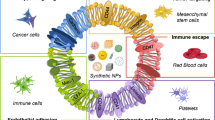
Abstract
Nanotechnology in cancer has been a boon to the translational science bringing advantages to the conventional drug delivery approaches. There are different types of nanoparticles such as liposomes, dendrimers, and mesoporous silica nanoparticles that are being employed to improve the overall biodistribution of the drug; however, this often fails in in vivo model due to the lack of stealth property, ultimately leading to immune rejection. PEG, chitosan, etc. are polymeric coatings that have been used as stealth covering around nanoparticles that prevent the nanoparticles from aggregation of proteins and opsonization. However, synthesis of polymeric coatings requires chemistries for conjugation that are often tedious and labor intensive. In this scenario, biomimetic nanoparticles have become convenient as they can be produced without much use of organic solvents. In addition, they can mediate natural targeting due to the virtue of homotypic interaction with membrane proteins present on the host cell. In addition, they can also prevent immune recognition due to the presence of marker proteins that are often recognized as “self” by the body. There have been several achievements in this field; still there are certain limitations that need to be dealt with. Techniques to produce biomimetic nanoparticles in a cost-effective manner in larger batches can lessen the burden in manufacturing process. Biomimetic nanoparticles possess immense benefits with better targeting and stealth property that can reduce the shortcomings of the traditional nanoparticles employed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
3.1 Introduction
Nanotechnology has revolutionized the field of medicine and health sciences. The amenability for wide and tailored functions has made them one of the potent used carriers for therapeutics. Nanosystems are now being recognized worldwide as versatile tools for various applications and have been started getting approved by U.S.F.D.A (Food and Drug Administration). Promising efforts are being continuously made to ensure the biocompatibility of these man-made synthetic nanoparticles. Conventional approaches for drug delivery have faced numerous problems that include renal clearance of the drug leading to its elimination. Although traditional methods like topical, parenteral, intravenous injections have proven to be effective at some point of time, these methods administer drugs systemically and not to the target area specifically with more than 90% of drugs are subjected to renal clearance. Hydrophobic drugs have poor solubility and often face issues in bioavailability when it comes to oral administration. In oral administration, the drugs remain at lower saturation level and are not absorbed properly [1]. In contrast to this, hydrophilic drugs face poor cellular penetration issues [2]. Protein/peptide and gene-based drugs are enzyme and acid sensitive leading to degradation. In such scenario, smart drug delivery approaches are becoming increasingly popular that leads to maximum accumulation of the drug at the target site leading to targeted drug delivery approach. Nanoparticles are usually the first choice for carrier-based drug delivery due to small size and effective cargo loading properties. Nanosystems aim for a controlled and sustained or steady release of the drug leading to maximum deposition in the diseased area. In addition to this, plasma half-life of the therapeutics is also considerably increased when compared to its native counterpart. Nanoparticles (NPs) fall in nanometer range (up to 1000 nm) with various shapes and sizes amenable for diverse purposes. Some of the most frequently used nanoparticles for research, diagnostic, and translation purposes are liposomes, biodegradable polymers, polymeric micelles, gold nanoparticles, mesoporous silica nanoparticles, dendrimers, metallic and carbon-based nanosystems, each serving an unique function of its kind as they have very different structural and functional aspects. Liposomes are primarily synthesized from lipids and proteins and mimic biological cell membranes. Liposomes have the ability to load both water-soluble and -insoluble compounds inside the aqueous core. The major demerit of liposomes is their physical and chemical instability. In some cases, drug leakage has also been reported [3]. Polymeric nanoparticles, on the other hand, include PLA (polylactic acid) and PLGA (polylactic glycolic acid) that have been even approved for human consumption by US FDA. They have improved biodistribution, stability, and biocompatibility as compared to liposomes. Due to small sizes of polymeric nanoparticles, there is scope for increased surface area to volume ratio which allows for facile conjugation of varieties of ligand and functional groups for specifically targeting the desired site [4]. While these are the advantages of polymeric nanoparticles, the major drawback is faster degradation kinetics and high variation from batch to batch production. Polymeric micelles are formed by spontaneous arrangement of amphiphilic block copolymers in aqueous solutions, basically containing a hydrophobic core and a hydrophilic shell that has the capability to load hydrophobic drugs [5]. Amphiphilic block copolymers self-assemble into stable structures, examples include triblock PCL-b-poly(2,4-dinitrophenylthioethyl ethylene phosphate)b-PEG. Polymeric micelles degrade very slowly in blood thus prolonging the circulation time. However, micelles face issues in loading water-soluble compounds that can be combated by using emulsification method. Still, the usage of organic solvents at industrial level poses a serious health hazard [6]. Dendrimers are monodisperse and highly symmetrical molecule having a perfect symmetry. Polymer brushes come under the high molecular weight dendron and dendrimer [7]. Generally, dendrimers are known for their high loading capacity of the drug by virtue of numerous functional surface groups and internal cavities. The high bioavailability of the attached drug is usually through covalent/non-covalent bonds [8]. Cationic amine dendrimers such as poly(amidoamine) (PAMAM) and poly(propylene imine) (PPI) have the ability to penetrate the negatively charged cell membrane therefore disintegrating the lipid bilayer [9]. However, the toxicity of different dendrimers is a major concern in biomedicine. Magnetic nanoparticle like iron oxide-based NP targets the desired target area via the aid of external magnetic field [10]. Drug localization to the desired area is mainly governed by competition of force generated by the blood compartment and the magnetic force produced from the magnetic core [11]. But, the major demerit of magnetic delivery is the absence of mechanisms for delivery into the depth of the body. Gold nanoparticles are also one of the non-toxic potential theranostic nanocarriers. High surface area to volume ratio enables one to utilize surface conjugation chemistries over the surface for efficient therapeutic purposes. The unique physicochemical properties like unique optics and surface plasmon resonance of the gold core is ideal for thermal ablation and allow for efficient diagnostic applications [12]. However, this field requires a multidisciplinary approach. Mesoporous silica nanoparticles (MSNs) constitute silica matrix that has numerous uniform pores ranging from 2 to 50 nm, each being tuned to the size of the drug to be loaded. MSNs can load multiple drugs at a time to the desired area. The one demerit mesoporous silica nanoparticles have is the speculated hemolysis caused by the interaction of the silanol groups of nanoparticle and the lipid membrane of red blood cell [13].
