Abstract
Major Histocompatibility Complex (MHC), firstly identified as the main factor determining the fate of organ transplantation, is involved in antigen presentation to T cells. Among the ever-increasing list of genes identified to be related to different autoimmune-inflammatory diseases, HLA loci have shown the strongest association. This is because of their role in antigen presentation to T lymphocytes and their involvement in the negative selection of auto-reactive thymocytes. One of the most striking associations between an autoimmune disorder and an HLA allele is for Ankylosing Spondylitis (AS) and HLA-B27. Several mechanisms have been proposed to explain how HLA-B27 can participate in the pathogenesis of AS. However, the association of other HLA loci and also HLA-related genes with AS has been evidenced. Epigenetics is another important player in the etiopathology of AS through affecting the expression of the genes involved in different physiologic and immunologic processes. In this chapter, we will explain the cutting-edge knowledge about the probable mechanisms through which, HLA-B27 plays a role in AS development. Then, we discuss other HLA and HLA-related genes shown to be associated with AS in different populations. At the end of the chapter, we describe the impact of epigenetics as another predisposing factor.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Biology of HLA Expression and Functions
In contrary to B cells, T lymphocytes have to recognize their specific antigens in the context of MHC molecules. MHC genes are the most polymorphic ones among the others. In humans, MHC is called Human Leukocyte Antigen (HLA) and is inherited as a co-dominant trait across the generations. As HLA alleles are inherited as haplotypes, therefore, each person has one maternal and one paternal HLA haplotype in his/her genome.
1.1 Genomic Organization
HLA alleles which are located on the short arm of chromosome 6, occupied a place approaches 3500 base pair length. There are two different classes of HLA named HLA-class I and HLA-class II. Class I HLA is responsible for presenting cytosolic (endogenous) peptides to CD8+ T cells and HLA-class II is dedicated to present endocytosed (exogenous) antigens to CD4+ T cells. Class I is located on the telomeric end of the chromosome and, from this end, consists of HLA-A, -C, and -B loci, respectively. Inside this area, there are some other genes that encode non-classical HLA class I or MHC class IB molecules, like HLA-G, -E, and -HEF, as well as MICA and MICB. These are structurally related to HLA-class I molecules but are not so polymorph and do not present antigenic peptides. On the centromeric site, HLA-class II area is located where the loci of HLA-DP, -DQ, and -DR are placed in order from the centromeric end. Throughout the HLA class II area, there are genes that encode necessary molecules in the process of antigen presentation by HLA-class I, such as Transporter in Antigen-Presenting (TAP) 1, TAP2, and Tapasin (Fig. 4.1).
Organization of HLA genes on chromosome 6p. Class I HLA loci are located on the telomeric side of the chromosome while on the centromeric side is the area for HLA class-II. Distributed among HLA class-I, there are several non-classical HLA-I genes (HLA class-Ib). On the other hand, genes encoding for effector molecules in antigen presentation are found in HLA class-II area. The area between class-I and class-II loci is the region of the genes encoding some cytokines, hormones, and complement proteins
1.1.1 HLA Nomenclature
The accepted nomination of HLA genes and their encoded proteins is based on similarities among their sequences and their molecular structure. As is depicted in Fig. 4.1, only one allele is present per each HLA-class I locus while for class II, every single locus consists of at least two different alleles. These alleles encode different HLA chains. For this, there are some differences in the way of HLA class I and II nomination.
Each locus for HLA-class I is named by a letter in capital format, like HLA-B while for class two, there are two capital letters indicating a locus, such as DR. To determine an allele (allelic group) for HLA class I, a number is added after the letter, like HLA-B27. For the nomination of an allele of HLA-class II, one letter and one number are added after the two-letter code for locus. This latte letter defines the type of HLA-class II chain. For example, HLA-DRB1 stands for the chain β1 from HLA-DR locus. In order to define a specific allelic subtype, after the capital letter determining an HLA-class I locus, an asterisk and a four-digit number are written. For example, HLA-B*2705 means that this is the fifth subtype of allele B27 from HLA-class I locus. Defining an HLA-class II allelic subtype is similar to what is used for class one. After the three letters and one number, an asterisk and a four-digit number are written. In this way, HLA-DRB1*1502 indicates for the second subtype of the allele 15 from the chain β1 in the HLA-DR locus.
1.2 Molecular Structure
MHC molecules are heterodimeric transmembrane proteins with a peptide-binding cleft that encompass polymorphic residues. As different HLAs bind to and present different peptides, the kind of immune response against the same antigen in distinct individuals is different. HLA molecules also have a non-polymorph region for binding to T cells co-receptors.
1.2.1 Molecular Structure of HLA-Class I
MHC-I molecules are expressed on all nucleated cells except sperm and trophoblast. Class I HLA consists of two different chains named α and β2-microglobulin (β2m). The light chain, β2m, is encoded in chromosome 15 which is out of the MHC locus. This chain has only one extracellular domain with no activity for antigen binding. Its role is to stabilize the structure of HLA-class I. In contrast, α chain is encoded inside the HLA locus and consists of three extracellular, transmembrane, and intra-cytoplasmic parts. The extracellular part includes three domains which numbered from N-terminal side as α1, α2, and α3, respectively. The peptide binding cleft (PBC) is formed between the α1 and α2 domains. Domain α3 is a non-polymorphic member of the Ig super-family which binds to the CD8 co-receptor of T lymphocytes (Fig. 4.2a).
Molecular structure of MHC. (a) Class-I MHC consists of one heavy chain named α and one light chain called β2-microglobulin (β2m). The α chain is comprised of three, extracellular, transmembrane, and intracellular, parts. The extracellular part consists of three domains which are numbered in order from the N-terminal end as α1, α2, and α3. The area between domains α1 and α2 is the peptide binding cleft. The α3 is the site of interaction with CD8 marker on T cells. (b) Class-II MHC is comprised of two almost similar chains named α and β. Both chains include extracellular, transmembrane, and intracellular, parts. The extracellular part of both chains is comprised of two domains which are numbered in order from N-terminal end as α1 and α2 for α chain, and β1 and β2 for β chain. The peptide binding cleft is formed by α1 and β1. The β2 domain is the site of interaction with CD4 marker on T cells
1.2.2 Molecular Structure of HLA-Class II
This molecule has a limited distribution and is expressed only by B lymphocytes, macrophages, and dendritic cells (professional APCs) as well as thymic epithelial cells. MHC-II molecules consist of two nearly similar chains, α and β, that both of them are encoded in the HLA locus. Both chains have three intracellular, transmembrane, and extracellular areas. The extracellular part includes two domains which are numbered from the N-terminal end of the chains as α1 and α2 as well as β1 and β2. The peptide binding cleft is made of α1 and β1 domains where the most polymorphic residues are located, especially in β1. The CD4 binding site of HLA-class II is the invariant β2 domain (Fig. 4.2b).
1.3 Pathways of Antigen Presentation
MHC molecules bind to peptide antigens in order to present them to a relevant T cell receptor (TCR) on specific T cells. The role of discrimination between self and foreign peptides is played by TCR as MHC molecules do not have such ability. For binding to MHC, the peptide must have specific structural properties like a defined size. Peptides with 8–11 amino acid length and hydrophobic or basic residues in their C-terminal end bind to MHC-I while those with 10–30 amino acid residues present with MHC-II. Some amino acid residues may have branched chains through them can be linked to the PBC packets via hydrophobic interactions. These branched-chain amino acids are named “anchor residues.” However, the overall affinity of MHC molecules for their relevant peptide is low (kd = 10−6 M) and their connection is made by non-covalent forces.
The residues which are recognized by TCR are different from those linked to MHC. In fact, TCR recognizes both antigenic peptide and MHC molecules and hence, possesses two specificities while having just one capacity. Positively selected T cells have been instructed to recognize peptides presented by self MHC molecules. Simultaneously, the non-polymorphic domains of MHC molecules are identified by CD4 or CD8 co-receptors.
1.3.1 Processing and Presentation of Endocytosed Antigens
On one hand, when an exogenous protein is engulfed by an antigen-presenting cell (APC), enters the cell in a vesicle called a phagosome. This phagosome then fuses to lysosomes in cytosol and forms phagolysosome in which, acidic pH and proteolytic enzymes disintegrate the protein antigen. Through this process, so-called “antigen processing,” different antigenic peptides are produced. On the other hand, synthesis of MHC-II chains is done in the endoplasmic reticulum (ER) linked ribosomes and newly formed chains enter the ER. In order to fold in the right way and assembling together, Calnexin, a chaperon, binds to them in ER. When the process of forming a two-chain MHC-II molecule is completed, an invariant protein called “Ii” binds to its PBC and prevents it from binding to endogenous peptides. After transferring to the Golgi complex, this three-peptide complex enters into secretary vesicles which then fuse to phagolysosomes containing the peptide antigens and forms the MHC-II compartment (MIIC). Here in MIIC, the “Ii” is proteolytically broken, and then, HLA-DM, a non-classical MHC class II, replaces its remnant with a specific peptide antigen. Finally, the vesicle goes out and the peptide: MHC-II complex emerges on the cell surface to be recognized by a specific CD4+ T cell.
