Abstract
Scale-up is the process of expanding a fermentation process from a smaller-scale fermenter, where operational and production parameters have been studied, to a larger scale. Scale-up is perhaps one of the hardest and most complex steps of any fermentation process for engineers. Engineers must take into account all aspects that affect the integrity of the fermentation during the scale-up process. These aspects include physical (namely, heat and mass transfer phenomena such as oxygen transfer rates and mixing time), biochemical (such as medium compositions and rheology), and process (such as conditions of the pre-culture and inoculum) factors. Usually, engineers focus on the most effective factor and take in one of the common scale-up strategies. A constant height to diameter ratio for the bioreactors is perhaps the simplest and most common strategy. A constant oxygen transfer rate coefficient is usually the path for engineers, where oxygen is crucial for the fermentation and oxygen concentration limits product secretions. Also, a constant mixing time during the scale-up comes into play, where engineers usually face a viscous sophisticated fermentation broth. Yet, there are many other approaches that engineers can take. Often they blend these strategies and improvise a new strategy that serves the fermentation in the larger scale. However, engineers’ ultimate goal is to maximize production and efficiency in plant-scale production.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara What You Will Learn in This ChapterScale-up is the process of expanding a fermentation process from a smaller-scale fermenter, where operational and production parameters have been studied, to a larger scale. Scale-up is perhaps one of the hardest and most complex steps of any fermentation process for engineers. Engineers must take into account all aspects that affect the integrity of the fermentation during the scale-up process. These aspects include physical (namely, heat and mass transfer phenomena such as oxygen transfer rates and mixing time), biochemical (such as medium compositions and rheology), and process (such as conditions of the pre-culture and inoculum) factors. Usually, engineers focus on the most effective factor and take in one of the common scale-up strategies. A constant height to diameter ratio for the bioreactors is perhaps the simplest and most common strategy. A constant oxygen transfer rate coefficient is usually the path for engineers, where oxygen is crucial for the fermentation and oxygen concentration limits product secretions. Also, a constant mixing time during the scale-up comes into play, where engineers usually face a viscous sophisticated fermentation broth. Yet, there are many other approaches that engineers can take. Often they blend these strategies and improvise a new strategy that serves the fermentation in the larger scale. However, engineers’ ultimate goal is to maximize production and efficiency in plant-scale production.
7.1 Introduction
It would be a misconception to think that an ideal bench-top bioreactor in the lab could simply be enlarged to hundreds of thousands of liters in a fermentation plant, to give the same production performances and yields. Surely, every bioprocess engineer wishes it was that simple! But, will a 100,000-liter bioreactor with yeast converting cornstarch to bioethanol give out exactly the same outcome as the 2-liter bench-top glass bioreactor does? In this chapter, main factors and issues surrounding bioreactor scale-up in different types of bioreactors are discussed. Furthermore, we will review mass and heat transfer phenomena with an emphasis on their role in scale-up.
7.2 Scale-Up
A fermentation process development usually starts with lab scale with bench-top bioreactors (1–50 L) or even shake flasks (100–1000 mL) and microbial cells (few mL). After optimization has convinced that a bioprocess is feasible, then a pilot-scale process (50–10,000 L) is designed and implemented to establish the optimal operating conditions. It is only then, after successful lab- and pilot-scale studies, that the bioprocess is implemented on a plant scale (> 10,000 L) for commercial productions. Therefore, the step of setting up a bioprocess from a small to a large scale is called scale-up. The objective of scale-up is to transfer the optimal conditions obtained in small-scale bioreactors to the large-scale bioreactor. Scale-up studies are indispensable for the development of any fermentation process, so that an appropriate criterion for changing the scale can be established without damaging the kinetic behavior of microorganisms and hence the process performance. However, the kinetic behavior of microorganisms is affected by local environmental conditions such as nutrient concentration, pH, temperature, dissolved oxygen, etc. It is well known that microorganisms are more sensitive to these environmental variables in a large scale. Therefore, small-scale trials have the tendency to overpredict the process performance at larger scales unless inconsistencies in scale-up are eliminated. This is where scale-up techniques become crucial. For this purpose, the environmental conditions affecting the bioprocess must be controlled. This is done by considering the physical, biochemical, and bioprocess factors. Physical factors include mass and heat transfer conditions, mixing (agitation) conditions, shear stress regimes, power consumption, pH, temperature, dissolved oxygen, etc. Biochemical factors mainly are medium components and their concentrations along with their physiochemical properties. Finally, process factors including pre-culture conditions, sterilization quality, and inoculation ratio also dictate how successful scale-up is implemented [1].
The traditional method for scale-up of a fermentation process involves determining the reactor geometry, impeller speed, and aeration rate of the large-scale bioreactor on the basis of the experimental results of the lab-scale bioreactor. The most common method of scale-up is based on maintaining geometric similarity of bioreactors. Once the volume of the large-scale bioreactor has been chosen, its geometric parameters, namely, tank height, tank diameter, and stirrer dimension, can be estimated. The typical methods of determining impeller speed and aeration rate are dependent on empirical correlations to keep relevant parameters constant with the change in scale. Evaluation of impeller speed is based on keeping agitation power input per unit volume (P/V), volumetric oxygen mass transfer coefficient (kLa), or impeller tip velocity constant, whereas the aeration rate is estimated by using those criteria such as keeping equal superficial gas velocity, specific gas flow rate, or gas flow number. Engineers often keep one or several parameter(s) constant through scale-up and build their strategy around it.
