Abstract
Many decellularized extracellular matrices have been developed and applied to various bioindustrial applications. Similar to these applications, decellularized extracellular matrices have started to be used for stem cell engineering. There are numerous efforts to unveil the effects of decellularized extracellular matrices on stem cell functions, and it has been demonstrated that decellularized extracellular matrices impact various stem cell functions, particularly differentiation, stem cell maintenance, and recovery of lost stemness (rejuvenation). In this chapter, stem cell functions on the decellularized extracellular matrices are summarized following a brief comparison of sources and a description of decellularized extracellular matrices preparation and characterization. Additionally, the present problems of decellularized extracellular matrices in stem cell applications are outlined in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Stem cells are a promising cell source for various bioindustrial applications (e.g., tissue engineering, regenerative medicine, and pharmacological studies) (Liu et al. 2020; Mohammadian 2018). The technology of regulating stem cell functions, including differentiation and stemness maintenance, is the key to their achievements. There are many efforts to regulate stem cell functions. One of the approaches is improving the culture conditions (Liu et al. 2020; Chen et al. 2011; Ng et al. 2008). Among the culture conditions, cell culture substrate is an important factor as well as a culture medium. Many substrates for stem cell culture have been developed with synthetic polymeric materials (Olivares-Navarrete et al. 2017; Duffy et al. 2014) and isolated proteins (Chen et al. 2011; Nakagawa et al. 2014), and stem cell functions are regulated to some degree.
In our body, stem cells are surrounded by a specific microenvironment, the so-called “stem cell niche.” The stem cell niche is composed of cells and extracellular matrix (ECM) (Mercier et al. 2002; Meran et al. 2017). In particular, the ECM plays pivotal roles in regulating many cell functions, such as cell adhesion, growth, migration, differentiation, and responses to soluble factors, through the activation of various intracellular signaling pathways (Harburger and Calderwood 2009; Geiger and Yamada 2011). It has been tried to reconstitute native ECM as culture substrates for regulating stem cell functions. ECM is constituted with many proteins and carbohydrates. However, there are over 300 types of ECM proteins (Hynes and Naba 2012), and their combinations vary according to cell/tissue/organ types and developmental and pathological stages (Naba et al. 2012; Lu et al. 2011; Manabe et al. 2008). Moreover, not all ECM molecules have been identified, and the complete compositions of the ECM have not yet been identified. Therefore, it is very difficult to reconstitute native ECM by combining identified ECM proteins and other conventional chemical methods. For this reason, the decellularization technique has been used to reconstitute native ECM as culture substrates in vitro. Currently, ECM reconstituted by the decellularization technique (decellularized ECM: dECM) are commercially available for clinical applications (Nakamura et al. 2017; Crapo et al. 2011), and dECM have started to be used as in vitro ECM models for cancer research (Hoshiba and Tanaka 2016; Hoshiba 2018, 2019; Castelló-Cros et al. 2009). dECM are now used as culture substrates for stem cell culture to induce specific stem cell functions. In this chapter, I summarize the methods for dECM preparations after comparing dECM sources. Additionally, stem cell functions exhibited on dECM are reviewed. Finally, the potential applications of dECM as culture substrates are discussed.
17.2 Preparation of dECM for Stem Cell Culture
17.2.1 Sources
dECM are generally prepared from two sources: ECM in tissues/organs and ECM produced by cultured cells. Both of them have advantages and disadvantages for the preparation, characteristics, and feasibility of stem cell culture (Table 17.1). Therefore, the sources of dECM should be carefully selected according to their purposes. Generally, dECM derived from tissues and organs can possess compositional, macro- and microstructural, and mechanical similarities with native ECM if decellularization is properly performed. Additionally, tissue-/organ-derived dECM can be obtained at larger scales than cultured cell-derived dECM. These are the most significant advantages of tissue-/organ-derived dECM to cultured cell-derived dECM. However, tissue-/organ-derived dECM tends to show significant batch-to-batch differences due to the individual differences in sources compared with cultured cell-derived dECM. More importantly, for stem cell culture, it is difficult to isolate ECM in limited regions (e.g., stem cell niche) as tissue-/organ-derived dECM due to the difficulties of their identification and isolation.
