Abstract
The robustness and hardness of biofiber composites produced of bamboo and tamarind fibers were investigated in this study. In contrast to synthetic fibers, these fibers are inexhaustible, lightweight, cost-effective, and biodegradable. While natural fibers have several advantages over synthetic fibers, one disadvantage is that they are hydrophilic. The hydrophilic aspect of fibers makes it difficult for the matrix and fibers to fuse well. So the fibers are chemically processed with sodium hydroxide, potassium permanganate, acrylic acid, and benzene diazonium chloride to improve their cohesiveness. These treatments change the surface texture and biocomposition, allowing fibers and resin to adhere properly. The fabrication process is completed using the hand layup method. The specimens were made with varying weight ratios of fiber composition (10, 20, 30, 40, and 50%). The specimens were tested for strength using ASTM standards. Chemical procedures changed the fiber structure and improved the composite’s performance, according to the study. 40% fiber weight composite specimen handled with benzene diazonium chloride had superior strength properties to untreated and chemically handled fibers. The moisture content of untreated and treated fibers was tested, and the results showed that the fibers treated with benzene diazonium chloride had less moisture. The dielectric strength test results revealed that 40% wt of fiber treated with benzene diazonium chloride has a higher dielectric strength.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Composites are the most appealing materials, since they are created by incorporating two or more natural or artificial products to create a composite that is stronger as a whole and adds to the most desirable characteristics such as weight, performance, and longevity. Due to advantageous properties such as strong damping power, high specific modulus, and high specific pressure, these are now widely utilized in the automotive industry. Due to their outstanding properties such as water resistance, light weight, high resilience, chemical resistance, electrical resistance, high strength, fire resistance, and corrosion resistance, fiber reinforced composites have been extremely common in the aircraft and automotive industries. The majority of FRP fibers are made up of synthetic fibers such as glass fibers and carbon fiber. However, there are a few drawbacks of using artificial fibers, including the reality that they are non-renewable, non-biodegradable, and noxious to the earth because the processing of these fibers release a large amount of carbon dioxide into the atmosphere, as well as their energy-intensive fabrication method. As a result, natural fibers must be adapted. These fibers are used to render composites lighter, and they have a number of benefits over artificial fibers, including their variety, low weight, reduced damage to manufacturing equipment, cost effectiveness, superior relative mechanical properties like flexural and tensile modulus, and superior surface strength of formed parts composites. The most important aspect in composites is insulation, which can endure the full load placed on the material. That it must withstand the weight, it must be porous and have superior hardness. Matrix is an adjoining structure that preserves and strengthens the reinforcement. We may obtain a wide variety of physical properties by combining the correct amount of matrix and reinforcing, lending composites to a myriad of applications. Polymer is used as the matrix for its extensive range of physical properties like strong power, low shrinkage, outstanding adhesion properties, simplicity of shape, and decent electrical and thermal insulation. Natural fibers are used as insulation to compensate for the polymer matrix’s poor strength and stiffness. Natural fibers have a number of benefits, including biodegradability, low expense, quick supply, no health effects, and renewability, but they still have a few disadvantages such as poor wettability and moisture tolerance, which decreases the bonding between the reinforcement and matrix. This lead to the evolutions of composites with poor mechanical properties. As a result, composites with weak mechanical properties evolved. To address this disadvantage, chemical treatment of the composites may be used to remove the repulsive quality and improve the properties of the composites by changing the microstructure and refining the wettability, tensile strength, and surface morphology of fibers, thus enhancing the adhesive property of fibers with matrix and making them less hydrophilic.
2 Experimentation
2.1 Materials
Fibers: Bamboo and tamarind fibers (Figs. 1 and 2, Table 1).
Resins: Vinyl ester and unsaturated polyester.
