Abstract
Groundwater is widely regarded as being among the freshwater natural resources with the lowest levels of contamination. Nevertheless, the saltwater intrusion has resulted in the contamination of groundwater in coastal regions with lower elevation. The rationale of the present work is to investigate the chemistry of groundwater, to identify the various facies of groundwater, to identify the processes that influence groundwater chemistry and saltwater intrusion, and to evaluate the groundwater’s aptness for use in drinking and farming. In order to gain an understanding of the groundwater quality as well as the salinization process that occurs in coastal aquifers as a result of hydrogeochemical processes, a total of 108 groundwater samples (54 each in pre- and post-monsoon) were taken and analyzed for several physiochemical parameters in the southern part of the Puri district in the Indian state of Odisha. The data has undergone analysis and examination to identify the factors (such as hydrological facies, potential solute source in water, and salinization process) that contribute to groundwater salinity. The result showed the chemistry controlling processes of rock-water interaction as per Gibbs diagram. The majority of shallow aquifers exhibit the Na-Cl type of facies as per the Piper plot. A total of 37% pre-monsoon and 33% post-monsoon samples having Na+/Cl− ratio below the threshold of 0.86 indicating the influence of saltwater intrusion. In both seasons, it was observed that 74% of the samples exhibited a Na+ concentration that exceeded the permissible limit set by the World Health Organization (WHO) for drinking purposes. The findings indicate that most groundwater failed to pass safe drinking water and irrigation standards due to saltwater intrusion. Consequently, the monitoring of coastal aquifer quality has become imperative in order to ensure the sustainability of aquifers and the development of groundwater resources. This is because coastal aquifers are highly vulnerable to saltwater intrusion, primarily as a result of the extensive extraction of groundwater for diverse purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While the Earth possesses an ample supply of water, the availability of potable freshwater is relatively limited. Thus, the lion’s share of water system employment is saline. This saltwater intrusion (SWI) is due to density and pressure differences in coastal aquifers, a common phenomenon. However, it has increased in recent years due to enhanced anthropogenic activities and industrialization (De Pippo et al. 2006; Huang et al. 2013; Kumar et al. 2023). Saline water ingress deteriorated the groundwater quality, especially around the coasts.
SWI is widespread in numerous coastal regions worldwide. It is one of the main reasons of destruction of the fresh coastal aquifers (Bear et al. 1999; Purnama and Marfai 2012). Groundwater quality mainly depends on the concentration of the dissolved ions (Raju et al. 2009; 2011 and 2014; Patel et al. 2016; Madhav et al. 2018 and 2021). The tremendous water demand has significantly intensified groundwater extraction, mainly in densely populated coastal areas (Famiglietti 2014). Excessive groundwater extraction compared to natural recharge causes lowering of the water table in coastal aquifers, resulting SWI (Sahagian 2000; Chidambaram et al. 2018; Selvakumar and Chandrasekar 2021). The intensification of groundwater salinity is due to the inflow saline water naturally as well anthropogenic causes. These coastal aquifers decaying is caused by various natural processes including the infringement of sea water, highly pressurized brines stabbing into fresh groundwater, and by underground dissolution of soluble salts inventing in neighboring country rocks and also by water–rock interaction (Vengosh and Rosenthal 1994; Glynn and Plummer 2005; Cloutier et al. 2008; Idowu and Lasisi 2020; Etikala et al. 2021). Moreover, increasing trend of temperature due to global warming leads to sea level rise and overexploitation of fresh water in overpopulated coastal region have led to SWI (Oude Essink 2001; Ferguson and Gleeson 2012). Groundwater quality declines due to SWI into coastal confined or unconfined aquifers due to a difference in fluid density (Nair et al. 2015). Identification of ‘saltwater-freshwater’ mixing in the coastal aquifer has been identified by various researchers using different ionic ratios like Na+/Cl− ratio (Vengosh and Rosenthal 1994), Ca2+/Mg2+ ratio (Moujabber et al. 2006), Cl−/Br− ratio (Nair et al. 2015), and Cl−/HCO3− ratio (Chidambaram et al. 2018). The saltwater mixing index is an identification of SWI in the freshwater aquifer (Mondal et al. 2011; Idowu et al. 2017). The current study examined and discussed the saline water intrusion processes in the region of Puri district.
Saltwater mixing remains the major degrading factor in the coastal aquifer of the Puri district. As a result, over 50% of fresh groundwater in the south Mahanadi delta including Puri, Konark, and Rabanagaon regions have become either brackish or saline (Radhakrishna 2001). A study carried out by Mohanty and Rao (2019) in Puri district, revealed that the TDS value increases from inland recharge region to the shoreline discharge region based on the groundwater flow path and the facies changes from Na–K-HCO3 to Na-Mg-Cl type. Mohapatra et al. (2011) and Vijay et al. (2011) studied the groundwater of Puri city; it was revealed that septic tank and soak pits are the cause of groundwater pollution along with the hydrological process of SWI in summers.
The similar research work was carried out earlier (Mohapatra et al. 2011; Prusty et al. 2018 and Vijay et al. 2011) but the studies on groundwater quality is a dynamic phenomenon. The earlier work covers some different parts of the Puri regions from the current work. The present work covers more wide area and provide recent data about the groundwater quality and saltwater mixing. The earlier studies were more focused on groundwater quality where the current study includes the saltwater mixing also. This study is being carried out to understand the groundwater pollution due to anthropogenic activities and SWI and the acceptability of water for diverse applications in the sectors of agricultural and domestic functions. In the current study, hydrogeochemical assessment of groundwater samples in the coastal region of Puri was carried out and the saltwater mixing was also examined in the freshwater aquifers.
Study area and geology
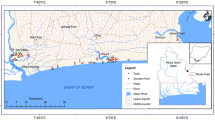
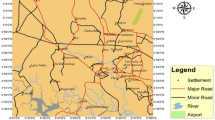
The study region chosen is between longitude 85° 25′ 30″ E to 85° 52′ 30″ E and 19° 40′ 0″ N to 19° 58′ 30″ N with a geomorphological area of 670 km2. Mainly, the Mahanadi delta covered this area (Fig. 1). The tributaries drain of the Mahanadi River in the Puri region, which supplies a maximum of the fresh surface water before it empties into the Bay of Bengal. However, the population explosion, overexploitation, intense urbanization, industrialization, and excessive use of fertilizers gave birth to a water crisis (Mohapatra et al. 2011; Vijay et al. 2011). The climate of the Puri district may be described as tropical, and the district receives an average of 1449 mm of rainfall during the monsoon season. The annual groundwater recharge in the study area is estimated by 55.1 to 348 mm/yr (Sahoo 2014). The southwest monsoon is the primary mechanism responsible for precipitation in the district. Flood is typical in this area due to high rainfall, frequent cyclones and storm surges (CGWB 2017). Physiographically, there are two natural divisions: the first is a saline marshy zone beside the coast, and the second is a tenderly sloping plain (alluvium with some patches of laterite). Geomorphology is broadly characterized by fluvial, coastal, lacustrine, and water bodies. The older and active floodplains indicate fluvial origin. Dune elevation ranges 15–27 m close to the coastline, while height remains less than 27 m above sea level, with a significant part representing the elevation range of 5–10 m (Sahu et al. 2018).
The quaternary formations are common in the study area, while Tertiary and Archean are over-lien by alluvium, coastal marshy plains, and laterite in patches. The older alluvium is exposed in the northwestern parts containing a sequence of sand, clay, and kankars of gray to brown. The younger alluvium occupies the remaining area, which consists of an admixture of silt, sand, gravel, and pebble to a changeable extent. These layers rise in width towards the sea (Radhakrishna 2001). The water table varies from 1 to 10 m below ground level (bgl) and its level shifts according to the rainfall pattern (Mohanty and Rao 2019). The ordinary groundwater extraction formations are dug wells and shallow and deep tube wells (CGWB 2017).