Although there are many synthetic nanoparticles that can be utilized for drug delivery, biocompatibility and immune acceptance are the major criteria which have led to the emergence of various stealth and targeted nanotherapeutics for evading immune evasion and specific delivery to the diseased area. Passive targeting is the route that guides these stealth-based nanoparticles. Passive targeting is based on EPR effect (Enhanced Permeability and Retention), term given by Matsumura and Maeda in 1908s. Nanoparticles easily pass through the leaky microvasculature and enter into the site of tumor tissue that allows molecules of definite size usually within 600 nm. This phenomenon is termed as the famous EPR effect. However, the major disadvantage of passive targeting is the lack of cell-specific interactions, thus decreasing the chance of the drug concerned to target the diseased area. Coatings of polymer that are most often used can be both natural and semisynthetic. Among natural polymers, polysaccharides originating from nature are used that include dextran, polysialic acid, hyaluronic acid, chitosan (CS), heparin while synthetic polymers include polyvinyl pyrrolidone (PVP), polyacrylamide (Pam), poly(ethylene glycol) (PEG), and PEG-based copolymers such as poloxamers, poloxamines, and polysorbates [14]. PEG-coated NPs evade the mechanism of opsonization and subsequently are rescued from macrophages uptake as studied by Garcia et al. [15]. Despite advancement in stealth coating, PEG-coated nanoparticles are not completely hidden from the immune cells and are engulfed by the mononuclear phagocytes. Anti-PEG antibodies have also been reported in some cases raising immunogenic reactions [16]. In an attempt to have an alternative polymer coating that is more hydrophilic than PEG, polyoxazoline-coated nanomaterials have been developed [17]. However, these polymer-coated nanoparticles require a tedious job of coating and conjugation chemistries that often is not that effective as projected. In addition to this, various other parameters are taken into consideration for increasing the residual time of the nanoparticles in the blood such as molecular weight and conformation of the polymers being used. While passive targeting includes mainly the EPR effect, active targeting aims for increased targeting to a diseased area. Certain moieties are readily employed in active targeting purposes. Antibodies are the most prominent of all the ligands as it utilizes the receptor-mediated endocytosis. It binds to the overexpressed antigens present on certain tumor cells and thus leads to engulfing of the particles that carries the drug and leads to enhanced accumulation of the therapeutics. Trastuzumab (Tmab)-coated lipid-polymer nanoparticles (hybrid nanoparticles) composed of PLGA; PEI (polyethylenimine) and lipids loaded with Docetaxel (DTX) have been developed in which Tmab is surface adsorbed onto the nanoparticle, designed for enhancing targeted delivery to HER-2 receptor-positive breast cancer cells. DTX-loaded-eTmab (e stands for electrostatically adsorbed)-PPLNs have proven to be more cytostatic to BT474 cells as compared to plain PPLNs [18]. Similarly, polyethylene glycol-poly(ε-caprolactone) NPs (PEG-PCL NPs) has been synthesized with conjugated programmed death-ligand 1 (PD-L1) monoclonal antibody. Drug-loaded-PD-L1 antibody-conjugated nanoparticles induce apoptosis arresting G2-M checkpoint, an indication of impairment in microtubule synthesis [19].
Among polysaccharides, hyaluronic acid (HA) is a polymer that is widely used as a targeting moiety in nanoparticulate systems. The concept of using HA as a targeting moiety has been taken from the idea that HA being the main component of ECM (extracellular matrix) beside collagen can bind effectively with CD44 receptors that are highly being overexpressed on tumor cells [20]. Hyaluronic acid nanoparticles are coated with PEG on the nanoparticles to evade RES and maximum retention in the blood. Tumor cells deliberately endocytose these nanoparticles by receptor-mediated endocytosis. Various small molecules have also been exploited as ligands such as folate that are folate receptors specific present on cancer cell membrane. PLGA NPs have been decorated with DPPC: DSPE–mPEG and folate as ligand encapsulating a photosensitizer, i.e., pheophorbide that kills cancer cells by producing free radicals. Folate-modified PLGA is usually preferably uptaken over the unmodified ones. In vivo MKN28 tumor-bearing mice model also has a higher accumulation of folate-decorated NPs during a period of 24 h after i.v. injection as compared to the unmodified NPs [21]. In the year 2017, Huo and the team improvized the melanoma Trp2 vaccine delivery along with Sunitinib. Sunitinib is a blocking agent for apoptosis and is known to inhibit tumors in melanoma. Huo and colleagues prepared Sunitinib base-loaded polymeric micelles (SUNb-PM) that was functionalized with anisamide. The concept being anisamide will bind to Sigma-2 receptors that are highly expressed on B16F10 skin melanoma cells. B16F10 injected to mice by i.v. injection and treated with Sunitinib-loaded polymeric micelles modified with anisamide to govern the maximum internalization by melanoma cells. Tumor inhibition was maximum for Trp2 and Sunitinib-loaded anisamide decorated polymer micelles. The groups receiving polymeric micelles containing drug and Trp2 showed higher CD8+ T cells, suggesting improvized elicitation of the immune response [22].
