Abstract
Nanomaterials and nanostructures have shown fascinating performances in various biomedicine fields, from cosmetic to cancer diagnosis and therapy. Engineered nanomaterials can encapsulate both lipophilic and hydrophilic substances/drugs to eliminate their limitations in the free forms, such as low bioavailability, multiple drug administration, off-target effects, and various side effects. Moreover, it is possible to deliver the loaded cargo to the desired site of action using engineered nanomaterials. One approach that has made nanocarriers more sophisticated is the “biomimetic” concept. In this scenario, biomolecules (e.g., natural proteins, peptides, phospholipids, cell membranes) are used as building blocks to construct nanocarriers and/or modify agents. For instance, it has been reported that specific cells tend to migrate to a particular site during specific circumstances (e.g., inflammation, tumor formation). Employing the cell membrane of these cells as a coating for nanocarriers confers practical targeting approaches. Accordingly, we introduce the biomimetic concept in the current study, review the recent studies, challenge the issues, and provide practical solutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Even though many treatments exhibit significant pharmacological action against particular disorders, their usage in their natural state is frequently constrained for practical reasons. The low stability, poor biodistribution, low barrier penetrating abilities, and lack of targeting qualities of active molecules are significant problems associated with free drug delivery (1,2,3). Cite constraints have encouraged the creation of nanoscale-controlled drug delivery devices, resulting from the introduction of nanoscience in medicine. With a significant decrease in drug discovery expenses, nanoparticles (NPs) for drug delivery efficiently lower barriers to free drugs, increasing the use of existing medications that have already been discovered and evaluated but are not currently used because of their produced side effects (4,5,6).
NPs are systems that conceal and shield physiologically unstable active molecules, maintaining pharmacological efficacy (7). Additional advantages of using nanoparticles for drug administration include their customizability and functional ability, which offer particular qualities to address concerns with solubility, off-target deposition in healthy tissues, and low bioavailability (8,9,10). In order to comprehend how nanoparticles work to treat diseases, extensive research on nanocarriers is being conducted on a global scale. Significant progress has been achieved for years in creating various NPs that are efficient medication delivery systems (11, 12).
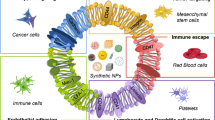
Despite significant advancements in the treatment of tumors, these nanocarriers still face several difficulties in clinical settings, including poor encapsulation efficiency, excessive cytotoxicity, and a difficult-to-manage release ratio in vivo (13, 14). To overcome these drawbacks, a novel strategy based on biomimicking natural components, such as cells, proteins, and other biological macromolecules, has gained popularity in the drug delivery sector. Otto Schmitt coined the term “biomimetic,” defining it in the context of biophysical science in 1957 (15). The term of “biomimetic” is also used to refer to “biomimesis,” the science that studies nature as a source of inspiration to solve the human problems that nature has already solved. The critical problems in medicine and the development of medications for incurable diseases can be accepted and understood through biomimetics. Scientists have concentrated on recent investigations on the biomimetic structures to understand the processes and biological system (16,17,18,19). The biomimetic concept can be applied to treat different diseases via the targeted delivery of therapeutic agents (Schematic 1).
Biomimetic nanocarriers are an emerging paradigm of nanomaterials whose surface is merged or synthesized with biomaterials capable of replicating the biological characteristics and functions of native cells. Due to this, biomimetic nanocarriers feature considerably improved cytocompatibility, high target selectivity, a prolonged waiting time, and little unwanted immune reactions. Cell membranes from red blood cells (RBCs), neutrophils, NK cells, macrophages, platelets, extracellular vesicles, and cancer cells are only some of the biomaterials that can be utilized to coat nanoparticles to enable their biomimicry capacity. Synthetic biomaterials like targeting peptides and aptamers complement nature-inspired biomaterials like monoclonal antibodies, natural proteins, and viral capsids (20,21,22,23,24,25).
The researchers have been motivated to imitate natural systems for a variety of biomedical applications by the properties of biomolecules and their characteristics at the cellular or molecular scale. The study of cell development, cell growth, cellular connections, hormone signaling pathways, and metabolic regulation are all covered within the field of biomimesis (26, 27). This review paper summarizes recent advancements in synthesizing biomimetic nanocarriers, including an overview of their current state of progress, utilization in clinical trials, and commercial products.
2 Biomimetic nanostructures as the targeted drug delivery vehicle
The NPs used as drug carriers are known as nanoparticles, and their sizes typically vary from 10 to 100 nm. This size and the sizes of proteins and DNA are in good agreement (28, 29). Biomaterials can self-assemble to create hollow, cylindrical nanotubes or wrap inorganic polymers like graphene. Three processes (self-assembly, electrospinning, and phase separation) are used to fabricate nanofibers from artificial or organic polymeric materials (30, 31). These nanomaterials (NMs) have excellent biocompatibility and biodegradability, in addition to having a good drug-loading capacity. Since 1986, a significant issue involving the circulation time of NPs in body fluid has been the enhanced permeability and retention (EPR) effect of NPs for drug leakages into tumor cells (32, 33). The reticuloendothelial system (RES) eliminates therapeutic NPs as aggressive foreign particles by the opsonization process (binding plasma proteins, including complement proteins and immunoglobulin G (IgG), onto their surfaces).
Numerous hydrophilic polymers have been used to cover NPs with a stealth coating shielding nanocarriers from the immune system and to enhance the EPR effect (34). For instance, one of the common techniques over the past 30 years has been PEGylation (surface coating of NPs with polyethylene glycol, PEG) (35). However, the cellular internalization of PEGylated NPs has not occurred and the encapsulated substances were destroyed in lysosomes because of the unfavorable interactions between polyethylene glycol and cells and low endosomal escape (this is known as the PEG dilemma) (36, 37). These PEGylated NP surface decoration with proteins, vitamins, peptides, antibodies, and aptamers as functional ligands is one biomimetic method for removing the steric hindrance.
On the basis of target cell properties, such as the overexpression of particular receptors on tumor cells, the kind of these ligands with a high affinity for receptor-mediated targeting on desired cells is chosen. As a result, biomimetic in cutting-edge drug delivery systems needed to modify the surface of biomimetic nanocarriers with a few amino acids, saccharides, and lipids to give them innate abilities. The two primary categories of targeting techniques based on BNPs are passive targeting and active targeting (as shown in Schematic 2). For this reason, paying close consideration to the basic building blocks, size, and shape of drug carriers is essential if you want them to be able to imitate real cells for cellular internalization (38,39,40).
