Abstract
A fermenter (bioreactor) is a closed vessel with sufficient aeration, agitation, temperature, and pH regulation arrangements and a drain or overflow vent to extract the waste biomass along with its products from cultured microorganisms. The fermenter is intended for the production of biological products. The fermenter’s design and mode of operation depend primarily on the production organism, the optimal working condition needed to create the target product, the product value, and the production scale. Fermenter is a consent to design criteria including sterilization ability; simple construction; easy calculation, monitoring, control techniques; scale-up; flexibility; long-term stability; upstream process compatibility, antifoaming steps. Fermentation is accomplished in several forms, including batch, fed-batch, and continuous fermentation process. The current chapter addresses the fermenter configuration, fermentation mechanism, fermenter types used in industries, heat and mass transfer in the fermenter, and the scaling up and regulation of the industrial fermentation process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
During the First World War (1914–1918), Chaim Weizmann, alongside his team, formed a methodology for processing acetone by deep liquid fermentation using Clostridium acetobutylicum, primary to the subsequent usage of initial large-scale fermentation vessels. Contamination has frequently remained a solemn difficulty, predominantly in the initial portion of the large-scale development phase. At first, no appropriate vessels existed, and challenges remained inadequate with lid-fitted alcohol fermenters as steam sterilizers because atmospheric pressure might not be accomplished. Large cylindrical mild-steel vessels with hemispherical tops and bottoms sterilized under pressure with steam were the primarily built fermenters. The difficulties of aseptic media or inoculum additions persisted. As a result, steps were taken to design and improve piping, joints, and valves that could achieve and maintain sterile conditions if necessary. Only the minor seed vessels were mechanically stirred, and the vessel’s contents were agitated continuously by the large capacities of gas produced during fermentation .
In the 1930s, Central Europe used the first real large-scale aerobic fermenters to manufacture compressed yeast (Becze and Liebmann 1944). These fermenters entailed large cylindrical air tanks at the base through perforated pipe systems. Motorized impellers replaced them, progressing the rate of mixing and air dispersion. This indeed led to the compressed-air necessities being decreased by a factor of 5. Baffles prevented vortex formation. Also, at this time, it was known that 10–20% of the overall production cost could be the cost for compressed air. In the early 1930s, water and steam were provided for sterilizing the aeration tubes. Before 1940, ethanol, glycerol, acetic acid, citric acid, other organic acids, enzymes, and sorbose were the other essential fermentation products besides baker’s yeast . These procedures used extremely discriminating surroundings such as acidic or anaerobic conditions or the usage of an unusual substrate , resulting in comparatively negligible contamination compared to acetone fermentation or subsequent fermentation of aerobic antibiotics. An essential factor in imposing the formation of sensibly constructed and purpose-built fermentation vessels was to practice submerged culture techniques for the production of penicillin, in which aseptic environments, good aeration, and agitation were critical. The Distillers Company’s solvent plant in Bromborough was the only required aeration equipment to make it ideal for penicillin production (Hastings 1971). On 15 September 1943, the large-scale manufacturing of penicillin by deep fermentation , with steel fermenters of volume 54,000 dm3, was initiated by Terre Haute in the United States of America (Callaham 1944).
5.2 Fermenter Systems
The fermenter, recognized as the heart of the fermentation process, endures numerous reactions and activities. Throughout the exploration of fermentation technology, tasks such as downstream, upstream, and midstream study are involved. Upstream processes involve selecting a microbial strain distinguished by synthesizing a particular commodity’s desired commercial value in industrial fermentation . In contrast, downstream processing requires appropriate techniques and methods for recovery, purification, and characterization of the desired fermentation product in the different stages that accompany the fermentation phase. It has the primary objective of recovering the target product to the requirements (biological operation, purity) effectively, reproducibly, and safely while optimizing recovery yield and minimizing costs (Fig. 5.1) (Gudin and Chaumont 1991; Agrawal et al. 2019; Agrawal and Verma 2020; Bhardwaj et al. 2019; Kumar et al. 2018). The small distinction between the bioreactor and fermenter is that the fermenter is for microbial culture and the bioreactor is used for animal and plant cell mass culture (Gaikwad et al. 2018). Studies of fermentation technology comprise significant efforts from several fields of bioprocessing and chemical engineering, microbiology, biochemistry, genetics, and even physics and mathematics (Chandrashekhar and Rao 2010). The fermenter/bioreactor is a vessel that offers a biomechanical and biochemical environment that controls the movement of oxygen, nutrients to the cells, and metabolic products from the cell. The bioreactor vessels are constructed to use catalyst activity to achieve the anticipated chemical conversion (Sharma 2012). Bioreactors are also known as engineered devices designed to aid microorganisms, enzymes, biocatalysts, and plant and animal cells for the metabolic activity and optimal growth of organisms. For example, by regulating a culture temperature profile, some enzymes are denatured, organizing the cells’ metabolic pathways over others. The fermenter or bioreactor is a cylindrical vessel with a hemispherical bottom and top shape and is mainly made of glass and stainless steel.
To some extent, bioreactors are distinct from conventional chemical reactors. The fermenter device is sufficient to control contamination when the organism is less stable and more susceptible than chemicals. To maximize shelf life and preserve acceptability, the development from natural art to commercial practice of these goods needs process enhancement. The consistency of the parameters of the product, equipment, and process control stayed simple. Generally, an aerobic system is supplied with sufficient substrate , salts, oxygen, suspended solids, and water. Products and by-products of gas production during the fermentation process need proper protection to be followed. Scale-up, controlling techniques, typical construction and calculation, sterilization, operational versatility, process control systems compatible with upstream and downstream processes, and antifoaming processes are considerations regarding fermenter design attributes specifications (Sharma 2012).
5.2.1 Functions of Fermenter
The primary purpose is to provide a regulated environment to obtain the desired product to grow microorganisms or animal cells. There are some criteria to be considered in designing and building a fermenter:
-
1.
A vessel should work aseptically for some days with stable conditions and comply with the specifications of containment legislation for long-term service.
-
2.
The metabolic requirements, adequate aeration and agitation of the microorganism’s are important. The mixing does not, however, harm the organism.
-
3.
Consumption of power should be as minimal as possible.
-
4.
There should be a scheme for temperature control.
-
5.
pH control is required.
-
6.
Sampling facilities are needed.
-
7.
Losses from evaporation at the plant.
-
8.
In service, harvesting, cleaning, and repair, the vessel should be built to make minimum use of labor.
-
9.
Preferably, the vessel should be appropriate for various processes, but this may be limited because of containment regulations.
-
10.
The vessel design with even internal surfaces, wherever possible, using welds instead of flange joints.
-
11.
The vessel’s geometry in the pilot plant should be identical to smaller and larger vessels or installation to promote scale-up.
-
12.
Use the cheapest material for better results.
-
13.
Adequate service arrangements should be in effect for individual plants.
5.3 Parts of Fermenter
The fermenter design requires enhanced efficiency and authentication of the desired parameters with a high-quality product at a low cost. The fermenter’s strategy and mode of operation are focused on the output of microorganisms, the product’s cost, the condition required for the anticipated product development, and the scale of production. The successful fermenter has a beneficial effect on the biological reaction and avoids contamination from the outside. The essential considerations to be taken into account in fermenting capital expenditure and operating costs are adequate mixing, uniform shear rates, and monoseptic conditions to be preserved during the fermentation process. Culture aeration is accomplished by one of the succeeding approaches: raising ambient pressure or increasing the limited oxygen pressure and direct sparging, indirect and membrane aeration, or medium perfusion aeration (Eibl and Eibl 2008). The bioreactor enterprise must control pH, temperature, oxygen tension, aseptic feature, and shear stress. Several aspects of the biotechnological process require special care in the design of the bioreactor (Narendranathan 1986). Bioreactor conditions, such as pH, temperature, the velocity of agitation, dissolved oxygen level, development of foam, low gas rates, etc., are essential to regulate and closely track (Chen and Hu 2006). Figure 5.2 illustrates the parts of a basic laboratory-scale fermenter.
5.3.1 Construction Materials
Fermenters are constructed with materials that can resist constant steam sterilization and cleaning cycles. Substances that trace the fermentation medium and the broth must be nonreactive and nonabsorptive. For the development of fermenters with a capacity of up to about 30 L, glass is a good option. Smooth, nontoxic, corrosion-proof, and transparent are the advantages of glass. In common, glass fermenters are equipped with stainless steel head plates comprising several screw fittings since medium, inoculum, air, pH, and temperature sensors need entry ports. Most pilot and extensive fermenters are corrosion-resistant stainless steel, while mild steel with stainless steel cladding can also be used. In fermentation areas that influence the culture, copper and copper-containing ingredients must be ducked as they are toxic to cells. Internal steel surfaces are refined to a superior mirror surface to enable cleaning and sterilization of the reactor; welds on the vessel’s inner are ground flushed prior to polishing. Electropolishing is constantly chosen over mechanical polishing, which leaves minute ridges and trenches in the metal to accrue dirt and microorganisms.
5.3.2 Temperature Control
Generally, an appropriate temperature control provision in the design is required. Microbial action and mechanical agitation will produce heat, and if the heat produced by these two methods is not ideal for the precise production process, then heat will have to be applied to the system or removed from it. Usually, little heat is generated on a laboratory scale, and additional heat must be given by retaining the fermenter in a thermostatically operated bath or using inner heating coils or a heating jacket in which water circulates through a silicone heating jacket.
A dual silicone rubber mat by means of heating wires among the two mats contains a silicone jacket enfolded around the vessel and detained by Velcro strips. When a specific size is selected, the jacket’s surface area becomes too small to extract heat from the fermentation . When this situation happens, it is essential to use internal coils and circulate cold water to accomplish the correct temperature. Diverse forms of fermentation will affect the maximum vessel size that can be used for jackets alone. The fermenter’s required cooling surface cannot be precisely defined because the cooling water temperature, the process of sterilization, the temperature of cultivation, the kind of microorganism, and the energy provided by stirring resolve significantly in different processes.