Peptides are also the most used targeting ligands for TDDS. They are known to display several advantages: low cost related to productivity, higher stability, and easy conjugable chemistries over NP surface [20]. In the year 2014, Mei et al. showed interest in glioma for gliomas are not that easy to reach because of the blood–brain barrier (BBB), so they developed a dual-targeted nanoparticulate system that can reach and target BBB and glioma, effectively and simultaneously. They designed a cell-penetrating peptide, (E8)-6-aminohexanoyl-PLGLAG-(R8) modified onto PEG-PCL nanoparticle that effectively targets glioma cells as the MMP-2 expression level is quite high in gliomas [23]. In the blood, cationic R8 is usually protected by E8 via ionic bonds because of which the penetration is inhibited as R8 is shielded. PLGLAG is usually used as a linker which is degraded by MMP-2. Thus, E8 is detached from R8 at the site of the glioma, recovering R8 from E8. Low-density lipoprotein-related protein 1 (LRP 1) is a highly expressed receptor on both the BBB and glioma. Angiopep-2, derived from the Kunitz domain of aprotinin (aprotinin also known as bovine pancreatic trypsin inhibitor that breaks down blood clots), binds with a high affinity towards LRP1 [24, 25]. The targeting ability of angiopep-2 and ACP dual-modified NPs (AnACNPs)-loaded Docetaxel(DTX) was investigated in this study (anti-glioma effects). Angiopep-2 was conjugated to the nanoparticle by EDC-NHS(AnNPs) and for the R8 or ACP modification (R8 or ACP modification (CNPs, ACNPs, and AnACNPs), ACPR8 or ACP added to the NP or Angiopep-2-conjugated NP suspension. ACP and R8-modified NPs showed an increased uptake by both BMEC and C6 cells. After 24 h, the distribution of all the NP formulation increased in the brain. AnNPs accumulated to a larger extent in the brain region than that of only NPs and R8/ACP modified NPs, clearly depicting that angiopep-2 effectively crosses BBB and targets glioma interacting with LRP1 which justifies the hypothesis of the study.
Aptamers are short stretches of DNA or RNA oligonucleotides that can selectively bind to specific target molecules and that can fold into 3D structures. They are employed in TDDS because of their lower immunogenicity, low molecular weights, and effortless availability. For example, AS1411 aptamer-functionalized PEG-PLGA nanoparticles mediated drug delivery systems encapsulating paclitaxel for anti-glioma therapy is a novel approach. AS1411 binds strongly to nucleolin which is highly expressed on the cancer plasma membrane and thus an effective anti-glioma therapy [26]. Aptamer-conjugated NP is found to have a longer residual duration in circulation leading to enhanced paclitaxel accumulation succeeded by tumor inhibition and enhanced longevity of rats bearing C6 glioma xenografts. HPA aptamers on PEGylated PLGA NPs encapsulating paclitaxel are also designed that preferably binds to Heparanase on tumor cells [27].
All these active targeting moieties that mediate the specific targeting to the diseased area, have still some disadvantages as they often fail to target the cell due to the presence of a single targeting moiety that can also be redundant to any other cell. For such reasons, biomimetic nanoparticles have evolved taking the advantage of both active and passive targeting.
3.2 Biomimetic Nanoparticles
Mimicking nature is a powerful tool for the development of newer nanosystems for targeted drug delivery approaches. Nanosystems functionalized with moieties that mimic the structure and chemical nature of the biological entities are somewhat termed Biomimetic NPs. Conventional polymeric stealth coatings and liposomes may serve as biomimetic nanosystems, but they suffer from antigenic responses and stability issues, respectively. The emergence of biomimetic nanosystems was due to the need for such nanosystems that will evade the immune cells and naturally mimic the structural aspects of the certain biological molecule such as protein, protein fragments, and whole cell membrane cloaked onto nanocores. This section will mostly cover the types of biomimetic nanoparticles that are playing a major role in biomedical science.
3.2.1 Protein or Peptide-Based Biomimetic Nanoparticles
Recently, protein/peptide-based biomimetic mineralization is known for their efficient biomimetic property and environment-friendly technology. Biocompatibility, high polarity, and surface area for conjugation of certain chemical groups are the hallmarks of this group of biomimetic nanomaterial. Albumin, ferritin, lipoproteins, enzymes, and peptides are the various biomimetic templates that can be good candidates for being biocompatible nanomaterials. Albumins are universal and robust proteins that can maintain the intact structure at higher (60 °C) temperatures. The nanoparticles from albumin can be easily prepared by emulsion, desolvation, or coacervation method under mild conditions. The low size and controlled release pattern are as good as liposomes and it also provides better patient compliance. Low cost for getting abundant albumin and ease of purification techniques have enabled Albumin as one of the most used biotemplates for preparing biomimetic nanoparticle. Bovine serum albumin (BSA) biotemplate has been used for making gold (Au) nanoclusters (NCs) via a simple, one-pot, and “green” synthetic route. BSA template-Au NCs are highly stable both in solutions (aqueous or buffer) and in solid form. Also, chloroauric acid (HAuCl4·3H2O) along with hydrazine monohydrate (N2H4·H2O) acts as a reducing agent in the existence of BSA under constant vigorous stirring was prepared to composite gold NPs via one-pot synthesis method [28]. In order to reduce the toxicity profile in vivo while maintaining the stability, human serum albumin is nowadays employed. The albumin paclitaxel (PTX) nanoparticle (Abraxane®) is FDA approved for the treatment of metastatic breast cancer. Use of toxic organic solvents however is the most common demerit of the conventional method for Abraxane® preparation. Therefore, salting-out greener technique is commonly used [29] (Fig. 3.1).