In contrast to tissue-/organ-derived dECM, cultured cell-derived dECM can be prepared with smaller batch-to-batch differences than tissue-/organ-derived dECM if the culture conditions (e.g., the compositions of a culture medium, initial culture substrates, and cell passages) are properly controlled. Additionally, cultured cell-derived dECM can mimic ECM in limited regions, such as the stem cell niche, which is the most significant advantage of cultured cell-derived dECM to tissue-/organ-derived dECM. However, compositional, macro- and microstructural, and mechanical similarities of cultured cell-derived dECM tend to be lower than those of tissue-/organ-derived dECM. Therefore, these similarities should be checked more carefully when cultured cell-derived dECM are used as ECM models. Additionally, cultured cell-derived dECM are challenging to prepare at large scales even though the batch-to-batch differences can be controlled at a small level. Tissue-/organ-derived dECM tend to be used for stem cell differentiation and for the reconstitution of engineered tissues and organs with stem cells. On the other hand, cultured cell-derived dECM tend to be used to maintain and differentiate stem cells and are mainly applied for in vitro applications. The above functions and applications of dECM are further reviewed in the following sections.
17.2.2 Preparation
17.2.2.1 Decellularization
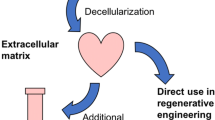
The decellularization process is critical for many properties and functions of the dECM. Decellularization is performed by various methods: treatment with detergents (e.g., sodium dodecyl sulfate, sodium deoxycholate, Triton X-100), chemical methods (e.g., alkaline or acid treatments and chelating), physical treatments (e.g., hypotonic and hypertonic pressures, ultrahydrostatic pressure, and freeze-thawing), enzymatic treatments (e.g., DNase, RNase, and proteinases), and their combinations (Gilbert et al. 2006; Keane et al. 2015; Nakamura et al. 2017; Crapo et al. 2011). For the decellularization of tissues and organs, the solution for decellularization can be perfused through their blood vessels, enabling decellularization while keeping macro- and microstructures of original tissues and organs (Ott et al. 2008; Uygun et al. 2010). For this structural advantage, whole tissue-/organ-derived dECM have been developed as scaffolds for the in vitro reconstruction of new tissues and organs. In addition to whole tissue/organ decellularization, tissues and organs can be decellularized after physical treatments, such as cutting and milling, for fabrication.
17.2.2.2 ECM Formation by Cultured Cells
For the preparation of cultured cell-derived dECM, ECM should be formed by the cells prior to decellularization. ECM formation is influenced by various factors: culture medium compositions (influencing ECM compositions and formation rates) (Satyam et al. 2014; Furuyama and Mochitate 2000; Furuyama et al. 1999), initial culture substrates (influencing ECM compositions, formation rates, and macrostructures) (Mochitate et al. 2020; Hoshiba and Tanaka 2015; Prewitz et al. 2013), and cell types (influencing ECM compositions) (Hoshiba and Tanaka 2015; Hoshiba et al. 2011a, 2012a) (Fig. 17.1). ECM formation conditions should be carefully optimized for cultured cell-derived dECM because the formed ECM strongly influences cell functions. Additionally, decellularization (described above) and post-decellularization (described below) processes impact the functions of cultured cell-derived dECM. Detailed effects of ECM formation conditions are discussed in a previous review (Hoshiba 2017).
Schematic illustration of the simple decellularization process. (Reproduced from Hoshiba (2017) by permission from RSC publishing)
17.2.2.3 Modification
Prepared dECM are often modified with several methods. ECM components and ECM fragments (e.g., ECM-derived peptides) can be easily modified by immersion in these molecule-containing solutions (Mahara et al. 2019). Additionally, treatments with enzymes, such as proteinases and enzymes degrading carbohydrates, can remove specific ECM components (Hoshiba 2018; Hoshiba et al. 2011b). Cross-linking of the dECM can change the mechanical properties to modulate mechanical signal transduction (Kim et al. 2018b). These modifications can improve dECM functions and can add new functions to the original dECM. Additionally, dECM is sometimes treated with pepsin for solubilization. Solubilized dECM can be used as a supplement to the culture medium (Crapo et al. 2012) and coating materials to the substrate surface (Aguado et al. 2016). More importantly, the solubilized dECM form gels after neutralization, making it possible to use solubilized dECM as injectable gels (Singelyn et al. 2012; Freytes et al. 2008).