2.2 Surface Alteration of Natural Fibers
Just a few issues occur at the interface during the fortification of natural fibers into the resin mix due to the proximity of natural fibril hydroxyl groups. Since natural fiber absorbs water, it is tough to hold strands in a resin mix. Pectin, oil, waxy dirt, and grease present in the fibers act as a buffer between the resin blend and the reinforcement. To increase interfacial holding capability between fibers and resin, fiber surfaces must be treated with various chemicals.
2.3 Chemical Treatments
Various chemical therapies, binding agents, and reaction supplements must be added to the fiber surface to boost interfacial adhesiveness. Surface handling creates redundant reflex sections on the fiber surface, making matrix union possible.
2.3.1 Alkali Treatment
The fibers were dewaxed after being soaked in a 2:1 combination of benzene and ethanol for 72 h. At 30 to 32 °C, these fibers were immersed in a 6% sodium hydroxide solution for 1 h. The solution’s total amount is 15 times the wt of the fibers. They are then dissolved in an alkaline solution for 36 h at 30 to 32 °C, washed, and neutralized with a 2% (CH3COOH) acetic acid solution. Finally, they are washed under water to remove any residual acid that has adhered to them, bringing the pH of the fiber to nearly 7. Eventually, alkali-treated fibers are acquired by dehydrating them at room temperature for 48 h.
2.3.2 Acrylic Acid Treatment
Acrylic acid has been used for treatment. Bamboo and tamarind fibers were soaked in a 1% acrylic acid solution for 1 h at 50 °C, then thoroughly washed with seltzer water until being shriveled in a 70 °C oven for 24 h.
2.3.3 Permanganate Treatment
After 30 min of immersion in 6% sodium hydroxide, the fibers were thoroughly cleansed with seltzer water. These alkali-treated fibers are treated with 0.5% potassium permanganate in C3H6O solution for 2 min, then cleaned using water and dehydrated at 80 °C in an oven.
2.3.4 Preparation of Benzene Diazonium Chloride
8cm3 of concentrated HCL was applied to a boiling tube comprising 10cm3 of water and 3cm3 of aniline, and the blend was shaken until the amine was dissolved. The mixture was then cooled in an ice bath at 5 °C, and then a solution of NaNO3 (3 g in 8cm3 of water), formally luke warmed to 5 °C, was poured at the time of blending the blend temperature was conserved to less than 10 °C.
2.3.5 Benzene Diazonium Treatment
The bamboo and tamarind fibers were cut to 10 mm lengths, cleaned using seltzer water, and dehydrated at 70 °C in an oven for 24 h. The fibers were then soaked in a 2L glass beaker containing a 6% sodium hydroxide solution for 10 min at 5 °C. The newly made diazo solution was then allowed into the beaker and continuously stirred. Finally, the fibers were stripped and cleaned with clean water and soap solution, followed by dehydration in the open air for 48 h.
2.3.6 Fabrication of Blended Hybrid Biocomposites.
For easy removal of composites, the mold cavity is filled with a dense layer of solid wax. Lean liquor arranged POLYVINYL ALCOHOL (PVA) was added immediately after the wax was re-established. The hand layup method is used to assemble the bio-fibrils crossover composite. Cross-breed biostrands of treated and untreated tamarind bamboo fibers are reinforced to network mix to required biocomposite execution and mechanical requirements at that stage. After that, air bubbles are gently removed with delicate spinning rollers. The objective of the post-cure in the temperature of 800 oC for about two hours by putting the specimen in a hot oven is to cure composite samples fully. Biocomposite specimen samples are checked after they have been completely cured, both raw and refined (Figs. 3 and 4).
3 Chemical Treatment of Fibers
3.1 Alkali Treatment
The morphology of bamboo and tamarind is significantly altered by alkali therapy, which helps in the expulsion of hydrogen bonding in the fiber network, resulting in a reduction in moisture absorption. It decreases the width of the thread, resulting in a higher aspect ratio. The interfacial adhesion is improved as the aspect ratio is increased, which increases the mechanical properties. Enhancing the surface roughness improves mechanical interlocking and the amount of cellulose on the fiber surface. This allows for increased fiber wetting by increasing the number of appropriate reaction sites. It also increases the amount of amorphous cellulose, resulting in a decrease in crystalline cellulose.