Schematic representation sustained/steady release of Docetaxel (DTX) for prolonged half-life in blood circulation. High cellular uptake and high maximum tolerated dose (MTD) are the cues for anti-tumor activity along with reduced systemic toxicity [29]
Besides these two categories of albumin, the third category of albumin is also widely used in the nanoparticles preparation, i.e., ovalbumin, also known as egg albumin, a glycoprotein used as vector for drug delivery approaches because of easy availability and cheap production. Ovalbumin forms gel networks and leads to stabilization of emulsions and foams. Owing to its pH- and temperature-sensitive properties, OVA has a promising potential for being applied as a carrier for sustained drug release [30]. One-pot approach has been used to synthesize OVA-conjugated Ag NPs, in which OVA acted as an active template for the spontaneous reduction of Ag ions. Biomimetic NPs prepared with this facile, cost-effective, and eco-friendly process is proving to be biocompatible through in vitro cell arrays [31].
Ferritin is a ubiquitous intracellular protein that can self-assemble into a cage-like nanostructure with an external diameter of about 12–13 nm, consisting of 12 or 24 subunits which has enabled researchers to synthesize nanomaterials out of it. Ferritin constitutes an efficient protein nanoplatform for in vivo antigen delivery, immune modulation, and antigen presentation. Ferritin NPs can be easily taken up by dendritic cells (DCs) for antigen presentation. Also, ferritin NP elucidates thermal and chemical stability which is amenable for ease of purification process. RGD4C-modified ferritin (RFRT) has been developed as a delivery vehicle to transport a hydrophobic photosensitizer named hexadecafluorophthalocyanine (ZnF16Pc) [32]. Drugs like doxorubicin (DOX) can also be encapsulated to RGD-modified apoferritin nanocages having high loading efficiency. Therefore, it is imperative to put forward ferritin as an ideal nanoplatform for drug delivery approaches.
Similarly, lipoproteins such as high density (HDL) and low density lipoproteins (LDL) have an immense application in targeted drug delivery due to their innate mechanism to evade immune system and easy loading capacity for amphiphilic compounds, much like liposomes. A hybrid HDL/polymer NP made up of a polymeric core coated with lipid/apolipoprotein showed not only a typical slow release profile of PLGA NP, but also natural characteristics of HDL, including specific accumulation by macrophages [33]. LDLs have also tendency to accumulate near tumors due to high demand for cholesterol in the tumor area, many of them even have been used to effectively load hydrophobic photosensitizers as these poorly water-soluble compounds interact better with lipoproteins, especially with LDL. Other lipoproteins, such as apolipoprotein A-I, are also in used in research to exploit it as a nanoplatform for pH-responsive drug delivery applications [28].
Inspired by the structure of the natural multi-enzymes, researchers are now also interested in designing enzyme complexes. Diverse enzyme/protein nanoparticles are immobilized onto electrode or a matrix for the fabrication of biosensors. HRP, uricase, cholesterol oxidase, and hemoglobin are some of them that can be used as biosensors. The enzyme-based NP can be produced by desolvation technique. NPs exhibit exceptional properties like optical, electronic, electric, thermal, chemical, mechanical, and catalytic. Trypsin single enzyme nanoparticles have been used to improve stability of enzymes at higher temperature, while chymotrypsin SENs improve cellulose degradation. In addition, the nanocomplexes from the enzymes can be used to specifically target tumors as the surface area is amenable for designing receptors proteins [34].
Peptide NPs are another class of NPs that have the intrinsic capacity to fold into 2D nanostructure that can have wide range applications in biomedical science due to the shape criteria and the design of amino acids that may serve in electrochemical catalysis, nanobiosensor fabrication, and even retroviral transduction. Protein/peptide-template biomimetic NPs have deeper tissue penetration that can be used for targeting cancer cells with enhanced therapeutic efficacy and in-built biocompatibility. Tryptophan–phenylalanine dipeptide is one such example of a peptide nanoparticle that has the property to diagnose tumor due to their intrinsic criteria of shifting fluorescence spectra from UV to the visible range [35].
3.2.2 Cell Membrane-Coated Nanoparticles (CMCNPs)
The story began when Zhang et al. in 2011 developed the first cell membrane-coated nanoparticle from red blood cell (RBC) membrane. The RBC membrane was coated onto the PLGA polymeric core. Cell membrane-coated NPs offer an autogenous option which is not possible in the case of certain polymeric coatings that can excite the immune system. The cell membrane proteins present on the surface of cell membrane-coated nanoparticles bind to cells expressing the same membrane protein due to the virtue of hemophilic or heterophilic interaction. CMCNPs can be both passively targeted (avoiding mononuclear phagocytes and consequently high retention time) and actively targeted (proteins of CMCNPs binding to the adhesion proteins on the desired cell of interest). By utilizing various nanocores such as polymeric, gold, mesoporous silica NPs, liposomes, and magnetic and coating them with various cell membranes, a variety of functions can be achieved depending on what one needs, targeting, or diagnosis (Fig. 3.2).