Typical fermenters are made cylindrical and have a height to diameter ratio (H/D) between 2/1 and 3/1. This ratio can be kept constant as the simplest scale-up strategy. However, even that would not be so simple in reality. If diameter is increased by a factor of 5 and the ratio is kept constant, the vessel volume increases 125-fold, which would undoubtedly make fermentation in the larger scale quite distinct. Also, there are other parameters and ratios that can be considered besides the H/D ratio, which can be solely considered or in combinations. ◘ Table 7.1 presents some of these parameters and how the rest of them are affected when each is kept constant in scaling up from an 80-L pilot-scale fermenter to a plant-scale 10,000 L fermenter. As ◘ Table 7.1 indicates, if the impeller speed is maintained constant, the energy input of the impeller(s) will be 3,125 times higher in the large-scale fermenter. It is safe to presume that such a significant increase can change markedly the performance of the larger fermenter, e.g., with oxygen transfer rates, temperature gradient, etc.
Example Problem 7.1
Lysozyme is an anti-bacterial and anti-fungal enzyme widely present in animals, plants, and microorganisms. Recently, bioprocess engineers have focused on the production of human lysozyme via genetically modified microorganisms owing to the health concerns associated with egg lysozyme. The bioprocess parameters of the recombinant strain of Kluyveromyces lactis were studied in a lab-scale fermenter giving 110 IU/mL of lysozyme within 43 h. Engineers plan to produce annually 3 × 1012 IU of the enzyme. Thus, what should the main and inoculum fermenters look like if a H/D = 3 and 20% headspace are assumed and if the fermenters are to be operated for 11 months per year?
Solution:The complete batch period is an important parameter used to determine the number of batches that can be processed and hence, the total production per year. This time period includes medium preparation, sterilization time, fermentation time, product harvest, and cleaning time, which in this case can be assumed to be 10 h. Therefore, each batch will take roughly 53 h to complete. Thus we have:
The number of units of lysozyme produced per month = \( \frac{3\times {10}^{12}\kern0.28em \mathrm{IU}}{11\kern0.28em \mathrm{months}}=2.7\times {10}^{11}\kern0.28em \mathrm{IU}/\mathrm{month}. \)
Volume of the broth required to achieve the projected production per month
the number of batches per month = 30 days per month × 24 hours per day/53 hours per batch ≈ 13 batches per month ≈ 156 batches per year; and
Since the volume is high, two bioreactors will be used to produce the projected amount of lysozyme. Volume of each bioreactor =192,307/2 = 96,154 L
Thus, for a working volume of 96,154 L and assuming 20% headspace, the total volume of the bioreactor is the following:
Assuming that the height to diameter ratio is 3:1, the reactor dimensions are calculated as follows:
The width of baffles can be assumed to be 10% of the diameter of the bioreactor. Therefore, the width is
Impeller diameter is calculated assuming that it is 20% of the tank diameter.
As for the pre-fermenter, since the strain used is yeast with a relatively long lag-phase time, the inoculation percentage is assumed as 5%. The working volume of the pre-fermenter is thus 4808 L, and with 20% headspace and the total prefermenter volume, we have:
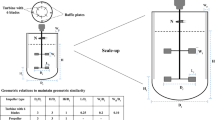
Similarly, the baffle width will be 13.5 cm and impeller diameter will be 27 cm (◘ Fig. 7.1).
Schematic drawing of prefermenter and main fermenter for ► Problem 7.1
7.2.1 Physical Properties
Mass and heat transfer along with mixing conditions (or flow behavior) are the physical properties that affect scale-up strategies. For instance, in most fermentation processes, the heat generated by catabolism is taken into account with heat transfer rates in large-scale bioprocesses. Also, oxygen transfer rate (OTR) that controls oxygen uptake rates (OUR), especially in aerobic fermentations where oxygen is limiting, is another crucial factor in scaling up. Here we will discuss the effects of these mass transfer phenomena on scale-up strategies.
7.2.1.1 Aeration and Agitation
The oxygen uptake rate (OUR) in a fermentation process is expressed as
where x is the biomass concentration (g/L), \( {q}_{O_2} \) is the specific oxygen uptake rate (gmol O2/g. h), CL∗ is the saturation oxygen solubility under the given conditions, and CL is oxygen concentration at the given time. The combination of these two variables determines the total oxygen demand of the biomass. ◘ Table 7.2 depicts typical specific oxygen uptake rates for some common cells.
Note how the uptake rates can be different from strain to strain. Candida bombicola and Saccharomyces cerevisiae are both yeast strains, but as you can see, Saccharomyces cerevisiae can consume over eight times as much oxygen under aerobic conditions. Also, it is interesting to note how bacterial strains can adapt to different conditions and how the uptake rates differ accordingly. See how the uptake rate can be over 23- and 10-folds for Escherichia coli and Bacillus acidocaldarius, respectively (◘ Table 7.2) [2].