Material and methods
A total of 108 groundwater samples were taken from shallow aquifers of the study area, with 54 taken before the monsoon season began in May 2017 and 54 taken after monsoon season in November 2017. Standard procedures of the American Public Health Association were used for collecting, preserving, and succeeding examination of the groundwater samples (APHA 2012). The samples for major ions were collected in ½-L sample bottles (Tarsonspolyethene), pre-treated in a solution of nitric acid diluted 1:1 over 24 h. Sampling bottles were washed with deionized water to avoid any redundant contamination. The samples were cleaned of suspended sediments before the chemical test. In order to remove the suspended sediments, each sample was run through vacuum filtration equipment and filtered using filter paper with a pore size of 0.45-μm Millipore.
Physical constituents pH and electrical conductivity (EC) were calculated in the field with portable electrodes (pH/Cond 340i SET 1 Electrode). The measurement of Ca2+, HCO3−, Cl−, and hardness was carried out by titration method. Mg2+ and alkalinity were calculated by the calculation method, while F− and Br− were measured by the ISE method (ion selective electrode, Thermo-scientific Orion 4-star). Na+ and K+ were analyzed by a flame photometer (ELICO model CL-378). SO42−, NO3−, and SiO2 were quantified by a UV–visible double beam spectrophotometer. The precision of the investigation was confirmed by calculating ionic equilibrium. The electro-neutrality (EN %) of the data within the ± 5. The map preparation was done in a GIS environment. Microsoft Excel and (Statistical Package for Social Sciences) SPSS 21 were used for various statistical calculations, Gibbs diagram, and saturation indices. PHREEQC Interactive 2.12 and Aquachem 4.0 have been used for geochemical modelling to generate the saturation indices value. The SI, or saturation index, is a mathematical representation of the relationship between the ion activity product (IAP) and the mineral equilibrium constant (Ksp) at a specific temperature. This definition was established by Freeze and Cherry in 1979. Saltwater mixing index (SWMI) were calculated using major ions in seawater including Na+, Mg2+, Cl−, and SO42−. The threshold value were estimated by the inflection point scaled on cumulative probability curve (Kumar et al. 2016).
Result and discussion
General geochemistry
The pH value ranges from 5.76 to 7.64 with a mean value of 6.91 in the pre-monsoon and from 6.34 to 8.14 with a mean value of 7.31 in the post-monsoon, respectively. The results shows that the pH of the study area is slightly alkaline in post-monsoon which could be due to the influx of bicarbonate ions in groundwater aquifer system through rainwater percolation (Masood et al. 2022). All the values of pH in both the seasons is under the permissible limit recommended by WHO (2006) and BIS (2012). The concentration of EC in the groundwater ranges from 223 to 7600 mg/L with a mean value of 2677.93 mg/L in the pre-monsoon and from 125 to 5500 mg/L with a mean value of 2389.57 mg/L in post-monsoon, correspondingly (Table 1). High values of EC are observed in the pre-monsoon as compared to post-monsoon, which could be due to the evaporation dominance character and halite enrichment in the groundwater in the pre-monsoon. Approximately 54% samples fall under very high salinity hazard (> 2250 µS/cm). The concentration of TDS in the groundwater ranges from 149 to 3513 mg/L with a mean of 1552 mg/L in the pre-monsoon season and from 125.96 to 3377 mg/L with a mean of 1532 mg/L in the post-monsoon season (Table 1).
Among the cationic constituents, the dominant cation was Na+ with a range of 33.4 to 950.6 mg/L with a mean of 397.35 mg/L in pre-monsoon and 35.7 to 890.4 mg/L with a mean of 414.01 mg/L in post-monsoon followed by Ca2+, Mg2+, and K+. The details are given for cationic constituents in the Table 1. Among major anionic concentrations, Cl− was the dominant ion having a range of 28 to 1650 mg/L with a mean of 611.17 mg/L in pre-monsoon and 46 to 1510 mg/L with a mean 613.2 mg/L in post-monsoon followed by HCO3−, SO42−, and NO3–(Table 1). Moreover, Br− ranged from 0.54 to 33 mg/L with a mean value of 8.95 mg/L in pre-monsoon and 0.5 to 22 mg/L with mean value of 8.39 mg/L in post-monsoon (Table 1). For the cations chemistry, 72% and 85% samples show Na+ > Ca2+ > Mg2+ > K+ type of water and the remaining 28% and 19% samples show Na+ > Mg2+ > Ca2+ > K+ type of water in pre- and post-monsoon, correspondingly. Further, the anion chemistry indicates 63% and 81% of samples show Cl− > HCO3− > SO42− > NO3− type of water and the remaining 37% and 19% of samples show HCO3− > Cl− > SO42− > NO3− type of water in pre- and post-monsoon, correspondingly.
Hydrogeochemical facies and water type
Groundwater occurrence modes within an aquifer that vary in chemical composition are referred to as “hydrogeochemical facies” (Patel et al. 2016). The lithology, solution kinetics, and aquifer flow patterns influence the water facies. When considered alongside distribution maps and hydrochemical sections, hydrochemical diagrams help to analyze evolutionary tendencies, particularly in groundwater systems (Raju et al. 2011).
Piper’s diagram
The Piper diagram (Piper 1944) was used to classify the hydrochemical facies of water according to their ionic dominance. Na+ and Cl− are predominant among all ions in the groundwater samples. For cationic facies, it is noted that 85% and 91% of samples fall under Na+ + K+ type, whereas 15% and 9% of samples fall under no dominance type in pre- and post-monsoon, correspondingly. For anionic facies, it was noted that 85% and 91% of samples fall under Cl− type, 11% and 4% of samples fall under HCO3− type of water, and 4% and 5% of samples fall under no dominance type in pre- and post-monsoon, correspondingly (Fig. 2).
The results of the Piper diagram showed that the Na-Cl type of facies was present in 81% of pre-monsoon samples and 89% of post-monsoon samples, correspondingly. In the diamond plot, the Na-Cl composition of the water served as an indicator of the salinization process. Rest of the samples lie in Na-HCO3-Cl and Na-HCO3 type of water.
Gibbs diagram
Gibb’s value for cations (Gibb’s I) is a plot between TDS (mg/L) and (Na+ + K+)/(Na+ + K+ + Ca2+) (meq/L). Gibb’s I ranged from 0.531 to 0.968 with a mean value of 0.829 in pre-monsoon and from 0.571 to 0.978 with a mean value of 0.841 in post-monsoon. Moreover, Gibb’s value for anions (Gibb’s II) is a plot between TDS (mg/L) and Cl−/(Cl− + HCO3−) (meq/L). Gibb’s II ranged from 0.271 to 0.949 with a mean value of 0.701 in pre-monsoon and from 0.268 to 0.915 with a mean value of 0.733 in post-monsoon. According to Gibb’s I, approximately 67% and 61% of the samples show evaporation dominance and 33% and 39% of the samples show rock dominance as the principal controlling processes for groundwater chemistry in pre- and post- monsoon, correspondingly (Fig. 3a). In Gibb’s II, approximately, 67% and 63% of the samples show evaporation dominance and 33% and 37% of the samples show rock dominance in pre- and post-monsoon, correspondingly. The Gibbs diagram suggest that evaporation dominance followed by rock dominance are the primary chemistry controlling process for cationic and anionic composition of water (Fig. 3b) (Suguna and Sherene 2019). An increased level of evaporation or human activities contributes to higher total dissolved solids (TDS) in water samples, causing a transition from rock-dominated areas to the evaporation zone (Krishna Kumar et al. 2014; Satheeskumar et al. 2021). The movement of sampling locations towards the evaporation zone, away from the rock-water interaction zone, demonstrates an elevation in Na+ and Cl− ions. Additionally, the increased total dissolved solids (TDS) levels could perhaps be attributed to the intrusion of seawater (Satheeskumar et al. 2021).