Source cells can be fused to various nanocores to produce cell membrane-coated NPs (CMCNPs) having a broad range of applications [36]
Synthesis of cell membrane-coated nanoparticles includes two crucial steps, i.e., isolation of membrane fragments (or vesicles) and vesicle/membrane and nanoparticle fusion. Coating membrane vesicles to various nanoparticle cores are the most commonly employed method in comparison to membrane fragments as the fusion of fragments to particles do not necessarily facilitate the presence of all the proteins required. The synthesis method differs from cell to cell as in RBCs and platelets nucleus is absent and the isolation of membrane vesicles or fragments is quite straightforward not involving sophisticated steps unlike other eukaryotic cells RBCs are made free from its buffy coat and hemoglobin via employing high-speed centrifugation followed by sonication and then polycarbonate porous membranes (preferably 100 nm) are used to get definite size RBC vesicles which are usually stored at 4 °C for preservation. Throughout the entire process of extraction of membrane fragments, it is made sure that the isolation process is as gentle as can be to assure minimal protein denaturation. Apart from this fact, there is always the use of protease inhibitors for preventing the action of proteases that may act on the membrane proteins. Complex eukaryotic cells like WBCs, cancer cells, stem cells, etc. undergo various complex biochemical processing like hypotonic lysis, ultrasonication along discontinuous sucrose density centrifugation to completely clear the intracellular contents from the cell. The freeze-thaw method and physical homogenization techniques are some other ways of membrane extraction other than hypotonic lysis. In the freeze-thaw method, cells are subjected to cold shock at −80 °C followed by thawing at 37 °C or room temperature as a result of which ice crystals are formed that leads to the disintegration of the membrane [36]. Electroporation enables formation of enough pores in the cell membrane to create flaccidity due to electrical fields [37]. However, this method may also lead to changes or fluctuations in membrane potential. All of these methods have some demerits in them however the most approachable method of cell membrane extraction includes the hypotonic buffer treatment followed by mild sonication for membrane disorientation. Sucrose density centrifugation or ultracentrifugation is usually needed to completely making devoid of nucleus and other intracellular components. For cancer cells, mild lysis followed by ultracentrifugation is needed as compared to RBCs. The difference in the extraction of membrane proteins occurs as a result of the difference in size, granularity, and lipid bilayer of cells that vary from cell to cell. The core nanoparticles can then be fused to membrane vesicles by various approaches to synthesize the cell membrane-coated nanoparticles. The membrane is usually oriented in the right side out position with all the receptor proteins exposed. Most of these used methods depend on the net attraction between the oppositely charged molecules between the inner core particle and the membrane vesicle thus forming a core-shell structure with the proteins of the membrane facing towards right-side-out conformation making it more energetically favorable [38]. The various methods used for fusing nanoparticle cores to membrane vesicles are as follows:
-
(a)
Co-extrusion approach: The nanoparticle solution and the vesicle mixture undergo co-extrusion via polycarbonate porous membranes and then sonicated to achieve CMCNPs of tunable sizes. Physical extrusion dictates the principle of strong force to pull off the vesicles to wrap around the nanoparticle core.
-
(b)
Sonication method: This is the most reliable method as in the co-extrusion method, large-scale synthesis of nanoparticles remains a challenge. Sonication uses the energy of specific disruptive frequencies to fuse membrane and nanoparticle cores. The frequency, sonication time, and amplitude are the major factors governing the effective fusion process.
-
(c)
Microfluidic electroporation: In this method, electromagnetic energy creates pores in the cell membranes creating an imbalance of dielectric field enabling the NPs to be coated by vesicles. This method is a novel approach and is becoming popular among researchers. Also, the stability of particles is unaffected.
-
(d)
Cell membrane-templated polymerization technique: This technique relies on the interfacial interactions between the core and the membrane. The polymeric core is grown in situ within the cores unlike in old conventional processes preformed polymers are used which cannot handle the homogeneity of sizes. Acrylamide polymers have been produced within the membrane vesicles with the cell membrane vesicles acting as a nanoreactor containing reaction mixture of polymers, initiators, crosslinkers etc. To prevent any further macrogelation of unencapsulated polymers outside the membrane vesicles, the reaction an is stopped by an inhibitor, i.e., (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl (TEMPO), thus forming cell membrane coated nanogel [39].
The CMCNPs need various characterizations after synthesis that can enable the integrity of membrane coating onto the material cores. Transmission electron microscopy (TEM) and dynamic light scattering (DLS) are the physicochemical procedures that are usually done for the verification of membrane coating on nanoparticles [40]. Morphology of coated nanoparticles shows a halo around the NPs while the uncoated ones do not show such structural features. There is usually an increase in size of the particle due to coating with a shift in zeta potential similar to membrane potential of the cell membrane that confirms the successful coating. Zeta potential of the final CMCNPs formed is similar to membrane vesicles as the membrane vesicles are the ones that are coated onto nanoparticle. Flow cytometric gives the signal fluorescence for antibodies specific to membrane, for example, signal fluorescence signal becomes relatively higher for CD47 when RBCNPs are stained with CD47 antibody. Western blotting also helps for the confirmation of the coating onto particles. Antibodies specific to membrane under consideration are taken for validation of the integrity and right-side-out coating of membrane vesicles. Preparation and characterization procedures are usually more or less generalized that can be taken for confirmation of the membrane fusion to particles [41, 42].
There are various mechanisms by which CMCNPs can be produced; however, the major goal is always to produce intact and stable nanoparticles that can be used for robust functions inside body fluids in vivo and in patient samples. The contribution of each type of membrane-coated particles has an immense effect in biomedical research. In the upcoming sections, there will be discussion on various types of cell membrane-coated nanoparticles including their advantages and disadvantages in the field of targeted drug delivery approaches.