The other side of Eq. 7.1, however, represents the oxygen supply to the fermenter, which, like any other mass transfer phenomenon, is composed of a driving force (CL∗ − CL) (g/L) combined with a constant value, which is the volumetric oxygen transfer coefficient kLa (h−1). For the cases where aeration is critical, it is most common to monitor and consider a constant oxygen transfer rate (OTR) throughout the scale-up. This is, for instance, mostly the case in novel applications of biofilm reactors for value-added products, where higher cell densities are utilized for higher production rates but at the same time oxygen diffusion into the biofilm matrices becomes critical. Thus, in such aerobic fermentations, kLa becomes the crucial factor in scale-up. As it is obvious from Eq. 7.2, kLa is the dominant factor that reflects the effects of agitation, viscosity, impeller(s) and bubbles’ dimensions and shapes, rheological properties of the liquid phase, and even the working volume. This is due to the fact that the term in the parenthesis (CL∗ − CL) is mainly a function of temperature and oxygen partial pressure only. Of course, nowadays it is possible and even preferable to directly measure the dissolved oxygen (DO) levels in bioreactors and simply maintain them through the scale-up process; yet, this is not usually the option that bioprocessing engineers prefer. Rather, engineers follow a constant kLa coefficient, which they can empirically estimate using Eq. 7.2 [8].
where k, α, β,and γ are empirical constants, Pg is the gassed power output, Vw is the working volume, vs is the superficial exit gas velocity, and N is the speed of impeller(s). The constant k in the equation for lab-scale stirred-tank bioreactors with Newtonian fluid regimes is 0.001–0.005 for kLa expressed in mmol/L-h-atm, depending on the geometry of the vessel and impeller(s). In common small-scale reactors, α = 0.4 and β = γ = 0.5 are good assumptions. As it can be seen, besides the aeration rates, the agitation rates also play a direct effect on the oxygen transfer rate and mass transfer phenomena in the process in general. Again, in small-scale Newtonian regimes, the dependency on agitation rates is negligible and therefore the (N)0.5 term is eliminated. Then the constant term varies even more from geometry to geometry. The term \( \frac{P_{\mathrm{g}}}{V_{\mathrm{w}}} \) can be defined as the volumetric agitation power output. Sometimes, it is more convenient to use a scale-up model based on keeping this parameter constant, since the data obtained in bench-top or pilot-scale fermenters are often available and can be easily used for scale-up. The other reason is that other effective rheological properties such as viscosity are incorporated in the term \( \frac{P_{\mathrm{g}}}{V_{\mathrm{w}}} \). When there is a need for a higher DO level, engineers choose either a stronger agitator motor or a smaller working volume. Such a model simplifies the scale-up strategy; however, there is still a need to have an estimate of the required power input. It is usually easier to determine the power input for a similar ungassed fermenter and then empirically transcend it to the aerated vessel. For this purpose, an empirical equation such as the following is used:
where K is a constant, Pu is the power required in the ungassed vessel, d is the impeller(s) diameter, and Q is the volume of air supplied per minute per volume of the liquid in the vessel for which the unit is often referred to as vvm. The constant term in this equation is strictly dependent on the geometry of the tank and impeller(s) and the operational conditions.
Although these empirical correlations give fair estimations on how oxygen uptake rates are affected by scale-up, they are only estimations. For many cases of Newtonian or non-Newtonian systems, these correlations are unable to cope with the significant effects resulting from changes in viscosity that usually occur in fermentation processes or medium compositions. For such effects unfortunately, it is not easy to make such estimations. Also, many times when viscosity increases to high levels as the fermentation process proceeds, engineers simply water down the composition to counter it.
Moreover, the presence of salts and surfactant or antifoam agents in the fermentation broth, which is very common, not only can significantly affect kLa but also affect oxygen solubility in the broth. Yet, the driving force term in Eq. 7.1 (CL∗ − CL) is not controllable but providing good mixing to avoid the increasing liquid film resistance around the gas bubbles or keeping the operating temperatures as low as possible will work. The example problems below show how kLa can be measured in real-time fermenters to help the scale-up or design strategies.
Example Problem 7.2
Assume you have a 2-L bench-top bioreactor for production of L-asparaginase by Candida utilis and you would like to scale it up to a pilot scale of 100 L using a conventional medium composition. You have two different impellers. How can you determine which impeller is better for aerobic fermentation?
Solution:Candida utilis is a yeast that excretes the enzyme L-asparaginase under highly aerobic conditions. It is safe to presume that oxygen transfer rates are limiting and thus critical in this case. Therefore, whichever impeller that provides higher kLa values with the same power inputs is the winner. Also we must determine kLa values in both bioreactors.
There are basically three methods for determining kLa in a bioreactor: unsteady-state, steady-state, and the sulfite method.
In the unsteady-state method, we fill fermenters with the medium (or as an easier estimation with DI water) and accurately measure the CL∗ and CL using a calibrated DO probe. By sparging the medium with nitrogen for an ample period of time, we make sure that there is no oxygen left in it. At this time CL is zero. Then, we start sparging it with air and measure CL values over time until we eventually reach CL∗. If mixing is sufficiently robust, whichever impeller enables us to reach the C∗value faster is essentially better. Nonetheless, we have
and so knowing that CL∗ is constant with constant temperature we have:
thus
Therefore, a plot of ln(CL∗ − CL) versus time can be drawn, whose slope is the kLa term. In other words, the steeper slope means higher kLa values.