1.3.2 Processing and Presentation of Cytosolic Antigens
Every single endogenous protein that enters the cytosol will be ubiquitinated after a while. In this way, they are marked to be trapped by a cylinder form organelle named “proteasome” where they are converted to small peptides via proteolytic degradation. These small peptides will then enter the ER through a heterodimer gate-operating protein called transporters in antigen presenting (TAP). Thereafter, longer peptides will be further cleaved by ER aminopeptidase (ERAP) 1 and ERAP2 to approach the length which is appropriate for antigen presentation. ERAP1/2 efficiently cleaves these newly entered peptides to oligopeptides with 8 or 9 residues in length, which is optimal for binding to HLA class I. Simultaneously, newly synthesized chains of MHC-I come into the ER, fold and join together by Calnexin, and link to Tapasin by Calreticulin. As Tapasin is also connected to TAP, brings the new MHC-I molecule into the vicinity of the TAP. Now, small peptides which come into ER are trapped by these MHC-I molecules. Then, these peptides: MHC-I complexes exit the ER and will be transported from the Golgi apparatus to the plasma membrane of the cell. Here, these complexes are ready to be recognized by specific CD8+ T cells.
2 Association of HLA loci with Ankylosing Spondylitis
2.1 HLA-B27
Ankylosing spondylitis (AS) is a chronic inflammatory rheumatic disease from the category of seronegative spondyloarthritis (SpA). AS is inherited as an intricate genetic trait in which 4–10 predisposing genes have been estimated [1, 2]. Genetic studies have prepared invaluable knowledge to our perception of AS etiology. Familial aggregation studies have clarified a substantial heritability of AS susceptibility as the occurrence risk of AS in monozygotic twins is 63% and in first-degree relatives is 8.2% in comparison with 0.1% in the general population. In addition, the risk of AS is appraised as 16 times higher in the HLA-B27 positive relatives in comparison with isolated HLA-B27 positive individuals in general population. Unlike most complex genetic diseases, a significant genetic predisposition has been established by the discovery of one of the strongest associations among HLA alleles and diseases between AS and HLA-B27 in 1973. The correlation between HLA-B27 and AS is one of the most significant associations between a disease and an HLA molecule, as though its contribution is approaching 50% of genetic susceptibility to AS [2,3,4,5,6,7]. Among the populations with a high frequency of HLA-B27, the prevalence of AS and other SpAs is raised up. For instance, in the Haida Native Americans, the HLA-B27 frequency in the population is 50%, and the frequency of AS is 6.1%, while in the Japanese, HLA-B27 frequency is less than 1%, and the AS prevalence is only 0.0065% [8]. Not only has the HLA-B27 been shown as a predisposing factor, a lot of studies have suggested that HLA-B27 positive patients with AS show more severe clinical and radiological manifestations than those who do not express HLA-B27. It means that HLA-B27 may affect many features of AS, such as the age of onset, sex, and family history. For example, several studies have shown a younger age of onset in HLA-B27 positive patients. Moreover, several studies have shown that the presence of HLA-B27 is associated with increased occurrence of uveitis, cardiac involvement, worse sacroiliitis, hip joint involvement, peripheral arthritis, and significantly more symptoms of involvement of spinal column such as increased dorsal kyphosis, and decreased cervical slope, in AS patients. In fact, HLA-B27 expressing AS patients have significantly extended disease duration and shows worse Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), and Ankylosing Spondylitis Quality of Life (ASQoL) in comparison with HLA-B27 negative AS patients. Although AS is an intricated genetic disease and a proportion of HLA-B27 negative AS patients have been reported, B27 is still considered as the main factor in the development of AS [2, 3, 6].
About 80–98% of white patients with AS are HLA-B27 positive, while the prevalence of this gene is less than 8% in the general population. However, in different races and ethnic groups, the degree of this association is different. For example, only 50% of American blacks with AS are HLA-B27 positive and in Iran, HLA-B27 has been reported in 70% of AS patients [2, 5,6,7, 9, 10]. Differences in the distribution of HLA-B27 subtypes among distinct populations and ethnic groups might be the main reason for such a different amount of association with AS.
The genome-wide association studies and technological advances in high throughput genotyping of single nucleotide polymorphisms (SNPs) have enabled us to identify many risk alleles predisposing to AS. Based on what is reported by the Anthony Nolan Trust database (http://hla.alleles.org/alleles/class1.html) and IMGT (https://www.ebi.ac.uk/ipd/imgt/hla/) there are 187 distinct HLA-B27 subtypes (designated HLA-B*27:01 to-B*27:188; the subtype B27:22 has been withdrawn), making B27 one of the most polymorphic alleles among HLA-B alleles. The difference among these subtypes is the occurrence of one or more mutations, generally affecting the peptide-binding cleft which is the polymorphic part of the molecule [2, 4, 11].
The geographic and ethnic distribution of the HLA-B27 alleles is considerably different. The most prevalent subtype which is found in all populations around the world is B*27:05 and accounts for about 90–96% of HLA-B27 positive individuals in the Euro-Caucasian (Aryan) tribes (odds ratio = 62.41, CI = 95%) [5, 6, 12]. Although HLA-B*27:05 has been found to be associated with AS virtually in all populations studied, it does not appear to abdicate risk in West Africa and American blocks. The predominant subtype in these populations is B*27:03 which is less strongly associated with the disease than B*27:05 [6, 9, 11]. The relatively common subtypes including B*27:05, B*27:08 (Caucasians), B*27:02 (Mediterranean), B*27:07 and B*27:04 (Asians) are present in multiple populations have been associated with AS. In contrast to B*27:05 which is over-represented in circumpolar and sub artic regions of Eurasia, B*27:02 is the predominant subtype among the Middle East and the Jewish populations. This allele is the second most AS-associated subtype among European-descent subjects (odds ratio = 43.41, CI = 95%). Based on studies on Asian populations, it has been suggested that B*27:04 might be more strongly associated with AS than B*27:05 in areas where both alleles coexist, such as in Taiwan, China or India. The two alleles, B*27:06 and B*27:09, appear to be unassociated or perhaps weakly associated with the disease and have been reported to have a possible protective role in Thais and Sardinians, respectively [4, 5, 9, 11, 12]. However, reports of B*27:06 and B*27:09 presence in patients with AS are emerging [3, 6, 9]. These cases confirm that B*27:09 and B*27:06 have a weaker association with AS, and are not absolutely protective for the disease [11]. In Caucasians, the AS-related subtype B*27:01 is the less frequent allele of HLA-B27. In China and Singapore, only B*27:04, B*27:05, and B*27:06 have been found which is completely different from those in Caucasians [9]. An uncommon European allele that was first observed in Britain is B*27:08. Its association with AS in a large family from the Azores Islands of Portugal has been reported. In Brazilians and Western Indians, B*27:13 and B*27:14 have been found to be associated with AS, respectively. More recently, B*27:10, B*27:12, B*27:15, B*27:17, B*27:19, B*27:24, B*27:27, B*27:30 and B*27:49 have also been reported to be predisposing factors for AS. However, the epidemiologic evidence for their association has remained unreliable [4, 5, 11].
Apart from these pieces of information, the molecular basis for such an association has not yet been convincingly explained. Currently, there are three main hypotheses presented to explain the mechanism of the association between HLA-B27 alleles and AS:
-
The arthritogenic peptide hypothesis: based on this theory, it is assumed that the presence of a similarity (molecular mimicry) between pathogens and self-derived peptides could lead to T cells cross-reactivity, resulting in AS.
-
The HLA-B27 misfolding hypothesis: it is proposed that the accumulation of unusually folded HLA-B27 chains or β2m accumulation and deposition in the endoplasmic reticulum (ER) provoke intracellular signaling, ends up to a stress in ER and unfolded protein response (UPR).
-
The HLA-B27 dimer hypothesis: the generation of HLA-B27 homodimers on the cell surface could lead to an unusual immune response. These dimers could be recognized by an activating type of a group of natural killer (NK) and CD4+ T cells receptors named killer immunoglobulin-like receptor (KIR) [2,3,4].
2.1.1 Arthritogenic Peptide Hypothesis
From the date that the association between HLA B27 and AS has firstly been described, great attempts have been done to elucidate the molecular biology of HLA-B27 molecules and their role in the presentation of antigenic peptides to T cells. When the molecular structure of HLA B27 was clarified, it was revealed that peptides composed of nine amino acids in length with a highly conserved binding motif, including the existence of an arginine at position 2 and another basic residue located at position 9 were typically presented by this molecule. However, the precise mechanism of the association between AS and HLA B27 remains obscure [13].
The “molecular mimicry” theory recommends that a microbial pathogen-derived cross-reactive peptide provokes T cells responses. Consequently, the T cells could respond to a “self-peptide” which is associated with HLA-B27, or to peptides directly derived from HLA-B27 per se. Based on this, a great deal of self and foreign antigen peptides has been interrogated and sequenced to find candidate peptides. However, no convincing evidence indicating that any of these peptides are self-peptides or cross-reactive has been found [3].