Base exchange indices
Base exchange indices are typically used in regional hydrogeochemical studies to indicate salinizing or freshening phenomena of aquifers that have been freshened or salinized in the past. Generally, base exchange indices are represented as salinized or freshened facies or into a state of equilibrium (Stuyfzand 2008).
Schoeller classification (chloroalkaline indices)
Chloroalkaline indices are helpful to understand the evolution of groundwater chemical composition and rock-water interaction (Gowd 2005; Islam et al. 2017). To validate the ion exchange processes that take place in an aquifer medium, Scholler (1965) developed the chloroalkaline indices, and he has provided the following computation of CAI-I and CAI-II
The units for measuring ionic concentrations are meq/L.
CAI-I ranged from − 2.59 to 0.39 with a mean − 0.20 in pre-monsoon and − 2.95 to 0.36 with a mean − 0.16 in post-monsoon, correspondingly. However, CAI-II values varies from − 0.98 to 2.74 with a mean 0.05 and − 0.94 to 1.31 with a mean − 0.12 in pre- and post-monsoon, correspondingly (Table 1). It has been revealed that 63% and 69% of samples show negative ratios, whereas 37% and 31% of samples showed positive ratios for both CAI I and CAI II indices in pre-and post-monsoon, correspondingly (Figs. 4a, b, and 5a, b). The positive value of CAI indicates base exchange reaction whereas negative value for the chloroalkaline disequilibrium. This study demonstrated that the indirect cation exchange process is prevalent during both seasons (Table 1).
Base exchange index (r1)
The mathematical demonstration of the base exchange index, which is used for groundwater type categorization, is presented as follows:
The units for measuring ionic concentrations are meq/L.
The value of the base exchange index ranged − 86.10 to 29.08 with a mean − 0.36 in pre-monsoon and − 4.18 to 10.18 with a mean − 1.19 in post-monsoon (Table 1). The water samples are categorized into two categories according to the base exchange index: those with a Na+-HCO3−water type (r1 > 1) and those with a Na+-SO42− water type (r1 < 1). 43% and 52% of samples have r1 values greater than 1 (r1 >), in pre- and post-monsoon, correspondingly (Fig. 6a, b). Therefore, Na+-SO42− a type of water dominant in pre-monsoon and Na+-HCO3− water type in post-monsoon. The enrichment of HCO3− may be due to the formation and leaching of bicarbonate produced by the reaction of CO2 and rainwater.
Meteoric genesis index (r2)
The meteoric genesis index is another method that can be used to categorize the types of groundwater. The following equation is used to give an expression for these meteoric genesis indices:
The units for measuring ionic concentrations are meq/L.
Base exchange index grouped the groundwater as a shallow meteoric water type (r2 > 1) or a deep meteoric water type (r2 < 1) based on its numeric value. It ranged − 84.91 to 29.24 with a mean − 0.20 in pre-monsoon and − 4.17 to 10.22 with a mean 1.26 in post-monsoon (Table 1). then, 44% and 52% of samples with r2 values higher than one are categorized as shallow meteoric groundwater types, whereas 56% and 48% of samples with an r2 value less than one were classified as deep meteoritic groundwater in pre- and post-monsoon, correspondingly (Fig. 7a, b).
Sources of ions
Natural sources
Saltwater intrusion (SWI) can contribute significantly to the salinity in groundwater. This is mostly owing to the presence of a natural mixing zone and the geographical conditions. Other factors that contribute to salinity include evaporation dominance, rock-water interaction, and precipitation. In addition, human activities that increase non-point sources, such as the return flow from agriculture, the excessive use of fertilizers and pesticides in paddy fields and aquaculture, and the wastewater infiltration, might have an impact on water chemistry.
Saturation index (SI)
The SI explain the variation of water from equilibrium with regard to the origin of groundwater salinization and the dissolved minerals quantitatively (Jalali 2007, 2010). It is estimated on the basis of the equation that is provided by Lloyd and Heathcote (1985):
where IAP stands for ion activity product and Ks is the mineral’s solubility product.
The positive SI with iron-bearing minerals reveals the presence of a high concentration of iron in groundwater (Borah et al. 2009; Chakrabarty and Sarma 2011). Graphs of SI of minerals with TDS indicate that 89% and 57% of samples are undersaturated for calcite in pre- and post-monsoon, correspondingly. And, for dolomite, 80% and 52% of samples are undersaturated in pre- and post-monsoon, correspondingly (Fig. 8). The SI value of calcite ranged from − 2.45 to 0.33 with a mean − 0.57 in pre-monsoon and from − 2.46 to 0.40 with a mean − 0.29 in post-monsoon. In contrast, for dolomite, it ranged from − 4.74 to 0.92 with a mean − 0.98 in pre-monsoon and from − 5.49 to 0.75 with mean − 0.45 in post-monsoon. Ca2+-bearing minerals (gypsum, anhydrite, fluorite, aragonite, and calcite), Mg2+-bearing minerals (dolomite, chrysolite, talc, and sepiolite) are undersaturated (SI < 0), while silicate minerals (quartz and chalcedony) and Fe-containing minerals (goethite and hematite) are found in saturation state (SI > 0) (Fig. 9a, b). The lack of Ca- and Mg-bearing minerals can be inferred from the surrounding lithology, which does not contain rocks rich in Ca and Mg, or if they are present, they are not in a form that can dissolve as Ca and Mg into the water system.
Saltwater intrusion processes
Ionic ratio comparison, saltwater mixing index, and seawater fraction have been used to acknowledge the potential source of salinity in coastal aquifers.
-
1. Ionic ratios
SWI significantly adds more ions to coastal aquifers (Etikala et al. 2021). High salinity and relatively higher concentrations of Na+, Mg2+, Br−, and Cl− indicate SWI in coastal aquifers. Some good ionic ratios are used to identify SWI processes. Some imperative ionic ratios (Na/Cl−, Ca2+/Mg2+, K+/Cl−, Br−/Cl−, and Cl−/HCO3−) have been compared as a potential cause of groundwater salinity. Salinization of groundwater in the coastal zone is predominantly due to SWI and profound deep saline upconing (overexploitation of coastal aquifer) (Vengosh and Rosenthal 1994; Nair et al. 2015; Patel et al. 2016; Sudaryanto and Naily 2018; Satheeskumar et al. 2021).
-
Na+/Cl− ratio
The salinity of water caused by the seawater intrusion is distinguished by a comparatively low Na+/Cl− ratio when compared to both freshwater and seawater (Asare et al. 2021). Saline water has Na+/Cl− ratio equal to 0.86, freshwater has this value > 1, and the value from 0.86 to 1 indicates the seawater invasion into the freshwater aquifers. Na+/Cl− ratio ranged 0.61 to 3.58 with mean 1.19 and 0.64 to 3.94 with mean 1.16 in pre- and post-monsoon, correspondingly (Table 1 and Fig. 10a, b). In the present study, 37% in pre-monsoon and 33% in post-monsoon samples show Na+/Cl− ratio < 0.86, which indicates SWI into the coastal groundwater (Vengosh and Rosenthal 1994; Nair et al. 2015). On the other hand, 63% of pre-monsoon and 67% of post-monsoon samples show a Na+/Cl−molar ratio > 1, revealing freshwater aquifers in the coastal aquifer.
-
Ca2+/Mg2+ ratio
A high concentration of Mg2+ compared to Ca2+ with a high TDS value shows saltwater mixing (Kura et al. 2018). If the Ca2+/Mg2+ratio of < 1 indicates SWI (Moujabber et al. 2006). Ca2+/Mg2+ ratio ranged 0.21 to 6.06 with mean 1.21 and from 0.05 to 14.71 with mean 1.62 in pre- and post-monsoon, correspondingly (Table 1). In the present study, 61% and 63% samples in pre-and post-monsoon, correspondingly showed < 1 value of Ca2+/Mg2+ ratio. Thus, the majority of the samples were affected by SWI (Fig. 11a, b).