Similarly, in the steady-state method, the fermenter is filled with the broth and oxygen is monitored as fermentation takes place. Oxygen is uptaken by the biomass and at the same time it is provided by aeration. Thus, from Eq. 7.1 we have
Assuming that the fermentation process is slow enough that the OUR values at the time of measurements are constant, it is possible then to measure the OUR value in the bioreactor or externally in a respirometer, and then CL∗ and CL must be accurately measured at the same time to calculate the respective kLa value. However, this requires exact measurement of OUR and oxygen concentrations. Moreover, if later on we decide to scale up the process to industrial-scale fermenters with tens or hundreds of thousands of liter volumes, turning the huge fermenter into a real-time respirometer is easier said than done! In such large volumes, even oxygen concentration measurements become a challenge when the mixing is never ideal anymore and the liquid depth and hydrostatic pressure are significant at the sparger level at the bottom making the CL∗values significantly different at the bottom compared to the headspace zones.
As a result of such complications, the more common sulfite method comes into play, where the fermenter is filled with the medium along with sulfite anions (\( {\mathrm{SO}}_3^{2-}\Big) \). Sulfite anions irreversibly and readily react with dissolved oxygen and are converted to sulfate (\( {\mathrm{SO}}_4^{2-}\Big) \) until CL reaches zero.
Then, we start aerating the mixture and as oxygen is dissolved, it is instantly consumed. As the stoichiometry of the above reaction dictates, the rate of sulfate formation doubles the rate of oxygen consumption and considering Eq. 7.4a we have
and
Thus, by monitoring the sulfate concentration in the mixture over a short period of time and calculating the rate of change we can calculate the kLa value. Note that determining sulfate concentration in the mixture is essentially easier than monitoring dissolved oxygen concentrations or finding OUR values, but, obviously the sulfite method cannot be applied to a real-time fermentation process unlike the steady-state method [5].
Example Problem 7.3
In order to scale up a prototype fermenter design for citric acid fermentation using Yarrowia lipolytica from bench-top to pilot scale, we wish to use a constant kLa approach. To obtain information about the coefficient, the bench-top bioreactor was filled with DI water at 30°C. Nitrogen was sparged into the vessel with 500 rpm agitation to take out all the dissolved oxygen. Then, air was introduced instead of nitrogen and agitation was set at 200 rpm and another time at 400 rpm. Percentage of DO saturation was recorded versus time as shown in ◘ Table 7.3. Using these findings and oxygen solubility, determine the kLa values for each agitation regime.
From the oxygen solubility table, we can see that CL∗ = 7.559 mg/L. Therefore, the data for 200 and 400 rpm can be processed to prepare ◘ Tables 7.4, 7.5, and 7.6, respectively.
Then, plotting CL and ln(CL∗ − CL) changes versus time at 200 and 400 rpm we have:


and from Eq. 7.4b, for 200 rpm, we have:
And for 400 rpm we have
As it can be seen, by doubling the agitation rate from 200 to 400 rpm, the kLa coefficient and thus OUR is almost doubled as well. This is the simplest example of a bench-top bioreactor with DI water. Things can be much more complex as size increases and complex broth compositions are used. Still, this example clearly shows how complicated operational physical properties can be formed in a bioreactor, and all without exception must be taken into account carefully while designing or scaling up a fermentation process. See how nicely the 200 rpm points in the second plot fall into a straight line and 400 rpm ones don’t… why??
Another common parameter for scale-up is the impeller power consumption per volume P/V. This strategy is perhaps the oldest scale-up strategy that has been used ever since penicillin production revolutionized the early twentieth century. Usually a ratio of 1.0:2.0 KW/m3 is simply maintained. However, such simplification in massive scale-up operations leads to significant energy inefficiency, which is certainly unacceptable. Therefore, more complex alterations emerge. For instance, instead of a constant P/V ratio, impeller power number (Np) is defined, measured, and held constant.
In the above equation, M is torque (with full working volume of DI water) (N·m), Md is torque (empty vessel) (N·m), ρ is broth density, N is agitation speed (rpm), and d is impeller diameter. Note that as the impeller gets larger, the power number decreases drastically. The only perquisite to this strategy is that the torque must be carefully measured and it is important to measure the net impeller torque without bearing resistance. A constant power number scale-up strategy in some cases may prove more energy efficient than the constant P/V ratio strategy. Nevertheless, the power number can be obtained alternatively to calculate the P/V ratio as:
As the flow regimes change with the Reynolds number, the power number also changes empirically for different impeller geometries. ◘ Figure 7.2 shows the dependency of power number in model bioreactors with some most common impellers. Such a graph is handy for engineers to estimate the power input needed in the large-scale fermenter based on the Reynolds number.