3.3 Cell Membrane-Coated Nanoparticles in the Field of Drug Delivery
3.3.1 Red Blood Cell Membrane-Coated NPs (RBCNPs)
RBCs are blood cells that are predominantly found among all the cells in humans that has role in transporting oxygen to all the body sites via hemoglobin. RBCs are amenable for isolation as they are in the circulatory system. RBC-coated NPs were the first among all to be chosen as a delivery vehicle due to the presence of self-marker proteins such as CD47, CD59, complement factor 1, decay-accelerating factor, and C8 binding protein that avoid the immune system [43].
RBCs and stem cells often fail to specifically target cancer cells specifically as there are no such cell adhesion molecules (CAMs) on the plasma membrane that enhances targeting via homotypic adhesion with like CAMs. For making it more target specific, RBC membranes are decorated with ligand moieties like folate, mannose, transferrin, etc. for entry to desired cells [44]. Certain methods are available that are readily used for conjugating chemical moieties to RBC membrane [45]. Chemical methods interfere with the integrity of intact proteins on the membrane and as a consequence can lead to unsatisfactory results. To rule out such scenario, a non-disruptive lipid insertion approach is usually followed for conjugating ligand or targeting moieties, known as lipid insertion approach. In this method, ligand moieties were incorporated on RBC membranes via lipid tethers and PEG linkers [40].Various conventional chemotherapeutic agents such as doxorubicin, paclitaxel, and cisplatin are encaged inside RBC-coated nanomaterials in both surface modified and unmodified forms. Transferrin, folate, nucleolin-targeting aptamer, AS1411, mannose, etc. are the various receptors that are surface modified on RBC membrane for efficient targeting (Fig. 3.3).
RBC has a long life span of approximately about 120 days and property of evading the immune proteins of body, which can be well implicated in various aspects like targeting, imaging, photodynamic therapy apart from only conventional drug delivery purposes.
3.3.2 White Blood Cell Membrane-Coated NPs (WBCNPs)
WBCNPs are also prominent among various types of biomimetic nanoparticles taking account of inherent homing property to tumor and inflammation prone zones in the body. White blood cells have five major types depending on their granularity and morphology, i.e., monocytes/macrophages, neutrophils, eosinophils, basophils, and lymphocytes. Macrophages have the ability to home to inflamed and hypoxic area due to chemoattractants like CSF-1 (colony-stimulating factor) and chemokine-ligand-2 (CLCL-2) [46]. Receptors such as Tf (Transferrin) can be conjugated to macrophage membrane that is fused to nanoparticle has usually high targeting capability as compared to only membrane coating. Neutrophil-coated NPs have also been developed for targeting cartilages to inhibit synovial fluid inflammation thereby improving the condition of arthritis [47]. Natural killer cells (NK cells) have the ability to target cancer cells releasing granzymes and perforins. NKsome have been produced by fusing cholesterol-liposome with NK cells NK-92 for breast cancer therapy. NKsomes have the inherent ability to be retained in the blood for a longer duration and thus useful in stealth property of coated particles [48]. T cell-coated lipid-PLGA hybrid NPs have also been employed in research as they effectively target Burkitt’s lymphoma [49]. Neutrophil membrane-coated nanoparticles (NNPs) have also been developed to overcome the blood–pancreas barrier using poly(ethylene glycol) methyl ether-block-poly(lactic-co-glycolic acid) (PEG-PLGA) nanoparticles, celastrol being the therapeutic agent. Celastrol-loaded NNPs inhibited tumor as well as liver metastases and overall survivability of tumor-bearing mice [50] (Fig. 3.4).
WBC-coated NPS have the excellent property of targeting tumors due to the intrinsic property of immune; however, it is restricted to certain and not all tumors. The circumvention of these demerits can be countered by other biological-derived cell membrane coating.
3.3.3 Platelet Membrane-Coated Nanoparticles (PMNPs)
Platelet membrane contains some important cell adhesion molecules like CD47, CD44, and p-selectin. P-selectin binds with higher affinity for circulating tumor cells involving CD44, CD55/59. The adhesive glycoprotein membrane proteins are exploited for coating platelet membrane onto NPs, thus rescuing from macrophages resulting in better targeting ability [51]. NPs coated with platelet membrane bind to CD44+ tumor cells via p-selectin interaction. PLGA NP cloaked by platelet membrane has also been known to reduce the condition of atherosclerosis. The enhanced targeting ability of platelet membrane-coated NPs in joints of collagen-induced arthritis (CIA) model of mice was due to p-selectin and GVPI receptors [52]. Platelet membrane-coated NPs have demonstrated to be a potential biomimetic candidate as they incite low immunogenic response with enhanced biocompatibility that target injuries and inflammation prone area. However, there is one demerit that platelet membrane proteins can also activate immune system that may lead to release of various pro-inflammatory cytokines. To combat the immune evasion, tumor cells that can also be coated onto nanoparticles is discussed in the succeeding Sect. 3.4.