Mechanisms for targeted delivery focusing on biomimetic nanocarrier in a schematic form. a Drug-loaded biomimetic nanocarriers were i.v. administered to the organism. b The passive targeting of biomimetic nanocarriers by the EPR effect towards tumors. Due to the leaky vasculature seen in tumor vessels, biomimetic nanocarriers are permitted to pass through pathological rather than normal walls, and because of the distinctive size, shape, and surface charge of the nanoparticles, this results in the accumulation of biomimetic nanocarriers within the tumor. c Biomimetic nanocarriers can be taken in by the ligand-mediated pathway and the stimulus-responsive pathway when active targeting is used. By maintaining them in contact with the targeting ligands, ligand-mediated targeting takes advantage of the high expression of particular receptors on the surface of targeted cells. Biomimetic nanocarriers aim to accumulate in disease tissue microenvironments in the presence of intrinsic and/or extrinsic triggers in order to achieve environment-responsive medication release. Reproduced with permission from (40)
Additionally, by biomimetic surface tailoring of these NPs with particular biomolecules analogous to the composition and functionality of specific cell membrane, a localized/targeted drug delivery within intended cells may be obtained. Additionally, there are two main classifications of biomimetic nanocarriers on the basic fundamental biomaterial: synthetic nanocarriers, which are biomimetic engineered NPs with characteristics similar to biological materials, and existing biological building bulks like passivated viral vectors and bacteria vectors (41,42,43).
2.1 Biomimetic nanoparticles
Recent years have seen a considerable increase in interest in nanotechnology as a potential route for the synthesis of potent carriers with multifunctional characteristics. For instance, AgNP-loaded chitosan/alginate structures with biomedical applications were developed, according to Bilal et al. (44). Due to the simplicity of surface modification, cytocompatibility, and optical properties, AuNPs have also been employed as nanocarriers and imaging agents in the life sciences (12). For instance, AuNPs functionalized with RBCs, according to Gao et al. The created RBC-AuNPs contained a membrane covering made of proteins from RBCs that could exhibit immunosuppressive functions that prevented macrophage absorption (45). Two varieties (needle and sheet forms) of hydroxyapatite (HA)-based biomimetic nanocrystals were created by Palazzo et al. to incorporate physicochemical and morphological features in a drug carrier. It was examined how well three drugs—cisplatin, di(ethylenediamineplatinum) medronate, and alendronate—adsorb to and desorb from HA-NPs (46). Similar to this, three distinct HA-based biological matrices were investigated by Sheikh et al. Collagen, polyvinyl alcohol, and BSA were used in the hydroxyapatite-mediated matrices that the group showed. The biomimetic NPs that have been created behave like the ECM. In conclusion, pH-responsive nanocrystals fabricated from polyvinyl alcohol (PVA) are the best options for targeted medication delivery (47).
Recent researchers have reported using the cell membrane (CM) infusion method as an alternative to PEG in the targeted drug delivery concept (48). In order to create CM-covered PLGA (poly(lactic-co-glycolic acid) NPs that may impede cancer cell movement toward mammary fibroblasts, Jin et al. created biomimetic PLGA NPs covered with cancer cell membrane fractions (49). By focusing on both circulating tumor cells (CTC) and the premetastatic microenvironment, Kang and colleagues synthesized neutrophil-mimicking NPs that reduced the metastatic load (50). Similarly, Pitchaimani and colleagues created “NKsome,” an envelope-camouflage fusogenic liposomal nanocarrier, for the immunosurveillance of sick/stressed cells from NK cells-ghost (51). Qin and colleagues combined mesoporous silica (MS) and graphene oxide to create bio-inspired nanocarriers applicable to cancer treatment. In order to allow cellular internalization through folate receptors on cancer cells, a lipid bilayer was also self-assembled on the surface of the GO-based nanocomposites. The implemented GO in nanocarriers was then used as a photothermal agent to transform light energy into heat, which significantly increasing the release of the loaded drug (DOX) into cancer cells (52).
2.2 Biomimetic liposomes
Liposomes, which take the form of spherical vesicles, are made up of one or several phospholipid bilayers and have a very similar structure to that of cell membranes (53). Because of their ability to contain both hydrophilic and hydrophobic medicines, liposomes have shown to be a valuable drug delivery mechanism. In the cosmetics and pharmaceutical industries, liposomes play an important role as carriers for a wide variety of compounds. The use of liposome packaging to develop delivery methods that can encapsulate unstable substances (such as antimicrobials, antioxidants, flavors, and bioactive components) and safeguard their activity has also been intensively investigated by various industries. Liposomes can encapsulate hydrophobic and hydrophilic substances, protect them from degradation, and then release them at specific sites (54, 55).
Conjugation with different compounds can alter the surface composition of liposomes, increasing their usefulness by increasing their blood circulation time and decreasing their opsonization (adsorption of blood proteins). Alginate, chitosan, pectin, collagen, and fibroin are all biological matrices that can coat liposomes (56,57,58); these biomaterials are biocompatible, biodegradable, and bioavailable; they can also be used to simulate the architecture of biological cells. These polymeric components also act as a steric hindrance to stop the aggregation of liposomes, opsonization, and RES clearance. In order to transport medications or biomolecules inside cells, biomimetic liposomes could be modified to bind to specific moieties or ligands. Biomimetic liposomes may also facilitate the slow or rapid release of their payload in response to predetermined stimuli in the cellular microenvironment (56, 59).
As early as 2001, Westhaus et al. developed thermally responsive liposomes that encapsulate CaCl2 and release Ca+2 at body temperature. Ca+2 and polysaccharides, such as alginate and protein, gel rapidly, which inspired the creation of these biomimetic liposomes. CaCl2-loaded liposomes could be injected locally as a medication or biomaterial for on-the-spot tissue healing (60). Using the reverse phase evaporation method, they successfully enclosed nano-Pt within the liposomes’ interior aqueous chamber (Schematic 3). Hydrophobic, clinical photosensitizer verteporfin was loaded into the lipid bilayer (VP). RAW264.7 macrophage (M) cell membrane was hybridized with the resulting nano-Pt/VP@Lipo to produce nano-Pt/VP@MLipo (61).
A Nano-Pt/VP@MLipo and B chemophototherapy effectiveness in tumors depicted schematically. (1) An organic solvent (light yellow) was used to disperse lipid and VP, and nano-Pt was then dissolved in water (light blue). (2) An emulsion was formed by sonicating the mixture. The gel-like condition was (3) removed by evaporating the organic solvent. Adding phosphate-buffered saline (PBS) caused the development of liposomes with a lipid bilayer and nano-Pt in the aqueous cavity. (4) Finally, vesicles derived from the M cell membrane (CM) were introduced so that the second round of hybridization could take place. (5) Freeze–thaw cycles and extrusion were used to create the biomimetic nano-Pt/VP@MLipo, and then, the unentrapped components were removed.