It appeared that few residues in the α1 and α2 domains of the very first HLA-B27 alleles, forming the peptide-binding cleft (PBC) of the molecule, are different from other HLA-B alleles. These include His-9, Glu-45, Cys-67, Lys-70, and Ala-71. Interestingly, all of these unique disease-specific residues are located around the B-pocket of the PBC, where the second anchor residue of the peptide antigen accommodates [4]. Following this observation, the “arthritogenic” peptide hypothesis on the role of HLA-B27 in AS predisposition emerged.
The “arthritogenic” peptide theory, proposed by Benjamin and Parham, suggests that the pathogenicity of HLA-B27 may be due to its unique capability to present a particular repertoire of peptides to pathogenic CD8+ T cells [4, 6]. As is argued in this hypothesis, the apparently unique peptide binding specificity of HLA-B27 could be, in large part, due to its unusual B pocket. This might be responsible for selecting self-peptides that are similar to pathogen-derived peptides that might induce an autoreactive T cell response [6]. In comparison with most of the alleles, it seems that the HLA-B27 heavy chain is unusually slow to fold. In order to correct the problem, a hybrid HLA-B27 molecule consists of an HLA-A2-like B pocket transplant (referred to as B27.A2B) is formed and causes the heavy chain of HLA-B27 (B27.A2B) to fold, bind β2m, and then, exit the ER faster than the “wild type” B27 allele, more similar to other A and B alleles. This hybrid class I molecule can select and present a unique set of antigenic peptides. The B27.A2B molecule does not present peptides that are naturally restricted to HLA-B27 unless they are modified at position 2 in a way that the side chain of amino acid could fit appropriately into an HLA-A2-like B pocket [6].
Nevertheless, there are several problems with this theory. The first one is the failure to recognize any peptide specially presented by disease-associated HLA-B27 subtypes. The second problem is that apparently CD8+ T cells are not necessary for disease development in the HLA-B27 transgenic rat model of spondyloarthropathies (SpA) [4].
2.1.2 HLA-B27 Misfolding Hypothesis
In recent years, a considerable body of researches has focused on uncommon features of HLA-B27, including a tendency to misfold in HLA-B27 heavy chain and stable BiP binding in the ER before its assembly with peptide and β2m and to make disulfide-linked homodimers after arriving at the cell surface [6]. As mentioned before, there are a few residues such as Glu-45, Cys-67, and Lys-70 which are virtually unique to HLA-B27 (www.anthonynolan.com/HIG/). Mutagenesis experiments have defined that Glu-45 and Cys-67 in B pocket are necessary for HLA-B27 misfolding. Thus, it could be assumed that misfolding is extremely uncommon in other HLA-B alleles if not unique to HLA-B27. Moreover, it has been shown that, HLA-B27 needs about 30-fold higher concentration of antigen peptide to attain the same half-maximal binding capacity as B27.A2B and other class I alleles. This lower binding capacity of wild-type B27 allele suggests that B27 folding abnormality may affect its peptide-binding ability [1].
A reduced rate of folding in the majority of the disease-related forms of HLA-B27 subtypes (HLA-B*2705 , HLA-B*2704, and HLA-B*2702 ) has been demonstrated compared with HLA-B*2706 and HLA-B*2709 and most of other non-AS related MHC class-I molecules. Increased duration for assembly of the disease-associated HLA-B27 subtypes appears to cause the accumulation of misfolded HLA-B27 molecules in the ER. The accumulation of unfolded and/or misfolded proteins may disturb ER function and lead to ER stress and activation of the unfolded protein response (UPR), in order to rescue from the burden of misfolded proteins [3]. ER stress and activation of UPR in the result of HLA-B27 misfolding might be involved in AS pathogenesis. Cells exhibiting UPR activation has altered response to innate immune stimuli which in turn alters the cytokine production pattern. In fact, the UPR activation or its subsequent outcomes such as autophagy may work as additional signals that promote the production of particular pro-inflammatory cytokines by macrophages in response to TLR agonists such as LPS and other bacterial products. In this way, innate immune stimuli polarize macrophages toward increased production of IFN-β, IL-23, and IL-6. As has been demonstrated before, IL-23 and IL-6 are important for the differentiation of Th17 cells. This recommends a novel mechanism that may describe, at least in part, the pathologic role of HLA-B27, as the IL-23/IL17 axis could be important as an upstream factor in AS pathogenesis. In addition, implications of HLA-B27 misfolding during the response to other inflammatory cytokines such as TNFα and IFNγ may play a more highlighted downstream role which contributes to the AS phenotype. It has been indicated that IFNγ upregulates several components of the MHC class I assembly pathway such as TAP1 and 2, Tapasin, ERAP1, β2m, and proteasome subunits LMP2 and LMP7. This, in turn, exacerbates HLA-B27 misfolding and UPR activation [1, 14, 15].
2.1.3 HLA-B27 Dimer Hypothesis
As is mentioned before, three main features of HLA-B27 that are known to distinguish it from other class I HLA molecules are proposed to describe how it may contribute to AS development. We have discussed in detail about arthritogenic peptide presentation and the predilection of HLA-B27 to misfold in the ER which triggers ER stress and provokes the UPR. The third hypothesis that try to describe the role of B27 in the pathogenesis of AS is a tendency for forming HLA-B27 heavy chain homodimers during cell surface recycling [14]. Usually, misfolded proteins may activate ER-associated degradation while, misfolded HLA-B27 heavy chains tend to form dimers or multimers [3]. Peptide-binding studies have revealed that newly synthesized B27 heavy chains oligomerize in disulfide-linked structures whose formation depends on the presence of unpaired cysteine at position 67 (Cys67) [14]. Cell surface expression of HLA-B27 dimers may be important in the pathogenesis of AS, because of their role in binding to immune cells receptors.
It has been shown that HLA-B27 homodimers could bind to immunoreceptors expressed on NK cells and other leukocytes and hence might be important in the immunopathogenesis of autoimmune diseases like AS [3]. These receptors include killer cell immunoglobulin-like receptors (KIR) and leucocyte immunoglobulin-like receptors (LILR). Enhanced survival and proliferation of KIR3DL2+ IL-17-producing CD4+ T cells and increased IL-23 receptor expression and the production of IL-17, TNF-α and IFN-γ have been observed in patients with AS following stimulation with HLA-B27 homodimers expressing antigen-presenting cells (APCs). Differential association of B27 alleles with AS might be due to the formation of these dimers from different HLA-B27 subtypes. For instance, it has been demonstrated that HLA-B*2705, which is significantly associated with AS, forms a higher number of B27 heavy chain dimers for KIR3DL2 in comparison with HLA-B*2709 that is almost not associated with AS [3].
Another proposed mechanism by which B27 dimers can be involved in AS pathogenesis is the “β2m over-expression” hypothesis. Based on this, following B27 dimers dissociation, β2m may accumulate and become trapped in the synovia, where they may bind to collagen and make deposits of amyloid or interact with synovial fibroblasts. In this manner, they can stimulate the production and secretion of proteins involved in tissue destruction, which in turn lead to AS development [3].
Apart from what was discussed in the above-mentioned theories, there are evidences of the role of other possible mechanisms which associate HLA-B27 to AS development. One of them is that the ER chaperone BiP transiently binds to many newly synthesized MHC class I heavy chains to fold them properly. Nevertheless, HLA-B27 heavy chains show a prolonged association with BiP in ER. It has been shown that BiP has a predilection to bind oligomers of HLA-B27 in cells. This prolonged association might be a consequence of oligomerization of HLA-B27 heavy chains. The biochemical details of this BiP/HLA-B27 association have not yet been explicated but it is considered as a potential factor for developing AS. As has been reported, expression of BiP protein in peripheral joints macrophages of subjects with ankylosing spondylitis is greater than in osteoarthritis. Moreover, a correlation between synovial fluid inflammatory cytokines, TNFα and IL-6, and expression of BiP in macrophages, as well as between serum level of IL-6, disease activity, and macrophage BiP level has also been found [14].
In addition, it has been demonstrated that the macrophages of individuals who are HLA-B27-positive have altered intracellular killing in particular infections. It has been observed that transfected cell lines with HLA-B27 but no other HLA allele show enhanced intracellular bacterial survival and replication. This could be important for the pathogenesis of SpAs as the p38 MAP kinase pathway may be disrupted in these cells. This evidence presumably represents an intracellular or at least a non-antigen presenting effect of HLA-B27 in the pathogenesis of AS [1]. This suggests that infection or altered immune response may work as a trigger of SpA in B27 expressing individuals [8]. Based on this, another proposed mechanism is the modified serological recognition of B27 by oxidation of the free sulfhydryl group of its Cys67. This might be attained by the interaction of HLA-B27 with a bacterial product, homocysteine. There are certain factors recognized to be able to alter the serological properties of HLA-B27, which in people could result in recognition of B27 as a non-self-antigen [6, 16].