17.2.2.4 Fabrication
Tissue-/organ-derived dECM can maintain the macro- and microstructures of original tissues and organs, which is an advantage for tissue and organ reconstruction. On the other hand, it is usually required for the fabrication of dECM into desirable shapes, including patch and tube shapes. dECM are sometimes solubilized and incorporated in other polymeric materials. Then, hybrid materials are fabricated into several shapes (Gao et al. 2017; Baiguera et al. 2014). According to the recent progress of three-dimensional (3D) printing technology, the combination of dECM and 3D printing technology has been applied to fabricate dECM into desirable shapes (Dzobo et al. 2019; Kim et al. 2018a). For this purpose, solubilized dECM are used as bioinks. For cultured cell-derived dECM, initial culture substrates act as templates and then determine dECM shapes. Therefore, initial culture substrates are prepared by 3D printing technology to form desirable shapes of cultured cell-derived dECM and initial culture substrate complexes (Hoshiba and Gong 2018). Moreover, initial culture substrates as templates can be removed if they are prepared with degradable materials, such as poly(lactic-co-glycolic acid) (PLGA) (Lu et al. 2011a, 2011b).
17.2.3 Characterization
Cell removal confirmation should be the first step for dECM characterization. Additionally, it is necessary to examine how ECM components and structures are retained in the prepared dECM. A summary of methods for these characterizations is shown in Table 17.2. Additionally, a general characterization of the materials is required.
17.2.3.1 Confirmation of Cell Removal
Detection of cellular components is always performed to confirm cell removal. In particular, cell nuclei and/or nuclear DNA are generally detected for the confirmation of cell removal. For tissue-/organ-derived dECM, three criteria are proposed for the confirmation of cell removal: (1) no visible nuclei are detected in tissue sections stained with DAPI or hematoxylin, (2) <50 ng dsDNA per mg ECM dry weight, and (3) <200 bp DNA fragment length (Crapo et al. 2011). However, these criteria have not been fully discussed, and a consensus should be built in the field. On the other hand, there are currently no criteria to confirm cell removal for cultured cell-derived dECM. For the cultured cell-derived dECM prepared in 2D culture, cell removal is easily confirmed by microscopic observation. For the dECM prepared in 3D culture, it seems proper to follow the criteria for tissue-/organ-derived dECM. In addition to nuclear detection, the detection of cytosolic proteins, such as cytoskeletal fibrillar actin, is often used. The detection of these components was performed by immunohistochemical analyses.
17.2.3.2 Confirmation of ECM Component Retention
The confirmation of ECM components is usually performed by immunohistochemical analyses with antibodies against specific ECM proteins and carbohydrates and with lectins against specific glycosaminoglycans. In addition to the detection of specific ECM components, denatured collagen can be detected with some collagen hybridizing peptides that might be used for the quality control of dECM (Li et al. 2012; Hwang et al. 2017). Additionally, several histochemical analyses, such as Sirius red staining and alcian blue staining, were performed. Recently, mass spectrometry has been used for exhaustive testing of ECM components (Piccoli et al. 2018).
17.2.3.3 Other Characterization
To examine the ECM microstructure, observations are usually performed with scanning electron microscopy (SEM) and transmission electron microscopy (TEM). In particular, the basement membrane, a special architecture of the ECM, can be identified as an electron-dense region by TEM. TEM should be used to check the integrity of the basement membrane after decellularization (Furuyama and Mochitate 2000). Additionally, fast Fourier transform analysis has been used to study fibril alignment (Harris et al. 2018).
17.3 Stem Cell Functions on dECM
Many studies have developed dECM for the culture of various stem cells. These studies reported that the dECM influenced many stem cell functions (Fig. 17.2). In particular, stem cell differentiation, stemness maintenance, and recovery from loss of stemness (rejuvenation) are important for bioindustrial applications with stem cells. In this section, the behaviors of stem cells on the dECM are summarized from the viewpoints of these functions.
17.3.1 Stem Cell Differentiation
The regulation of stem cell differentiation is the key for stem cell-based bioindustrial applications, including regenerative medicine and tissue engineering. Therefore, dECM have been applied for the regulation of stem cell differentiation. Many dECM derived from both tissues/organs and cultured cells have been developed. The partial lists of these dECM are shown in Tables 17.3 and 17.4. Proper ECM composition is required to fully exert cell functions (Hynes and Naba 2012). Indeed, it has been reported that the functions of somatic cells are strongly induced on the dECM originating from the same sources (Hoshiba et al. 2011a; Sellaro et al. 2007). Therefore, tissues/organs/cultured cells that are the same as the targets of differentiation are frequently used as sources for dECM preparation.