5.3.3 Aeration and Agitation
The primary aim of aeration is to afford adequate oxygen for metabolic requirements for microorganisms in submerged cultures. Simultaneously, agitation ensures that a constant holdup of microbial cells is attained in a homogeneous nutrient medium. Depending on the fermentation characteristics, the nature of the aeration-agitation device used in a specific fermenter differs. In fungal and actinomycete fermentations, mechanical agitation is generally necessary. Non-agitated fermentations are usually performed in vessels with a 5:1 height/diameter ratio. In such vessels, aeration remains adequate to create high turbulence, but in the processing of compressed air, a towering column of liquid requires more significant energy input. The structural components of the fermenter are:
-
(a)
The agitator (impeller)
-
(b)
Stirrer glands and bearings.
-
(c)
Baffles.
-
(d)
The aeration system (sparger)
5.3.3.1 The Agitator (Impeller)
The agitator is essential to attain a variety of mixing aims, e.g., bulk fluid and gas-phase mixing, air dispersion, oxygen transfer, heat transfer, stable particle suspension, and consistent atmosphere in the innards of the vessel. Agitators can be listed as disc turbines, vane discs, variable-pitch open turbines, and propellers. The disc turbine entails of a disc through a rectangular series around the circumference set in a vertical plane. A sequence of rectangular vanes is mounted vertically to the bottom of the vaned disc. Air from the sparger ranges the disc’s bottom and moves to the vanes, where the air bubbles break down smaller bubbles. On the agitator shaft, the vanes of an adjustable pitch open turbine, and a marine propeller’s blades are directly associated to the main. In this occurrence, earlier dispersion by the vanes or blades, the air bubbles do not originally trace any surface. The disc turbine in a fermenter is suitable because it might break down fast airstream deprived of flooding into air bubbles. When the vessel’s bulk flow arrangement is typically related through the agitator arrangement, it is misplaced and exchanged by a centrally smooth air-broth plume up the middle of the vessel through a liquid flow as an annulus (Nienow 1990). The propeller is least effective at breaking down a stream of air bubbles. The flow produced is axial rather than radial and flooded lower than the disc turbine. The disc turbine is essential to thrust the sparged air hooked on the agitator tip zone where bubble breakup occurs. If the agitator rapidity is sufficiently high, good gas dispersion will arise in low-viscosity broths (Smith 1985). Gas dispersion often occurs in high-viscosity broths from gas-filled cavities straggling behind the spinning blades but inadequate to safeguard adequate bulk mixing of all the vessel matters. The impeller tends to spin in a pocket of gas when the cavities are of full height, from which no actual dispersion takes place through the rest of the vessel. A dual impeller grouping implies suitable mixing and aeration in high-viscosity broths. The subordinate impeller performs like a gas disperser, and the upper impeller works predominantly as a device to aid the movement of the vessel’s contents.
5.3.3.2 Stirrer Glands and Bearings
One of the most challenging problems in constructing a fermenter that can be worked aseptically for extensive periods is the adequate sealing of the top plate’s stirrer shaft assembly. From the top, side (Richards 1968), or bottom of the vessel, the stirrer shaft gets into the vessel. The top entry is widely used, but if a different room is required on the top plate for entry ports, the bottom access is preferable, and the smaller shaft enables advanced stirrer speeds to be used by removing the issue of high-speed shaft whipping. The bottom entry stirrers are unacceptable as the bearings are submerged. Mechanical seals are used for bottom entry if sustained and replaced regularly at suggested intervals (Leaver and Hambleton 1992).
A porous bronze bearing is mounted in the top fermenter center and another in a yoke directly above it for a 13 mm shaft. The bearings are constrained into steel housings, secured into the yoke, and the fermenter’s top into place. A skirt-like shield protects the lower bearing and housing with a 6.5 mm overhang that rotates with the shaft and stopped airborne pollutants from settling on the bearing and making into the fermenter. The stuffing box, the simple bush seal, the mechanical seal, and the magnetic drive are used as four specific seal assembly forms. As an alternative to stuffing boxes and packed glands, most modern fermenter stirrer mechanisms now integrate mechanical seals. Mechanical seals are costlier but are more robust and less likely to be a point of entry for pathogens or a moment of leakage for the organisms or items to be enclosed. Magnetic drives are used in assured animal cell culture vessels, which are also expensive.
5.3.3.3 Baffles
Four baffles are usually inserted into agitated vessels of all sizes to circumvent a vortex and upsurge aeration performance. Six or eight baffles are used in vessels of larger diameter. Baffles are metal strips approximately one-tenth of the diameter of the vessel and radially connected to the wall. With wider baffles, the agitation effect is only marginally increased but drops sharply with narrower baffles (Winkler 1990). A scouring action on the baffles and the fermenter walls around and behind the baffles minimizes microbial development. To increase the cooling power, extra cooling coils are connected to baffles of the fermenter without unduly influencing the geometry.
5.3.3.4 The Aeration System (Sparger)
A sparger is a device for introducing air into the liquid. Three specific sparger types are used and can be defined as the porous sparger, the perforated pipe orifice sparger, and the nozzle sparger (an open or partially closed pipe).
5.3.4 Feed Ports
Nutrient and acid/alkali additions to minor fermenters are usually made through silicone tubes that are separately autoclaved and pumped after an aseptic connection by a peristaltic pump. The nutrient reservoirs and associated piping in large fermenter units are typically an integrated component sterilized with the vessel. There can also, however, be ports that are in use intermittently.
5.3.5 Sensor Probes
Double “O”-ring seals offer a sterile closure for glass electrodes in fermenters. This method is also sufficient for levels 1 and 2, as long as the release of microorganisms is reduced and adequate disinfection procedures are available to deal with leakage (Werner 1992). Probes are built-in with triple “G”-ring seals at containment levels 2 and 3/4 (Leaver and Hambleton 1992). While double “G”-ring seals are denoted with steam tracing, they are not naturally considered achievable. As a way of coping with probe failure instead of using a retractable electrode housing throughout the fermentation cycle, pre-inserted backup probes are advised because of the chance of broth leakage (Sharma 2012).
5.3.6 Foam Control
It is essential to reduce foaming during any fermentation . The chances of filters getting wet when foaming becomes excessive, resulting in pollution. There is also the risk of siphoning, leading to losing some or all parts of the fermenter’s content. Antifoams can cause aeration or downstream processing issues in some circumstances. The foam is guided over fixed turbine blades in a separator in certain fermenters, and the liquid fraction is reverted to the fermenter.
5.3.7 Valves and Steam Traps
Valves and ancillary devices connected to fermenters are used in several ways to regulate the flow of liquids and gases. The valves are required to:
-
1.
Be easy as regards switching them on/off that are either completely open or fully closed.
-
2.
Provide flow rates with coarse control.
-
3.
Be very precisely calibrated to regulate flow rates.
-
4.
Be safe so that liquids or gases flow in one direction only.
5.3.8 The Achievement and Maintenance of Aseptic Conditions
It is essential to sterilize and hold a fermenter and its contents sterile in a full growth cycle. The containment requirements depend on the size of the fermentation vessel. To achieve and maintain aseptic conditions during fermentation , the following operations are performed according to the specifications:
-
1.
The fermenter’s sterilization.
-
2.
Sterilization of the inlet air and exhaust gas.
-
3.
Agitation and aeration.
-
4.
The addition of nutrients, inoculum, and others with supplements.
-
5.
Sampling.
-
6.
Controlling foam.
-
7.
Monitoring and control of various parameters.
When conniving a fermenter to act as a contained device at level 1, B2, or higher containment levels, the following points must be considered:
-
1.
All vessels comprising live species must be sterile vent filters and appropriate for steam sterilization.
-
2.
Vessel exhaust gases can pass through sterile filters.
-
3.
At the lower stages of containment, flange joint seals should be fitted with a single “O”-ring. Double “O”-rings or double “O”-rings plus a steam barrier are desired on flange joints on vessels for containment levels 3 and B3/4.
-
4.
Seals for sensor probes, inoculum, sampling, medium addition, acid, alkali, and antifoam entry ports must be given.
-
5.
A double-acting mechanical seal with steam or condensate between the seals can be used to seal rotating shafts into a bolted device.
-
6.
A steam barrier must be recognized in all fixed piping prominent to the “contained” vessels during service.
-
7.
Adequate pressure relief conveniences must be provided.
5.3.8.1 Sterilization of a Fermenter
The medium may be sterilized both in the vessel and separately before being applied aseptically. To avoid the increase of large quantities of condensate, the temperature of the sterilized medium should be increased before injecting live steam. Steam may be injected into the fermenter coils or jacket to accomplish this. Since any entry point to and exit from the fermenter can be a source of pollution, steam must be added through all entry and exit points except the air outlet, which should be left open.
To confirm that steam enters all sections of the machinery and is not blocked by siphons or pockets of condensate or mash, all pipes should be installed as simply as conceivable and grade into drainage points. A steam trap should be installed at each drainage point in the pipework. Welded joints should be used wherever possible for long-term aseptic service, even if parts need to be cut out and re-welded during maintenance and repair.
5.4 Properties of an Ideal Fermenter
The fermenter is a device used to convert low-value materials into higher-value products through microorganisms’ controlled growth. Industrial production of highly valued brewed products, such as wine and beer , or other fermented products such as citric acid, acetic acid, etc. (Zhong 2010) would require a carefully choreographed sequence of events, which can be controlled by the various parts of the fermenter as described in the previous section. Working of a fermenter in ideal condition is essential for maximizing the profit from the industry . Some of the ideal properties of running a fermenter are shown below.
5.4.1 The Ability to Provide Contained Conditions
An ideal fermenter should be able to provide an aseptic condition for long periods. If the fermenter is not a closed system (except for components we inject deliberately), there can cause many complications such as the growth of unwanted microorganisms, which can decrease the overall product’s efficiency. It can also produce unwanted products and our desired product that can hinder the desired product’s ideal use. Such products being introduced into the system can make the downstream processing costlier. Microbial contaminations are known to cause considerable losses to companies since the whole batch needs to be discarded. Hence, providing a contained environment is top-notch for the fermenter’s ideal working (Yang 2011).
5.4.2 The Ability to Provide an Ideal Working Environment
Most microorganisms have unique needs to work with maximum efficiency; these needs can be temperature, pH, and other chemicals. An ideal fermenter will need ports for the insertion of probes for temperature and pH. Systems have been developed with computer programing to monitor these parameters and adjust the same to fit the requirement. A heating coil, water jacket, or steam jacket is installed in a fermenter to control the temperature. Predetermined concentrations of acid or alkali are fed into the fermenter to maintain the ideal pH. Other chemical agents or enzymes are introduced according to the reaction’s need, as they can be inserted into the reactor at selected times. Thus, an ideal reactor should sense and control the above parameters without compromising the vessel’s sterility (Yamuna Rani and Ramachandra Rao 1999).