Despite extensive studies being done to clarify the mechanism of function of HLA-B27 in AS development, the problem remains open and not fully answered [2]. In human, only as few as 1–5% of HLA-B27 positive individuals develop AS, and there are increasing evidences to support that susceptibility to AS is complicated and B27 is only one of several genes that are involved in predisposition and phenotype of the disease [6, 11]. One of the main non-HLA-B27 contributions to susceptibility to AS is proposed by the higher concordance rate of monozygotic twins (60–75%) in comparison with B27-positive dizygotic twin pairs (24%) [11, 17]. Thus, an attractive subject is to explain why only a proportion of individuals with HLA B27 develop AS [13]. Today, whole-genome association studies (GWAS) are applied to identify other human predisposing genes [1]. The GWAS has enabled us to identify non-MHC associations of AS with more velocity. GWAS findings have underlined a number of important pathways involved in the pathogenesis of AS including aminopeptidases and peptide presentation, the IL-23 pathway, stimulation of innate immune responses, and the interaction and homeostasis of normal microbial flora [17].
2.2 HLA Genes Other Than HLA-B27
Although HLA-B27 is evidently the main AS-associated MHC gene, is clearly not the only one and several studies have indicated that there are several other HLA-B and non-HLA-B alleles that are important in the risk of AS development [11]. In this way, both linkage and association of other class I and II HLA loci with AS have been demonstrated [8]. Possibly HLA-B40, specifically with B*40:01 (a DNA-defined allele that is serologically defined as HLA-B60) subtype, has the second most characterized and validated association after HLA-B27. This association was defined for the first time in HLA-B27-positive patients with AS from Europe and the USA in 1989 [8]. Thereafter, several studies demonstrated that the strong epistatic interaction between B27 and B40 multiplies the predisposition to AS in B27 positive Caucasians by 3.6 to 4.7-fold [2, 13]. However, further studies by many research groups confirmed the association of AS with HLA-B*40:01 as well as B*40:02 (serologically defined as B61) alleles in both B27-positive and B27-negative AS patients in European and East Asian populations (odds ratio = 1.22 and 1.59, respectively, CI = 95%). However, the power of association of HLA-B40 with AS is much weaker than the association with HLA-B27, having an odds ratio of 3.6 [4, 11, 12, 18].
HLA-B14 is another HLA-B allele described to be associated with AS in French families with SpA. In addition, recent studies have shown a remarkable association of AS with HLA-B*14:03 in several sub-Saharan African populations (Burkina Faso, Zambia, and Togo). This is a very low frequent allele that has been detected in 32% of SpA patients in those areas but in none of the healthy controls. Both SpA and HLA-B27 are rare in West Africa [4, 8].
In Tunisia, in Belgian patients with undifferentiated SpA as well as in Mexican patients with peripheral spondyloarthritis, an association has been reported with HLA-B15. An association between HLA-B39 and HLA-B27-negative AS patients has also been described [8, 18].
In addition to HLA-B alleles, using single-nucleotide polymorphisms (SNPs) studies to ascribe other HLA alleles, a significant association between HLA-A*02:01 (rs2394250) and AS susceptibility with odds ratios of 1.21 in B27 positive and 1.36 in B27 negative samples has been revealed. Thus, the association of the HLA-A*02:01 allele is independent of the HLA-B*27 genotype and no linkage disequilibrium with HLA-B27 has been explained [8, 18].
After controlling for the associated haplotypes in HLA class-I, independent associations with the class-II HLA alleles have also been observed [18]. Initial studies from the United Kingdom had implicated HLA-DRB1*01:01. Nevertheless, this was then attributed to its linkage disequilibrium with HLA-B*27:05-bearing haplotypes. Further studies have shown a significant association with HLA-DRB1 irrespective of whether the haplotype includes HLA-B27. These reports evidenced a predisposing role for HLA-DR4 (DRB1*04:04) in a French family-based study and subsequently, in a North American case–control study. The impact of these associations is considerable, as the attributable risk of these haplotypes is 34% [8, 11].
In a large case–control study performed in an HLA-B27+ Spanish population, a region placed around the HLA-DPA1 and HLA-DPB1 loci has been described as weakly but significantly associated with susceptibility to AS. This association is with the DPA1*01:03, DPA1*02:01, and DPB1*13:01 subtypes. Interestingly, the HLA-DPA1 and -DPB1 molecules make a heterodimer implicated in the presentation of extracellular antigens (e.g., microbial) to CD4+ T cells Afterward, the association of AS with HLA-DPA1*01:03 was found again in the Portuguese population, supporting its validity [4].
On the short arm of chromosome 6, the closest gene to the HLA-B locus is major histocompatibility complex class I chain A related (MICA). The MICA gene encodes a cell stress-inducible glycoprotein as a ligand for natural killer group 2D receptor (NKG2D) activation associated with the proinflammatory pathway. NKG2D is an activating receptor from the C-type lectin-like family which is expressed on natural killer (NK) cells, CD8+ T cells, and NK T cells [19]. In addition to HLA genes, there is strong evidence for the association of MICA alleles with susceptibility to SpA. A triplet repeat polymorphism in the transmembrane section of the MICA-A4 gene closely linked to the HLA-B locus has been reported to be associated with AS. In fact, a high frequency of MICA-A4 allele in HLA-B27 negative AS patients from Sardinia has marked this allele as a susceptibility factor albeit weaker than B27 for AS. Other data have proposed that MICA is a strong predisposing factor for AS in the US white population [8]. It is well established that the two MICA alleles reported as positively associated with SpA are MICA*007:01 (in Caucasian and Han Chinese populations) and MICA*019 (in Chinese patients). Interestingly, these associations have been found both in B27-negative and B27-positive patients that indicate them as independent of HLA-B27 [4].
It has been evidenced that MICA 129 Val/Val constitutes a negative genetic association with SpA and indeed, protects against PsA. This polymorphism in exon 3 results in the replacement of methionine by valine which can influence NKG2D signaling. In fact, the presence of methionine instead of valine at position 129 of the α2-heavy chain domain of MICA results in strong binding to the NKG2D receptor, while MICA-129 Val/Val leads to a weak binding affinity. Therefore, SpA patients with MICA129 Val/Val polymorphism show lower levels of inflammation close to their sacroiliac joint and hence suffer less injury from sacroiliitis. Present studies have shown that MICA129 val/val, MICA A5.1 and NKC3 C/C polymorphisms (the latter is related to low level of NK cell cytotoxic activity) protect against SpA. On contrary, MICA129 met/met, NKC3 G/G, and G/C polymorphisms (related to an increased level of cytotoxic activity) are considered as risk factors for SpA. NKC3 G/G polymorphism could be used to improve the diagnosis of SpA, especially when the patient is HLA-B27 negative [19].
3 Association of HLA-Related Genes with Ankylosing Spondylitis
Genome-Wide Association Studies (GWAS) have identified 31 non-HLA genetic loci In AS patients with European ancestry [20]. However, as is estimated, all of these non-HLA predisposing loci account only for 4.3% of heritability in AS, while HLA-B27 alleles contribute more than 20%, indicating that a majority of predisposing loci have still been undefined [21].
3.1 Transporters in Antigen Presenting (TAP)
It has been proposed that differences in susceptibility to AS among different B27-positive individuals may be due to the differences in the peptides presented by HLA-B27. Interestingly, there are evidences that some B27-positive persons fail to present viral epitopes which are restricted to B27 in other individuals. One possible elucidation for this could be the involvement of the well-defined polymorphisms of a group of genes encoding components of the pathway by which peptide fragments are produced as the result of breaking down of the complex antigens and transported to the ER. TAP 1 and TAP 2 are two examples of these genes [13]. The TAP1 and TAP2 genes code for subunits of the functional TAP gate, which transport proteasome processed cytosolic derived peptides into the ER. Mutations in TAP1 or TAP2 genes entail to structural alterations that inhibit TAP heterodimer formation and affect antigen recognition and presentation [22]. It is hypothesized that there is a linkage disequilibrium between particular alleles at the TAP loci and HLA B27 in both the HLA-B27 positive healthy controls and AS patients. Finding an association with specific TAP alleles, TAP1B, TAP1C, and TAP2E have been shown to be rare in the general population, while they are increased in B27 positive individuals, either healthy or patient. Thus, this may not have a profound effect and is not of primary importance in AS susceptibility [8, 13].
Although the importance of TAP in the pathogenesis of autoimmune disorders like AS is almost unknown, many studies have demonstrated its direct or indirect participation in the development of AS. It is possible that TAP alleles may affect the selection of epitopes that play a role in the pathogenesis of AS and other autoimmune inflammatory diseases [22].