Power number versus Reynolds number in a model bioreactor. (Adapted from Bates et al. [3])
7.2.1 Case Study 7.1
Xanthan gum is a natural polysaccharide heavily used in food and cosmetic industries for a number of important reasons, including emulsion stabilization, temperature stability, compatibility with food ingredients, and its pseudoplastic rheological properties [6]. Xanthan gum is produced by the bacterium Xanthomonas campestris through aerobic fermentation. Shortly after the fermentation starts, the gum production excels and the broth viscosity increases dramatically. Although, the produced gum needs to be dewatered and dried in the downstream processing, engineers have no choice other than adding water to the broth to dilute it so that the heat and oxygen transfer and even agitation are not impaired by the viscosity jump. It is possible to defeat viscosity to some extent, by heating the beer up in the downstream steps, but it is not feasible during the fermentation since the temperatures for optimum production are around 30°C. Now can you imagine how such a viscosity jump that deeply affects production itself may affect your scale up strategy? Which strategy would be the best?
7.2.1.2 Shear Rate
Shear stress in a fermenter depends on the rheological properties of broth, which are defined for broth viscosity during the fermentation process and shear rate, which is a function of impeller geometry and impeller rotational speed. For Newtonian fluids they are defined as
where τave is the average shear stress between impeller blades and fermenter inner wall (N. m−2), μ is dynamic viscosity (N. s. m−2), νave is the average shear rate between impeller blades and fermenter inner wall (s−1), κ is a constant that depends on the system geometry only for Newtonian fluids, and N is the impeller rotational speed (rps). These equations can now estimate the shear rates in agitated systems with viscosities similar to water and with perfect Newtonian behaviors. With deviations from these conditions, which is usually the case in most fermentation broths, complex equations must be used. Most mold strains due to filamentous growth and mammalian cells are very sensitive to shear rates for which there is a threshold. Higher shear rates are simply fatal or reduce product yields. Thus, in these cases, the highest feasible shear rate is calculated and kept constant, which depends on the impeller tip speed and thus on the agitator speed. Since agitation is critical in fermentation, especially in aerobic and/or viscous conditions, increasing the impeller diameter or the number of impellers may provide robust agitation without creating overstress. The case study below shows that overstress may not always be a conspicuous matter of life and death and yet very problematic [14].
7.2.1 Case Study 7.2
Bacillus subtilis natto is a highly aerobic Gram-positive bacteria that excretes menaquinone-7, a potent form of vitamin K, under aerobic conditions [10]. This form of vitamin K2 is the most expensive vitamin. Thus, this bacterium has been used to produce supplementary vitamin K2 for decades. Scientists have discovered that this strain has a high potency to form biofilm matrices that have a positive effect on vitamin secretion. Furthermore, engineers are working to perform the fermentation in agitated and aerated liquid states, since conventional static solid states are not easy to scale up. Although robust agitation and aeration do not affect growth or metabolism in B. subtilis but actually improve them, the shear stress caused by them decimates the biofilm formations and therefore knocks out vitamin secretion. To overcome this dilemma, engineers are working on biofilm reactors where mature biofilm formations are allowed to form on suitable support surfaces, which would be resilient enough to tolerate robust agitation and aeration up to feasible extents. The downside to biofilm reactors, however, is that oxygen molecules need to diffuse all the way into the biofilm to reach the production sites [4]. Would optimization methods be helpful to find optimum conditions for maximum vitamin secretion and solve such trade-off equations? How do these considerations come into play when optimum conditions in lab-scale studies are supposed to be scaled up?
7.2.1.3 Mixing Time
For highly viscous, non-Newtonian broths, the conventional equations are not valid. In these cases, robust agitation becomes the ultimate goal. For instance, when acid or base solutions, antifoam agents, or fed-batch ingredients are to be added to the broth periodically, it is essential to have robust mixing. Providing robustness with a larger impeller or higher numbers of impellers is easier and yet much less energy efficient. Alternatively, we can opt for a taller fermenter where the impeller diameter does not need to increase for robustness; however, a deep stack of broth can definitely be more troublesome. For example, oxygen solubility at the bottom of the deep fermenter near the sparger will be significantly higher than the surface. This inevitably not only creates an undesirable oxygen gradient, but also significantly increases the gas power input (P/Vα d3) and thus power requirements become prohibitive in large-scale fermenters. Thus, there is a trade-off. In this case, engineers may choose to scale up based on equal mixing or blending time. Mixing time can be defined as
where V is the working volume (m3), N is the impeller rotational speed (rpm), and d is the impeller diameter (m).
7.2.2 Biochemical Factors
So far, we have discussed how fermentation at a large scale can be distinct from a lab scale, backing it up through physical and design point of views. But that is not all of it. The media compositions used for plant production scarcely include any pure or lab-grade components. Rather, economy is engineers’ first priority and therefore low-grade abundant and natural resources are used. For instance, if scientists find out that glucose is the key nutrient to producing an enzyme, they should undertake a series of lab-scale experiments to figure out how glucose affects enzyme production and what the optimum conditions are and perhaps use pure glucose only for clarity purposes of the results. Yet, it is quite impractical to use pure glucose for plant-scale production, because it would be too expensive. It is replaced with either unrefined molasses or unrefined dextrose extracts from inexpensive sources. Now, the best way to tackle such a situation is to carry out some lab experiments using the exact plant-scale composition and thus reiterate the conditions. But, even this may not be sufficient as the composition, purity, and physical properties of these natural resources may change from batch to batch or over time. The emergence of a trace toxic or inhibitory component in the resources may halt the process or result in changes in purity, and amounts of the nutrients may take the metabolic paths sideways. Therefore, engineers do keep lab-scale fermentation studies on the side even after the plant starts its work specifically to monitor and tackle such unprecedented changes. The incoming nutrient purity and composition are monitored using analytical chemistry techniques such as high performance liquid chromatography (HPLC) or gas chromatography (GC), and changes in the composition of the working medium are implemented accordingly to keep the fermentation process smooth [7, 11].