5.4.3 The Construction Material
The construction material should be corrosion resistant, preventing the accidental insertion of trace metals into the system. The material should be able to withstand repeated sterilization. Usually, the vessel is a pressure vessel. For volumes less than 10 L, glass can be used, while vessels with larger capacity are made using various grades of stainless steel (302, 304, 316, 318). The material is usually polished to a mirror finish for ease of cleaning and preventing any dust and other components. The joints are welded and are smoothened out to prevent any sharp corners.
5.4.4 The Shape of the Fermenter
The fermenter is designed to withstand high pressure. The ideal shape is a cylinder with an arched bottom. The walls and bottom are made curved to withstand higher pressure and ease of cleaning. It also helps in the excellent mixing of the contents in the system. Aspect ratio is the ratio between the height of the reactor to its diameter, which should be kept between 1 and 3 for a continuous stirred tank reactor, whereas, for a tower reactor, the value can go up to 10.
where:
-
Ht is the height of the tank
-
Dt is the diameter of the tank
5.4.5 The Dimensions of Fermenter Parts
The various parts of the fermenter play a vital role in the system’s stability and unhindered working. Standards have been established on ratios of various parts of the reactor such as impeller and baffles. The ratio of the diameter of the impeller to the diameter of the tank should be kept in between 1/3 and ½, the ratio of the diameter of the baffles to the diameter of the tank should be 0.08–0.1, the ratio of impeller blade height to diameter of the impeller should be 0.2, the ratio of impeller blade width to the diameter of the impeller should be 0.25, and the ratio of the distance between the middle of impeller blade to impeller blade height should be 1. These values are established in international standards for a stirred tank fermenter (Bates et al. 1963).
5.4.6 Aeration and the Impeller Velocity
The aeration rate should be fixed depending on the fermenter and organism substrates causing the fermentation (Rajavathsavai et al. 2014). If enough rate of aeration is not provided, it can prevent cell proliferation. If the rate of aeration is higher than required, it can lead to unnecessary foaming and other side reactions, which will decrease the reaction’s efficiency. The impeller’s velocity varies widely depending on the type of cells (animal cells or microbial organisms), reactor’s size, the impeller (Rushton disc turbine, marine propeller, hydrofoil), nature of the product, etc. Ideally, the impeller’s velocity is kept stable so that it does not harm the cells by shearing or destroying the end product.
5.5 Types of Industrial Fermenters/Bioreactors
Fermenters are suitable for producing a wide variety of products, from products as simple as ethanol using Saccharomyces cerevisiae to products as complicated as vaccines, interferons, and antibodies. A fermenter’s design depends on the source of cells, the product, the production scale, etc. Depending on the requirements, various types of reactors are designed. Some of the different bioreactors are continuous stirred tank reactor, batch fermenter, tower fermenter, gas lift fermenter, deep jet fermenter, fluidized bed bioreactor , airlift bioreactor , bubble column bioreactor , photobioreactor, wave bioreactor , membrane bioreactor , and sparged tank bioreactor (Table 5.1) (Ali et al. 2018).
5.5.1 Continuous Stirred Tank Reactor
Continuous stirred tank reactor (CSTR) is the most utilized bioreactor . Almost 70% of the industrial bulk production using fermentation is performed using this type of bioreactor . In a CSTR, the media, cells, and products are continuously mixed with an impeller ’s help (Fig. 5.3b). This would mean that the composition of the media present in the reactor will be the same as the output media, making the CSTR ideal for research studies and calculations. The CSTR can be operated in the principles of chemostat and turbidostat. The chemostat involves using flow rates of the reactor to maintain a chemical equilibrium in the system, which is ideal for the fermenting microbes to produce maximum yield . Turbidostat is maintaining constant turbidity in the vessel. The turbidity measures the number of microorganisms present in the reactor (Wang and Zhong 2007; Ali et al. 2018). Stirred type bioreactors are discussed in detail in the next section.
Types of bioreactors. (a) Wave bioreactor (1, base; 2, pivot; 3, cell culture media in bag; 4, inflated plastic bag; 5, exhaust vent filter; 6, inlet air filter). (Adapted with permission from Zhong 2011). (b) CSTR. (Adapted with permission from Wang and Zhong 2007). (c) Packed bed reactor. (Adapted with permission from Watanabe et al. 2005). (d) Membrane bioreactor . (Adapted with permission from Wang and Zhong 2007)
5.5.2 Batch Fermenter
A batch fermenter is a system where all the components required for the cell growth and production of products are added initially before starting the process and are run until the time of harvest. It is a closed vessel system. Batch fermentation is seldom performed industrially for biological products since the product in bulk quantities would cause a change in various parameters, which needs to be adjusted as required. This process is used for processes like crystallization, dissolution of multiple components, polymerization, etc. Another property of the batch fermenter is that it is performed in discreet batches. After completing each process, the product is harvested, and the process has to start again from scratch. This type of fermenter is used for high-value, low quantity yield products. A significant advantage of this type of fermenter is that in case of contamination, unlike a continuous process, only a single batch of significantly less quantity is lost (Cardello and San 1988; Yeh et al. 2006).
5.5.3 Tower Fermenter
In simple terms, the tower fermenter is a tube with a tapering bottom with facilities to control various parameters like temperature and aeration. Tower fermenters are used mainly for continuous fermentation . The overall aspect ratio for the tower fermenter is 10, implying that the reactor’s height is significantly higher compared to its diameter. The tower fermenter usually has a sparger at the bottom of the tower from which air is pumped in resulting in a nonmechanical stirring and ample aeration. This type of reactor is used for brewing beer . Owing to its high diameter, the tower fermenter can be used to create a gradient (yeast and wort gradient in the case of beer brewing). In beer production, the raw materials float toward the surface (Jones et al. 1984). This type of reactor usually has a perforated baffle sitting parallel to the ground level, unlike the CSTR as it is the aeration that causes the mixing in the tank.
5.5.4 Gas Lift Fermenter
A gas lift fermenter is a vessel that has a central inner tube through which compressed gas is pumped. The air pumped will cause the mixing of the components in the media and bring about a loop circulation. Since it is using air for mixing, it is a non-mechanically stirred reactor. Heat transfer is also a result of the air circulated. The airlift fermenter is almost similar to the gas lift fermenter and bubble column fermenter. As compared to the bubble column fermenter, the airlift fermenter has a central tube that helps in proper mixing and circulation (Fig. 5.4a). An airlift reactor has two zones, the riser and the comer. The riser is the column where the air is pumped. There is no air pumped in the comer, which will result in circulation through the riser and comer. An adequate flow of air maintains the circulation. There are two types of airlift reactors, internal loop airlift reactor and external loop airlift reactor. The internal loop airlift reactor has a single central draft tube, which creates the circulating media loop that the external loop airlift reactor has separate channels for circulation. The advantage of using an airlift is it has very low shear stress making it ideal for animal cells. Due to the height of the reactor, there is an increased pressure toward the bottom, helping in easier mass transfer (Burkert et al. 2005).
5.5.5 Deep Jet Fermenter
A high-powered pump is useful in circulating the medium in a deep jet fermenter. An injector nozzle pumps in a jet of medium with compressed air. The gas forms giant bubbles that are removed from the port present on the top. The media then passes through the ejector nozzle, which degasses the media. The cooled aerated medium is finally injected into an air entrainer above the reactor (Jafari et al. 2012).
5.5.6 Packed Bed Reactor
Packed bed reactor/fixed bed reactors are reactors with packed (immobile) components such as enzymes or biocatalysts in a tubular or cylindrical arrangement (Fig. 5.3c). In general, the media will be pumped from the bottom of a packed column, and the effluent is taken out through the top simultaneously. Aeration can be provided from the bottom of the reactor, and the air outlet is placed at the top of the vessel. A packed bed reactor is used in wastewater treatment as the microbe forms a biofilm on the packed material (usually a biocatalyst ), enhancing the process. A significant advantage of the fixed bed reactor is that the conversion rate per unit mass is higher since it has biocatalysts. Separation of catalyst from the product can easily be achieved in these types of reactors. The packed bed reactor also provides the possibility of operation at higher pressures and temperatures (Shojaosadati and Babaeipour 2002; Watanabe et al. 2005; Zhong 2011).
5.5.7 Fluidized Bed Reactor
In a fluidized bed reactor, unlike the packed bed reactor, the materials are distributed in a fluid by its flow, i.e., the biocatalysts are suspended in the reactor due to the fluid flow rate acting themselves as fluid (Fig. 5.4b) (González et al. 2001). Like the packed bed reactor, the catalysts are held on a porous plate, which helps fluid pass through the material. At a lower fluid flow rate , the force imparted on the materials is not sufficient to lift the particles, and this system will act similar to a packed bed reactor. As the flow rate increases, the particles tend to lift up, and a stage is achieved where the particles’ weight is suspended in the fluid and balances out due to the fluid flow. This process is referred to as incipient fluidization. After the incipient fluidization, as the fluid flow rate is increased further, the particles become fluidized and behave similar to a fluid, hence the name, fluidized bed reactor. Aeration can also be provided along with the fluid flow; however, the particle distribution is no longer uniform in the presence of gases. The fluidized bed reactor can be classified as a tower reactor as the aspect ratio is similar. These types of reactor has better temperature distribution and has significantly high contact with the particles, hence increases in the product yield (Özkaya et al. 2019). The reactor is used for the production of various chemicals like vinyl chloride, polypropylene, etc. It is also used in the brewing industry for the production of beer (Krishnan et al. 1999).
5.5.8 Photobioreactor
Photobioreactors are utilized to grow organisms that require light sources for their survival (phototrophic microorganisms). The reactor provides an artificial environment, cyanobacteria, mosses, algae, and plants by providing light apart from other nutrients. Photobioreactors can be classified as open type and closed type. The open-type photobioreactors are either natural open ponds or artificially created ponds with controlled conditions to grow the organisms. A closed photobioreactor is a closed laboratory system that provides all the nutrients and light with a highly reduced risk of contamination (Fig. 5.4c). Depending on the configuration, closed photobioreactors are divided into six categories: tubular glass photobioreactor, Christmas tree photobioreactor, plate photobioreactor, horizontal plate photobioreactor, foil photobioreactor, and porous substrate bioreactor . Interestingly a closed photobioreactor provides higher cell growth (Hoekema et al. 2002; Singh and Sharma 2012; Fu et al. 2019).