3.3.4 Cancer Cell Membrane-Coated Nanoparticles (CCMNPs)
Tumor cells possess unique property of evading immune cells such as NK cells and macrophage/monocytes, i.e., in simple terms cancer cell membrane-coated NPs does not disturb the immune system of the body. CCM (cancer cell membrane) also possesses certain adhesion glycoproteins. These adhesive anchor proteins help in the attachment of cell to cell. N-cadherin, Epcam, carcinoembryonic antigen, Galectin-3, etc. are the adhesion proteins that facilitate homotypic binding [42]. The CCMNPs therefore can be internalized into cells expressing proteins on the membrane that homotypically target the cells expressing similar protein, thereby releasing the drugs inside the desired cell of interest. The cancer cell membrane can also be decorated on the core nanoparticle with cancer-specific antigens for immunotherapy. The antigens are low immunogenic in nature. Likewise, plethora of research work is accomplished by various scientists to study on cancer cell membrane-camouflaged NPs. There has been successful implementation of therapeutic and imaging agents utilizing homotypic binding to receptors present on the cancer cell membrane in mice model and even in some cases, patient samples. Mesoporous silica NP core cloaked by PEGylated liposome yolk/cancer cell membrane coating have been developed by scientists encapsulating doxorubicin and a PARP inhibitor mefuparib hydrochloride that have potential cytotoxicity than free drug. The higher cytotoxicity is due to higher accumulation of CCMNPs [53].
To summarize, it can be inferred that homotypic targeting of cancer cell membrane-coated NPs can be put in various areas to generate anti-tumor therapeutics be it chemotherapy, PDT, and starvation therapy or immunotherapy.
3.3.5 Stem Cell Membrane-Coated NPs (SCMNPs)
Stem cells have the characteristic property of circulating in the bloodstream for a prolonged duration enabling its stealth property to escape the macrophages of the immune system. MSC-coated NPs circulate for a prolonged duration that impedes the lacuna of tumor cell-coated NPs having property to cross the endothelial barrier. MSCs can be synthesized from a broad range of tissues thus creating opportunities for therapeutic applications. Stem cell-coated gelatin nanogels encapsulating Dox have been produced recently that has displayed higher cytotoxicity and uptake profile than the bare counterpart in Hela cell with higher regression of tumor in mice model. CXCR4 antibody has also been conjugated to stem cell membrane for higher specificity. Stem cell membrane coated NPs however, lack a little specificity towards cancer cells. Compensation for low targeting ability can be achieved by conjugating ligands [54].
Apart from all these cell membranes, others can also be used for various translational approach. As single cell membrane coated NP might lack an advantage, hybrid cell membrane-coated NPs are nowadays synthesized, for example, RBC (for stealth property) and MCF (targeting breast cancer) and RBC (immune evasion—platelet (for tumor homing). Bacterial and viral cell membranes have also been in use as bacteria contains peptide immunogens or epitopes that have the potency to elicit immune response against a specific pathogen. Many of these cell membrane-coated NPs have also been applied for patients as clinical trials have given fruitful result. For convenience, different types of cell membrane-coated NPs are listed in Table 3.1.
3.4 Advances and Limitations
Biomimetic NPs are mostly in the third phase of a clinical trial due to their excellent biocompatibility. Various model drugs as described above are of enhanced therapeutic efficacy. Still, some obstacles such as poor pharmacokinetics and biodistribution are some of the major concerns in this field. Tumor cells most of the time show resistance towards such conventional drugs. Natural targeting mediated by the proteins on the CMCNPs has the potential for both active and passive mode of action. Minimum labor-intensive approach on the preparation of CMCNPs is the biggest advantage as compared to that of a single antibody-conjugated particle (immune-nanoparticle). However, the field is in its pilot stage, and it needs easy scalable and manufacturing practices for better therapeutic translatability in biomedical sciences.
3.5 Conclusion
Biomimetic nanoparticles are a boon to the field of biomaterials as they suffer from minimum resistance in the in vivo system (increased biocompatibility), and the synthesis procedure is also environment friendly. There is no use of organic solvents and hazardous chemicals in the production process. Sources of cells that are used for cell membrane extraction can be disambiguous and the stability of the biomimetic nanoparticles can be questioned. However, with increasing demands of biomimetic systems and higher number of patients flooding in, it is becoming clear that though this field is naïve, it needs attention because of the immense therapeutic scope.
References
Tambosi G et al (2018) Challenges to improve the biopharmaceutical properties of poorly water-soluble drugs and the application of the solid dispersion technology. Matéria (Rio de Janeiro) 23
Vrignaud S, Benoit J-P, Saulnier P (2011) Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 32(33):8593–8604
Olusanya TOB et al (2018) Liposomal drug delivery systems and anticancer drugs. Molecules 23(4):907
Masood F (2016) Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C 60:569–578
Xu W, Ling P, Zhang T (2013) Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv 2013:340315
Wakaskar R (2017) Polymeric micelles for drug delivery. Int J Drug Dev Res 9:3
Antoni P et al (2009) Bifunctional dendrimers: from robust synthesis and accelerated one-pot postfunctionalization strategy to potential applications. Angew Chem Int Ed Engl 48(12):2126–2130
Shadrack DM, Mubofu EB, Nyandoro SS (2015) Synthesis of polyamidoamine dendrimer for encapsulating tetramethylscutellarein for potential bioactivity enhancement. Int J Mol Sci 16(11):26363–26377
Beddoes CM, Case CP, Briscoe WH (2015) Understanding nanoparticle cellular entry: a physicochemical perspective. Adv Colloid Interf Sci 218:48–68
Price PM et al (2018) Magnetic drug delivery: where the field is going. Front Chem 6(619)
