (1998). Directive of the European Parliament and of the council relating to the quality petrol and diesel fuels and Amending Council Directive 93/12/EEC.
Rengaraj, S., Elampooranan, T., Elango, L., & Ramalingam, V. (1996). Groundwater quality in suburban regions of Madras City, India. Pollution Research, 15(4), 325–328.
Ravi Prakash, S., & Krishna Rao, G. (1996). Delineation of salt water contamination zones by chemical parameters in Parvada Area, Vishakapattnam, AP. Journal of Indian Water Research Society, 2, 63–38.
Acknowledgements
The authors are thankful to GITAM Management to carry out the work. The authors also thanking Prof. Sabu Joseph, Department of Environmental Sciences, University of Kerala for GIBBS software assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Mamatha, K., Kumar, K.S., Srinivas, N., Gopamma, D., Ziauddin, A. (2022). Preliminary Study on Seawater Intrusion in Coastal Aquifers of Visakhapatnam. In: Siddiqui, N.A., Khan, F., Tauseef, S.M., Ghanem, W.S., Garaniya, V. (eds) Advances in Behavioral Based Safety. Springer, Singapore. https://doi.org/10.1007/978-981-16-8270-4_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-8270-4_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8269-8
Online ISBN: 978-981-16-8270-4
eBook Packages: EngineeringEngineering (R0)