5.5.9 Wave Bioreactors
Wave bioreactors are comparably newer technology used for culturing both animal and plant cells. The bioreactor system is simple and cost-effective . The bioreactor can be made with polymers yet providing all the utilities of a regular stainless steel bioreactor . The polymer bag (bioreactor ) is placed on a rocking platform. The platform’s rocking motion creates waves that provide very little shear stress without compromising on the mixing (Fig. 5.3a). The wave action also prevents the accumulation of cells. A pre-sterilized bag is an advantage in a polymer bioreactor , and hence further sterilization is not required. The wave bioreactor also shows relatively less foaming due to its less vigorous wave action (Zhong 2011).
5.5.10 Membrane Bioreactor
Membrane bioreactors are mainly used in biological wastewater treatment. The membrane bioreactors can retain the microorganisms and other catalysts in the process tank while pushing out the treated product (Fig. 5.3d). In a water treatment process, the contaminated water comes in contact with the microbes inside the process tank, and only the clean water leaves out. The sludge along with the microbes remains in the process tank, which can be later removed. The membrane is usually made using polyamide, polysulfonate, and cellulose acetate. This bioreactor is employed for the formation of alcohols and acids (Wang and Zhong 2007; Chang et al. 2019).
5.5.11 Sparged Tank Bioreactor
Sparged tank bioreactors introduce air into a metal vessel from the bottom through a porous plate. This air non-mechanically agitates the system and also takes away the heat in the system. The gas bubbles get dispersed with the help of a baffle arranged horizontally. Since it is agitated by air, the system does not require an agitator and has comparatively less shearing stress. This type of reactor uses significantly low power since it has limited moving parts.
5.5.12 High-Density Bioreactor
A high-density bioreactor (HDBR) is a novel reactor design used to treat sewage wastewater with microorganisms’ help. After the secondary wastewater treatment, the mixed liquor goes to a clarifier where the sludge settles down in the conventional activated sludge process. The practical and complete removal in a separate step is essential. The use of HDBR will not require this additional step. The HDBR system is a modified fluidized bed reactor in which the rate of the recycled stream is less with aeration. This will maintain the biomass zone in the reactor without disruption. The reactor in itself is similar to a fluidized bed reactor with an inlet port on the bottom of the reactor, pumping in calculated quantities of influent with aeration, and an outlet port situated on the top to remove the feed which is partially recirculated into the inlet port (Sales and Shieh 2006). The HDBR is used for the high-density cultivation of algae to produce biofuels (Price et al. 2015).
5.5.13 Microbioreactors
Microbioreactors are a novel idea that lets us collect precise data using tiny quantities of media and microorganisms. Microbioreactors are scaled-down CSTRs and shake flask cultures. This type of bioreactor helps in screening for many numbers of microorganisms in a very cost-effective manner. A microbioreactor is especially helpful in animal cell cultures where the media and other components are significantly costlier than the microbial growth medium. These reactors can be used for growth profiling, running parallel cultures, standardization of fermentation parameters, media screening, toxicity screening, and phenotyping. The above parameters can be studied using fluorescence, pH, dissolved oxygen, spectroscopy, etc. Quantities of less than 1 mL are only required for all the above testing. After studying the microbioreactor parameters, the test can be carried out on a lab-scale quickly, followed by scale-up to pilot plant and industrial-scale cultivation (Ravindran et al. 2019). The bioreactor is a commercial microbioreactor manufacturer providing bioreactors for testing for multiple parameters (Toeroek et al. 2015).
5.5.14 Rotating Bed Biofilm Reactor
Rotating bed biofilm reactor (RBBR) was invented by Prof. Hallvard Ødegaard in the late 1980s (Ødegaard et al. 1994). It is a reactor with an aerated tank with a cylindrical carrier partially immersed in the growth medium. The cylindrical carrier is capable of rotation around an axis. The carrier forms a surface for the microorganisms to form a biofilm. The rotating carrier provides alternate exposure to media and air. They are mainly used for wastewater treatment. An advantage of RBBR is that it provides significantly higher areas for biofilms to form. It is also highly cost-effective and is easily scalable. It can also be used for the production of algae, in turn helping in biofuel production and for the production of products like ethanol (Shen et al. 2017).
5.6 Stirred Tank Reactors
Continuous stirred tank reactors (CSTR) are vessels made from glass or stainless steel with a motor-driven shaft with impellers that rotate to cause uniform mixing of components in the vessel supporting fermentation and thus producing high yields of value-added products. The vessel will also have ports for various purposes such as sampling, acid inlet, alkali inlet, temperature, pH sensing, etc. The CSTR is the most employed fermenter for research purposes. This fermenter can be easily scaled up, and the mixture inside the reactor is assumed to be uniform, thus making the calculations more comfortable.
5.6.1 Geometry of CSTR
A CSTR is a pressure vessel capable of continuous sterilization and decontamination. It is a cylindrical vessel with an outward curved base. There will be no sharp joints inside the reactor; this will prevent the accumulation of any components in these sharp joints. The curved base helps in the uniform mixing of components. The aspect ratio of CSTR is kept between 1 and 3. The motor-driven shaft is usually fixed in the center of the reactor horizontally, with impellers radially outwards (depending on the type of impeller). The vessel is made using glass or stainless steel. Glass is used to construct vessels below 10 L, while stainless steel was used to construct reactors above 10 L. The outer and inner surfaces are usually polished to a mirror finish for easy cleaning and to prevent any accumulation. The vessel material and inside components are corrosion-resistant air.
5.6.2 Impeller
As the name suggests, the impeller is an essential component in a CSTR. It helps in the mixing of the components in the vessel. There are many types of impellers such as blade paddles, spiral propeller blades, ribbon blades, anchor blades, screws with draft tubes, etc. Selection of the impeller blade is crucial as each type of blade’s properties are different, making it unique to different requirements. Blade paddle or paddle agitator or Rushton turbine is the most commonly used impeller blade. There is a two-blade and four-blade configuration. This blade is apt for slow operations. Paddles at high speeds can cause heavy shear stress on the cells, thus decreasing the efficiency. The axial flow hydrofoil impeller is a way to reduce shear rates. In this impeller, the liquid is either drawn up or down with lower energy requirements. This blade can thus be used in shear-sensitive processes involving animal cell culture. Another example of an axial flow impeller is the marine propeller (Ameur 2015; Gu et al. 2017).
The impellers need to be positioned ideally to achieve maximum oxygen transfer rate . If the impellers are too close to each other, the mixing quality is decreased drastically. If the impellers are placed too far apart, the uniformity of the overall solution is affected. One to 1.5 impeller diameter is the spacing that gives ideal mixing in most cases.
The selection of the impeller blades depends on the fluid’s rheology inside the reactor. If the broth is too viscous, a helical ribbon is useful. Also, it is observed that the use of a Rushton turbine in non-Newtonian broth is seen to increase the viscosity of the broth near the central shaft, thus impeding proper mixing (Sossa-Echeverria and Taghipour 2015). To solve such types of issues, the impeller should be custom-designed for the broth being used. Novel impellers such as Mixco A-315 have shown to give homogeneous mixing with almost the same power requirement as the Rushton turbine.
Hence the Impeller should be so selected to maximize air dispersion and oxygen transfer with ideal fluid-gas mixing. The blade type and rotation speed should not cause any shear stress on the organisms cultivated. The blade dimensions should provide homogeneous mixing and avoid any stagnant zones in the vessel. It should use less power for the same oxygen transfer rate it provides. A proper impeller with a precise rotation should be selected based on the parameters mentioned above.
5.6.3 Modelling of Ideal CSTR
A CSTR is useful for most of the research calculations, and in practical industrial applications, it is still a much-preferred system. It has several advantages like homogeneous mixing of contents in the vessel, fair heat distribution , good yield , etc. The following assumptions are made to model the design equation of an ideal CSTR (Liu 2017):
-
1.
The mixing is entirely homogeneous, and, hence a sample taken from practically anywhere in the bioreactor will have the same composition.
-
2.
The fermenter is in a steady state, i.e., there is no accumulation of contents in the reactor.
\( \left(\frac{\mathrm{d}\mathrm{NA}}{\mathrm{d}t}=0\right) \) where NA is the number of moles of species A.
-
3.
The boundaries of the vessel are closed, and there is no mass transfer between the boundaries.
-
4.
It is assumed that there is a constant fluid density.
-
5.
There is a constant temperature.
-
6.
The reaction is considered to be irreversible.
-
7.
All the reactants are converted to products by the reaction.
Net accumulation
where:
-
FAo is the molar flow rate of the inlet of species A
-
FA is the molar flow rate of outlet of species A
-
VA is the stoichiometric coefficient
-
rA is the reaction rate
$$ 0=\mathrm{FAo}-\mathrm{FA}+V\ast \mathrm{rA}\ast \mathrm{VA} $$
Assuming steady state, VA = − 1
Thus, 0 = FAo − FA − V ∗ rA
We know that
where:
-
CAo is the concentration of inlet species A
-
CA is the concentration of outlet species A
-
Q is the fluid flow rate
$$ 0=Q\ast \mathrm{CA}\mathrm{o}-Q\ast \mathrm{CA}-V\ast \mathrm{rA} $$
where T is the theoretical residence time \( \left(T=\frac{V}{Q}\right) \).
Residence time is the total amount of time wherein a quantity of compound will be retained in a bioreactor .
From Eq. ((5.3), the design equations from zeroth order, first order, second order, and nth order reactions can be calculated.
The size of the reactor (volume) can be shown using the Levenspiel plot, which is represented by \( \frac{\mathrm{FAo}}{-\mathrm{rA}} \) on the X-axis and conversion in the Y-axis.
The reaction rate is most significant at the start of the reaction, which reduces in practicality.
5.6.4 Gas Delivery System
A gas delivery system or aeration system consists of a device called a sparger. It is used to introduce air into a bioreactor vessel. A perforated ring-type sparger is the most used sparger in a CSTR since it provides the most uniform air distribution . Aerobic culture requires significantly high air inputs; for a 100 m3 reactor, almost 100 m3 air is required to be pumped per minute. Continuous stirring in a CSTR provides ample air distribution in the system (Karimi et al. 2013). All the air that goes into the system needs to be sterilized. Details of the same are given in the sections below.