17.3.1.1 Differentiation on Tissue-/Organ-Derived dECM
Tissue-/organ-derived dECM have been applied for the differentiation culture of both multipotent somatic stem cells and pluripotent stem cells (i.e., embryonic stem (ES) cells and induced pluripotent stem (iPS) cells) (Table 17.3). Myoblasts were cultured on skeletal muscle-derived dECM (Chaturvedi et al. 2015; Stern et al. 2009). The dECM supported the growth of a murine myoblast cell line, C2C12, even in a serum-free medium (Chaturvedi et al. 2015). Moreover, myotube formation was promoted on these dECM.
Human liver stem-like cells (HLSCs), which can differentiate into hepatocytes, were also cultured in whole liver-derived 3D dECM (Navarro-Tableros et al. 2015). The dECM can support liver stem cell viability with EGF and FGF and can induce differentiation into hepatocytes. Additionally, some HLSCs differentiated into epithelial cells and tubular structure-formed endothelial cells. The reconstructed tissues with HLSCs and dECM showed urea production activity. Therefore, it is expected that this reconstructed tissue can be used as a liver-like tissue. When neural stem cells (NSCs) were cultured on brain-derived dECM, contradictory results were reported (Waele et al. 2015; Crapo et al. 2012). Waele et al. reported that an NSC line that was originally established on brain-derived dECM suppressed the differentiation of neural cells (Waele et al. 2015). On the other hand, Crapo et al. reported that PC12 cells (an NSC model cell line) on brain-derived dECM promoted differentiation into neural cells (Crapo et al. 2012). It is not clear why these opposite results were obtained after using similar brain-derived dECM. It is possible that the differences in cell types used in the studies, decellularization methods, region of the brain for decellularization, the provided form of dECM to the cells, and other culture conditions led to these conflicting results.
Tissue-/organ-derived dECM have also been applied for the differentiation culture of pluripotent stem cells, such as ES and iPS cells. Higuchi et al. prepared heart and liver-derived dECM, and these dECM were applied for the cardiac differentiation culture of ES cells (Higuchi et al. 2013). Cardiac differentiation marker expression was higher on heart-derived dECM than liver-derived dECM. This report suggested that dECM from different sources have different effects on stem cell differentiation. Additionally, tissue-/organ-derived dECM have been used for ES/iPS cell differentiation to reconstruct new tissues and organs with minor cell populations in vitro because these pluripotent stem cells can differentiate into almost any cell type. This is one of the biggest challenges in tissue engineering and regenerative medicine with stem cells. Bruno et al. prepared dECM from mammary tissues and solubilized the dECM (Bruno et al. 2017). Then, ES cells were transplanted in cleared mammary fat pads with solubilized dECM. The solubilized dECM inhibited teratoma formation in all transplanted cases but directed differentiation into mammary epithelial cells with gland-like structures.
Cortiella et al. tried to culture ES cells in dECM derived from the whole lung with the trachea (Cortiella et al. 2010). The dECM supported cell viability and growth and, surprisingly, the dECM directed site-specific differentiation into lung and tracheal cells. In the upper tracheal region, there were no occlusions, and the cells expressing cytokeratin were lined along the tracheal wall to form a sheet structure. Additionally, cytokeratin-18-expressing cells (i.e., ciliated epithelial cells) were found in the upper tracheal region. Clara cell protein 10-expressing cells (i.e., Clara cells) were found in the lower tracheal region. In the distal lung regions, pro-surfactant protein C-expressing cells (i.e., type II pneumocytes) formed hollow epithelial cyst-like structures. Moreover, CD31-expressing cells (i.e., endothelial cells) were found in lung and tracheal regions, suggesting that the reconstructed tissues were vascularized. This report suggests that tissue-/organ-derived dECM provide powerful platforms for the reconstruction of large tissues and organs with ES/iPS cells. However, further studies are required for cell seeding methods, teratoma formation suppression, and site-specific differentiation in other tissue-/organ-derived dECM. For the reconstruction of new tissues and organs with ES/iPS cells, trials to seed cells differentiated in vitro into specific cells into tissue-/organ-derived dECM have also been performed (Takeishi et al. 2020; Ghaedi et al. 2013).