It is hypothesized that the liposome’s biomimetic features, such as extended circulation and inflammatory endothelium (such as tumor vasculature) targeting, will be enhanced by its camouflage with M membrane proteins. Injecting nano-Pt/VP@MLipo intravenously (i.v.) allows the VP-based PDT impact to be amplified by the nano-Pt catalyzing the breakdown of large levels of H2O2, so providing oxygen to the tumor location (61) (Fig. 1). PDT’s production of 1O2 can also rupture liposomal membranes [19, 21], releasing tumor-penetrating, nano-sized Pt that can more effectively be injected into tumors and used in chemotherapy. Nano-Pt/chemophototherapeutic VP@MLipo’s activity is established in vitro and in vivo, and its physicochemical features are explored.
In vivo cancer treatment. A The therapeutic regimen. B A 20-day tumor volume growth curve. A comparison of nano-Pt/VP@MLipo + L and nano-Pt/VP@Lipo + L, or VP@MLipo + L and nano-Pt@MLipo, is shown. Contrast (b) Nano Pt/VP@MLipo + L to (a) MLipo (empty) or (c) saline. The curves of mouse survival, exhibit C. Mice are light in weight (letter D). F Images of the 20-day tumor samples that were taken for analysis. The mass of the tumor on day 20. G Lung metastasis imaging using ex vivo bioluminescence. The incidence of metastases is depicted graphically via a heat map. Pimonidazole-positive hypoxic regions in 4T1 cancers. HIF-1 expression in 4T1 tumors was evaluated by Western blotting 4 h after i.v. injection of liposomes, as shown in figure 1 (I). Paraffin tumor slice with H&E staining (J). Necrotic tissue is shown by the dashed line. Using an anti-PCNA antibody, we observed brown staining in the nuclei of tumor cells that are actively dividing. For B, C, and D, n = 8 (D). n = 5 in (F). n = 3 in (I).
In conclusion, liposomes’ capabilities in targeted drug delivery were improved through the enhancement of the EPR effect due to biomimetic modification of liposome surface and biomimetic techniques of liposome manufacture (53, 62). In animal models, the fundamental drawback of unilamellar liposomes is the limited stability of drug-loaded carriers against enzyme breakdown. An additional liability is that encapsulated medications sometimes leak out before they reach their intended cells. A compartmentalization technique can create biomimetic multilamellar liposomes, which boosts the EPR effect. These structures can also encapsulate a variety of hydrophobic and hydrophilic medicines as well as gene therapy agents, allowing for the creation of dual cancer cell targeting carriers. In order to facilitate transport across the cellular membrane, the fluidity of liposomal membranes may be improved (63).
2.3 Biomimetic micelles
Micelles, amphiphilic structures, can efficiently transport medications to their intended sites of action. Amphiphilic molecules self-assemble into micelle in aqueous media. Both hydrophilic/polar (head) and hydrophobic/nonpolar (body) regions are present in the structures. When formed in water, micelles have their nonpolar regions at their centers and their polar regions on their exteriors. Micelles are capable of transporting hydrophobic as well as hydrophilic substances. Such structures are capable of delivering macromolecules due to the stability of the encapsulated molecules, the enhancement of drug pharmacokinetics and favorable tissue distribution, and the enhancement of drug bioavailability. Above the critical micelle concentration, micelle formulation can be accomplished. Micelles are typically prepared through one of four standard procedures: aqueous solvent evaporation, solid dispersion, dialysis, or oil-in-water emulsion (64,65,66). Drug bioavailability and half-life can be improved by encapsulating hydrophobic medicines into the hydrophobic core of micelles. Micelles have gained interest as a drug delivery vehicle for low water-soluble medicines. Micelles are created when amphiphilic molecules assemble into a sphere. Polymeric micelles, a type of colloidal delivery method, have attracted much interest because of their practical drug-loading efficacy (64, 65).
The self-assembly of amphiphilic di or triblock copolymers in water often produces nanoscale spheres known as polymeric micelles. It is also possible to create biomimetic micelles, which allow for more precise localized drug administration, through the incorporation of biological moieties or biomimetic base-block copolymers during the self-assembly of unique biomimetic amphiphiles in a micellar architecture. In 2005, Xu and colleagues utilized PEO to produce a biomimetic amphiphile that mimicked natural membrane fluidity regulators called cholesterol moieties (Chol). These Chol-PEO micelles were designed to encapsulate the lipophilic anti-cancer medication adriamycin, and their lipophilic cholesterol cores were surrounded by biocompatible PEO coverage. In vitro investigations have shown that Chol-PEO nanocapsules are capable of delivering both efficient drug loading and sustained release characteristics (66).
Hu et al. synthesized cancer theranostic agent based on redox-responsive biomimetic polymeric micelles as the aggregation-induced emission (AIE) imaging agents and biomimetic nanocarrier for doxorubicin. They observed that the synthesized and biomimetic nanocarrier exhibited significant antifouling property and biocompatibility. Moreover, they reported on-demand drug release in the presence of high levels of glutathione. They suggested that the synthesized biomimetic nanocarrier can be applied ad cancer theranostic agent (67). To attack tumor-associated macrophages, Wang et al. developed a unique RBC-cancer cell hybrid membrane covered copolymer micelle. When comparing the in vivo anti-tumor activity of the free drug (inhibition rate: 21.3%) with that of the hydrophobic interaction (inhibition rate: 43.6%), the DH@ECm was shown to be the most effective (inhibition rate of 64.5%). They proposed that the innovative multiple cell hybrid membrane therefore held great promise in the treatment of tumors (68).
3 Improving targeting properties
The targeting capabilities of bio-hybrid NPs can be improved, thanks to the biological components that make up the NPs. The multifaceted properties of NPs derived from cells and cell membranes make them well-suited for NP-based cancer therapy (69, 70). More and more research has focused on cell membrane-coated NPs (CMCNPs), designed to mimic cell surface functionality (71, 72), which are designed to mimic the functionality of cell surfaces. This can help to dampen the immune responses of synthetic NPs in vivo and introduce the ability to combine natural and synthetic materials in a single, compact package, as shown in Fig. 2 (73). Overcoming the difficulties encountered by synthetic NP-based drug carriers has been demonstrated that cell- and cell-membrane-based drug carriers exhibiting intrinsic features of in vivo biology can attain tolerable toxicity and more excellent biocompatibility than their synthetic counterparts. The ability to generate desired cytotoxic immunomodulatory effects via cell surface engineering, leading to tumor recurrence, and the availability of immune escape afforded by the cell membrane proteins, resulting in increased EPR in treating cancer, are all key benefits of using cells and cell membranes as drug delivery carriers (74,75,76).