5.7 Strategies for Gases, Nutrient Solution, and Media Sterilization for Industrial Fermentation
Sterilization is the process of elimination of all viable organisms from a system. This process is of utmost importance to lab scale, pilot scale, and industrial fermenters. This failure would lead to the competitive growth of both our organism of interest and the invading species, which would mean that a significantly lower amount of nutrients will be available for the target organism, which will reduce the system’s overall productivity. The contaminant organism can also produce unwanted products into the system leading to contamination of the product. These unwanted products can also make the downstream processing much more complicated, decreasing the firm’s revenue. The contaminants may all ruin the whole process calculations for the systems, i.e., the contaminant organisms may have a completely different morphology (such as mycelia or more rigid cell wall) or can have a different growth curve. It may produce more heat, which will render the cooling system ineffective, thus killing the target organism. Another factor that influences the need for contamination is if the process is batch or continuous process. Contamination in a batch process is timely resolvable compared to a continuous process. In a continuous process, the contaminant organism will outgrow the target organism if not monitored closely. The loss of revenue by the loss of a continuous process is significantly higher than the batch processes. Hence, the need for sterilization is very high in any industry or research facility involving the use of fermentation (Stanbury et al. 2013).
To achieve a sterile environment, all the bioreactor components and vessels are needed to be sterilized. The sterilization strategies for the different components are different. For example, a vessel’s sterilization strategy cannot be used to sterilize gases that enter the system. Similarly, a standard sterilization method is not useful for the sterilization of all media types; for example, we cannot sterilize the media for microbial culture and animal cell culture in a similar strategy. Thus, sterilization strategies for various components in an industrial fermenter are included in this chapter.
5.7.1 Strategy for Medium Sterilization
In theory, media sterilization can be performed by methods such as radiation, chemical treatment, filtration, ultrasonic treatment, and heat. But considering the pros and cons, heat sterilization is the most widely preferred method of sterilization for microbial cell cultures; on the other hand, filtration is the most sought-after method for media sterilization in animal cell culture since the media is heat-labile. In heat sterilization, sterilization using steam is the most preferred method mainly because of its high latent heat (Sternfeld and Paulus 1993).
Sterilization using steam can be much more comfortable in a laboratory-scale fermenter (less than 10 L). The whole system can be disassembled and can be taken to an autoclave where almost all the components are sterilized. This is not practical for a pilot-scale plant or an industrial-scale plant that will have huge parts that cannot be moved; hence clean-in-place (CIP) and sterilization-in-place (SIP) approach are used. In this approach, the cleaning and sterilization are performed at the place where the fermenter is installed (Pettigrew et al. 2015).
The sterilization-in-place approach is a widely used system in which the heat for sterilization is brought to the vessel, usually in the form of steam. These two approaches, either a steam jacket to heat the whole system or the steam, are pumped into the vessel and are often preferred. This strategy used depends on the process required. Sterilization with steam is usually carried out at 120 °C at 2 bar pressure, with a holding time of 60–70 min. The kinetics for sterilization is calculated according to the organism used and the product used. The sterilization time and temperature regime are pre-calculated.
The sterilization can be performed either as a batch process or a continuous process. Batch sterilization is preferred for laboratory and pilot-scale plants (Singh et al. 1989), while industries prefer continuous sterilization. This is mainly because in huge plants (100,000 L), it will be difficult to heat the media batch-wise. The core temperature will be different as compared to the borders. Robust impellers also will need to be employed for uniform heat distribution .
In comparison, the motors running the impellers will need less power, hence decreasing the industry’s running cost. Studies have shown that 60–80% less water and steam are utilized for continuous sterilization. Continuous sterilization also helps in lowering turnaround time, implying more productivity (Pfeifer and Vojnovich 1952).
Continuous sterilization plate heat exchangers were used initially, but because there were immense chances of gasket failures (thus mixing sterile and non-sterile streams of fluids) and blocking of the heat exchangers due to particulates in the media, the system was replaced by spiral heat exchangers. This solved the problem of gasket failures and the two streams were separated by steel. The flow inside the spiral heat exchangers is self-cleaning, hence reducing the chances of blockage. A major disadvantage of the heat exchanger is the temperature regimes need to be controlled accurately. This is much easier in batch sterilization (since it can be controlled manually). Sophisticated computer programs have been designed to take over the control of these heat regimes. The computer-controlled continuous sterilization is so accurate that the technique can be used to sterilize heat-labile media components with controlled heating and cooling regimes (Deindoerfer and Humphrey 1959).
As mentioned before, most of the sterilization is performed using steam (heat sterilization). Heat-labile nutrient feeds used in animal cell culture cannot be sterilized using this method as it decreases the nutritional availability in the media. The preferred method for sterilizing heat-labile media without any particulate matter is filtration (Coté 1999). The filtration is usually described by the following mechanisms: inertial impaction, diffusion, electrostatic attraction, and interception. In inertial impaction, the suspended particles’ momentum causes them to collide with the filter membrane, while the fluid passes on through the membrane. Diffusion is caused by Brownian motion. The suspended particles, owing to their Brownian motion, tend to get stuck in the membrane. Charged particles get attracted by oppositely charged surfaces, such as filtration membranes. Interception is how the filter membrane will stop the suspended particles if the pore size is smaller than the particle itself (Tien and Ramarao 2011). In most of the filters, the pore size will be smaller than the particles’ size to be filtered. These filters are arranged to form a sheet of cartridge accommodated in the housing. On a laboratory scale, a standard disc or capsule filter can be used for filtration. This can be achieved using a syringe to provide the necessary pressure. Industrial filtration requires more complicated filtration cartridges for this purpose. Zeta Plus™ Encapsulated System is a scalable depth filter that can be used for media sterilization. Most of the industries use membrane filters in cartridges with a pore size of 0.2 μm for filtration. The disadvantage of using membranes with such small sizes is that it can get easily clogged; hence, usually a prefilter is equipped in an industrial fermenter. A prefilter is generally a filter with a much bigger pore size to remove any coarse particles suspended in the solution.
5.7.2 Strategies of Gas Sterilization
Aerobic fermentation needs significant quantities of air, which is injected into the reactor through a sparger. A fermenter requires one volume of air per volume of medium per minute. This implies that vast quantities of air need to be pumped in to maintain the culture. This air also needs to be sterilized to maintain a contamination-free environment inside a bioreactor . The most commonly used method for sterilization of gases is filtration. As mentioned previously, the filtration method employed for gas sterilization is similar to the methods employed in media sterilization. The air is passed through a fixed pore membrane filter. The membranes are arranged to form a cartridge which is housed in plastic housings (for laboratory scale and pilot scale) or stainless steel housings (for industrial-scale bioreactors). The most commonly used filtration material is polytetrafluoroethylene (PTFE) (Wikol et al. 2008). The PTFE in itself forms a complicated mesh that reduces the pore size of the whole membrane; apart from this, it is also arranged in loops inside a cartridge to provide a complicated path for the gas to travel, thus filtering it. Another advantage of using a PTFE membrane is that it is hydrophobic, thus repelling water from the membrane. In gas filtration, as in media filtration, there is a requirement of a prefilter to remove significantly larger debris. The prefilter is usually made of glass microfiber, polypropylene, etc.
The strategy usually followed by an industry in the sterilization of fermenter is by pumping steam into the reactor once the media is loaded. This would sterilize both the media and the vessel. But if the fermenter needs to be sterilized separately, the vessel may be heated using steam in heating jackets or electrically heated coils. Steam directly pumped into the reactor can also be used for vessel sterilization. The steam pressure is held at 15 psi at 121 °C for 20 min to sterilize the vessel. Once the sterilization is complete, sterilized air should only be pumped inside the bioreactor , and the bioreactor should always be kept at positive pressure. If this step is not performed carefully, air from surroundings may creep into the system due to the vacuum created because of the exit of steam and compromise the whole bioreactor ’s integrity.
5.8 Industrial Fermentation Processes
Industrial fermentation methods start with selecting suitable microorganisms and conditions, such as careful nutrient concentration adjustment. The by-product is diverse: alcohol, glycerol, and carbon dioxide from yeast fermentation of various sugars; butyl alcohol, acetone, lactic acid, monosodium glutamate, and diverse bacterial ACEs; and citric acid, gluconic acid, and small quantities of antibiotics, and vitamin B12 and riboflavin.
Five important commercial fermentation groups exist:
-
(a)
Those that generate as a result microbial cells (or biomass )
-
(b)
The manufacturers of microbial enzymes
-
(c)
Those which generate microbial metabolites
-
(d)
Those producing recombinants
-
(e)
Those who change the transformation phase of a compound added to the fermentation
5.8.1 Microbial Biomass
Commercial expansion of microbial biomass is split up into two main processes: yeast production for baking and microbial cells’ production for human and animal use (single-cell protein). Since the beginning of the 1900s, baker’s yeast has been developed on a wide scale, and in Germany, in the course of the First World War, yeast has been produced as human food. Nevertheless, until the 1960s that microbial biomass was extended as a food protein source. These developments were focused on feedstock hydrocarbons, which could not compete against other high protein animal feeds (Sharp 1989). However, the decline of animal biomass feed fermentations was composed by establishing the method for manufacturing fungal biomass .
5.8.2 Microbial Enzymes
Commercially plant, animal, and microbial source enzymes are developed. However, microbial enzymes have the enormous advantage that stable fermentation techniques can be produced in great numbers. Infinitely simpler than in a plant or animal system is the enhancement of a microbial system’s productivity. Also, the introduction of recombinant DNA technology allowed for synthesis by microorganisms having animal origin enzymes. Development in microorganisms is controlled, and these controls can be generated over several phases of the growth curve to increase productivity. The products formed during the log growth process are essential for cell growth and include amino acids, nucleotides, proteins, nucleic acids, lipids, carbohydrates, etc. These goods are called the basic metabolism products, and a step (log equivalent or exponential) in which the metabolism is formed is referred to as the trophophase.
The industrial microbiologist is responsible for modifying the wild organism and creating cultural conditions to increase such compounds’ productivity. Some microbial cultures synthesize in the deceleration and stationary phases compounds that have not been generated during the trophophase and do not seem to be apparent to cells’ metabolism. These are considered secondary compounds and their phase (stationary phase equivalent) as an idiophase. It is necessary to note that secondary metabolism is a feature of the slow-growing and the nongrowing cells in continuous cultivation at low growth rates. Suppose the microorganisms evolve in their natural environments at relatively low growth rates; it is tempting to say that idiophase prevails, not a trophophase in nature, which is more a culture property of microorganisms.