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7(1):144
Singh P et al (2018) Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci 19(7)
Shahbazi MA, Herranz B, Santos HA (2012) Nanostructured porous Si-based nanoparticles for targeted drug delivery. Biomatter 2(4):296–312
Salmaso S, Caliceti P (2013) Stealth properties to improve therapeutic efficacy of drug nanocarriers. J Drug Deliv 2013:374252
Garcia-Fuentes M et al (2005) A comparative study of the potential of solid triglyceride nanostructures coated with chitosan or poly(ethylene glycol) as carriers for oral calcitonin delivery. Eur J Pharm Sci 25(1):133–143
Garay RP et al (2012) Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv 9(11):1319–1323
Bludau H et al (2017) POxylation as an alternative stealth coating for biomedical applications. Eur Polym J 88:679–688
Zhang X et al (2019) Trastuzumab-coated nanoparticles loaded with docetaxel for breast cancer therapy. Dose Response 17(3):1559325819872583
Xu S et al (2019) PD-L1 monoclonal antibody-conjugated nanoparticles enhance drug delivery level and chemotherapy efficacy in gastric cancer cells. Int J Nanomedicine 14:17–32
Yoo J et al (2019) Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers (Basel) 11(5)
Poltavets YI et al (2019) In vitro anticancer activity of folate-modified docetaxel-loaded PLGA nanoparticles against drug-sensitive and multidrug-resistant cancer cells. Cancer Nanotechnol 10(1):2
Huo M et al (2017) Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J Control Release 245:81–94
Mei L et al (2014) Angiopep-2 and activatable cell penetrating peptide dual modified nanoparticles for enhanced tumor targeting and penetrating. Int J Pharm 474(1–2):95–102
Shao K et al (2010) Angiopep-2 modified PE-PEG based polymeric micelles for amphotericin B delivery targeted to the brain. J Control Release 147(1):118–126
Ché C et al (2010) New angiopep-modified doxorubicin (ANG1007) and etoposide (ANG1009) chemotherapeutics with increased brain penetration. J Med Chem 53:2814–2824
Guo J et al (2011) Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 32(31):8010–8020
Duan T et al (2019) HPA aptamer functionalized paclitaxel-loaded PLGA nanoparticles for enhanced anticancer therapy through targeted effects and microenvironment modulation. Biomed Pharmacother 117:109121
Li B et al (2018) The potential of biomimetic nanoparticles for tumor-targeted drug delivery. Nanomedicine (Lond) 13(16):2099–2118
Qu N et al (2019) Docetaxel-loaded human serum albumin (HSA) nanoparticles: synthesis, characterization, and evaluation. Biomed Eng Online 18(1):11
Wongsasulak S et al (2010) Electrospinning of food-grade nanofibers from cellulose acetate and egg albumen blends. J Food Eng 98:370–376
Iravani S et al (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9(6):385–406
Zhen Z et al (2013) Ferritin nanocages to encapsulate and deliver photosensitizers for efficient photodynamic therapy against cancer. ACS Nano 7(8):6988–6996
Sanchez-Gaytan BL et al (2015) HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug Chem 26(3):443–451
Liu Y et al (2013) Biomimetic enzyme nanocomplexes and their use as antidotes and preventive measures for alcohol intoxication. Nat Nanotechnol 8(3):187–192
Fan T et al (2017) Peptide self-assembled nanostructures for drug delivery applications. J Nanomater 2017:4562474
Dash P, Piras AM, Dash M (2020) Cell membrane coated nanocarriers - an efficient biomimetic platform for targeted therapy. J Control Release 327:546–570
Rao L et al (2017) Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 11(4):3496–3505
Zhai Y et al (2017) Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics 7(10):2575–2592
Zhang J et al (2015) Synthesis of nanogels via cell membrane-templated polymerization. Small 11(34):4309–4313
Liu Y et al (2019) Cell membrane coating technology: a promising strategy for biomedical applications. Nano-Micro Lett 11(1):100
Luk BT, Zhang L (2015) Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release 220(Pt B):600–607
Fang RH et al (2014) Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 14(4):2181–2188
Hu CM et al (2011) Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A 108(27):10980–10985
Bidkar AP, Sanpui P, Ghosh SS (2020) Transferrin-conjugated red blood cell membrane-coated poly(lactic-co-glycolic acid) nanoparticles for the delivery of doxorubicin and methylene blue. ACS Appl Nano Mater 3(4):3807–3819
Xia Q et al (2019) Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B 9(4):675–689
Dong Y et al (2018) The survival of fetal and bone marrow monocyte-derived alveolar macrophages is promoted by CD44 and its interaction with hyaluronan. Mucosal Immunol 11(3):601–614
Sun K et al (2020) Saikosaponin D loaded macrophage membrane-biomimetic nanoparticles target angiogenic signaling for breast cancer therapy. Appl Mater Today 18:100505
Pitchaimani A, Nguyen TDT, Aryal S (2018) Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 160:124–137
Han Y et al (2019) T cell membrane mimicking nanoparticles with bioorthogonal targeting and immune recognition for enhanced photothermal therapy. Adv Sci 6(15):1900251
Cao X et al (2019) Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm Sin B 9(3):575–589
Li Z, Hu S, Cheng K (2018) Platelets and their biomimetics for regenerative medicine and cancer therapies. J Mater Chem B 6(45):7354–7365
Song Y et al (2019) Platelet membrane-coated nanoparticle-mediated targeting delivery of Rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE(−/−)) mice. Nanomedicine 15(1):13–24
Nie D et al (2020) Cancer-cell-membrane-coated nanoparticles with a yolk–Shell structure augment cancer chemotherapy. Nano Lett 20(2):936–946
Gao C et al (2016) Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small 12(30):4056–4062
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dash, P., Dash, M. (2022). Biomimetic Nanosystems in Targeted Drug Delivery. In: Dash, M. (eds) Biomimetic Biomaterials for Tissue Regeneration and Drug Delivery. Springer, Singapore. https://doi.org/10.1007/978-981-16-4566-2_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-4566-2_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4565-5
Online ISBN: 978-981-16-4566-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)