Techniques for improving drug delivery using nanoparticles in cancer therapy and immunomodulation, including current and potential cell types employed in this context (strategies include nanoparticle hitchhiking, autocrine signaling via cell membrane-bound nanoparticles, cell surface engineering, and cell membrane-coated nanoparticle-based drug delivery). The barrier between the blood and brain is sometimes known as the blood–brain barrier.
Using bio-hybrid NPs functionalized with WBC cell membranes as a cancer treatment is promising (Fig. 2). Janus NPs that were partially coated with WBC membrane were shown to increase absorption in cancer cells by He and colleagues. This resulted in preferential recognition between the various types of cancer cells. A system very similar to this one in photothermal cancer treatment demonstrated positive results (77). Similarly, the CM of macrophages was utilized in order to coat MSNPs. This resulted in an increase in the amount of time the NPs spent in circulation, as well as an increase in the amount of tumor accumulation, in addition to active targeting in cancer cells for the delivery of DOX (78).
Recent research conducted by Palomba and colleagues has shown that nanoporous silica NPS (NSNPs) that are covered with a WBC cell membrane have elevated tumor vasculature penetration. This is because immune cells have the innate ability to target inflamed vasculature and penetrate across the endothelium. NPS that are coated with the membranes of cancer cells presents an additional challenging method for targeting cancer. This unique CM provides an exciting functionalization of the specific molecules (homologous binding adhesion molecules), which are promising for the synthesis of theranostic NPs for the photothermal therapy of cancers (78). Additionally, the homologous binding adhesion molecules have been shown to have anticancer properties. Additionally, the homologous binding adhesion molecules have been shown to have anticancer properties. Although this method has yet to be utilized to its full potential in drug delivery applications, researchers’ substantial findings should be applied to nanoparticles of this particular type. In this area of research, Fang and colleagues recently released an important work in which they proposed an anticancer vaccine that is based on biodegradable PLGA NPs covered with mouse melanoma cells. The synthesized NPs demonstrated the same level of cell adherence as the donor cells while also exhibiting enhanced cell-specific targeting (79).
4 Biocompatibility of the biomimetic NPs
The biocompatibility of the biomimetic NPs synthesized through different methods from various sources must be clarified before clinical translation (27, 80, 81). Even though CM-coated NPs eliminate problems associated with conventional free drugs and nanocarriers, such as the administration of free therapeutic molecules. These problems include low solubility in an aqueous environment, non-specific, and off-target for cancer cells, and the subsequent adverse effects on normal cells (82, 83). Recent research by Xuan and colleagues describes the use of cancer photothermal therapy utilizing AuNPs that have been coated with the macrophage cell membrane. Through the use of macrophages as a camouflage, coated particles were able to accumulate in cancer cells in vivo. This method permitted active drug targeting, which was made possible by the nanoparticle envelops’ particular identification of tumor endothelium. Additionally, this method resulted in more efficient accumulation compared to RBC-coated nanoparticles. PLGA NPs loaded with gambogic acid (a poorly water-soluble substance) and covered with RBC membranes were tested on colorectal cancer cells by Zhang et al. (84).
In addition to investigations on absorption and anticancer activity, the cytocompatibility of these NPs, with or without the CM-covering, was examined by incubating the NPs with macrophages. This was done both with and without the cell membrane coating. They were able to resist phagocytosis, thanks to the presence of particular proteins on the RBC membrane, which resulted in an increased amount of time spent in circulation. As a result, they demonstrated a decreased rate of absorption. In order to cure breast cancer and reduce the medicine’s toxicity at the same time, Sun and colleagues created PLGA NPs coated with macrophage membrane and then loaded with the anticancer agent saikosaponin D. These nanoparticles targeted breast cancer cells, which exhibited overexpression of the transferrin receptors. This was made possible by the presence of the T7 peptide on the membrane of macrophages. Studies comparing cancer cells’ uptake to that of healthy cells revealed that cancer cells had a higher rate of uptake than healthy cells (85).
In addition, cytotoxicity research revealed that cancer cells experienced a higher toxicity level than healthy cells, which did not experience any toxicity. Synthesized PLGA NPs loaded with the anticancer chemical bufalin and covered with platelet membrane were shown in a recent study to evade the uptake of macrophages and to increase the binding with target cancer cells. Compared to the free drug, these biomimetic nanoparticles were shown to reduce the cellular viability of H22 hepatoma cells more efficiently. In addition, cellular absorption was found to be more significant when using NPs coated with platelet membrane as opposed to those that were left uncoated. After that, the hemolysis assay was carried out to determine whether or not their blood was compatible with one another. The results showed that just 3.85% of the red blood cells were lysed, establishing that the blood was compatible (43).
In order to investigate the biosafety of the substance in vivo, these biomimetic NPs were given to a mouse model of the H22 tumor. When compared to the group that acted as the control, there was no evidence of any toxicity. In a different recent study that was very similar to this one, biocompatibility assays were performed on PLGA nanoparticles that carried the drug PTX and were covered with cytotoxic T-lymphocyte membranes for the treatment of gastric cancer. The results showed that macrophage uptake was decreased in comparison to the same NPs that did not have hCTL membrane coverage. The fact that these biomimetic structures are able to escape detection by the immune system was demonstrated by this study (86).
In addition, cytotoxicity testing revealed a parallel decline in cell viability throughout the course of time and in proportion to the increasing drug concentration. Furthermore, Corbo et al. (87) reported that biomimetic NPs play a role in the reduction of systemic toxicity in drug delivery. This was published alongside the promising biocompatibility and biosafety profiles published by Evangelopoulos et al. [75], which demonstrated a minimal accumulation of biomimetic NPs in the liver, lung, and spleen. Studies on the bio-compatibility of NPs continue to be a significant topic that must be investigated in depth for each biomimetic NP formulation, despite the encouraging results that have been obtained from the works that have been discussed. This is due to the fact that these nano-bio hybrid NPs are intended to attain a prolonged circulation period and are designed to bypass RES filtration. As a result, they are more likely to evoke potentially harmful effects (88).
The experimental techniques taken to synthesize biomimetic NPs need to be thoroughly standardized across different labs to obtain reproducible nanocarriers. This will allow us to overcome these possible issues and speed up their clinical translation. As was discussed in the preceding paragraphs, the experimental procedures that are required for the synthesis of biomimetic nanocarriers have the potential to change the biochemical and biophysical characteristics of the applied membrane through the modification of the membrane proteins' properties, including the composition, orientation, third structure stability, and glycosylation. This poses the potential risk of an unexpected immune response as well as adverse side effects. In point of fact, reports demonstrated an increase in the toxicity of biomimetic NPs, which was found to correlate with conformational changes of the proteins that were adsorbed on the surface of the NPs.