Polymorphism in the TAP1 gene was first identified in the early 1990s. The main polymorphic positions in TAP1 are 333 (Val) and 637 (Asp) which corresponds to cDNA positions 1069 and 1982. In TAP2, the main polymorphic site is 565 (Thr) which corresponds to cDNA position 1693. Interestingly, strong associations have been detected between the TAP1-333, -637, and TAP2-565 and AS compared with total and HLA-B27 negative, but not with HLA-B27 positive controls. It has been demonstrated that TAP1-333Val, TAP1-637Gly, and TAP2-565Thr are potential risk factors for AS in HLA-B27 negative cases. In the British population, the frequency of allele TAPIC (333 Val-637 Asp) has been shown to be significantly increased in the AS patients compared with normal controls. In addition, it has been reported that the frequency of the TAP1B allele is significantly elevated in AS patients with the extra-spinal disease compared with AS patients without extra-spinal disease or normal controls. In a Chinese population, TAP1-637 and TAP2-565 have been demonstrated to be associated with AS in comparison with HLA-B27 negative control. Nevertheless, no significant association has been identified between TAP1 and TAP2 alleles in Japanese, Spanish, and Caucasian populations [22].
In the genotype analyses, allele G and genotype A/G at TAP1 site 1910 have been described as possible risk factors for AS in HLA-B27 negative controls. Interestingly, this position has also been described to be associated with infection with hepatitis B virus, dengue virus, allergic rhinitis, and hypersensitivity pneumonitis. Both TAP1 position 1910 SNP A/G and TAP2 position 1693 G/A led to Asp637Gly and Ala565Thr replacements, respectively. The presence of both Asp and Gly at the position of amino acid 637 for TAP1 may influence peptide transport to ER and contribute to AS susceptibility, while being homozygous for either Gly or Asp does not. With regard to TAP2 SNP 1693, only the presence of Thr and not Ala at the position of amino acid 565 may play a role in AS development [23].
3.2 M1 zinc-Aminopeptidases
Antigenic peptides are obligatory for oligomerization and expression of HLA-B27. Thus, it could be logical to assume that occurrence of polymorphisms in intracellular peptidases can affect the quality and quantity of antigenic peptides available for presentation with MHC class I molecules and influence the susceptibility to AS [14]. In this way, aminopeptidases play an essential role in peptide trimming which is a required step for the generation of suitable peptides for HLA class I-binding [20]. In mammals, the M1 zinc-aminopeptidase family contains 5 integral transmembrane proteins and 4 non-transmembrane enzymes. From these, the endoplasmic reticulum aminopeptidase (ERAP)-1, ERAP2, and insulin-regulated aminopeptidase (IRAP or LNPEP) belong to the oxytocinase subfamily and are encoded in human chromosome 5p15 locus. This enzyme family is recognized by the HEXXH(X18) E Zn-binding and GAMEN motifs which are necessary for their activity. NPEPPS gene which encodes puromycin-sensitive aminopeptidase is located on the chromosome 17q21 locus. ERAP1 is a type II integral membrane enzyme, while ERAP2 and NPEPPS are non-transmembrane enzymes [17, 24]. ERAP1 and ERAP2 have been reported as important components of the final processing of antigenic peptides for MHC-I in the ER. In particular, they are recognized to trim peptides that are going to be presented by MHC class I molecules [24, 25]. Alterations within several aminopeptidases, such as ERAP1, ERAP2, LNPEP, and NPEPPS, have been identified.
3.2.1 Endoplasmic Reticulum Aminopeptidase (ERAP)-1
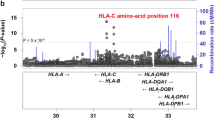
Studies done on patients with HLAB27 negative AS patients prepared a wider view of non-HLA-B27 genetic predisposition factors associated with AS. However, a number of these genes have been shown to have a significant relationship with HLA-B27 [2]. Abnormal peptide processing and presentation might be involved in the pathogenesis of AS owing to the interaction between ERAP1 and HLA-B27 [3]. Both susceptible and protective variants of ERAP1 associated with AS have been recognized by genome-wide association studies (GWAS). The relative attributable risk of ERAP1 for AS is around 25%, while that of HLA-B27 is around 50%. These two genes are the two strongest disease risk factors for AS development. Interestingly, the association of ERAP1 is limited to HLA-B27 positive AS patients [21]. The relationship between HLA-B27 and ERAP1 supports the hypothesis that asserts the mechanism of action of B27 in AS development involves abnormal processing and presentation of antigenic peptides. The discovery of the mechanism of the contribution of ERAP1 and HLA-B27 interaction in the pathogenesis of AS will open a door to better understanding and management of AS [2]. However, the exact fundamental molecular mechanisms of this process have not been fully clarified [25].
The protein ERAP1 has four domains (I to IV). Domain II includes the catalytic site. On the top of domain II, domain I is located and contains binding residues for the N-termini of the substrate. The Gln181 and Glu183 are such binding residues whose mutations influence the enzyme’s specificity and activity. Domain III is a small one with a β-sandwich structure that connects the catalytic domain II with the large C-terminal domain IV. Domain IV is a big α-helical domain that extends away from the catalytic part and makes a large cavity between domains II and IV which is probably a substrate-binding site accommodating different length peptides for catalysis [24, 25].
Preliminary studies have shown that the association of AS with ERAP1 occurs only among HLA-B27 positive cases as shown in Caucasians and Taiwanese populations [25]. In fact, those data demonstrating genetic interaction between ERAP1 and HLA-B27 as well as the involvement of ERAP1 mutations in protein changes propose that abnormal peptide presentation might be influential in AS susceptibility. Genetic variants associated with diminished function of ERAP1, and loss of ERAP2 expression, are protective for AS. Presumably, these genes work in AS by influencing the quantity of MHC class I peptide presentation or by a qualitative impact on the peptide repertoire presented. It has also been shown that down-regulation of ERAP1 and ERAP2 expression declines the cell-surface expression of class-I MHC molecules [8, 25].
HLA class-I molecules enforce strict limitations to peptide length, binding mainly 9 or 10-mers peptides. Thus, the N-terminal peptides entering the ER must be trimmed to achieve optimized length for HLA class-I binding. This is done by ERAP1, presumably in concert with the action of ERAP2. Apparently, a unique property of ERAP1, called the “molecular ruler” mechanism, is to cleave substrates longer than 9-mers efficiently, while doing that with less efficiency for 9-mers and almost is unable to cleave 8-mers or shorter peptides. This feature makes ERAP1 ideal for generating optimal HLA class-I ligands [25]. It is also recognized to cleave substrates with a hydrophobic C-terminal residue, more efficiently [24]. In fact, positively charged and nonpolar residues, except Trp and Val, are preferred over polar, basic, and acidic residues. Anywhere in the peptide sequence, Glu, Gly, or Pro disfavored cleavage [25].
Variants of ERAP1 have been widely reported by GWA studies in East Asians and in populations of white European ancestry. ERAP1 is known as a highly polymorphic molecule and certain ERAP1 haplotypes, or combinations of particular SNPs, are predisposing for AS susceptibility. However, very tight linkage disequilibrium between ERAP1 variants complicates the assignment of a given SNP as a predisposing factor for AS [25]. Fine-mapping studies on ERAP1 have indicated that the SNP rs30187 (R528 or Lys528Arg), as the major allele, and the minor allele of rs10050860/rs17482078 (N575/Q725 or Asp575Asn/ Arg725Gln) are significantly associated with AS [17]. In addition, another AS-associated SNP (rs27434) in ERAP1 has been reported in the Chines population [26]. The risk of AS in B27-positive homozygous carriers of ERAP1 protective variants in combination is three- to fourfold lower [24]. Haplotype evolution studies have evidenced that among the most strongly associated haplotypes with AS, the haplotype of rs30187-rs10050860-rs27044 (AGG) is predisposing, while the haplotype of rs26653-rs26618-rs30187 (CAG) is protecting against AS [18]. Apart from a very strong linkage disequilibrium between rs10050860 and rs17482078, in vitro studies of peptidase activity of ERAP1 variants have indicated that the protective variants of rs17482078 and rs30187 are associated with a 40% decrease in peptidase function, while rs10050860 has no impact [17, 24]. Indeed, the polymorphisms associated with protection from AS are related to decreasing ERAP1 activity. It has also been suggested that a main mechanism of the functional ERAP1 and HLA-B27 interaction is the variations induced in the balance between epitope generation and degradation by ERAP1 polymorphisms [25]. In this way, ERAP1 may degrade some antigenic peptides and hence, prevent their presentation [17]. The SNP rs30187 of ERAP1 has been shown to change a highly conserved residue (Arg528Lys, located near the entrance of the substrate pocket) which entails to a dramatic decrease of the enzyme catalytic activity. The SNP rs17482078 (Arg725Gln), on the other hand, reduces enzyme activity by influencing the substrate sequence specificity as it is exposed on the inner surface of the C-terminal cavity. Therefore, ERAP1 variants could affect AS susceptibility through both the amount of antigenic peptide presented by HLA-B27, as well as the length and sequence of the presented peptides [24]. More recently, the interaction between the rs30187 SNP and HLA-B40 (B*40:01) which is another AS-associated HLA-B allele has been demonstrated. This has been confirmed by the observation of rs30187 association with AS among HLA-B27 negative/HLA-B40 positive patients, but not among HLA-B27 negative/HLA-B40 negative patients [18, 24].