3.2 Acrylation Treatment
This treatment made the fibers more hydrophobic by reducing the absorption of water through them. Bamboo and tamarind fibers treated with acrylic acid absorbed less moisture. It is because the hydroxyl group has been replaced by hydrophobic water groups.
3.3 Permanganate Treatment
Extremely, reactive permanganate ions interact with cellulose hydroxyl groups to form cellulose manganate in this treatment. Chemical interlocking at the surface improves, as a result, resulting in better matrix-fiber attachment. It lowers the fiber’s hydrophilic value.
3.4 Benzene Diazonium Chloride Treatment
This treatment aids in the reduction of fiber water absorption by improving matrix-fiber adhesion. The coupling reaction of benzene diazonium chloride with OH groups produces diazo cellulose in this treatment.
4 Results and Discussion
4.1 Tensile Strength Properties
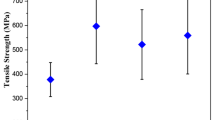
Composite specimens with measurements of 150 × 15 × 3 mm3 were prepared, in accordance with ASTM D 3039 requirements (Universal Testing Machine). The interfacial bonding between matrix and fiber would not be accurate due to the existence of hydroxyl groups in fiber and the inclusion of undesirable substances such as oil, grease, pectin, and waxy powder. Chemical treatments were used to resolve these issues such as, alkali treatment decreases fiber diameter, raises aspect ratio, enhances stress transfer capability, and improving tensile strength, while permanganate treatment roughens the surface, offering strong interlocking at the interface and increasing adhesion between fiber and matrix, increasing tensile strength. The stress transmission quality at the fiber interface improves as a result of the acrylation of the fiber by increasing the tensile power. Chemical treatments have an effect on fiber strength, as seen in Fig. 5 the analysis shows that at 10, 20, 30, 40 and 50% fibril quantity biocomposites, [C6H5N2] Cl-treated fiber composites exhibit higher and optimum values than sodium hydroxide treated, acrylic acid-treated, potassium permanganate treated, and untreated fiber composites [1, 4, 9]. The strength values increased from 10 to 40% due to rich fiber distribution. Owing to a lack of adequate adhesion between the matrix and fibers, inadequate stress transfer, weak fiber distribution, less fiber splitting, and tensile strength drop after 40%. As opposed to all chemically cured fiber composites, the untreated fiber composites have poor tensile power, as seen in Fig. 5. The hydrophilic design of the untreated fibers explains this. As a result, the matrix and fiber are incompatible. In either case, alkali-treated tamarind- bamboo fibrils composites had a strong visible tensile power, and [C6H5N2] Cl-treated tamarind-bamboo fibrils composites had a vital increment. According to the findings, chemical processes generated a strong interface with fibril cell walls, and pairing response between [C6H5N2] Cl and fibril cellulose results in the development of diazo cellulose compounds, which are supposed to watch the essential increase of composite tensile property. Surface treatment with [C6H5N2]Cl, sodium hydroxide, allows one to expel unwanted materials, thus improving fibril distribution in matrix blends, resulting in improved mechanical properties [1, 3, 4, 6, 10].