5 Market research and clinical trials
A growing number of clinical and preclinical studies can be attributed to development of more efficient production processes. These developments are allowing for a more rapid transition of drug-delivery nanoparticles from the laboratory to the patient’s bedside. Studies are essential for gaining a fundamental understanding of the particle-cell interactions that occur in people as well as the efficient functioning of the developed systems. Since the middle of the 1990s, the typical number of nanoparticle delivery systems that have been approved is around 13, whereas there have been 51 unique items developed. In the majority of cases, liposomal and first-generation polymer nanoparticles are taken into consideration in approved systems for clinical studies (89).
The clinical development of long-circulating nanoparticles has reached an advanced stage. [Clarification needed] In a recent report (90), researchers analyzed open clinical studies of long-circulating nanoparticles. Most cancer treatments are administered via clinically tested systems, which often include chemotherapeutic drugs currently in widespread clinical use.. For the therapy of chronic kidney illnesses, there is just one example of a non-PEGylated stealth NPs system, and that is Renagel (Sanofi S.A., Gentilly, France), which is made of PEI (poly (allylamine hydrochloride)) [108]. On the other hand, only a select few biological carriers have been given the green light for use in clinical studies. These biological carriers are bacteria-derived nano cells. The TargomiRs is an intriguing biomimetic technology that is currently being tested in a clinical Phase I experiment (91).
This system is made up of targeted bacterial minicells that are capable of producing miRNA. It is intended to be used as replacement therapy for individuals suffering from thoracic cancer. The EDVTM nanocell technology (EnGeneIC Ltd) is being used in this investigation (trial ID: NCT02369198), which has already been evaluated in animal and preclinical studies for the administration of chemotherapeutic drugs, siRNAs, and miRNAs. EDV containing mitoxantrone is currently being investigated in the Phase I trial for the treatment of refractory solid and CNS tumors in children. The same drug delivery carrier, loaded with DOX and containing EGFR, is also in Phase I clinical trial for the treatment of recurrent glioblastoma multiform (trial ID: NCT02766699) (trial ID: NCT02687386) (92,93,94,95). Table 1 summarizes the conducted clinical trials.
6 Conclusion
The sector of delivery systems has been extensively studied for cancer treatment and several illnesses, resulting in researchers seeking an approach that can meet the criteria of compatibleness for human use and, most remarkably, effectiveness in terms of health care, so as to reduce treatment with a freely systemic drug or with traditionally used treatments that often lead to side effects. Utilizing biomimetic carriers allows for the circumvention of physiological barriers and the reduction of off-target drug deposition, both of which are important goals. An ideal carrier would be one that is nontoxic, biodegradable, and exceedingly safe, exhibiting features related to size, surface charge, and generally the membrane that allow it to engage with the particular target without being identified as “not self” by the immune system. Researchers’ efforts to create biomimetic drug carriers have led to a noticeable improvement in the targeted drug delivery process. Increases in encapsulation efficiency, cellular uptake, and sustained drug release within target cells have all been observed. Preclinical data show promise for enhancing medication pharmacokinetics, which is exciting. The discoveries of novel materials and biological mechanisms from materials science and cell biology aid the improvement of biomimetic qualities. This emerging topic of nanotechnology offers a potential new approach to treating significant, widespread diseases. It is inevitable that the works on computer simulation and modeling in investigations of many different types of new nanomaterials will develop and rise in prominence as modern computational modeling of simulation and structure and property calculations of nanomaterials advance, as this will reduce the cost for their design and significantly increase the efficiency with which they are created. The development of novel materials with specified characteristics is currently dominated by the use of computer modeling and simulation. Our ability to forecast the physical properties, features, and behavior of biomimetic nanomaterials under varying situations, as well as identify the ideal parameters for the technologies required for their practical fabrication, can be possible by this method.
Data Availability
Not applicable.
Abbreviations
- AIE:
-
Aggregation-induced emission
- CM:
-
Cell membrane
- CMCNPs:
-
Cell membrane–coated NPs
- Chol:
-
Cholesterol
- CTC:
-
Circulating tumor cell
- EPR:
-
Enhanced permeability and retention
- HA:
-
Hydroxyapatite
- MS:
-
Mesoporous silica
- NPs:
-
Nanoparticles
- NK:
-
Natural killer cells
- NMs:
-
Nanomaterials
- NSNPs:
-
Nanoporous silica NPs
- PEG:
-
Polyethylene glycol
- PLGA:
-
Poly (lactic-co-glycolic acid)
- PEO:
-
Polyethylene oxide
- RBCs:
-
Red blood cells
- RES:
-
Reticuloendothelial system
References
K.A. Akulo, T. Adali, M.T.G. Moyo, T. Bodamyali, Intravitreal injectable hydrogels for sustained drug delivery in glaucoma treatment and therapy. Polymers 14(12), 2359 (2022)
I. Raya, S. Chupradit, Y.F. Mustafa, H. Oudaha, K, M Kadhim M, TurkiJalil A, et al., Carboxymethyl chitosan nano-fibers for controlled releasing 5-fluorouracil anticancer drug. J Nanostruct 12(1), 136–43 (2022)