AS-associated variants of ERAP1 alter the amount, length, and stability of peptides presented by the MHC molecule. The high level of alterations in the cell’s peptidome induced by polymorphism of ERAP1 may affect other aspects of HLA-B27 related ERAP1 pathogenetic roles in AS, such as stability, folding, and generation of heavy chain homodimers at the cell surface [17, 25]. For instance, if the presence of an ERAP1 variant with diminished activity disrupts the optimization of the HLA-B27 peptidome, this could result in retention of HLA-B27 in the endoplasmic reticulum and increased misfolding level in an AS-protective context. This is in contrary to the misfolding hypothesis of the pathogenesis of AS which was mentioned earlier. Nevertheless, this prediction has not been yet established experimentally. In addition, the expression of heavy chain homodimers at the cell surface might also happen as the result of decreased HLA-B27 stability. This means that, in the presence of less functional ERAP1 variants, higher amounts of suboptimal HLA-B27/peptide complexes could be exported to the cell surface and dissociate under the acidic situation of the endosome which favors homodimer formation. Again, it seems that the effect of AS-associated polymorphism of ERAP1 which leads to a decrease of surface expression of HLA-B27 homodimers contradicts their above-mentioned pathogenetic role [25]. Some studies have demonstrated that ERAP1 suppression as the result of genetic polymorphism could lead to a B27-subtype-specific effect ending up to increased intracellular free B27 heavy chain which influences the cells expressing HLA- B*27:04 or B*27:05 but not B*27:06 and B*27:09. These observations confirm that alterations in the ERAP1/HLA-B27 interactions entail variable peptide presentation and provide a possible explanation for the absence of, or at least weak, the association of the HLA-B27 subtypes B*27:06 and B*27:09 with ankylosing spondylitis (AS) [12, 24].
In addition to its function to trim peptides before loading into newly synthesized class-I HLA molecules, ERAP1 may affect disease susceptibility through its role in cleaving receptors of pro-inflammatory cytokine from the cell surface, such as IL-1 receptor 2, TNF receptor 1, and IL-6 receptor. The effect of cytokine receptor cleavage has been argued but there are no in vivo evidences to support this function as of yet [11]. Altogether, these suggest that inhibition of ERAP1 could potentially be applied as a promising therapeutic target both in Ankylosing Spondylitis and in other HLA/ERAP1 associated diseases [24].
3.2.2 ERAP2
ERAP2 is another zinc-dependent M1 aminopeptidases member in ER which is structurally related to ERAP1 and other members of this family. ERAP1 and ERAP2 share around 50% identity in amino acid sequence with many similarities in their three-dimensional structure. ERAP1 and 2 are an integral part of the class-I MHC presentation pathway in ER and it is evident that ERAP1 and ERAP2 can form heterodimers more efficient trimming activity in comparison with ERAP1 or ERAP2 homodimers [17, 24, 27]. In this manner, ERAP1 and ERAP2 together are responsible for trimming peptides to optimize their length for presenting by HLA class-I. Therefore, they have a central role in the regulation of immune responses. It has been evidenced that there is a strong association of ERAP2 with AS, inflammatory bowel disease (IBD), and psoriasis [12, 24].
The most recent GWAS outcomes have shown an association of AS in HLA-B27-negative cases with two functionally important variants of ERAP2 [21]. The most important variant is the synonymous SNP rs2248374 G allele which alters the stability of the exon 10 splice site, leading to a larger exon 10 transcript with 2 stop codons [18]. This leads to transcription of a truncated mRNA that undergoes nonsense-mediated decay causing the loss of ERAP2 protein production. This loss-of-enzyme variant phenomenon is associated with decreased HLA class I surface expression and is protective of AS, in a similar manner to the loss-of-function variants in ERAP1 which is also protective of AS [17]. Another variant of ERAP2 is rs2549782 (Asn392Lys) which alters both the speed of action and specificity of ERAP2 trimming activity. This variant is also associated with AS, IBD, and psoriasis and is in significant linkage disequilibrium (LD) with rs2248374 [17, 18, 20, 24]. It has been demonstrated that an ERAP1/ERAP2 haplotype (rs27044[G] rs30187[T] rs2549782[T]) is in association with familial AS. As mentioned before, ERAP1 variants rs27044 and rs30187 affect the peptide-trimming activity and ERAP2 SNP rs2549782 changes its specificity and activity of ERAP2 [21]. Regarding the strong LD between ERAP1 and HLA-B27, as well as between ERAP1 and ERAP2, it is almost impossible to determine whether ERAP2 is associated with AS in HLA-B27 positive samples. However, as mentioned earlier, its association with AS is evident in HLA-B27 negative cases [17].
3.2.3 NPEPPS
NPEPPS, also known as puromycin-sensitive aminopeptidase or cytosol alanyl aminopeptidase, is the only cytosolic aminopeptidase that cleaves polyQ sequences and hydrolyzes N-terminal amino acids from its peptide substrates. NPEPPS prefers hydrophobic and basic residues in the peptide substrate. There is rarely a knowledge on the impact of mutations on NPEPPS functional activity and its functional variants. Genome-wide association studies demonstrated a significant association at synonymous SNP rs9901869 of NPEPPS at chromosome 17q21 with AS [24]. Whether NPEPPS operates in AS through influencing autophagy or via alternate effects on intracellular peptide handling needs further explorations [17, 20].
3.2.4 Ubiquitinases
Ubiquitination is another factor that plays an important role in determining what antigenic peptides are presented to the immune system. GWA studies have revealed two ubiquitinases, UBE2E3 and UBE2L3, in association with AS. UBE2E3 and UBE2L3 encode the UbcH9 and UbcH7 enzymes, respectively. UbcH9 cannot form bonds with ubiquitin but is able to do that with a similar protein SUMO. In contrary to ubiquitination, when a protein undergoes sumoylation it will not be degraded and instead, it may increase the protein's stability, change its position, or direct involvement in other cellular processes such as signal transduction. On the other hand, UBE2L3 variants are involved in the regulation of NF-κB and it has been associated with a number of other inflammatory disorders [17, 18]. Collectively, these findings further emphasize the importance of antigen presentation in AS pathogenesis [20].
3.2.5 LMP2
It has been demonstrated that the large multifunctional protease (LMP) 2 and LMP7 subunits which are coded in the HLA class-II region act to amplify specific endopeptidase functions of the proteasome. IFNγ and the expression of the LMP2 and LMP7 genes could favor the generation of peptides almost exclusively with hydrophobic or basic residues by proteasomes [28, 29]. It has previously been shown that LMP2 is associated with both AS and uveitis in patients with AS. Based on its association with uveitis, LMP2 could be considered as a marker representing a more aggressive form of AS which may result in more structural damage. In addition, the proteasome breaks down the β-catenin, and hence, aberrations in LMP2 may lead to excess Wnt/β-catenin signaling and osteoblastic activity [21, 30].
4 Role of Epigenetics in Ankylosing Spondylitis Pathogenesis
Epigenetics is typically recognized as potentially reversible but heritable changes in gene expression without aberration in nucleotide sequence. Therefore, this phenomenon is largely responsible for controlling the profile of the cell-specific expression of genes during the cell cycle, development, and in response to biological or environmental conditions. This explains that how a limited number of genes can result in the differentiation of a cell into several different cell types. Epigenetic modifications involved in regulating the gene expression include modification of histones proteins by acetylation, DNA methylation, and microRNAs functions. Environmental factors can affect some epigenetic processes like acetylation and methylation, while other epigenetic factors are inherited [20]. Histone acetylation facilitates gene expression by converting a heterochromatin structure to euchromatin form and therefore, promotes the accessibility of DNA regulatory regions to the transcriptional factors or to other DNA binding proteins, while methylation of DNA in the promoter region of genes hampers gene transcription activity. Considering several surveys, it has been established that epigenetics is involved in the etiopathogenesis of AS [18, 31].
4.1 DNA Methylation
The most extensively studied epigenetic mechanism is DNA methylation which is mediated by two DNA methyltransferase (DNMT) enzymes, with S adenosyl-methionine (SAM) as the methyl donor. There are five members of DNMT enzymes categorized into two main classes: de novo DNA methyltransferases (DNMT3a, DNMT3b, and DNMT3L) and maintenance methyltransferases (DNMT1, DNMT2). Methyltransferases transfer the methyl group (CH3) onto the C5 position of cytosines to generate the 5-methylcytosine (5mC). Regulation of genomic imprinting, gene expression, genome defense, and X-chromosome inactivation are done by DNA methylation. Cytosine–guanine dinucleotides (CpG) are the regions of DNA where methylation frequently occurs and are termed CpG islands (CGIs). Four general types of DNA methylation based on the target sites are the methylation of CpG islands clustered within gene promoter regions, the methylation of the edge of CpG islands (CpG island shores), the methylation throughout the whole gene, and the methylation of repetitive sequences. DNA methylation is a highly stable marker so that is maintained throughout mitotic cell division [32]. DNA Methyltransferases have dual activities as are involved in the addition of methyl groups to DNA and also its removal. By the addition of methyl groups to DNA, transcription is blocked through diminished binding of transcription factors and enhanced binding of methyl-CpG-binding domain (MBD) proteins. In such a situation, the chromatin structure is affected and a co-repressor complex is generated. By contrast, removal of methyl groups from DNA leads to the euchromatin structure formation and allows binding of transcription factors to the correct regions [18].