7.2.3 Process Conditions
Processes that are undertaken prior to inoculation of a plant-scale fermenter and officially starting the production may affect how well the fermentation continues. Basically, these processes are sterilization of the working medium and pre-culture fermentation to produce the inoculum. Sterilizing the working medium for a large fermenter is quite different from the lab-scale counterpart. For the large-scale medium, a longer sterilization time is required to ensure a complete sterilization, as heat transfer rate into the large fermenter is always lower. Also, as mentioned earlier, the industrial medium may contain complex components. As sterilization temperatures reach 121.1 °C (250 °F), many spontaneous chemical reactions take place between these components (i.e., reducing sugar and amino groups end up with Maillard reactions). These unwanted reactions not only degrade valuable nutrients in the medium, but also may create substances which are harmful to microorganisms and biosynthesis of the product. Thus, efficiency of the fermentation process is lowered. Engineers often sterilize different medium components such as carbon sources, nitrogen sources, and minerals separately to minimize these undesirable side reactions. Usually growth rates (which are very important) in the media that are sterilized as a whole are significantly lower than those that are sterilized separately. However, before deciding to sterilize components separately, studies on the lab scale must prove it to be practical [16].
Another factor that affects the growth and condition of the main fermentation process is the condition of the inoculum. Engineers always keep a keen eye for the integrity and condition of the inoculum. Cell concentration, age, and phase of the inoculum cells even by an hour, morphology of the cells, and the metabolic trait from which the inoculum comes are imperative parameters that can determine the success or failure of a fermentation process [12].
Therefore, it’s good to remember the following tips:
-
If there are not enough cells in the inoculum despite a constant inoculation volumetric ratio, lag phase of the main fermentation will be prolonged. This not only imposes higher operational costs but also may decrease the product yields drastically as the optimum window for harvest is lost.
-
Usually, the inoculum cells are best when they are in their late exponential phases of the growth (e.g., for enzyme production). This is true for all scales of production, and that is when they should be harvested from the pre-cultures.
-
The number of pre-cultures may have significant effects on how fast and robust the inoculum is. This is a sensitive effect because the number of pre-cultures needed for a large fermentation process may be several more than a lab-scale one.
-
For fermentation of filamentous microorganisms like fungi, in addition to all of the above parameters, it is also important whether the inoculum is in pellet or filamentous form as metabolism in these forms is quite distinct especially for biosynthesis of complex materials such as enzymes and other secondary metabolites. The case study below investigates these effects in an important industrial application of filamentous strains [17].
7.2 Case Study 7.3
Citric acid is a weak organic acid that naturally occurs in citrus fruits and gives them the special taste. It has a very vast and diverse range of industrial applications in food and drink, detergent, cosmetic, pharmaceutical, dietary supplement, and even steel industries. For over a century, bioprocess engineers have produced citric acid using filamentous bacterial and fungal strains such as Aspergillus niger. They have learned by experience that broth pelleting and morphology in the seed stages strongly influence the outcomes. For instance, it was found that broth morphology influences broth thickness, which affects not only mixing but also aeration resistance and coating of instrument sensors, which can be a huge problem, especially in large-scale fermenters. Thus, they learned that identification and consideration of this phenomenon, which has close correlations to shear in the pre-cultures and the main fermenter, in developing scale-up conditions and interpreting scale-up behavior can be extremely beneficial for better production [9, 15].
7.3 Summary
The ultimate goal in fermentation process development is the large-scale commercial implementation. Plant-scale fermenters give us the opportunity to produce numerous valuable products through microbial fermentation. Before plant-scale fermenters are designed and put to work, bioprocess engineers must closely study and optimize the process in lab-scale and pilot-scale fermenters. An optimized lab-scale process can then be transferred to pilot scale following the established scale-up strategies. In doing so, engineers must consider all factors that make the fermentation process distinct in larger scales. These factors primarily include physical properties of the broth and the fermenter itself such as heat and mass transfer (especially oxygen transfer in aerobic fermentations) that are affected by agitation, aeration, broth rheology and fermenter geometry and design. Secondly, the broth or medium biochemical properties such as deviations from ideal and pure compositions in small-scale fermentations followed by the physical factors are also an aspect to consider. Finally yet importantly are process conditions that include sterilization step(s) and inoculum conditions. Considering these factors, the scale-up strategies can simply be based on a constant height to diameter ratio (H/D) up to constant impeller power input to working volume ratio (P0/V), power number (Np), or in most aerobic fermentations a constant volumetric oxygen transfer coefficient (kLa). As process conditions get more complicated and the rheological properties of the medium deviate from ideal Newtonian fluids, a combination of these strategies may be considered. A constant H/D ratio is perhaps the simplest scale-up strategy and can easily miss some special cases. On the other hand, keeping a constant P0/V ratio is perhaps the most common scale-up strategy due to good flexibility and simplicity and yet is usually not energy efficient and so following a constant Np may prove a better option. In aerobic fermentation, where oxygen mass transfer rates prove to be limiting, engineers try to provide the best aeration efficiency by implementing a constant kLa strategy. In some more exclusive cases, where shear rate is crucial, such as fermentation with filamentous microorganisms, engineers focus on agitation rates and impeller designs to carry out a successful scale-up. Of course, there may be more specific combinations and conditions that can be applied to pre-defined cases; yet, in this chapter, the most common and basic scale-up strategies that have been implemented by bioprocessing engineers are covered.