Z.S. Madhi, M.A. Shallan, A.M. Almaamuri, A.A. Alhussainy, S.S.S. AL-Salih, A.K. Raheem et al., Lipids and lipid derivatives for delivery of the CRISPR/Cas9 system. J. Drug Deliv. Sci. Technol. 78, 103948 (2022)
S. Chupradit, A.T. Jali, Y. Enina, D.A. Neganov, M.S. Alhassan, S. Aravindha S et al., Use of organic and copper-based nanoparticles on the turbulator installment in a shell tube heat exchanger: a CFD-based simulation approach by using nanofluids. J. Nanomater. 2021, 3250058 (2021)
E. Alhomaidi, S.A. Jasim, H.I.M. Amin, M.A. Lima Nobre, M. Khatami, A.T. Jalil et al., Biosynthesis of silver nanoparticles using Lawsonia inermis and their biomedical application. IET Nanobiotechnol 16(7–8), 284–294 (2022)
S.A. Jasim, I. Patra, M.J.C. Opulencia, K. Hachem, R.M.R. Parra, M.J. Ansari et al., Green synthesis of spinel copper ferrite (CuFe2O4) nanoparticles and their toxicity. Nanotechnol Rev 11(1), 2483–2492 (2022)
Y.D. Taghipour, A. Zarebkohan, R. Salehi, F. Rahimi, V.P. Torchilin, M.R. Hamblin et al., An update on dual targeting strategy for cancer treatment. J Control Release 349, 67–96 (2022)
S.M. Hosseini, M. Taheri, F. Nouri, A. Farmani, N.M. Moez, M.R. Arabestani, Nano drug delivery in intracellular bacterial infection treatments. Biomed Pharmacother 146, 112609 (2022)
A. Firouzi-Amandi, M. Dadashpour, M. Nouri, N. Zarghami, H. Serati-Nouri, D. Jafari-Gharabaghlou et al., Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: Possible application in tissue regeneration. Biomed Pharmacother 105, 773–780 (2018)
M. Shahgolzari, H. Dianat-Moghadam, S. Fiering, Multifunctional plant virus nanoparticles in the next generation of cancer immunotherapies. Semin. Cancer Biol. 86(Pt 2), 1076–1085 (2022)
M. Mehrabi, N.M. Dounighi, S.M. RezayatSorkhabadi, D. Doroud, A. Amani, M. Khoobi et al., Development and physicochemical, toxicity and immunogenicity assessments of recombinant hepatitis B surface antigen (rHBsAg) entrapped in chitosan and mannosylated chitosan nanoparticles: as a novel vaccine delivery system and adjuvant. Artif Cells Nanomedicine Biotechnol 46(sup1), 230–240 (2018)
M. Azizi, H. Dianat-Moghadam, R. Salehi, M. Farshbaf, D. Iyengar, S. Sau et al., Interactions between tumor biology and targeted nanoplatforms for imaging applications. Adv Func Mater 30(19), 1910402 (2020)
W. Jiang, B.Y. Kim, J.T. Rutka, W.C. Chan, Advances and challenges of nanotechnology-based drug delivery systems. Expert Opin Drug Deliv 4(6), 621–633 (2007)
T. Yih, M. Al-Fandi, Engineered nanoparticles as precise drug delivery systems. J Cell Biochem 97(6), 1184–1190 (2006)
J.F. Vincent, O.A. Bogatyreva, N.R. Bogatyrev, A. Bowyer, A.-K. Pahl, Biomimetics: its practice and theory. J R Soc Interface 3(9), 471–482 (2006)
C. Guido, G. Maiorano, B. Cortese, S. D’Amone, I.E. Palamà, Biomimetic nanocarriers for cancer target therapy. Bioeng 7(3), 111 (2020)
J. Liu, S.S. Liew, J. Wang, K. Pu, Bioinspired and biomimetic delivery platforms for cancer vaccines. Adv Mater 34(1), 2103790 (2022)
A. Parodi, D. Kostyushev, S. Brezgin, A. Kostyusheva, T. Borodina, R. Akasov et al., editors. Biomimetic approaches for targeting tumor inflammation. Semin. Cancer Biol. 86, 555-567 (2022)
N. Khatoon, Z. Zhang, C. Zhou, M. Chu, Macrophage membrane coated nanoparticles: a biomimetic approach for enhanced and targeted delivery. Biomater. Sci. 10, 1193–1208 (2022)
Y. Wang, K. Zhang, X. Qin, T. Li, J. Qiu, T. Yin et al., Biomimetic nanotherapies: red blood cell based core–shell structured nanocomplexes for atherosclerosis management. Adv Sci 6(12), 1900172 (2019)
C.-M.J. Hu, L. Zhang, S. Aryal, C. Cheung, R.H. Fang, L. Zhang, Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci 108(27), 10980–5 (2011)
Q. Zhang, D. Dehaini, Y. Zhang, J. Zhou, X. Chen, L. Zhang et al., Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol 13(12), 1182–1190 (2018)
E.L. Siegler, Y.J. Kim, X. Chen, N. Siriwon, J. Mac, J.A. Rohrs et al., Combination cancer therapy using chimeric antigen receptor-engineered natural killer cells as drug carriers. Mol Ther 25(12), 2607–2619 (2017)
H. Wang, J. Wu, G.R. Williams, Q. Fan, S. Niu, J. Wu et al., Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J Nanobiotechnol 17(1), 1–16 (2019)
J. Gan, G. Du, C. He, M. Jiang, X. Mou, J. Xue et al., Tumor cell membrane enveloped aluminum phosphate nanoparticles for enhanced cancer vaccination. J Control Release 326, 297–309 (2020)
M. Sheikhpour, L. Barani, A. Kasaeian, Biomimetics in drug delivery systems: a critical review. J Control Release 253, 97–109 (2017)
H. Serati-Nouri, A. Mahmoudnezhad, M. Bayrami, D. Sanajou, M. Tozihi, L. Roshangar et al., Sustained delivery efficiency of curcumin through ZSM-5 nanozeolites/electrospun nanofibers for counteracting senescence of human adipose-derived stem cells. J Drug Deliv Sci Technol 66, 102902 (2021)
S. Nummelin, J. Kommeri, M.A. Kostiainen, V. Linko, Evolution of structural DNA nanotechnology. Adv Mater 30(24), 1703721 (2018)
M. Nikolova, R. Slavchov, G. Nikolova, Nanotechnology in medicine. Drug Discov. Eval. 533–46 (2020)
M. Khalili, A. Zarebkohan, H. Dianat-Moghadam, M. Panahi, H. Andre, E. Alizadeh, Corneal endothelial cell sheet bioengineering from neural crest cell-derived adipose stem cells on novel thermo-responsive elastin-mimetic dendrimers decorated with RGD. Chem Eng J 429, 132523 (2022)
H. Sadeghzadeh, A. Mehdipour, H. Dianat-Moghadam, R. Salehi, A.B. Khoshfetrat, A. Hassani et al., PCL/Col I-based magnetic nanocomposite scaffold provides an osteoinductive environment for ADSCs in osteogenic cues-free media conditions. Stem Cell Res Ther 13(1), 1–18 (2022)
Z. Amoozgar, Y. Yeo, Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip Rev: Nanomedicine Nanobiotechnol 4(2), 219–233 (2012)
F. Danhier, To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release 244, 108–121 (2016)