-
K+/Cl− ratio
K+/Cl− ratio below 0.019 show deep saline upconing (Patel et al. 2016). K+/Cl− ratio ranged from 0.001 to 0.058 with a mean value of 0.01 and from 0.001 to 0.063 with a mean value of 0.007 in pre- and post-monsoon, correspondingly (Table 1 and Fig. 12a, b). Then, 81% in pre-monsoon and 90% post-monsoon samples deciphered deep saline upconing.
-
Br−/Cl− ratio
An elevated concentration of halogens, specifically Cl− and Br− in groundwater, indicates SWI. The Br−/Cl− is considered a reliable tracer of saline water intrusion as both Cl− and Br− are conservative elements, except in the occurrence of a very high amount of organic matter (Vengosh et al. 1999; Kim et al. 2003; Montety et al.2008; Jia et al. 2017; Gomes et al. 2019; Satheeskumar et al. 2021). The Br−/Cl− ratio ranged 0.008 to 0.026 with mean 0.016 and 0.002 to 0.030 with a mean 0.014 in pre-and post-monsoon, correspondingly (Table 1 and Fig. 13a, b). The Br−/Cl− ranged from 0.0033 to 0.0037, with a mean value of 0.0036 in seawater (Kim et al. 2003). The ratio of around 0.02 indicated the agricultural return flow (Montety et al. 2008). Then, 45% and 43% of the samples showed around 0.02 value of Br−/Cl− in pre- and post-monsoon, correspondingly. The elevated Br−/Cl− ratio deciphered some anthropogenic inputs other than saltwater invasion.
-
Cl−/HCO3− ratio
The Cl−/HCO3− ratio is also one of the essential tracers to indicate the saltwater mixing into freshwater aquifers and the origin of salinity. Based on Cl−/HCO3− ratios, there are three categorizations of groundwater that are unaffected (0.5), slightly or moderately (0.6–6.6), and strongly affected (> 6.6) affected by the salinization process (Chidambaram et al. 2018; Sudaryanto and Naily 2018). The ratio ranged from 0.053 to 2.70 with a mean of 0.58 in pre-monsoon and from 0.09 to 2.73 with a mean of 0.44 in post-monsoon (Table 1, Fig. 14a and b). Based on the Cl−/HCO3−, 34% and 24% of samples were moderately affected by the salinization process in pre- and post-monsoon, correspondingly.
-
-
2. Saltwater mixing index (SWMI)
The saltwater mixing index is recognized (Park et al. 2005) to acknowledge the seawater mixing in the coastal aquifer using Na+, Mg2+, Cl−, and SO42− as they are abundant in seawater. The following equation can compute the SWMI:
$$\text{SWMI}=a\frac{CNa}{TNa}+b\frac{CMg}{TMg}+c\frac{CCl}{TCl}+d\frac{CSO_4}{TSO_4}$$where constant a (0.31), b (0.04), c (0.57), and d (0.08) denote the relative concentration proportion of Na+, Mg2+, Cl−, and SO42− in seawater (Park et al. 2005). Ci is the measured value of ions in the groundwater, and Ti is the regional threshold value of respective ions. The threshold values for pre-monsoon were calculated: 89 mg/L for Na+, 20.18 mg/L for Mg2+, 122 mg/L for Cl−, and 18.47 mg/L for SO42−. For post-monsoon, the threshold value were 102.1 mg/L for Na+, 8.75 mg/L for Mg2+, 70 mg/L for Cl−, and 19.49 mg/L for SO42−. The threshold value was estimated by the interpreting the cumulative probability curve for both the seasons. If the measured value of SWMI is greater than one, in that case, water may show the effect of seawater interaction with freshwater, whereas this value greater than 3 indicates SWI (Idowu et al. 2017; Idowu and Lasisi 2020; Aladejana et al. 2021).
The current study indicates that 90.74% of samples in pre-monsoon and 94.44% of samples in post-monsoon show the SWMI values greater than unity (= 1), indicating the interaction of seawater with freshwater aquifers. Moreover, 64.81% of samples in pre-monsoon and 72.22% of samples in post-monsoon have shown an SWMI value greater than 3, deciphering the SWI into the freshwater system. SWMI ranged 0.29 to 11.62 in pre-monsoon and 0.53 to 15.66 in post-monsoon (Table 1). The water samples nearby the coast show SWI. In contrast, the samples far from the coast indicate deep saline upconing due to excessive groundwater withdrawal for agriculture and aquaculture. The spatial distribution of SWMI values (Fig. 15a, b) shows the seawater mixing in coastal aquifers. There are three categorizations on the map based on SWMI, i.e., (i) freshwater, (ii) fresh-seawater interaction, and (iii) SWI into freshwater aquifer system, laterally through seawater ingression and vertically by deep saline upconing.
-
3. Seawater percentage (SW%) or seawater fraction (%f sea)
Seawater percentage or seawater fraction is a measurement of seawater percentage in the freshwater aquifers based on the conservative element Cl− and it can be estimated by using the following formula:
$$\mathrm{Seawater Fraction }(\mathrm{\% S}.{\text{W}}.)=\frac{CCl, sample-CCl, fresh}{CCl,, sea-CCl, fresh}(\mathrm{Concentration in meq}/{\text{L}})$$The seawater fraction can be calculated by assuming that all Cl− should come from seawater and no brine conditions should be available in the area. The water having low Na+ and Cl− has been taken as the freshwater samples for the Cl− ions. In this study, the seawater percentage ranged − 0.074 to 8.495 in pre-monsoon and 0.021 to 7.755 in post-monsoon (Table 1, Fig. 16a, b). The sample having a higher value might be contaminated more by SWI. Then, 21% and 17% of samples in pre- and post-monsoon, correspondingly, have SW% > 5. This could be the brine mixing with freshwater aquifers (Mohanty and Rao 2019).
Anthropogenic input of ions
Anthropogenic activities (abundant fertilizers input and its runoff, aquaculture, and wastewater invasion into aquifers from the urbanization and metropolitan cities) also increased the ionic concentration of the groundwater. Relatively high concentrations of NO3−, Cl−, Br−, and SO42− may result from diverse anthropogenic activities. Anomalous concentrations of Cl− may also be the product of pollution by sewage wastes, excessive use of fertilizers, common salt added to a coconut plantation, fish farming, and leaching of saline residues entrapped within the soil (Raju 2012; Srinivasamoorthy et al. 2014). The presence of high levels of Na+, Cl−, and SO42− in the aquifer may be attributed to the use of NaCl and Na2SO4 salts in fish farming (Barclay et al. 2002). The excess of Na+ in groundwater might also come from wastewater infiltration. Na2SO4 is used instead of NaCl for commercial production of aquaculture because it has a corrosive effect on the equipment and reduces the production of microflora in the ponds (Barclay et al. 2002; Jeevanandam et al. 2012). Dissolution of potash feldspar occurs when the molar ratio of K+/Cl− exceeds 0.2 values while all values in groundwater are below 0.2, indicating no potash feldspar dissolution (Prasanna et al. 2011).
The concentration of Br− in freshwater ranged from 0.5 to 1 mg/L (WHO 2006). In the present study, all the samples had a Br− concentration are more than 1 mg/L. Fertilizers such as ethyl-dibromide and methyl-bromide may also be the source of Br− concentration other than SWI (Cartwright et al. 2006). The degradation of organic matter could be the reason behind the slight increase of the Br− in most groundwater samples (Montety et al. 2008).
The value of NO3− in groundwater samples is more than 3 mg/L, indicating human-affected values (Pastén-Zapata et al. 2014). In the present study, 59.25% of pre-monsoon and all the samples in post-monsoon showed more than 3 mg/L of NO3− values, which indicates human influence on NO3− concentration. The high value of NO3− in post-monsoon may be due to agricultural runoff, poor drainage, and untreated domestic waste. Although the distribution of NO3− is relatively uniform, some patches of high concentration, near Puri temple area, where degradation of flower waste could be the source of NO3−. High concentrations of NO3− in drinking water could be the reason for methemoglobinemia (blue baby syndrome) in newborns and subsequently leads to death by asphyxiation and stomach cancer in adults (Schwendimann et al. 2021).