Problems
-
1.
Which of the H/D ratios of a fermenter is better for oxygen transfer efficiency, a tall-narrow or short-squat fermenter? Briefly explain.
-
2.
A stirred tank reactor is to be scaled up from 0.1 m3 to 10 m3. The dimensions of the small tank are Dt = 0.64 m, Dimpeller = 0.106 m, and N = 470 rpm. Thus:
-
(a)
Determine the dimensions of the large tank (DL, Dimpeller, HL) by using geometric similarity.
-
(b)
What would be the required rotational speed (N) of the impeller in the large tank for a constant impeller speed taken as N × Dimpeller = constant?
-
(a)
-
3.
A strain of Azotobacter vinelandii is cultured in a 15-m3-stirred fermenter for the production of alginate. Under current conditions, the mass transfer coefficient, kLa, is 0.18 s−1. Oxygen solubility in the fermentation broth is 8 × 10−3 kg/m3. The specific oxygen uptake rate is 12.5 mmol/g. h. What is the maximum cell density in the broth?
-
4.
A value of kLa = 30 h−1 has been determined for a fermenter at its maximum practical agitator rotational speed with air being sparged at 0.5 L gas/L reactor volume/min. E. coli with qO2 of 10 mmol O2/g dry wt./h are to be cultured. The critical dissolved oxygen concentration is 0.2 mg/L. The solubility of oxygen from air in the fermentation broth is 7.3 mg/L at 30°C. Thus:
-
(a)
What maximum concentration of E. coli can be sustained in this fermenter under aerobic conditions?
-
(b)
What concentration could be maintained if pure oxygen was used to sparge the reactor?
-
(a)
-
5.
E. coli has a maximum respiration rate, qO2max, of about 240 mg O2/g dry wt./h. It is desired to achieve a cell mass of 20 g dry wt./L. The kLa is 120 h−1 in a 1000 L reactor (800 L of working volume). A gas stream enriched in oxygen is used (i.e., 80% oxygen) which gives a value of CL∗ = 28 mg/L. If oxygen becomes limiting, growth and respiration become slow. For these conditions it is safe to presume:
where CL is the dissolved oxygen concentration in the fermenter. What is CL when the cell mass is at 20 g/L?
-
6.
Calculate the oxygen transfer rate and kLa of an air-water system in a fermenter, in which the experimental work was carried out with a working volume of 20 L of water at 30°C. The pressure inside the fermenter was kept constant at 5 psig while two agitation rates of 100 and 300 rpm were employed. The results for the two runs are presented in the table below.
Time
%DO
Time
% DO
100 rpm
300 rpm
100 rpm
300 rpm
0
3.2
0
5.5
79.2
99.2
0.5
6.2
25.7
6
82.2
99.4
1
16.8
65.3
6.5
85
100
1.5
27.1
83.9
7
87.3
100
2
37
91.8
7.5
89.2
100
2.5
45.5
95
8
90.9
100
3
52.9
96.7
9
93.7
100
3.5
59.3
97.6
10
95.6
100
4
64.8
98.2
11
97.1
100
4.5
71.3
98.6
12
98.2
100
5
75.6
98.9
13
99.1
100
-
7.
Bacterial fermentation was carried out in a bioreactor containing broth with average density of 1200 kg/m3 and viscosity of 0.02 N.s/m2. The broth was agitated at 90 rpm and air was introduced through the sparger at a flow rate of 0.4 vvm. The fermenter was equipped with two sets of flat-blade turbine impellers and four baffles. Tank diameter is Dt = 4 m, impeller diameter is Di = 2 m, baffle width is Wb = 0.4 m, and the liquid depth is H = 6.5 m. Therefore, determine:
-
(a)
Ungassed power, P
-
(b)
Gassed power, Pg
-
(c)
KLa
-
(a)
-
8.
A medium containing 105 spores per liter needs to be sterilized before starting the fermentation. By assuming that the death rate for spores (kd) at 121 °C is 0.903 min−1, determine the sterilization time for 10 L and 10,000 L fermenters for 10−3 probability of failure. Ignore effects of heat-up and cool-down periods.
-
9.