It has been evidenced that abnormal DNA methylation can play an important role in several diseases in humans [33]. However, little has been explored with regard to the association of DNA methylation with AS, so far [18]. Whole-blood transcriptional profiling studies on AS have demonstrated that the expression level of DNMT1 is down-regulated and CpG islands at the promoter of DNMT1 are highly methylated in the PBMCs from patients with AS in comparison with the healthy controls. Based on the role of the DNMT1 enzyme in preserving the methylation pattern of DNA, it is expected that a decrease of DNMT1 can influence the level of DNA methylation of downstream genes that could probably contribute to the development of AS [34, 35].
DNMT3A and DNM3TB are de novo methyltransferases that have been shown to be involved in genomic imprinting and inactivation of X-chromosome, to impress development of hematopoietic stem cells, and activation of UBE2 ubiquitin ligases, which are also known to be associated with AS. The functions of these genes have led to the suggestion that they might be responsible for the male gender bias in AS [12].
Methylation in other genes has been reported as associated with AS. It has been shown that the methylation of SOCS-1 can only be found in the serum of HLA-B27-positive patients with AS but not in any of B27-positive healthy controls. Interestingly, the amount of methylated SOCS-1 has been shown to be significantly correlated with the degree of inflammation as measured through sacroiliitis, acute phase reactant, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), as well as cytokine level of IL-6 and TNF-α in AS patients [18, 20, 33]. In addition, down-regulation of BCL11B expression as well as its CpG island hyper-methylation in PBMCs from AS patients compared with healthy subjects has been observed. More detailed studies would be advantageous to explain the exact mechanisms by which both DNMT1 and BCL11B hyper-methylation are involved in the AS etiopathogenesis [18, 35]. One important finding of a study is that the HLA-DQB1 gene is involved in AS development. Interestingly, a significant level of hyper-methylation of the HLA-DQB1 gene in AS PBMCs has been identified [36].
4.2 Histone Modifications
Histones are conserved proteins involved in packaging and organizing DNA to form nucleosome structure. Histones are classified into two main groups of cores (H2A, H2B, H3, and H4) and linker (H1 and H5) proteins. Histone proteins can undergo a series of post-translational modifications such as acetylation, phosphorylation, sumoylation, ubiquitination, and methylation. These mechanisms regulate the transcription of the genes in two major ways. On one hand, histone modification changes chromatin conformation and hence makes the gene promoters accessible for transcription factors. On the other hand, histone modification may affect the binding of chromatin-associated factors. Histone methylation does not change the charge of histone proteins while acetylation does. Histone acetylation is necessary for the regulatory factors access to the transcription factors and for the following gene expression [32]. Histone acetylation is mediated by two enzymes named histone acetyltransferases (HATs) and histone deacetylases (HDACs). Histone acetyltransferases transfer an acetyl group to the amino group of lysine side chains from acetyl CoA. This alteration neutralizes the positive charge of lysine and diminishes the interaction between the DNA strand and the histone and hence, entails a less integrated and accessible chromatin structure, which rises the gene expression. By contrast, histone deacetylases remove an acetyl group from the acetylated lysine side chain, adding a positive charge and leading to an intense chromatin configuration, which reduces the gene expression [18]. It has been reported that in AS, HAT and HDAC activities are significantly decreased and HDAC inhibitors are capable of down-regulating TNF production by PBMC in patients with AS [20].
4.3 MicroRNAs
Another element of the epigenetic machinery is microRNA (miRNA). MicroRNAs are endogenous short non-coding RNAs that contain 18–23 nucleotides that play important roles in regulating gene expression at the post-transcriptional level. Their genes are located throughout the genome and are transcribed from intronic regions of protein-coding genes, within intronic or exonic sequences of noncoding RNAs, and approximately 30% in intergenic regions [32]. The miRNAs are generated in the nucleus and then moves to the cytoplasm. Primary miRNAs (pri-miRNAs), as long primary transcripts, are transcribed by RNA polymerase II from mono- or polycistronic miRNA genes in the nucleus. These pri-miRNAs fold into hairpin structures and then, processed by a multiprotein complex, which comprises an identifier component named RNA-binding protein DiGeorge syndrome critical region 8 (DGCR8), and a catalyzer component named RNase III type endonuclease Drosha (or RN3). The Drosha catalyzes the conversion of pri-miRNA to precursor miRNA (pre-miRNA), which subsequently will be transported to the cytoplasm via Exportin 5. In the cytoplasm, another RNase III type endonuclease, called Dicer, processes the pre-miRNA into a mature miRNA duplex. Finally, at the core of a multiprotein RNA-induced silencing complex (RISC), the guide (antisense) strand is selectively separated to bind to the 3′UTR of complementary mRNA sequences, which leads to degradation or translational suppression. The passenger (sense) strand is generally released and rapidly degraded (Fig. 4.3) [37]. Silencing of gene expression by miRNAs is mediated by two pathways. First, induction of degradation of target mRNA when a part of its 3’UTR sequence is completely matched with the miRNA. Second, steric hindrance which prevents translation when miRNA strand is matched incompletely with target mRNA 3’UTR. In addition, miRNAs can influence the gene expression either positively or negatively by the means of modulating promoter DNA methylation as well as histone modifications [18].
The microRNA biogenesis pathway. Pri-miRNA is the first product of transcription of miRNA genes in the nucleus. This is then processed by Drosha and DGCR8 to generate pre-miRNAs. Pre-miRNAs exit from the nucleus toward cytosol via exportin 5 (Expo 5). Inside the cytosol, pre-miRNA is further processed by Dicer and a double-stranded RNA with about 21–23 nt length is generated. Then, miRNA is incorporated into a multicomponent enzyme complex named RNA Induced Silencing Complex (RISC). The RISC complex, using the antisense strand of the miRNA as a guide, searches for the mRNA target and degrades it. In the case of incomplete matching between miRNA and target mRNA, steric hindrance prevents the translation of the target mRNA. If the antisense strand is completely matched with complementary mRNA, antisense hybridizes to the target mRNA and induces its degradation by the intracellular enzyme RNase H
As fine-tuning regulators of gene expression, microRNAs target numerous mRNAs participating in different physiological processes of cells, including proliferation, development and differentiation, cell metabolism, and apoptosis. Therefore, any abnormality in their expression profile can lead to dysregulation of the normal functions of the cell. MiRNAs are also important players in regulating the development of the immune system, normal immune function, autoimmunity, and osteoblast differentiation [15, 20]. Therefore, their deregulation can be associated with the pathogenesis of many human diseases [15, 18]. The pathological role of miRNAs has recently been studied most intensively in malignant diseases and also in autoimmune disorders. In recent years, some researchers have been engrossed in the potential role of miRNAs in autoimmune disorders and several unusual expression profiles have been defined as biomarkers of certain autoimmune disorders [37].
There are many studies evaluating the role of different miRNAs in AS. Expression profile studies of miRNAs in AS patients has revealed upregulation of several miRNAs including miR-16, miR-17-5p, miR-31, miR-32, miR-34a, miR-99b, miR-130a, miR-146a, miR-150, miR-221, miR-342-5p, let-7b, let-7i, miR-155-5p, miR-210-3p, and miR-513-5p, while down-regulation of miR-10b, miR-27b-3p, miR-29a, miR-30a, miR-30e, miR-126-3p, miR-154, miR-199a-5p, miR-215, miR-218, and miR-409-3p compared with healthy controls. On the contrary, the down-regulation of miR-16 and miR-150 has been shown in AS patients in one study [37,38,39,40]. Among the overexpressed microRNAs, miR-221 and let-7i have been confirmed to be correlated positively with the Bath Ankylosing Spondylitis Radiology Index (BASRI) of the lumbar spine. The contribution of the elevated expression of let-7i in T cells AS patients in the immunopathogenesis of AS via increasing the IFNγ production and Th1 inflammatory response has been indicated [18, 20, 37].
A remarkable higher level of miR-21 in AS patients, positively correlated with programmed cell death 4 (PDCD4) and C-telopeptide (CTX) mRNAs in patients with AS who are taking sulfasalazine has been demonstrated. By contrast, the high expression level of miR-21 has been seen to be negatively correlated with PDCD4 mRNA expression in AS patients who are taking neither NSAID nor DMARD. Positive correlations of miR-21 and CTX expression level have also been observed in AS patients with less than 7 years of disease duration and in active disease. The binding of miR-21 to PDCD4 can suppress the expression of PDCD4 and further induce the osteoclasts activation. It seems that the expression of miR-21 might be important in the AS development [18, 20].