Statistical approach
Multivariate statistical techniques, correlation matrix, and principal component analysis (PCA) are efficient means of manipulating, understanding, and symbolizing data concerning groundwater contaminant, quality, and geochemistry (Belkhiri et al. 2011; Emenike et al. 2018; Jampani et al. 2018).
A perfect positive correlation between Na+ and Cl− (0.96 in pre- and 0.91 in post-monsoon) could be the indication of halite dissociation (Tables 2 and 3). A good correlation between Ca2+ and SO42− (0.59 in post-monsoon) shows gypsum dissolution. Although K+ is low in concentration but a good correlation between K+ and NO3− (0.64 in post-monsoon) could be the agricultural runoff as a potential source of K+ and NO3− in the groundwater in post-monsoon.
Factor analysis
Factor examination is utilized as an arithmetical means to find more significance and define the nature of hydrochemical processes. The study of factor scores discloses the degree of influence of each factor on overall water chemistry (Yidana et al. 2018; Emenike et al. 2018; Busico et al. 2018; Madhav et al. 2020). In the current study, four factors are sufficient to explain 79.53% and 81.51% of the variance for the compound matrix in pre- and post-monsoon, correspondingly (Table 4). The total variance of pre-monsoon and post-monsoon is explained by four principal components with its eigenvalue (> 1). The details of the various extracted factors are given below:
-
Factor 1
explained 44.61% and 42.23% of the total variance in pre- and post-monsoon, correspondingly. Both seasons have high positive loading for EC, hardness, Ca2+, Mg2+, Na+, Cl−, Br−, and SO42. It shows that Na+, Ca2+, Mg2+, Cl−, Br−, and SO42− are responsible for high EC. The high value of hardness is directly associated with Ca2+, Mg2+, Cl−, and SO42−. The positive relation between Na+ and Cl− indicates SWI and, to some extent, halite weathering. The high amount of Br− is clearly indicating about SWI in the study area. Ferromagnesian minerals might be the source of the high amount of Mg2+ in groundwater (Rao 2012). The high value of hardness is directly associated with Ca2+, Mg2+, Cl−, and SO42−.
-
Factor 2
explain 12.60% of the total variance and showed high loading for K+, HCO3−, and NO3− in pre-monsoon. This factor deciphered intensive agricultural activities. NPK fertilizers used in paddy fields may be the source of K+ and NO3−. Fish farming might also be accountable for high HCO3− in groundwater (Barclay et al. 2002). The post-monsoon water samples show 16.89% of the total variance and increased loading of pH, HCO3−, and F−. It shows the source of ions as biological and natural geogenic sources. In both, the season’s high concentration of HCO3− was observed by leaching of HCO3− produced by the reaction of rainwater and CO2 present in the soil (Raju 2012).
-
Factor 3
explains 12.40% of the total variance. It was high negative loading of F− and high positive loading of SiO2 in pre-monsoon, showing the lithologic source of silica and fluoride. The high temperature in the pre-monsoon season favors silica weathering in water (Umar and Alam 2012). The post-monsoon water samples show 14.58% of the total variance and increased K+ and NO3− loading. NPK fertilizers are the source of the high concentration of K+ and NO3− extensively used in the study area to produce rice.
-
Factor 4
explains the 9.41% of the total variance and high loading of pH and HCO3−in pre-monsoon. High loading of HCO3− revealed the dissociation of H2CO3 from the soil by the reaction of rainwater and CO2 (Raju 2012). The post-monsoon water samples show 7.87% of the total variance and high positive loading of SiO2. This could be the silicate weathering due to the geogenic factor.
Groundwater quality
Classification of groundwater for drinking purpose
To comprehend the groundwater suitability for drinking and community health exercise, hydrogeochemical parameters are compared (Table 5) with the guiding principle prescribed by WHO (2006) and BIS (2012). According to Davis and De Wiest (1966) categorization, 65% and 63% of samples are inappropriate for drinking purposes in pre- and post-monsoon, correspondingly. The spatial diagram of TDS based on the Davis and De Wiest (1966) is given in Fig. 17a, b. In the context of salinity, 65% and 63% of samples lie in the brackish water category in pre- and post-monsoon, correspondingly (Freeze and Cherry 1979). Based on the Sawyer and Mc. Cartly (1967), 7% and 11% of samples are soft, 20% and 11% of samples are slightly hard, 27% and 47% of samples are moderately hard, and 46% and 31% of samples are very hard in pre- and post-monsoon, correspondingly. As per Na+ values, 74% and 70% of samples are beyond the permissible limit of WHO (2006) and BIS (2012) in pre- and post-monsoon, correspondingly.
Classification of groundwater for irrigation purpose
The significant chemical parameters for determining the degree of the usefulness of groundwater for irrigation are EC, %Na, salinity hazard, SAR, RSC, Kelly’s index, and magnesium hazard.
Electrical conductivity (EC) and percent sodium (%Na)
The EC and Na+ are important parameters to classify water quality for agricultural use. The %Na is weighed concerning relative proportions of cations (Na+, K+, Ca2+, and Mg2+), and a higher %Na negatively impacts soil permeability. Normally, higher %Na in irrigation water should not exceed 60% (Singh et al. 2015). It is calculated according to the given formula:
The units for measuring ionic concentrations are meq/L.
Based on the %Na classification, 3.7% of samples fall under the good category in both seasons, 16.7% and 13% of samples fall under the permissible category, 50% and 41% of samples fall under the doubtful category, while 29% and 43% of samples fall under unsuitable category in pre- and post-monsoon, correspondingly (Table 6). The water suitability can also be examined by plotting the values of %Na against corresponding values of EC on the Wilcox diagram (Fig. 18). Wilcox diagram (1948) represents that 5.6% and 7.4% of samples lie under excellent to good, 17% and 20% of samples are good to permissible, 11% and 7% of samples lie in permissible to the doubtful range, 33% and 32% of samples lies in doubtful to unsuitable, and 33% and 33% of samples lies in unsuitable class in pre- and post-monsoon, correspondingly.
Groundwater classification based on electrical conductivity and percent sodium (Wilcox diagram 1948) ((A) excellent to good, (B) good to permissible, (C) permissible to doubtful, (D) doubtful to unsuitable, and (E) unsuitable)
Salinity (EC) hazard
The groundwater has also been classified based on EC (Table 6) and categorized accordingly. As per Richards (1954) classification, 2% of samples fall under the excellent class (C1) in both the seasons, 7% and 6% of the samples lie in good category (C2) in pre- and post-monsoon, correspondingly, while 37% of samples lie in fair class and 54% of samples in poor class in both seasons.
Sodium adsorption ratio (alkali hazard)
The most frequent method to evaluate sodicity in water and soil is called the SAR. The following equation estimates the SAR
The units for measuring ionic concentrations are meq/L.
The SAR values ranged from 1.17 to 34.2 meq/L with a mean 10.6 meq/L in pre-monsoon and 1.27 to 23.05 meq/L with a mean 11.45 meq/L in post-monsoon (Table 6). This indicates that in both seasons, all samples came under the S1 category, considered excellent and could be used for irrigational and agricultural activities. According to Richards (1954) categorization on SAR values (Table 5), 37% of samples fall under the excellent category in both seasons, 59% and 48% of samples fall under good quality, 2% and 15% of samples fall in fair class in pre- and post-monsoon, correspondingly, and 2% of samples were poor class in pre-monsoon season.