Suppose we want to produce vitamin K from Bacillus subtilis natto in a fed-batch biofilm reactor using glycerol as a limiting nutrient. The biofilms are placed on straight-blade Rushton turbine rings. Since the vitamin is produced in the biofilm matrices; in order to preserve the biofilm formations from overstress, we need to hold Reynold numbers below 10,000. Since the glycerol medium is quite viscous, engineers have suggested a constant mixing time strategy to scale up from a 2-L bioreactor to a 10,000-L pilot-scale one. If the mixer for the pilot-scale fermenter is 1000 times stronger than the one in the model fermenter, what should be the impeller rotational speed and diameter for maximum mixing robustness?
Take Home Messages
-
Every value-added product that comes from fermentation in bioreactors starts with studying the process in small-scale flask fermentations and bench-top bioreactors in labs. Once they know enough about the bioprocess, biological engineers need to expand the process from those lab-scale modules to pilot-scale fermenters and finally plant-scale ones. This is called scale-up.
-
The bioprocess behavior in small-scale bench-top bioreactors in labs that can be only a few liters in volume are mostly distinct from the ones in plant-scale fermenters that can be up to hundreds of thousand liters of volume.
-
To address complexities during the scale-up process, engineers often follow certain and well-examined scale-up strategies while fully considering physical, biochemical, and process factors that dictate such complexities in the process.
-
Some of the most commonly used scale-up strategies include maintaining a key parameter fixed throughout the scale-up process. These key parameters are selected considering the nature of the bioprocess and to minimize distinctions in behavior to keep optimum conditions as much as possible.
-
Most common parameters are volumetric oxygen transfer coefficient kLa (in aerobic fermentations of course), volumetric power consumption of the impeller(s) P/V, impeller power number (Np), shear stress τ, and mixing time tm.
-
Sometimes engineers are not content with these conventional strategies and end up blending them together or crafting a process-specific strategy, all to ensure best of production conditions on the final scale.
Change history
04 June 2020
On page 224, Chap. 7—Bioreactor Scale-Up, the numbers calculated for kLa were erroneously published. This has been corrected in this version and it should be read as:
References
Bailey JE, Ollis DF. Biochemical engineering fundamentals. Chemical Engineering Education; 1976.
Baltz RH, Demain AL, Davies JE, editors. Manual of industrial microbiology and biotechnology. Washington, DC: American Society for Microbiology Press; 2010.
Bates RL, Fondy PL, Corpstein RR. Examination of some geometric parameters of impeller power. Industrial & Engineering Chemistry Process Design and Development. 1963;2(4):310–4.
Ercan D, Demirci A. Current and future trends for biofilm reactors for fermentation processes. Crit Rev Biotechnol. 2015;35(1):1–14.
Garcia-Ochoa F, Gomez E, Santos VE, Merchuk JC. Oxygen uptake rate in microbial processes: an overview. Biochem Eng J. 2010;49(3):289–307.
Garcıa-Ochoa F, Santos VE, Casas JA, Gomez E. Xanthan gum: production, recovery, and properties. Biotechnol Adv. 2000;18(7):549–79.
Hsu YL, Wu WT. A novel approach for scaling-up a fermentation system. Biochem Eng J. 2002;11:123–30.
Islam RS, Tisi D, Levy MS, Lye GJ. Scale-up of Escherichia coli growth and recombinant protein expression conditions from microwell to laboratory and pilot scale based on matched kLa. Biotechnol Bioeng. 2008;99(5):1128–39.
Junker BH, Hesse M, Burgess B, Masurekar P, Connors N, Seeley A. Early phase process scale-up challenges for fungal and filamentous bacterial cultures. Appl Biochem Biotechnol. 2004;119(3):241–77.
Mahdinia E, Demirci A, Berenjian A. Production and application of menaquinone-7 (vitamin K2): a new perspective. World J Microbiol Biotechnol. 2017;33(1):2.
Najafpour GD. Biochemical engineering and biotechnology. Amsterdam: Elsevier; 2007.
Oliveira SC, De Castro HF, Visconti AES, Giudici R. Bioprocess Eng. 2000;23:51–5.
Shuler ML, Kargi F, DeLisa M. Bioprocess engineering: basic concepts, vol. 576. Englewood Cliffs, NJ: Prentice Hall; 2017.
Silva-Santisteban BOY, Maugeri Filho F. Agitation, aeration and shear stress as key factors in inulinase production by Kluyveromyces marxianus. Enzym Microb Technol. 2005;36(5–6):717–24.
Tang YJ, Zhang W, Liu RS, Zhu LW, Zhong JJ. Scale-up study on the fed-batch fermentation of Ganoderma lucidum for the hyperproduction of ganoderic acid and Ganoderma polysaccharides. Process Biochem. 2011;46:404–8.
Vogel HC, Todaro CL. Fermentation and biochemical engineering handbook, Principles, Process Design, and Equipment. 2nd ed. Westwood: Noyes Publications; 1997.
Yang H, Allen G. Model-based scale-up strategy for mycelial fermentation processes. Can J Chem Eng. 1999;77:844–54.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mahdinia, E., Cekmecelioglu, D., Demirci, A. (2019). Bioreactor Scale-Up. In: Berenjian, A. (eds) Essentials in Fermentation Technology. Learning Materials in Biosciences. Springer, Cham. https://doi.org/10.1007/978-3-030-16230-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-16230-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16229-0
Online ISBN: 978-3-030-16230-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)