5.8.3 The Recombinant Products
The invention of recombinant DNA technology expanded the spectrum of possible product fermentation . Microbial cells can be inserted into genes from the higher organisms to allow recipients to synthesize “outside” proteins. A wide variety of microbial cells were used as hosts for the use of Escherichia coli, Saccharomyces cerevisiae, and filamentous fungi, and interhuman serum albumin, epidermal growth factor receptor vIII, growth factor, calf chymosin, and bovine soroatostatilo.
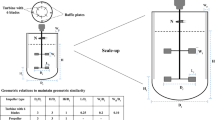
5.9 Scaling Up of Industrial Fermenters
Scale-up is the rise in fermenter level or dimensions, e.g., laboratory scale to pilot scale or a large production scale. Microbial processes are usually conducted for a commercial purpose, primarily to give consumers quality advantages and produce financial returns to investors. A technique produced in laboratories (e.g., fermenters of 0.5–10 L), with a scale range from thousands to millions, has to be transformed into a complete production process (e.g., 20,000–2,000,000 L fermenters). The critical feature to be restrained on the scale-up is the volume of the fermenter. Increasing the surface area by the square function, airflow rate , carbon dioxide, oxygen transfer, agitation rate , and foaming will be affected. If some parameters such as impeller speed endure perpetually at the fermentation process as power output, it surges to a widespread assortment and becomes economically unviable. Thus it is not easy to control the same level of mixing on scale-up as it primes to less satisfactory mixing behavior (Schmidt 2005).
Scale-up can also modify the generation of foam, shear forces, and rate of elimination of carbon dioxide. Scaling up using continuous oxygen transfer rate (OTR) can calculate or evaluate the volumetric mass transfer coefficient, kLa. One set of principles is extensively used to uphold the resemblance of the three different power input parameters per unit volume, geometry, and superficial air velocity. The consequence of the latter is to decline the comparative volumetric airflow rate as scale increases.
If a high degree of originality arises in the process and the commercial product, large industrial processes are ideally accelerated over two levels. The first stage is a pilot plant (pilot scale) with fermenters of 100–10,000 L and equipment upstream. Their goal is to convert the laboratory process into a practical, downgraded version of the production process. In utmost cases, the method is not entirely integrated; e.g., each device’s process is operated in a batch manner. The pilot scale selected is a measurement constructed on the measurement, accessibility, and cost of descriptive downstream equipment and the sample sizes required. The second phase of the scale-up is a demonstration plant (demo scale) with fermenters between 10,000 and 100,000 L and downstream equivalents. The risk is to be reduced by further validating the operation, supply chain (from the raw materials to the commercial good application), and consumer demand for significant capital investment in the complete manufacturing plant.
The financial expenditure to increase the production size of a microbial process is typically higher than improving production microbes and laboratory processes. The production plant’s annual operating costs are in the same order. Usually, it takes 3–10 years for the transition from laboratory to development. Under those conditions, the financial risk is high. This could be costly and lead to a loss of process efficiency during growth, likely leading to a project failure. So it is sufficient to correct and get it right for the first time when scaling up microbial processes.
5.9.1 Key Considerations for Fermentation Scale-Up
Low fermentation output is always regarded as a priority risk. Fermentation efficiency also affects the enactment of all downstream unit operations and process waste quantity/nature. Fermentation is also an intricate unit procedure (Yang 2011). The output of these parameters is subjected to modification during scale-up. The cost limitations or the equipment’s design and size will alter these parameters and directly affect process efficiency and overall plant economy. To minimize fermentation as an increased risk, the realization and attention to scale-dependent fermentation parameters are necessary. For those who follow a consistent approach, large-scale fermentation will be rewarded to meet the output standards and provide good downstream performance. A successful, scalable fermentation , because of the plant’s continuous knowledge from years of operational experience, eventually should achieve healthier in the production plant than in the lab. A scaled-down laboratory process diminishes the hazard of performance degradation during the scale-up procedure but does not remove the need for an intermediate scale validation, particularly in the case of a first-of-a-kind procedure. Piloting, usually involving 1–10% of total production, offers specific benefits and reduces more risks:
Process parameter differs as fermenters expand in size and volume, so there are difficulties around the scale-up process. In essence, compromises must be reached in the degree to which other parameters (e.g., speed of pump tip) are constant, for example, if fermentation as a power input is increased widely and economically unviable. Therefore, the mixing level at scale-up cannot be sustained, resulting in a less desirable mixing. Scale-up studies are laboratory studies or even pilot-plant fermenters to generate data for large industrial fermenters’ extrapolation and development. Scale-up studies are accessible. In scale studies, several rules are applied as follows:
-
1.
Similarity in geometry and the configuration of fermenters used to scale up.
-
2.
Minimal increase in scaling up of fermentation studies by three or four steps.
-
3.
Each leap in size is increased by magnitude or power and not a few liters. The slight increase in the amount of work does not provide essential data for the operation.
In the case of scale-up, inoculum development and environmental parameters including the availability of nutrients, pH, temperature, dissolved oxygen, dissolved carbon dioxide, and shear conditions are required. Design and development of a pilot plant, including the pilot plant’s construction, indicate product economics based on estimated market sizes and competitive pricing and provide guidelines for the permissible production costs.
5.10 Regulation of Industrial Fermentation Via Advanced Instrumentations and Computations
The framework will combine three layers of process control. Each higher level contains more complicated programs and needs to be better understood. The first level of control, used in the chemical industries, is already sequencing, for instance, controlling valves or start or stop pumps, recalibrating instruments, online maintenance, and fail-safe shutdown processes. At least in minutes in utmost operations, high-speed manipulation is not necessary. Sterilization cycles and medium batching are two applications of fermentation processes. The next stage of device control includes temperature control, pH control, foam control, etc., as sensors are interfaced directly with your computer. Separate controller units are not required when this is completed. The program determines the value of the given point, and control algorithms (e.g., PID) are included in the software kit. Enhanced regulator is promising because the control procedures are functions relative than electrical functions mathematically processed. This technique makes a policy of process management more flexible and more detailed. Computer failure will lead to significant problems without manual backup. The alternative solution is to use a computer for the sole control feature. The attached computer records only sensor data and sends signals to modify computer programs and manually change points. This system is called the Set-Point Supervisory Control (SSC). When using the SSC, control modes are restricted to proportional, integral, and derivative, because the electronic controller provides uninterrupted control to the fermenter. However, the process controller is controlled independently in case of machine failure.
Process optimization is the most advanced degree of control. This involves understanding a process, observing what happens, and controlling it to achieve and sustain optimal conditions. First of all, sufficient online sensors are required to track the operation continuously. A few now are usable for dissolved oxygen and specific metabolites (infrared spectroscopy, mass spectroscopy, and near infrared spectroscopy). Second, a mathematical model that effectively designates the dynamic conduct of a process ought to be created. These models’ critical role in the batch, fed-batch, and continuous processes for biomass and metabolites has been optimized to enhance yeast development, industrial antibiotic processes, and lactic acid production. Even though a lot is done to regulate a process, few sensors are existing to track the number of metabolites or other parameters in fermentation broth online. To achieve better control, an artificial nerve network is used because of those limitations. Unlike known systems based on experience, neural networks do not require evidence in the method of a collection of rules but acquire from the procedure examples that give them their own rules. This allows nonlinear structures and minimal data to be dealt with. However, it has previously been used in case studies of ethanol production, in the real-time variable estimation, and control of the fermentation and recombinant fermentation of Escherichia coli. The system is still at an early stage of growth. There are constricted time constraints for process optimization in industrial systems where vital online and offline data can be obtainable; the possibility of creating a reasonably accurate model of nerve networks within short time scales becomes very attractive (Glassey 1994).
5.10.1 Monitoring Software
Biomass monitoring and growth rates were developed to calculate the overall energy demand for animal cells in cultivation by online estimating the ATP production rate of oxygen intake and the lactic acid production rate (Dorresteijn et al. 1996). A redox-based software sensor is used to monitor substrate level in Thiobacillus ferrooxidans cultures for online estimation of the substrate concentration and biomass . In several cases (San and Stephanopoulos 1984; Iversen et al. 1994; Castrillo et al. 1995), estimated, the introductory rate of growth (μ) based on pH titration was presented. The sensor is constructed on the assumption that the ammonia used for pH checking is converted into biomass with a (relatively) constant proton production per ammonium ion consumed.
5.10.2 Monitoring of Stress Responses in Recombinant Fermentation Processes
Software sensors are not widely used to monitor stress reactions, but increased respiratory activities are a metabolic burden from recombinant protein development. Studies are carried out to track promoters’ online regulation associated with stress when fusions with a reporter protein, such as green fluorescent protein (GFP), are used. The monitoring of off-gas from a bioreactor combined with the pattern recognition methods for predicting metabolite and biomass concentration has been carried out with various chemical gas sensors, often called an electronic nose (Bachinger et al. 2001). This method is useful for metabolic burden identification during fermentation processes.
5.10.3 Multi-bioreactor Systems
The need for enormous data and relatively slow rate of ferments are a bottleneck in research into the link between analysis and process efficiency. Many bench-scaling multi-bioreactor systems provide parallel fermentations by several bioreactor manufacturing companies. These systems are ideal for a good experimental design, whereby statistically significant associations are formed between the restrained variable and the progression quality parameters. Also ideal for process optimization is the combination of multi-bioreactor systems and online analyses. Food profiles, induction techniques, and stress builders are essential parameters for process optimism with a multi-bioreactor framework. Multifunctional cultures need a high degree of automation because the job is highly labor-intensive. The automation of fermentation processes requires individual reactor surveillance and control to enable, for example, automated feed starting or inducing processes. Software sensors may aid the instinctive monitoring of such crops. The requirement for individual reactor control and monitoring results in very widespread data sets for process evaluation (Bachinger et al. 2001).