S.Y. Fam, C.F. Chee, C.Y. Yong, K.L. Ho, A.R. Mariatulqabtiah, W.S. Tan, Stealth coating of nanoparticles in drug-delivery systems. Nanomater 10(4), 787 (2020)
H. Otsuka, Y. Nagasaki, K. Kataoka, PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev 55(3), 403–419 (2003)
Y. Fang, J. Xue, S. Gao, A. Lu, D. Yang, H. Jiang et al., Cleavable PEGylation: a strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv 24(2), 22–32 (2017)
D. Zhang, H. Xu, M. Hu, Y. Deng, “PEG dilemma” for liposomes and its solving approaches. Yao Xue Bao Acta Pharm Sinica 50(3), 252–60 (2015)
K. Jin, Z. Luo, B. Zhang, Z. Pang, Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sinica B. 8(1), 23–33 (2018)
Z. He, Y. Zhang, N. Feng, Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review. Mater Sci Eng C 106, 110298 (2020)
L. Chen, W. Hong, W. Ren, T. Xu, Z. Qian, Z. He, Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther 6(1), 1–25 (2021)
S. Drotleff, U. Lungwitz, M. Breunig, A. Dennis, T. Blunk, J. Teßmar et al., Biomimetic polymers in pharmaceutical and biomedical sciences. Eur J Pharm Biopharm 58(2), 385–407 (2004)
D. Mendanha, J.V. de Castro, H. Ferreira, N. Neves, Biomimetic and cell-based nanocarriers—new strategies for brain tumor targeting. J Control Release 337, 482–493 (2021)
S.S. Kunde, S. Wairkar, Platelet membrane camouflaged nanoparticles: biomimetic architecture for targeted therapy. Int J Pharm 598, 120395 (2021)
M. Bilal, T. Rasheed, H.M. Iqbal, C. Li, H. Hu, X. Zhang, Development of silver nanoparticles loaded chitosan-alginate constructs with biomedical potentialities. Int J Biol Macromol 105, 393–400 (2017)
W. Gao, C.M.J. Hu, R.H. Fang, B.T. Luk, J. Su, L. Zhang, Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater 25(26), 3549–3553 (2013)
B. Palazzo, M. Iafisco, M. Laforgia, N. Margiotta, G. Natile, C.L. Bianchi et al., Biomimetic hydroxyapatite–drug nanocrystals as potential bone substitutes with antitumor drug delivery properties. Adv Func Mater 17(13), 2180–2188 (2007)
L. Sheikh, S. Tripathy, S. Nayar, Biomimetic matrix mediated room temperature synthesis and characterization of nano-hydroxyapatite towards targeted drug delivery. RSC Adv 6(67), 62556–62571 (2016)
L. Rao, B. Cai, L.-L. Bu, Q.-Q. Liao, S.-S. Guo, X.-Z. Zhao et al., Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 11(4), 3496–3505 (2017)
J. Jin, B. Krishnamachary, J.D. Barnett, S. Chatterjee, D. Chang, Y. Mironchik et al., Human cancer cell membrane-coated biomimetic nanoparticles reduce fibroblast-mediated invasion and metastasis and induce T-cells. ACS Appl Mater Interfaces 11(8), 7850–7861 (2019)
T. Kang, Q. Zhu, D. Wei, J. Feng, J. Yao, T. Jiang et al., Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano 11(2), 1397–1411 (2017)
A. Pitchaimani, T.D.T. Nguyen, S. Aryal, Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 160, 124–137 (2018)
C. Qin, J. Fei, P. Cai, J. Zhao, J. Li, Biomimetic membrane-conjugated graphene nanoarchitecture for light-manipulating combined cancer treatment in vitro. J Colloid Interface Sci 482, 121–130 (2016)
H. Dianat-Moghadam, M. Heidarifard, R. Jahanban-Esfahlan, Y. Panahi, H. Hamishehkar, F. Pouremamali et al., Cancer stem cells-emanated therapy resistance: implications for liposomal drug delivery systems. J Control Release 288, 62–83 (2018)
M. Li, C. Du, N. Guo, Y. Teng, X. Meng, H. Sun et al., Composition design and medical application of liposomes. Eur J Med Chem 164, 640–653 (2019)
S. Shah, V. Dhawan, R. Holm, M.S. Nagarsenker, Y. Perrie, Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev 154, 102–122 (2020)
P. Kumar, P. Huo, B. Liu, Formulation strategies for folate-targeted liposomes and their biomedical applications. Pharm 11(8), 381 (2019)
W. Yan, S.S. Leung, K.K. To, Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine 15(3), 303–318 (2020)
İ Eroğlu, M. İbrahim, Liposome–ligand conjugates: a review on the current state of art. J Drug Target 28(3), 225–244 (2020)
S. Mojarad-Jabali, S. Mahdinloo, M. Farshbaf, M. Sarfraz, Y. Fatahi, F. Atyabi et al., Transferrin receptor-mediated liposomal drug delivery: recent trends in targeted therapy of cancer. Expert Opin Drug Deliv 19(6), 685–705 (2022)
E. Westhaus, P.B. Messersmith, Triggered release of calcium from lipid vesicles: a bioinspired strategy for rapid gelation of polysaccharide and protein hydrogels. Biomater 22(5), 453–462 (2001)
X.L. Liu, X. Dong, S.C. Yang, X. Lai, H.J. Liu, Y. Gao et al., Biomimetic liposomal nanoplatinum for targeted cancer chemophototherapy. Adv Sci 8(8), 2003679 (2021)
S.Z. Vahed, R. Salehi, S. Davaran, S. Sharifi, Liposome-based drug co-delivery systems in cancer cells. Mater Sci Eng C 71, 1327–1341 (2017)
T. Rasheed, F. Nabeel, A. Raza, M. Bilal, H. Iqbal, Biomimetic nanostructures/cues as drug delivery systems: a review. Mater Today Chem 13, 147–157 (2019)
B. Ghosh, S. Biswas, Polymeric micelles in cancer therapy: state of the art. J Control Release 332, 127–147 (2021)
N.A. Hanafy, M. El-Kemary, S. Leporatti, Micelles structure development as a strategy to improve smart cancer therapy. Cancers 10(7), 238 (2018)
J.P. Xu, J. Ji, W.-D. Chen, J.C. She JC, editors. Biomimetic amphiphiles for polymeric micellar carrier system. Key Eng. Mater.; Trans. Tech. Publ. 288, 465–468 (2005)
J. Hu, W. Zhuang, B. Ma, X. Su, T. Yu, G. Li et al., Redox-responsive biomimetic polymeric micelle for simultaneous anticancer drug delivery and aggregation-induced emission active imaging. Bioconjug Chem 29(6), 1897–1910 (2018)
Y. Wang, Z. Luan, C. Zhao, C. Bai, K. Yang, Target delivery selective CSF-1R inhibitor to tumor-associated macrophages via erythrocyte-cancer cell hybrid membrane camouflaged pH-responsive copolymer micelle for cancer immunotherapy. Eur J Pharm Sci 142, 105136 (2020)
K. Wang, R. Shen, T. Meng, F. Hu, H. Yuan, Nano-drug delivery systems based on different targeting mechanisms in the targeted therapy of colorectal cancer. Mol 27(9), 2981 (2022)