A significant upregulated expression of miR-29a in PBMCs of AS patients compared with RA patients and healthy controls has been reported. The miR-29a regulates TNF-α mediated bone resorption mainly by targeting Dickkopf homolog 1 (Dkk-1) and GSK3b, and hence, activates the Wnt signaling pathway. Negative regulation of Dkk-1 in Wnt signaling may contribute to new bone formation in AS. However, no correlation between miR-29a expression and level of CRP and ESR, as well as Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and Bath Ankylosing Spondylitis Functional Index (BASFI) score of patients has been reported [18, 20]. Although a specific miRNA expression profile has not yet been established for AS, miR-29a could be considered as a helpful marker for diagnosis and monitoring the new bone formation in AS and might be a potential therapeutic approach in the future. Nevertheless, miR-29a is not specific for AS and could be involved in other disorders [18].
It has been evidenced that ANTXR2 is down-regulated and miR-124 expression is upregulated in AS patients. Given that ANTXR2 suppression by miR-124 promotes JNK activation and induces autophagy, it has been suggested that miR-124 may stimulate autophagy to participate in AS pathogenesis [20, 37].
Diminished miRNA-130a and elevated TNF-α and HDAC3 expression levels have been observed in PBMCs from AS patients. Down-regulation of TNF-α mRNA in PBMCs has been shown in overexpression of miRNA-130a, while inhibition of miR-130a led to an overexpression of TNF-α. On the other hand, knockdown or silencing of HDAC3 has been shown to be associated with both overexpression of miR-130a and down-regulation of TNF-α. These findings conclude that HDAC3 through establishing a negative feedback loop with miR-130a and promotion of TNF-α expression plays an important role in the molecular etiopathology of Ankylosing Spondylitis [18].
A remarkable higher frequency of the G and GG alleles in the miR-146a rs2910164G > C SNP has been demonstrated in the AS patients compared to the healthy controls in a Han Chinese population but not in other Chinese populations [37, 39]. In Chinese Han people, down-regulation of miR-143, miR-146a, miR-9-3, miR-205, and overexpression of miR-301a and miR-23a has been shown to be associated with susceptibility to acute anterior uveitis (AAU) in AS patients but not in AS negative cases [37, 41].
References
Colbert RA, et al. HLA-B27 misfolding and spondyloarthropathies. Prion. 2009;3(1):15–26.
Akassou A, Bakri Y. Does HLA-B27 status influence ankylosing spondylitis phenotype? Clin Med Insights: Arthritis and Musculoskeletal Disorders. 2018;11:1179544117751627.
Chen B, et al. Role of HLA-B27 in the pathogenesis of ankylosing spondylitis. Mol Med Rep. 2017;15(4):1943–51.
Breban M, et al. Revisiting MHC genes in spondyloarthritis. Curr Rheumatol Rep. 2015;17(6):40.
Nicknam MH, et al. Determination of HLA-B27 subtypes in Iranian patients with ankylosing spondylitis. Iran J Allergy Asthma Immunol. 2008;7(1):19–24.
Colbert RA, et al. From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev. 2010;233(1):181–202.
Gran JT, Husby G. HLA-B27 and spondyloarthropathy: value for early diagnosis? J Med Genet. 1995;32(7):497.
Reveille JD. An update on the contribution of the MHC to as susceptibility. Clin Rheumatol. 2014;33(6):749–57.
Nicknam M, et al. Association between HLA-B27 antigen and ankylosing spondylitis in Iranian patients. Hakim. 2005;8(1):29–34.
Nicknam M, et al. Comparison of val idity of microlymphocytotoxicity and flowcytometry methods with PCR for HLA-B27 antigen typing. Med J Islamic Republic Iran (MJIRI). 2003;17(1):75–9.
Brown M. Progress in studies of the genetics of ankylosing spondylitis. in clinical and experimental rheumatology. In: Clinical & exper rheumatology via santa maria, vol. 31. Italy: PISA; 2010. p. 56126.
Hanson A, Brown MA. Genetics and the causes of ankylosing spondylitis. Rheum Dis Clin. 2017;43(3):401–14.
Burney R, et al. Analysis of the MHC class II encoded components of the HLA class I antigen processing pathway in ankylosing spondylitis. Ann Rheum Dis. 1994;53(1):58–60.
Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014;57(1):44–51.
Ganjalikhani Hakemi M, et al. Optimization of human Th17 cell differentiation in vitro: evaluating different polarizing factors. In Vitro Cell Dev Biol Animal. 2011;47(8):581.
Ringrose J. HLA-B27 associated spondyloarthropathy, an autoimmune disease based on crossreactivity between bacteria and HLA-B27? Ann Rheum Dis. 1999;58(10):598–610.
Robinson PC, Brown MA. Genetics of ankylosing spondylitis. Mol Immunol. 2014;57(1):2–11.
Mahmoudi M, et al. New insights toward the pathogenesis of ankylosing spondylitis; genetic variations and epigenetic modifications. Mod Rheumatol. 2017;27(2):198–209.
Fechtenbaum M, et al. MICA and NKG2D variants as risk factors in spondyloarthritis: a case–control study. Genes Immun. 2019;20:599–605.
O’rielly DD, Uddin M, Rahman P. Ankylosing spondylitis: beyond genome-wide association studies. Curr Opin Rheumatol. 2016;28(4):337–45.
Tsui FW, et al. The genetic basis of ankylosing spondylitis: new insights into disease pathogenesis. Appl Clin Genet. 2014;7:105.
Qian Y, et al. Genetic association between TAP1 and TAP2 polymorphisms and ankylosing spondylitis: a systematic review and meta-analysis. Inflamm Res. 2017;66(8):653–61.
Feng M, et al. TAP1 and TAP2 polymorphisms associated with ankylosing spondylitis in genetically homogenous Chinese Han population. Hum Immunol. 2009;70(4):257–61.
Agrawal N, Brown M. Genetic associations and functional characterization of M1 aminopeptidases and immune-mediated diseases. Genes Immun. 2014;15(8):521.
Alvarez-Navarro C, de Castro JAL. ERAP1 structure, function and pathogenetic role in ankylosing spondylitis and other MHC-associated diseases. Mol Immunol. 2014;57(1):12–21.
Zhai J, et al. Immunogenetic study in Chinese population with ankylosing spondylitis: are there specific genes recently disclosed? Clin Dev Immunol. 2013;2013:419357.
de Castro JAL. How ERAP1 and ERAP2 shape the peptidomes of disease-associated MHC-I proteins. Front Immunol. 2018;9:2463.
Driscoll J, et al. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature. 1993;365(6443):262.
Gaczynska M, Rock KL, Goldberg AL. γ-Interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature. 1993;365(6443):264.
Westman P, et al. TAP1 and TAP2 polymorphism in HLA-B27-positive subpopulations: no allelic differences in ankylosing spondylitis and reactive arthritis. Hum Immunol. 1995;44(4):236–42.
Soleimanifar N, et al. Effect of food intake and ambient air pollution exposure on ankylosing spondylitis disease activity. Adv Rheumatol. 2019;59(1):9.
Roberts S, et al. Epigenetics of osteoarticular diseases: recent developments. Rheumatol Int. 2015;35(8):1293–305.
Lai N-S, et al. Association between cytokines and methylation of SOCS-1 in serum of patients with ankylosing spondylitis. Mol Biol Rep. 2014;41(6):3773–80.
Aslani S, et al. Evaluation of DNMT1 gene expression profile and methylation of its promoter region in patients with ankylosing spondylitis. Clin Rheumatol. 2016;35(11):2723–31.
Karami J, et al. Promoter hypermethylation of BCL11B gene correlates with downregulation of gene transcription in ankylosing spondylitis patients. Genes Immun. 2017;18(3):170.
Hao J, et al. Genome-wide DNA methylation profile analysis identifies differentially methylated loci associated with ankylosis spondylitis. Arthritis Res Ther. 2017;19(1):177.
Mohammadi H, et al. MicroRNA implications in the etiopathogenesis of ankylosing spondylitis. J Cell Physiol. 2018;233(8):5564–73.
Perez-Sanchez C, et al. Circulating microRNAs as potential biomarkers of disease activity and structural damage in ankylosing spondylitis patients. Hum Mol Genet. 2018;27(5):875–90.
Li Z, et al. The role of MicroRNAS in ankylosing spondylitis. Medicine. 2016;95(14):e3325.
Wang M, et al. Overexpression of miR-31 in peripheral blood mononuclear cells (PBMC) from patients with ankylosing spondylitis. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2017;23:5488.
Yang L, et al. miRNA copy number variants confer susceptibility to acute anterior uveitis with or without ankylosing spondylitis. Invest Ophthalmol Vis Sci. 2017;58(4):1991–2001.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ganjalikhani Hakemi, M. (2022). Association of HLA and HLA-Related Genes with Ankylosing Spondylitis. In: Nicknam, M.H. (eds) Ankylosing Spondylitis - Axial Spondyloarthritis. Springer, Singapore. https://doi.org/10.1007/978-981-16-4733-8_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-4733-8_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4732-1
Online ISBN: 978-981-16-4733-8
eBook Packages: MedicineMedicine (R0)