-
U.S. salinity laboratory diagram
U.S. salinity diagram (USSL Diagram 1954) has been applied for water classification regarding crop productivity. On the basis of this categorization, 2% of samples fall into the low salinity and low sodium (C1S1) zone in both seasons. 7% of samples fall in medium salty and low sodium (C2S1) in both seasons, which is suitable for all plants. 19% of samples in both seasons fall in high salty and low sodium (C3S1), which need proper drainage for irrigation. 19% of samples in both seasons lies in high saline and medium to very high sodium (C3S2, C3S3, C3S4), which are unsuitable for irrigation. 4% of samples in both seasons fall in very high salty and low sodium (C4S1), which requires a sound drainage system. 31% and 19% of samples fall under the very high salty and medium sodium category (C4S2) in pre- and post-monsoon, correspondingly, which was dangerous for soil. Whereas 19% and 31% of samples fall in very high salty and very high sodium (C4S3 and C4S4), in pre- and post-monsoon which were unsuitable for irrigation (Fig. 19).
Fig. 19 Groundwater classification on the basis of salinity hazard and alkali hazard (after USSL Diagram 1954)
Residual sodium carbonate (RSC)
Sodium adsorption in the soil is amplified to a greater degree when the RSC value is high. RSC has been deliberated to establish the dangerous impact of CO32− and HCO3− on water excellence for farming use (Eaton 1950). The RSC can be calculated using the following equation:
The units for measuring ionic concentrations are meq/L.
According to Eaton classification, 81% of samples were categorized within good quality (< 1.25 meq/L) in both seasons, 6% and 9% under medium quality (1.25–2.5 meq/L), and 13% and 9% under bad quality (> 2.5 meq/L) for irrigation purpose in pre- and post-monsoon, correspondingly (Table 6).
Kelly’s index
Kelly’s Index is one of the parameters to assess water quality for farming. For computing KI, Kelly (1940) and Paliwal and Singh (1967) have provided the following equation:
The units for measuring ionic concentrations are meq/L.
Categorization of water for irrigation based on Kelly’s ratio value:
-
1.
KI value < 1—suitable
-
2.
KI value (1–2)—marginally suitable
-
3.
KI value > 2—unsuitable
Based on Kelly’s index (KI), groundwater classifies into three classes. KI value is less than one indicates the suitability of water for farming, within 1–2 indicates marginally suitable. KI value > 2 shows the water is not suitable for agriculture. The KI values of groundwater samples ranged from 0.47 to 12.63 with a mean 3.57 and 0.60 to 14.89 with a mean 4.16 in pre- and post-monsoon, correspondingly (Table 6). Based on Kelly’s index, 74% and 67% of samples were unsuitable for irrigation in pre- and post-monsoon, correspondingly.
Magnesium hazard (MH)
The magnesium hazard was introduced by Szabolcs and Darab (1964) as a method for determining the quality of water worth framing. In most cases, the high intensity of Mg2+ in irrigated soil is caused by the exchangeable Na+ in the soils. Sodication in soils is intensified when there is a greater ratio of Mg2+ to Ca2+. It causes the scattering of clay particles. Since Mg2+ acts similarly to Na+, this causes damage to the structure of the soil and lowers it relative hydraulic conductivity (Raju 2012; Patel et al. 2016). Therefore, water having high Mg2+ reduces soil productivity.
The following equation calculates magnesium Hazard:
The units for measuring ionic concentrations are meq/L.
A magnesium ratio of more than 50 is regarded as hazardous and unsuitable for irrigation, as the water is considered unsafe and inappropriate for irrigation since it will harm crop production by rising the basic nature of the soil (Raju et al. 2011). The MH values of the sample ranged from 14.17 to 82.98 with a mean 51.35 in pre-monsoon and from 6.36 to 95.25 with mean 50.37 in post-monsoon. According to the MH, 61% and 63% of samples were not appropriate for irrigation in pre- and post-monsoon, correspondingly (Table 6).
Conclusions
Overexploitation of groundwater in coastal aquifers enhanced the SWI, which makes groundwater unsuitable for intake (drinking purposes), household and farming use. As per the Gibbs plots, all the samples came under rock dominance which is the principal controlling process for groundwater chemistry in both seasons. Piper diagram reveals Na+ and Cl− as dominant ions that were not only from SWI but also from the dissolution of evaporites and local anthropogenic activities (i.e. municipal waste and agricultural activity). Na+/Cl−, Ca2+/Mg2+, K+/Cl−, Br−/Cl−, and Cl−/HCO3− ratios are used to identify SWI. Around 35% of the samples show < 0.86 ratios of Na+/Cl−in both seasons. An increase of Cl− concentration in Br−/Cl− ratio indicates the source of salinization in aquifers is due to the mixing of seawater. According to Ca2+/Mg2+ ratio, 61% and 63% of samples were affected by SWI. Moreover, 64.81% and 72.22% of samples in pre- and post-monsoon, correspondingly, were greater than 3, indicating saline water intrusion into the freshwater system. SWI has been observed in all instances, indicating a pervasive occurrence. Factor analysis also verified that natural and anthropogenic activities are accountable for major ion contributions in the groundwater. A total of 65% and 63% of samples are unfit for drinking practice in pre- and post-monsoon, correspondingly. Irrigation water classified based on %Na, 79% and 84% of samples were doubtful to the unsuitable category in pre- and post-monsoon, correspondingly. SWI is a dynamic process, which is why the groundwater must be continuously monitored in the coastal aquifers. This study will help to differentiate the non-contaminated freshwater aquifers from the contaminated aquifers.
Competing interests
The authors declare no competing interests.
Data availability
All the data generated during the study are included in this research publication and reviewed by all authors. The data is available from the corresponding author, upon request.
References
Aladejana JA, Kalin RM, Sentenac P, Hassan I (2021) Groundwater quality index as a hydrochemical tool for monitoring saltwater intrusion into coastal freshwater aquifer of Eastern Dahomey Basin, Southwestern Nigeria. Groundw Sustain Dev 1(13):100568
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington
Asare A, Appiah-Adjei EK, Ali B, Owusu-Nimo F (2021) Assessment of seawater intrusion using ionic ratios: the case of coastal communities along the Central Region of Ghana. Environ Earth Sci 80:1–4
Barclay TM, Hicks RG, Lemaire MT, Thompson LK, Xu Z (2002) Synthesis and coordination chemistry of a water-soluble verdazyl radical. structures and magnetic properties of M (H 2 O) 2 (vdCO 2) 2· 2H 2O (M= Co, Ni; vdCO 2= 1, 5-dimethyl-6-oxo-verdazyl-3-carboxylate). Chem Comm (16):1688–1689
Bear J, Cheng AHD, Sorek S, Ouazar D, Herrera I (Eds.) (1999) Seawater intrusion in coastal aquifers: concepts, methods and practices, vol 14. Springer Science & Business Media
Belkhiri L, Boudoukha A, Mouni L (2011) A multivariate statistical analysis of groundwater chemistry data. Int J Environ Res 5(2):537–544
BIS (2012) Indian standard, drinking water-specification (second revision). IS: 10500, Bureau of Indian Standards, New Delhi
Borah KK, Bhuyan B, Sarma HP (2009) Heavy metal contamination of groundwater in the tea garden belt of Darrang district, Assam, India. E-J Chem 6(S1):S501-7
Busico G, Cuoco E, Kazakis N, Colombani N, Mastrocicco M, Tedesco D, Voudouris K (2018) Multivariate statistical analysis to characterize/discriminate between anthropogenic and geogenic trace elements occurrence in the Campania Plain, Southern Italy. Environ Pollut 1(234):260–269