5.11 Conclusion
Fermenters are used to make a variety of biological and high-value goods. They offer the opportunity to regulate and track the conditions of the fermentation process while also providing additional advantages. Fermenter design affects fermentation rate ; many factors such as fermenter size, vessel form, agitation, aeration, baffles, and so on all play a role in output. Various methods for increasing efficiency, as well as various controlling probes, have been developed to improve productivity. To provide a quantitative understanding of heat and mass transfer, appropriate calculation methods have been established. Innovative developments are being implemented in various types of fermenters in order to increase the efficiency rate .
To provide versatile and high-quality bioreactors, reliability and availability on new fermenter designs (bioengineering development) are needed worldwide. The sustainability of the process on a wide scale must consider achieving success in processes and bioproducts. It is important to invest in research and scale-up processes that ensure better performance in the plant’s optimum functioning and maintenance. As a consequence of this need, many efficient bioprocesses are established based on the best bioreactor design adapted to a particular production process and one that is economically profitable.
Abbreviations
- ATP:
-
Adenosine triphosphate
- CIP:
-
Clean in place
- CSTR:
-
Continuous stirred tank reactor
- GFP:
-
Green fluorescent protein
- HDBR:
-
High-density bioreactor
- OTR:
-
Oxygen transfer rate
- PTFE:
-
Polytetrafluoroethylene
- RBBR:
-
Rotating bed biofilm reactor
- SIP:
-
Sterilize in place
- SSC:
-
Set-point supervisory control
References
Agrawal K, Verma P (2020) Production optimization of yellow laccase from Stropharia sp. ITCC 8422 and enzyme-mediated depolymerization and hydrolysis of lignocellulosic biomass for biorefinery application. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00869-w
Agrawal K, Bhardwaj N, Kumar B, Chaturvedi V, Verma P (2019) Process optimization, purification and characterization of alkaline stable white laccase from Myrothecium verrucaria ITCC-8447 and its application in delignification of agroresidues. Int J Biol Macromol 125:1042–1055. https://doi.org/10.1016/j.ijbiomac.2018.12.108
Ali S, Rafique A, Ahmed M, Sakandar S (2018) Different type of industrial fermentors and their associated operations for the mass production of metabolite. Eur J Pharm Med Res 5(5):109–119
Ameur H (2015) Energy efficiency of different impellers in stirred tank reactors. Energy 93:1980–1988
Ashok V, Gupta SK, Shriwastav A (2019) Photobioreactors for wastewater treatment. In: Gupta SK, Bux F (eds) Application of microalgae in wastewater treatment: volume 1: domestic and industrial wastewater treatment. Springer International Publishing, Cham, pp 383–409
Asimakopoulos K, Gavala HN, Skiadas IV (2018) Reactor systems for syngas fermentation processes: a review. Chem Eng J 348:732–744
Bachinger T, Mandenius CF, Striedner G, Clementschitsch F, Dürrschmid E, Cserjan-Puschmann M, Doblhoff-Dier O, Bayer K (2001) Non-invasive detection of the metabolic burden on recombinant microorganisms during fermentation processes. J Chem Technol Biotechnol 76(8):885–889
Bajpai P (2017) Cultivation of microorganisms for single-cell protein production. In: Bajpai P (ed) Single cell protein production from lignocellulosic biomass. Springer Singapore, Singapore, pp 31–36
Baldi L, Hacker DL, Adam M, Wurm FM (2007) Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol Lett 29(5):677–684
Barker TW, Worgan JT (1981) The application of air-lift fermenters to the cultivation of filamentous fungi. Eur J Appl Microbiol Biotechnol 13(2):77–83
Bates RL, Fondy PL, Corpstein RR (1963) Examination of some geometric parameters of impeller power. Ind Eng Chem Process Des Dev 2(4):310–314
Becze GD, Liebmann AJ (1944) Aeration in the production of compressed yeast. Ind Eng Chem 36(10):882–890
Bello MM, Abdul Raman AA, Purushothaman M (2017) Applications of fluidized bed reactors in wastewater treatment—a review of the major design and operational parameters. J Clean Prod 141:1492–1514
Bhardwaj N, Kumar B, Agrawal K, Chaturvedi V, Verma P (2019) Purification and characterization of a thermo-acid/alkali stable xylanases from Aspergillus oryzae LC1 and its application in xylo-oligosaccharides production from lignocellulosic. Int J Biol Macromol 122:1191–1202. https://doi.org/10.1016/j.ijbiomac.2018.09.070
Burkert J FdM, Maldonado RR, Filho FM, Rodrigues MI (2005) Comparison of lipase production by Geotrichum candidum in stirring and airlift fermenters. J Chem Technol Biotechnol 80(1):61–67
Callaham JR (1944) Penicillin large-scale production by deep fermentation. Chem Met Eng 51(4):94–98
Cardello RJ, San K-Y (1988) The design of controllers for batch bioreactors. Biotechnol Bioeng 32(4):519–526
Castrillo JI, De Miguel I, Ugalde UO (1995) Proton production and consumption pathways in yeast metabolism. A chemostat culture analysis. Yeast 11(14):1353–1365
Chandrashekhar H, Rao JV (2010) An overview of fermenter and the design considerations to enhance its productivity. Pharmacologyonline 1:261–301
Chang H-M, Sun Y-C, Chien IC, Chang W-S, Ray SS, Cao DTN, Cong Duong C, Chen S-S (2019) Innovative upflow anaerobic sludge osmotic membrane bioreactor for wastewater treatment. Bioresour Technol 287:121466
Chen H-C, Hu Y-C (2006) Bioreactors for tissue engineering. Biotechnol Lett 28(18):1415–1423
Claus G, Kutzner HJ (1985) Autotrophic denitrification by Thiobacillus denitrificans in a packed bed reactor. Appl Microbiol Biotechnol 22(4):289–296
Coté RJ (1999) Sterilization and filtration. Curr Protoc Cell Biol 1(1):1.4.1–1.4.21
Deindoerfer FH, Humphrey AE (1959) Principles in the design of continuous sterilizers. Appl Microbiol 7(4):264–270
Dorresteijn RC, Numan KH, De Gooijer C, Tramper J, Beuvery EC (1996) On-line estimation of the biomass activity during animal-cell cultivations. Biotechnol Bioeng 51(2):206–214
Eibl R, Eibl D (2008) Design of bioreactors suitable for plant cell and tissue cultures. Phytochem Rev 7(3):593–598
Ensley BD, Kurisko PR (1994) A gas lift bioreactor for removal of contaminants from the vapor phase. Appl Environ Microbiol 60(1):285–290
Fink M, Cserjan-Puschmann M, Reinisch D, Striedner G (2021) High-throughput microbioreactor provides a capable tool for early stage bioprocess development. Sci Rep 11(1):2056
Fu J, Huang Y, Liao Q, Xia A, Fu Q, Zhu X (2019) Photo-bioreactor design for microalgae: a review from the aspect of CO2 transfer and conversion. Bioresour Technol 292:121947
Gaikwad V, Panghal A, Jadhav S, Sharma P, Bagal A, Jadhav A, Chhikara N (2018) Designing of fermenter and its utilization in food industries
Gargouri B, Karray F, Mhiri N, Aloui F, Sayadi S (2011) Application of a continuously stirred tank bioreactor (CSTR) for bioremediation of hydrocarbon-rich industrial wastewater effluents. J Hazard Mater 189(1):427–434
Ghasemi A, Bozorg A, Rahmati F, Mirhassani R, Hosseininasab S (2019) Comprehensive study on wave bioreactor system to scale up the cultivation of and recombinant protein expression in baculovirus-infected insect cells. Biochem Eng J 143:121–130
Glassey J (1994) Application of artificial neural networks to fermentation development and supervision. University of Newcastle upon Tyne, Newcastle upon Tyne
Glazyrina J, Materne E-M, Dreher T, Storm D, Junne S, Adams T, Greller G, Neubauer P (2010) High cell density cultivation and recombinant protein production with Escherichia coli in a rocking-motion-type bioreactor. Microb Cell Factories 9(1):42
González G, Herrera G, García MT, Peña M (2001) Biodegradation of phenolic industrial wastewater in a fluidized bed bioreactor with immobilized cells of Pseudomonas putida. Bioresour Technol 80(2):137–142
Gu D, Liu Z, Qiu F, Li J, Tao C, Wang Y (2017) Design of impeller blades for efficient homogeneity of solid-liquid suspension in a stirred tank reactor. Adv Powder Technol 28(10):2514–2523
Gudin C, Chaumont D (1991) Cell fragility—the key problem of microalgae mass production in closed photobioreactors. Bioresour Technol 38(2–3):145–151
Hastings JJ (1971) Development of the fermentation industries in Great Britain. Adv Appl Microbiol 14:1–45
Hoekema S, Bijmans M, Janssen M, Tramper J, Wijffels RH (2002) A pneumatically agitated flat-panel photobioreactor with gas re-circulation: anaerobic photoheterotrophic cultivation of a purple non-sulfur bacterium. Int J Hydrog Energy 27(11):1331–1338
Hülsen T, Sander EM, Jensen PD, Batstone DJ (2020) Application of purple phototrophic bacteria in a biofilm photobioreactor for single cell protein production: biofilm vs suspended growth. Water Res 181:115909
Iversen JJL, Thomsen JK, Cox RP (1994) On-line growth measurements in bioreactors by titrating metabolic proton exchange. Appl Microbiol Biotechnol 42(2–3):256–262
Jafari P, Mohammad ZG, Almasian F, Tajabadi EM (2012) Optimization of single cell protein (SCP) production in pilot plant scale with a deep jet fermenter of 1000 L capacity
Jones ST, Korus RA, Admassu W, Heimsch RC (1984) Ethanol fermentation in a continuous tower fermentor. Biotechnol Bioeng 26(7):742–747
Jürgensen L, Ehimen EA, Born J, Holm-Nielsen JB (2018) A combination anaerobic digestion scheme for biogas production from dairy effluent—CSTR and ABR, and biogas upgrading. Biomass Bioenergy 111:241–247
Karimi A, Golbabaei F, Mehrnia MR, Neghab M, Mohammad K, Nikpey A, Pourmand MR (2013) Oxygen mass transfer in a stirred tank bioreactor using different impeller configurations for environmental purposes. Iranian J Environ Health Sci Eng 10(1):6