G. Trapani, N. Denora, A. Trapani, V. Laquintana, Recent advances in ligand targeted therapy. J Drug Target 20(1), 1–22 (2012)
P. Dash, A.M. Piras, M. Dash, Cell membrane coated nanocarriers-an efficient biomimetic platform for targeted therapy. J Control Release 327, 546–570 (2020)
C.-H. Xu, P.-J. Ye, Y.-C. Zhou, D.-X. He, H. Wei, C.-Y. Yu, Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater 105, 1–14 (2020)
S. Yaman, U. Chintapula, E. Rodriguez, H. Ramachandramoorthy, K.T. Nguyen, Cell-mediated and cell membrane-coated nanoparticles for drug delivery and cancer therapy. Cancer Drug Resist (Alhambra, Calif). 3, 879 (2020)
W. Wuhao, Y. Zhang, Z. Lin, X. Wu, W. Fan, J. Chen, Advances, challenge and prospects in cell-mediated nanodrug delivery for cancer therapy: a review. J. Drug Target. 31, 1–13 (2023)
E. Nemati, Cell membrane coated nanoparticles for biomedical applications. Adv Appl NanoBio-Technol 3(1), 49–59 (2022)
M. Imran, L.A. Jha, N. Hasan, J. Shrestha, R. Pangen, N. Parvez et al., “Nanodecoys”—future of drug delivery by encapsulating nanoparticles in natural cell membranes. Int. J. Pharm. 621, 121790 (2022)
W. He, J. Frueh, Z. Wu, Q. He, How leucocyte cell membrane modified janus microcapsules are phagocytosed by cancer cells. ACS Appl Mater Interfaces 8(7), 4407–4415 (2016)
R. Kotcherlakota, A.K. Barui, S. Prashar, M. Fajardo, D. Briones, A. Rodríguez-Diéguez et al., Curcumin loaded mesoporous silica: an effective drug delivery system for cancer treatment. Biomater Sci 4(3), 448–459 (2016)
R.H. Fang, C.-M.J. Hu, B.T. Luk, W. Gao, J.A. Copp, Y. Tai et al., Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett 14(4), 2181–8 (2014)
M. Abdelkarim, L. Perez-Davalos, Y. Abdelkader, A. Abostait, H.I. Labouta, Critical design parameters to develop biomimetic organ-on-a-chip models for the evaluation of the safety and efficacy of nanoparticles. Expert Opin. Drug Deliv. 20, 13–30 (2023)
K.G. Gareev, D.S. Grouzdev, V.V. Koziaeva, N.O. Sitkov, H. Gao, T.M. Zimina et al., Biomimetic nanomaterials: diversity, technology, and biomedical applications. Nanomater 12(14), 2485 (2022)
U.H. Ibrahim, N. Devnarain, T. Govender, Biomimetic strategies for enhancing synthesis and delivery of antibacterial nanosystems. Int J Pharm 596, 120276 (2021)
Y.-k Gong, F.M. Winnik, Strategies in biomimetic surface engineering of nanoparticles for biomedical applications. Nanoscale 4(2), 360–8 (2012)
Z. Zhang, H. Qian, M. Yang, R. Li, J. Hu, L. Li et al., Gambogic acid-loaded biomimetic nanoparticles in colorectal cancer treatment. Int J Nanomed 12, 1593 (2017)
K. Sun, W. Yu, B. Ji, C. Chen, H. Yang, Y. Du et al., Saikosaponin D loaded macrophage membrane-biomimetic nanoparticles target angiogenic signaling for breast cancer therapy. Appl Mater Today 18, 100505 (2020)
L. Zhang, R. Li, H. Chen, J. Wei, H. Qian, S. Su et al., Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer. Int J Nanomed 12, 2129 (2017)
C. Corbo, R. Molinaro, F. Taraballi, N.E. Toledano Furman, K.A. Hartman, M.B. Sherman et al., Unveiling the in vivo protein corona of circulating leukocyte-like carriers. ACS Nano 11(3), 3262–3273 (2017)
M. Evangelopoulos, A. Parodi, J.O. Martinez, I.K. Yazdi, A. Cevenini, A.L. van de Ven et al., Cell source determines the immunological impact of biomimetic nanoparticles. Biomater 82, 168–177 (2016)
D. Bobo, K.J. Robinson, J. Islam, K.J. Thurecht, S.R. Corrie, Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 33(10), 2373–2387 (2016)
C.-M.J. Hu, R.H. Fang, B.T. Luk, L. Zhang, Polymeric nanotherapeutics: clinical development and advances in stealth functionalization strategies. Nanoscale 6(1), 65–75 (2014)
G. Reid, S.C. Kao, N. Pavlakis, H. Brahmbhatt, J. MacDiarmid, S. Clarke et al., Clinical development of TargomiRs, a miRNA mimic-based treatment for patients with recurrent thoracic cancer. Epigenomics 8(8), 1079–1085 (2016)
J.A. MacDiarmid, N.B. Mugridge, J.C. Weiss, L. Phillips, A.L. Burn, R.P. Paulin et al., Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell 11(5), 431–445 (2007)
J.A. MacDiarmid, N.B. Amaro-Mugridge, J. Madrid-Weiss, I. Sedliarou, S. Wetzel, K. Kochar et al., Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat. Biotechnol. 27(7), 643–651 (2009)
G. Reid, M. Pel, M. Kirschner, Y.Y. Cheng, N. Mugridge, J. Weiss et al., Restoring expression of miR-16: a novel approach to therapy for malignant pleural mesothelioma. Ann Oncol 24(12), 3128–3135 (2013)
J.R. Whittle, J.D. Lickliter, H.K. Gan, A.M. Scott, J. Simes, B.J. Solomon et al., First in human nanotechnology doxorubicin delivery system to target epidermal growth factor receptors in recurrent glioblastoma. J Clin Neurosci 22(12), 1889–1894 (2015)
Funding
This study was supported by the Al-Mustaqbal University College, Babylon, Hilla 51001, Iraq.
Author information
Authors and Affiliations
Contributions
Hussein Riyadh Abdul Kareem Al-Hetty: conceptualization, investigation, writing — original draft. Jabbar Hadsoon Zamil Al-Tamimi, Marwan Mahmood Saleh: investigation, writing — original draft. Maitha Sameer Kadhim, Naked Mahmood Ahmed, Mahmoud Kandeel, and Ruaa H. Abbas: contributed writing revised draft and figures improving. Abduladheem Turki Jalil: writing — review and editing, visualization, supervision, and project administration. All co-authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Research involving human participants and/or animals
Non applicable.
Informed consent
Non applicable.
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Hetty, H.R.A.K., Kadhim, M.S., Al-Tamimi, J.H.Z. et al. Implications of biomimetic nanocarriers in targeted drug delivery. emergent mater. 6, 1–13 (2023). https://doi.org/10.1007/s42247-023-00453-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00453-8