Cartwright I, Weaver TR, Fifield LK (2006) Cl/Br ratios and environmental isotopes as indicators of recharge variability and groundwater flow: an example from the southeast Murray Basin, Australia. Chem Geol 231(1–2):38–56
CGWB (2017) National Aquifer Mapping in the district of Puri, Odisha. Interim Unp. Rep. Central Ground Water Board, South Eastern Region, Bhubaneswar, Odisha. Ministry of Water Resources, RD & GR, Government of India
Chakrabarty S, Sarma HP (2011) Heavy metal contamination of drinking water in Kamrup district, Assam, India. Environ Monit Assess 179:479–486
Chidambaram S, Sarathidasan J, Srinivasamoorthy K, Thivya C, Thilagavathi R, Prasanna M V, Nepolian M (2018) Assessment of hydrogeochemical status of groundwater in a coastal region of Southeast coast of India. Appl Water Sci 8:1–14
Cloutier V, Lefebvre R, Therrien R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353(3–4):294–313
Davis SN, De Wiest RJM, Ferris JG, Knowles B, Brown RH, Stallman RW (1966) Groundwater in fractured rocks. New York, Hydrogeology 318
De Montety V, Radakovitch O, Vallet-Coulomb C, Blavoux B, Hermitte D, Valles V (2008) Origin of groundwater salinity and hydrogeochemical processes in a confined coastal aquifer: case of the Rhône delta (Southern France). Appl Geochem 23(8):2337–2349
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 69(2):123–134
Emenike CP, Tenebe IT, Jarvis P (2018) Fluoride contamination in groundwater sources in Southwestern Nigeria: assessment using multivariate statistical approach and human health risk. Ecotoxicol Environ Saf 156:391–402
Essink GH (2001) Improving fresh groundwater supply—problems and solutions. Ocean Coast Manag 44(5–6):429–449
Etikala B, Adimalla N, Madhav S, Somagouni SG, Keshava Kiran Kumar PL (2021) Salinity problems in groundwater and management strategies in arid and semi-arid regions. Groundw Geochem: Pollut Remediat Methods 25:42–56
Famiglietti JS (2014) The global groundwater crisis. Nat Clim Chang 4(11):945
Ferguson G, Gleeson T (2012) Vulnerability of coastal aquifers to groundwater use and climate change. Nat Clim Chang 2(5):342–345
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, Englewood Cliffs, 604
Glynn PD, Plummer LN (2005) Geochemistry and the understanding of ground-water systems. Hydrogeol J 13:263–287
Gomes OV, Marques ED, Kütter VT, Aires JR, Travi Y, Silva-Filho EV (2019) Origin of salinity and hydrogeochemical features of porous aquifers from northeastern Guanabara Bay, Rio de Janeiro, SE-Brazil. J Hydrol Reg Stud 22:100601
Gowd SS (2005) Assessment of groundwater quality for drinking and irrigation purposes: a case study of Peddavanka watershed, Anantapur District, Andhra Pradesh, India. Environ Geol 48:702–712
Huang G, Sun J, Zhang Y, Chen Z, Liu F (2013) Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China. Sci Total Environ 463:209–221
Idowu TE, Lasisi KH (2020) Seawater intrusion in the coastal aquifers of East and Horn of Africa: a review from a regional perspective. Sci Afr 8:e00402
Idowu TE, Nyadawa M, K’Orowe MO (2017) Hydrogeochemical assessment of a coastal aquifer using statistical and geospatial techniques: case study of Mombasa North Coast, Kenya. Environ Earth Sci 76:1–8
Islam ARMT, Ahmed N, Bodrud-Doza M, Chu R (2017) Characterizing groundwater quality ranks for drinking purposes in Sylhet district, Bangladesh, using entropy method, spatial autocorrelation index, and geostatistics. Environ Sci Pollut Res 24:26350–26374
Jalali M (2007) Salinization of groundwater in arid and semi-arid zones: an example from Tajarak, western Iran. Environ Geol 56:1479–1488
Jalali M (2010) Groundwater geochemistry in the Alisadr, Hamadan, western Iran. Environ Monit Assess 166(1–4):359–369
Jampani M, Huelsmann S, Liedl R, Sonkamble S, Ahmed S, Amerasinghe P (2018) Spatio-temporal distribution and chemical characterization of groundwater quality of a wastewater irrigated system: a case study. Sci Total Environ 636:1089–1098
Jeevanandam M, Nagarajan R, Manikandan M, Senthilkumar M, Srinivasalu S, Prasanna MV (2012) Hydrogeochemistry and microbial contamination of groundwater from lower ponnaiyar basin, cuddalore district, Tamil Nadu, India. Environ Earth Sci 67:867–887
Jia Y, Guo H, Xi B, Jiang Y, Zhang Z, Yuan R, Yi W, Xue X (2017) Sources of groundwater salinity and potential impact on arsenic mobility in the western Hetao Basin, Inner Mongolia. Sci Total Environ 601:691–702
Kelly WP (1940) Permissible composition and concentration of irrigated waters. In: Proceedings of the ASCF 66, p 607
Kim Y, Lee KS, Koh DC, Lee DH, Lee SG, Park WB, Koh GW, Woo NC (2003) Hydrogeochemical and isotopic evidence of groundwater salinization in a coastal aquifer: a case study in Jeju volcanic island, Korea. J Hydrol 270(3–4):282–294
Krishna Kumar S, Bharani R, Magesh NS, Godson PS, Chandrasekar N (2014) Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. App Water Sci 4:341–350
Kumar M, Panday DP, Bhagat C, Herbha N, Agarwal V (2023) Demystifying the decadal shift in the extent of groundwater in the coastal aquifers of Gujarat, India: a case of reduced extent but increased magnitude of seawater intrusion. Sci Total Environ 898:165451
Kumar M, Rahman MM, Ramanathan AL, Naidu R (2016) Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: health risk index. Sci Total Environ 539:125–134
Kura NU, Ramli MF, Sulaiman WN, Ibrahim S, Aris AZ (2018) An overview of groundwater chemistry studies in Malaysia. Environ Sci Pollut Res 25:7231–7249
Lloyd JW, Heathcote JAA (1985) Natural inorganic hydrochemistry in relation to ground water
Madhav S, Ahamad A, Kumar A, Kushawaha J, Singh P, Mishra PK (2018) Geochemical assessment of groundwater quality for its suitability for drinking and irrigation purpose in rural areas of Sant Ravidas Nagar (Bhadohi), Uttar Pradesh. Geol Ecol Landsc 2(2):127–136
Madhav S, Kumar A, Kushawaha J, Ahamad A, Singh P, Dwivedi SB (2020) Geochemical assessment of groundwater quality in Keonjhar City, Odisha, India. Sustain Water Resour Manag 6:1–1
Madhav S, Raju NJ, Ahamad A, Singh AK, Ram P, Gossel W (2021) Hydrogeochemical assessment of groundwater quality and associated potential human health risk in Bhadohi environs, India. Environ Earth Sci 80(17):585
Masood A, Aslam M, Pham QB, Khan W, Masood S (2022) Integrating water quality index, GIS and multivariate statistical techniques towards a better understanding of drinking water quality. Environ Sci Pollut Res 1–17
Mohanty AK, Rao VG (2019) Hydrogeochemical, seawater intrusion and oxygen isotope studies on a coastal region in the Puri District of Odisha, India. Catena 172:558–571
Mohapatra PK, Vijay R, Pujari PR, Sundaray SK, Mohanty BP (2011) Determination of processes affecting groundwater quality in the coastal aquifer beneath Puri city, India: a multivariate statistical approach. Water Sci Technol 64(4):809–817
Mondal NC, Singh VP, Singh S, Singh VS (2011) Hydrochemical characteristic of coastal aquifer from Tuticorin, Tamil Nadu, India. Environ Monit Assess 175(1–4):531–550
Moujabber ME, Samra BB, Darwish T, Atallah T (2006) Comparison of different indicators for groundwater contamination by seawater intrusion on the Lebanese coast. Water Resour Manag 20:161–180
Nair IS, Rajaveni SP, Schneider M, Elango L (2015) Geochemical and isotopic signatures for the identification of seawater intrusion in an alluvial aquifer. J Earth Syst Sci 124:1281–1291
Paliwal KV, Singh S (1967) Effect of gypsum application on the quality of irrigation waters. Madras Agric J 59:646–647