Koller AP, Wolf L, Weuster-Botz D (2017) Reaction engineering analysis of Scenedesmus ovalternus in a flat-plate gas-lift photobioreactor. Bioresour Technol 225:165–174
Krishnan MS, Nghiem NP, Davison BH (1999) Ethanol production from corn starch in a fluidized-bed bioreactor. In: Davison BH, Finkelstein M (eds) Twentieth symposium on biotechnology for fuels and chemicals: presented as volumes 77–79 of applied biochemistry and biotechnology proceedings of the twentieth symposium on biotechnology for fuels and chemicals held May 3–7, 1998, Gatlinburg, Tennessee. Humana Press, Totowa, NJ, pp 359–372
Kumar B, Bhardwaj N, Alam A, Agrawal K, Prasad H, Verma P (2018) Production, purification and characterization of an acid/alkali and thermo tolerant cellulase from Schizophyllum commune NAIMCC-F-03379 and its application in hydrolysis of lignocellulosic wastes. AMB Express 8(1):173. https://doi.org/10.1186/s13568-018-0696-y
Kurt M, Dunn IJ, Bourne JR (1987) Biological denitrification of drinking water using autotrophic organisms with H2 in a fluidized-bed biofilm reactor. Biotechnol Bioeng 29(4):493–501
Leaver G, Hambleton P (1992) Designing bioreactors to minimise or prevent inadvertent release into the workplace and natural environment. Pharm Technol Int 4(3):18–26
Leite JAC, Fernandes BS, Pozzi E, Barboza M, Zaiat M (2008) Application of an anaerobic packed-bed bioreactor for the production of hydrogen and organic acids. Int J Hydrog Energy 33(2):579–586
Li X, Zhang G, Zhao X, Zhou J, Du G, Chen J (2020) A conceptual air-lift reactor design for large scale animal cell cultivation in the context of in vitro meat production. Chem Eng Sci 211:115269
Listewnik HF, Wendlandt KD, Jechorek M, Mirschel G (2007) Process design for the microbial synthesis of poly-β-hydroxybutyrate (PHB) from natural gas. Eng Life Sci 7(3):278–282
Liu S (2017) Chapter 5—Ideal flow reactors. In: Liu S (ed) Bioprocess engineering (second edition). Elsevier, Amsterdam, pp 179–257
Liu K, Phillips JR, Sun X, Mohammad S, Huhnke RL, Atiyeh HK (2019) Investigation and modeling of gas-liquid mass transfer in a sparged and non-sparged continuous stirred tank reactor with potential application in syngas fermentation. Fermentation 5(3):75
McCarty PL, Smith DP (1986) Anaerobic wastewater treatment. Environ Sci Technol 20(12):1200–1206
Narendranathan T (1986) Designing fermentation equipment. Chem Eng 425:23–31
Nienow AW (1990) Agitators for mycelial fermentations. Trends Biotechnol 8:224–233
Niladevi KN, Prema P (2008) Immobilization of laccase from Streptomyces psammoticus and its application in phenol removal using packed bed reactor. World J Microbiol Biotechnol 24(7):1215–1222
Ødegaard H, Rusten B, Westrum T (1994) A new moving bed biofilm reactor-applications and results. Water Sci Technol 29(10–11):157
de Oliveira RL, Dias JL, da Silva OS, Porto TS (2018) Immobilization of pectinase from Aspergillus aculeatus in alginate beads and clarification of apple and umbu juices in a packed bed reactor. Food Bioprod Process 109:9–18
Özkaya B, Kaksonen AH, Sahinkaya E, Puhakka JA (2019) Fluidized bed bioreactor for multiple environmental engineering solutions. Water Res 150:452–465
Pagliolico SL, Verso VRML, Bosco F, Mollea C, La Forgia C (2017) A novel photo-bioreactor application for microalgae production as a shading system in buildings. Energy Procedia 111:151–160
Pettigrew L, Blomenhofer V, Hubert S, Groß F, Delgado A (2015) Optimisation of water usage in a brewery clean-in-place system using reference nets. J Clean Prod 87:583–593
Pfeifer VF, Vojnovich C (1952) Continuous sterilization of media in biochemical processes. Ind Eng Chem 44(8):1940–1946
Price JR, Shieh WK, Sales CM (2015). A novel bioreactor for high density cultivation of diverse microbial communities. J Vis Exp (106):e53443
Rajavathsavai D, Khapre A, Munshi B (2014) Study of mixing behavior of cstr using CFD. Braz J Chem Eng 31(1):119–129
Ravindran S, Singh P, Nene S, Rale V, Mhetras N, Vaidya A (2019) Microbioreactors and perfusion bioreactors for microbial and mammalian cell culture. Biotechnology and bioengineering. IntechOpen, London
Richards JW (1968) Introduction to industrial sterilization. Academic, London
Sales CM, Shieh WK (2006) Performance of an aerobic/anaerobic hybrid bioreactor under the nitrogen deficient and low F/M conditions. Water Res 40(7):1442–1448
San KY, Stephanopoulos G (1984) Studies on on-line bioreactor identification. IV. Utilization of pH measurements for product estimation. Biotechnol Bioeng 26(10):1209–1218
Schmidt FR (2005) Optimization and scale up of industrial fermentation processes. Appl Microbiol Biotechnol 68(4):425–435
Selden C, Fuller B (2018) Role of bioreactor technology in tissue engineering for clinical use and therapeutic target design. Bioengineering 5(2):32
Sharma KJBA (2012) Design and operational key factors of bioreactor
Sharp DH (1989) Bioprotein manufacture. E. Horwood, New York
Shen Y, Brown RC, Wen Z (2017) Syngas fermentation by Clostridium carboxidivorans P7 in a horizontal rotating packed bed biofilm reactor with enhanced ethanol production. Appl Energy 187:585–594
Shojaosadati SA, Babaeipour V (2002) Citric acid production from apple pomace in multi-layer packed bed solid-state bioreactor. Process Biochem 37(8):909–914
Singh RN, Sharma S (2012) Development of suitable photobioreactor for algae production—a review. Renew Sust Energ Rev 16(4):2347–2353
Singh V, Hensler W, Fuchs R (1989) Optimization of batch fermentor sterilization. Biotechnol Bioeng 33(5):584–591
Smith JM (1985) Dispersion of gases in liquids: the hydrodynamics of gas dispersion in low viscosity liquids. Gordon & Breach, New York
Sossa-Echeverria J, Taghipour F (2015) Computational simulation of mixing flow of shear thinning non-Newtonian fluids with various impellers in a stirred tank. Chem Eng Process Process Intensif 93:66–78
Stanbury PF, Whitaker A, Hall SJ (2013) Principles of fermentation technology. Elsevier, Amsterdam
Stephenson M, Grayson W (2018) Recent advances in bioreactors for cell-based therapies. F1000Res 7:F1000. Faculty Rev-1517
Sternfeld HJ, Paulus M (1993) Hydrogen/oxygen steam generators for sterilization processes and chemical engineering. Int J Hydrog Energy 18(11):945–951
Syed MS, Marquis C, Taylor R, Warkiani ME (2021) A two-step microengineered system for high-density cell retention from bioreactors. Sep Purif Technol 254:117610
Tien C, Ramarao BV (2011) Revisiting the laws of filtration: an assessment of their use in identifying particle retention mechanisms in filtration. J Membr Sci 383(1):17–25
Toeroek C, Cserjan-Puschmann M, Bayer K, Striedner G (2015) Fed-batch like cultivation in a micro-bioreactor: screening conditions relevant for Escherichia coli based production processes. Springerplus 4(1):490
Varavinit S, Srithongkum P, De-Eknamkul C, Assavanig A, Charoensiri K (1996) Production of single cell protein from cassava starch in air-lift fermenter by Cephalosporium eichhorniae. Starch 48(10):379–382
Vyrides I, Rivett DW, Bruce KD, Lilley AK (2019) Selection and assembly of indigenous bacteria and methanogens from spent metalworking fluids and their potential as a starting culture in a fluidized bed reactor. Microb Biotechnol 12(6):1302–1312
Wang S-J, Zhong J-J (2007) Chapter 6—Bioreactor engineering. In: Yang S-T (ed) Bioprocessing for value-added products from renewable resources. Elsevier, Amsterdam, pp 131–161
Watanabe T, Sugiura M, Sato M, Yamada N, Nakanishi K (2005) Diacylglycerol production in a packed bed bioreactor. Process Biochem 40(2):637–643
Werner R (1992) Containment in the development and manufacture of recombinant DNA-derived products. Safety in industrial microbiology and biotechnology. Elsevier, Amsterdam, pp 190–213
Wikol M, Hartmann B, Brendle J, Crane M, Beuscher U, Brake J, Shickel T (2008) Expanded polytetrafluoroethylene membranes and their applications. Drugs Pharm Sci 174:619
Winkler MA (1990) Problems in fermenter design and operation. In: Winkler MA (ed) Chemical engineering problems in biotechnology. Springer, Berlin, pp 215–350
Xiong W, Li X, Xiang J, Wu Q (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biotechnol 78(1):29–36
Yamamoto K, Hiasa M, Mahmood T, Matsuo T (1989) Direct solid-liquid separation using hollow fiber membrane in an activated sludge aeration tank. Water Sci Tehnol 21(4–5):43–54
Yamauchi Y, Okamoto T, Murayama H, Nagara A, Kashihara T, Yoshida M, Nakanishp K (1995) Rapid fermentation of beer using an immobilized yeast multistage bioreactor system. Appl Biochem Biotechnol 53(3):245–259
Yamuna Rani K, Ramachandra Rao VS (1999) Control of fermenters—a review. Bioprocess Eng 21(1):77–88
Yang S-T (2011) Bioprocessing for value-added products from renewable resources: new technologies and applications. Elsevier, Amsterdam
Yeh M-S, Wei Y-H, Chang J-S (2006) Bioreactor design for enhanced carrier-assisted surfactin production with Bacillus subtilis. Process Biochem 41(8):1799–1805
Zhong J-J (2010) Recent advances in bioreactor engineering. Korean J Chem Eng 27(4):1035–1041
Zhong JJ (2011) 2.14—Bioreactor engineering. In: Moo-Young M (ed) Comprehensive biotechnology (second edition). Academic, Burlington, pp 165–177
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Karthikeyan, A., Joseph, A., Subramanian, R., Nair, B.G. (2022). Fermenter Design. In: Verma, P. (eds) Industrial Microbiology and Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-16-5214-1_5
Download citation
DOI: https://doi.org/10.1007/978-981-16-5214-1_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5213-4
Online ISBN: 978-981-16-5214-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)