4.2 Compressive Strength Properties
ASTMD695–15 stipulations are used to prepare and test composite samples in order to determine their compressive power. Figure 6 shows the changes in estimations for compressive content properties for fibril volumes without and with surface modification. Similarly, [C6H5N2] Cl tested bio-fibrils composites have higher compressive properties and perfect conditions than alkali tested, acrylic acid-treated, potassium permanganate raw biocomposites [1, 4, 9]. The optimal and superior conditions are attributed to ideal fibril stacking and chemical handling, which advanced strong interfacial keeping between filaments framework mix, resulting in adequate stress transfer and execution [1, 4, 6]. Acrylic treated fiber composites remove more harmful materials than alkali, permanganate-treated fiber composites, but not as much as benzene diazonium chloride-treated fiber composites. The previous research has discovered that material treatment decreases the gap between fibrils and broadens the roughness of the surface [3, 7, 9]. At 50% fibril magnitude, there is a smaller drop in compressive properties of composites. The reduction in compressive properties at 50% fibril material handled and untreated composite (higher fibril loads) shows low fibril-grid interfacial griping and small scale breakage at interface prompt inadequate stress transfers. The compressive intensity of 40% fibril stackings was particularly impressive. The [C6H5N2] Cl therapy, on the other hand, fills the voids in the fibril resulting in higher compressive strength [1, 3, 5].
4.3 Flexural Strength Properties
To determine the flexural property, 150mmx15mmx3mm specimens were produced and checked according to the ASTMD 5943–96 standard. Figure 7 depicts the disparity of flexural content estimations of composites. As flexural properties of 40% fibril amounts, [C6H5N2] Cl-treated biofibrils composites were more compared to acrylic acid-treated, potassium permanganate treated, sodium hydroxide treated, untreated at 10, 30, 20, 40, and 50% fibril amounts composite [1, 3, 4] due to proper fiber distribution and load transfer. It was observed that the values increased from 10% fiber weight to 40% fiber weight and a decrease after 40% due to poor fiber distribution. Alkaline treatment improves the stress transmission capability of the fiber, while permanganate treatment improves the interlocking at the interface and increases fiber and matrix adhesion. Acrylic acid-treated biofibers composites had better fibril-matrix compatibility, collaboration, and handling than potassium permanganate and sodium hydroxide-treated biofibers composites [2, 4, 6, 7]. Compared to treated bio-fibrils composites and untreated bio-fibrils composites, [C6H5N2] Cl-treated bio-fibrils composites exhibited more significant and noticeable production of fibril dissemination matrix, stress, and aspect ratio transfer capability.
4.4 Moisture Content Testing
Figure 8 displays the rate of moisture ingestion by untreated fibril composites, alkali-treated composites, potassium permanganate, acrylic acid, and [C6H5N2] Cl-treated composites produced to ASTM D 543–87 standards. Bio-fibrils composites are made up of fibrils that have hydrophilic properties [7, 9]. In any case, alkali-responsive hydroxyl groups in molecules are dissolved, which combines with OH-H molecules at points and transfers from fibril formations. As a result, alkali and [C6H5N2] Cl treatment reduced hydrophilic hydroxyl groups and increased moisture blockage qualities outside the fibril. [1, 2, 4]. The presence of hydroxyl groups in fibers makes them capable for the formation of hydrogen bonding with water resulting in hydrophilic nature. These fibers when treated with alkali, ONa groups replace the hydroxyl groups resulting in the decrease of moisture absorption in [C6H5N2] Cl-treated fibers. Unprocessed fibril composites absorb more moisture than alkali-treated, acrylic acid-treated, potassium permanganate-treated, and [C6H5N2] Cl-treated composites [1, 2, 9].
4.5 Dielectric Strength
These composites samples were produced with ASTMD-149 steps to contemplate dielectric properties of untreated, acrylic acid, potassium permanganate alkali treated, and [C6H5N2] Cl-treated composites. A composite with dimensions of 120 × 120 × 3mm3 is reinforced with strands that are 120 mm long and stranded in one direction. For five of the specimens, dielectric splitting voltage is discovered and the mean value is used in the investigations. The test is performed at 50 Hz recurrence and ambient temperature. To determine the thickness of the composite at specific stages, a digital micrometer with a 0.001 mm least count was used. The fiber distribution is maximum at 40% wt of fiber, and the expulsion of unwanted materials is more at [C6H5N2] Cl-treated fibers at 40%, so the dielectric strength is maximum at 40% wt benzene diazonium-treated fibers. It is also worth noting that the dielectric content of crossover fibril composites improves when the percentage wt of fibril rises from 10 to 40%, but decreases at 50% fibril magnitude composites [3, 7, 9]. The lack of interfacial holding between strands and resins blend resulted in a decrease in dielectric quality at 50% fibril magnitude composites [1, 2, 4, 8] (Fig. 9).