Park SC, Yun ST, Chae GT, Yoo IS, Shin KS, Heo CH, Lee SK (2005) Regional hydrochemical study on salinization of coastal aquifers, western coastal area of South Korea. J Hydrol 313(3–4):182–194
Pastén-Zapata E, Ledesma-Ruiz R, Harter T, Ramírez AI, Mahlknecht J (2014) Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi-tracer approach. Sci Total Environ 470:855–864
Patel P, Raju NJ, Reddy BS, Suresh U, Gossel W, Wycisk P (2016) Geochemical processes and multivariate statistical analysis for the assessment of groundwater quality in the Swarnamukhi River basin, Andhra Pradesh, India. Environ Earth Sci 75:1–24
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25(6):914–928
De Pippo T, Donadio C, Guida M, Petrosino C (2006) The case of Sarno River (Southern Italy). Effects of geomorphology on the environmental impacts (8 pp). Environ Sci Pollut Res 13:184–191
Prasanna MV, Chidambaram S, Gireesh TV, Jabir Ali TV (2011) A study on hydrochemical characteristics of surface and sub-surface water in and around Perumal Lake, Cuddalore district, Tamil Nadu, South India. Environ Earth Sci 63:31–47
Prusty P, Farooq SH, Zimik HV, Barik SS (2018) Assessment of the factors controlling groundwater quality in a coastal aquifer adjacent to the Bay of Bengal, India. Environ Earth Sci 77:1–15
Purnama S, Marfai MA (2012) Saline water intrusion toward groundwater: issues and its control. J Nat Resour Dev 2:25–32
Radhakrishna I (2001) Saline fresh water interface structure in Mahanadi delta region, Orissa, India. Environ Geol 40(3):369–380
Raju NJ (2012) Arsenic Exposure through groundwater in the Middle Ganga Plain in the Varanasi Environs, India: a future threat. J Geol Soc 79:302–314
Raju NJ, Ram P, Dey S (2009) Groundwater quality in the lower Varuna river basin, Varanasi district, Uttar Pradesh. J Geol Soc 73:178–192
Raju NJ, Shukla UK, Ram P (2011) Hydrogeochemistry for the assessment of groundwater quality in Varanasi: a fast-urbanizing center in Uttar Pradesh, India. Environ Monit Assess 173:279–300
Raju NJ, Ram P, Gossel W (2014) Evaluation of groundwater vulnerability in the lower Varuna catchment area, Uttar Pradesh, India using AVI concept. J Geol Soc 83:273–278
Rao NS (2012) Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River Basin Vishakhapattanam District, Andhra Pradesh India. Environ Monit Assess 184:5189–5214
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. US Department of Agri. Hand book, no 60
Sahagian D (2000) Global physical effects of anthropogenic hydrological alterations: sea level and water redistribution. Glob Planet Chang 25(1–2):39–48
Sahoo S (2014) Assessment of groundwater recharge using water-table fluctuation method and water-balance model. Environ Sci Technol 1:27
Sahu JK, Das PP, Sahoo HK, Mohapatra PP, Sahoo S (2018) Geospatial analysis and hydrogeochemical investigation of a part of southern Mahanadi delta, Odisha, India. Himal Geol 39(1):92–100
Satheeskumar V, Subramani T, Lakshumanan C, Roy PD, Karunanidhi D (2021) Groundwater chemistry and demarcation of seawater intrusion zones in the Thamirabarani delta of south India based on geochemical signatures. Environ Geochem Health 43:757–770
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers. McGraw-Hill, New York
Schoeller H (1965) Qualitative evaluation of groundwater resources. Methods and techniques of groundwater investigations and development. UNESCO, Paris, pp 54–83
Schwendimann L, Sivaprakasam I, Buvaneshwari S, Gurumurthy GP, Mishra S, Ruiz L, Sekhar M, Fleiss B, Riotte J, Mani S, Gressens P (2021) Agricultural groundwater with high nitrates and dissolved salts given to pregnant mice alters brain development in the offspring. Ecotoxicol Environ Saf 224:112635
Selvakumar S, Chandrasekar N (2021) Impacts of beach placer mineral mining in the shallow coastal aquifers of Southern Tamil Nadu Coast, India. Water Sci Sustain 183–200
Singh S, Raju NJ, Ramakrishna C (2015) Evaluation of groundwater quality and its suitability for domestic and irrigation use in parts of the Chandauli-Varanasi region, Uttar Pradesh, India. J Water Resource Prot 7(07):572
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M, Sarma VS (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu, India. J King Saud Univ-Sci 26(1):37–52
Stuyfzand PJ (2008) Base exchange indices as indicators of salinization or freshening of (coastal) aquifers. In: 20th Salt Water Intrusion Meeting, Naples, Florida, USA. IFAS Research Gainesville
Sudaryanto, Naily W (2018) Ratio of major ions in groundwater to determine saltwater intrusion in coastal areas. IOP Conf Ser: Environ Earth Sci 118:012021
Suguna S, Sherene T (2019) Assessment of sea water intrusion in ground water samples of selected blocks of Cuddalore District, Tamil Nadu, India. Madras Agric J 106:7–9
Szabolcs I, Darab K (1964) Radioactive technique for examining the improving effect of CaCO3 on alkali (szik) soils
Umar R, Alam F (2012) Assessment of hydrogeochemical characteristics of groundwater in parts of Hindon-Yamuna interfluve region, Baghpat District, Western Uttar Pradesh. Environ Monit Assess 184:2321–2336
USSL (1954) Diagnosis and improvement of saline and alkali soils. USDA Hand Book, 60: 147
Vengosh A, Rosenthal E (1994) Saline groundwater in Israel: its bearing on the water crisis in the country. J Hydrol 156(1–4):389–430
Vengosh A, Spivack AJ, Artzi Y, Ayalon A (1999) Geochemical and boron, strontium, and oxygen isotopic constraints on the origin of the salinity in groundwater from the Mediterranean coast of Israel. Water Resour Res 35(6):1877–1894
Vijay R, Khobragade P, Mohapatra PK (2011) Assessment of groundwater quality in Puri City, India: an impact of anthropogenic activities. Environ Monit and Assess 177:409–418
World Health Organisation (WHO) (2006) Guidelines for drinking water quality; Geneva, World Health Organisation
Wilcox LV (1948) The quality of water for irrigation use
Yidana SM, Bawoyobie P, Sakyi P, Fynn OF (2018) Evolutionary analysis of groundwater flow: application of multivariate statistical analysis to hydrochemical data in the Densu Basin, Ghana. J Afr Earth Sci 138:167–176
Acknowledgements
First author is thankful to the Hydrogeology and Environmental Geochemistry Lab, School of Environmental Sciences for providing laboratory facilities for generating the data.
Funding
The author Jyoti Kushawaha has availed the funding from the Council for Scientific and Industrial Research (CSIR-NET, File No. 09/263(1148)/2018-EMR-I) during her research related to this publication.
Author information
Authors and Affiliations
Contributions
The conceptualization and methodology of the study were collective efforts of all of the authors. Jyoti Kushawaha has done the investigation and formal analysis, validation, data curation, interpretation, and writing of original draft preparation. Janardhana Raju Nandimandalam has made the investigation, validation, visualization, interpretation, editing, and supervision. Dr. Sughosh Madhav and Amit Kumar Singh have contributed in statistical analysis, validation, data curation, and editing. The final manuscript has been read and approved by all of the authors.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to publish
The authors have consent to publish this work.
Additional information
Responsible Editor: V.V.S.S. Sarma
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kushawaha, J., Nandimandalam, J.R., Madhav, S. et al. Evaluation of hydrogeochemical processes and saltwater intrusion in the coastal aquifers in the southern part of Puri District, Odisha, India. Environ Sci Pollut Res 31, 40324–40351 (2024). https://doi.org/10.1007/s11356-024-32833-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32833-w