5 Conclusion
From the above research by observing the results, we can conclude that the chemical treatments done on fibers imparted that the treatments impact their surface and their biochemical composition. The benzene diazonium chloride treatment has more impact on the fibers than the acrylic acid, potassium permanganate, NaOH treatments. In the tensile strength test, the benzene diazonium-treated fiber specimens have higher strength value followed by acrylic acid-treated specimens. The compressive results show that the benzene diazonium-treated fiber specimens have the highest value than other treated and untreated composites. Even in the flexural strength tests, the benzene diazonium-treated fibers have the highest value. Other than the strength tests, the dielectric strength results show that the benzene diazonium chloride treated specimens have higher strength. The moisture content results depict that the fibers treated with benzene diazonium chloride have less moisture. Hence, we can come to a point that benzene diazonium treatment has a better impact on the fibers to lose their hydrophilic nature and improve the overall strength of the composite.
References
Venkatesha Prasanna G, Venkata Subbaiah K, Varada Rajulu A (2012) Chemical resistance, impact, flexural, compressive properties and optimization of fibers of natural fibers reinforced blend composites. Scholarly J Eng Res 1(6):85–89
Kalia S, Kaith BS, Kaur I (2009) Pretreatments of natural fibres and their application as reinforcing material in polymer composites—a review. Polymer Eng Sci 49:1253–1272
.Venkatesha Prasanna G, Sunil Kumar V, Srilekha R, Sri Harsha AVN, Sai Abhi Chandan V (2021) Hybridization and influence of chemical treatment on the morphology and optimization of composites, Elsevier, Materials today proceedings, 4833–4837
Alamgi MK, Monimul MH, Islam RM, Bledzki AK (2010) BioResources 5:1618–1625
Rahman MR, Islam MN, Huque MM, Hamdan S, Ahmed AS (2010) BioResources 5:854
Punyamurth R, Sampath Kumar D, Bennehalli B, Badyankal P, Vekateshappa SC (2014) Surface modification of abaca fiber by benzene diazonium chloride treatment and its influence on tensile properties of abaca fiber reinforced polypropylene composites. CienciaTechnologia dos materials 26(2):142–149
Composites and its recycle. J Biol Sci 7:393-396 (2007)
Yu T, Ren J, Li S, Yuan H, Li Y (2010) Effect of fibre surface-treatments on the properties of poly (lactic acid)/ramie compo-sites. Composites Part A: Appl Sci Manuf 41:499–505
Venkatesha Prasanna G, Sri Harsha AVN, Srilekha R, Sunil Kumar V, Sai Abhi Chandan V (2020) Chemical treatment and fiber length,their effecton the mechanical properties of blended composites, Elsevier, materials today proceedings,4862–4866
John MJ, Anandjiwala RD (2008) Recent developments in chemical modification and characterization of natural fiber-reinforced composites. Polym Compos 29:187–207
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Venkatesha Prasanna, G., Sri Harsha, A.V.N., Sunil Kumar, V., Srilekha, R. (2022). Mechanical Testing and Optimization of Bamboo and Tamarind Fiber Composites. In: Dave, H.K., Dixit, U.S., Nedelcu, D. (eds) Recent Advances in Manufacturing Processes and Systems. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-7787-8_52
Download citation
DOI: https://doi.org/10.1007/978-981-16-7787-8_52
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7786-1
Online ISBN: 978-981-16-7787-8
eBook Packages: EngineeringEngineering (R0)