17.3.1.2 Differentiation on Cultured Cell-Derived dECM
Cultured cell-derived dECM have also been applied for the differentiation culture of somatic stem cells and ES/iPS cells (Table 17.4). Cultured cell-derived dECM are mainly used for research purposes and in vitro applications, such as pharmacological studies. In contrast to tissue-/organ-derived dECM, many stem cells have been cultured on the dECM derived from cells that are different from differentiation target cells. These trials will help to find alternative tissue/organ dECM sources for stem cell differentiation. When NSCs were cultured on glioma C6 cell-derived dECM with an inhibitor of GSK-3β, SB216763, NSCs were promoted to differentiate into neural cells (neurons, astrocytes, and oligodendrocytes) (Jian et al. 2015). MSCs were cultured on chondrocyte-derived dECM to promote chondrogenesis without hypertrophy (Choi et al. 2010). In addition to somatic stem cells, ES and iPS cells were grown on cultured cell-derived dECM. When ES cells were cultured on pancreatic RIN5F cell-derived dECM, ES cells differentiated into insulin-secreting β cells (Narayanan et al. 2014).
dECM derived from cells that are different from differentiation target cells have been used for stem cell differentiation. NSCs were cultured on embryonic fibroblast-derived dECM, and the cells differentiated into basal forebrain cholinergic neurons (Bai et al. 2018). SV40-immortalized type II alveolar epithelial cell-derived dECM have been used for tracheal basal cell culture, and tracheal basal cells are differentiated into ciliated cells (Hosokawa et al. 2007). Pancreatic lineage commitment was promoted by dECM derived from both HEK293 cells expressing laminin-511 and 804G bladder carcinoma cells (Kaitsuka et al. 2014; Higuchi et al. 2010).
17.3.1.3 dECM Mimicking Native ECM at Stepwise Developmental Stages
Stem cell differentiation proceeds step-by-step in vivo, and the compositions of the ECM surrounding differentiating cells are changed according to their differentiation stages (Hoshiba et al. 2009, 2010, 2011b, 2012b; Cai et al. 2015). It is difficult for tissue-/organ-derived dECM to be prepared as dECM surrounding differentiating stem cells because such ECM is difficult to identify and isolate. In contrast to tissue-/organ-derived dECM, it is possible for cultured cell-derived dECM to be prepared as dECM mimicking native ECM surrounding differentiating stem cells. Hoshiba et al. prepared dECM derived from MSCs differentiating into osteoblasts and adipocytes at each differentiative stage, and these types of dECM are termed stepwise tissue development-mimicking matrices (stepwise osteogenesis-/adipogenesis-mimicking matrices) (Hoshiba et al. 2009, 2010). The osteogenesis of MSCs was promoted on dECM mimicking ECM at an early osteogenic stage (osteogenic early-stage matrices) but not on other dECM mimicking ECM at a late osteogenic stage (osteogenic late-stage matrices), adipogenic early- and late-stage matrices (adipogenic early/late-stage matrices), and undifferentiation state (stem cell matrices) (Fig. 17.3a) (Hoshiba et al. 2012b). On the other hand, adipogenesis of MSCs was promoted on adipogenic early-stage matrices but not stem cell matrices, osteogenic early/late-stage matrices, and adipogenic late-stage matrices (Fig. 17.3b) (Hoshiba et al. 2012b).
Osteogenesis and adipogenesis of MSCs on stepwise osteogenesis-/adipogenesis-mimicking matrices. (a) Alkaline phosphatase staining of MSCs under osteogenic conditions on stepwise osteogenesis-/adipogenesis-mimicking matrices. (b) Glycerol-3-phosphate dehydrogenase (GPDH) activity in MSCs under adipogenic conditions on stepwise osteogenesis-/adipogenesis-mimicking matrices. Schematic models of the role of ECM in osteogenesis (c) and adipogenesis (d) of MSCs. (Reproduced with slight modification from the reference Hoshiba et al. 2012b by permission from Elsevier)
The mechanisms of MSC differentiation were examined from the viewpoint of transcription factor expression (Hoshiba et al. 2012b). The gene expression of an osteogenesis-promotive transcription factor, runt-related transcription factor 2 (RUNX2), was increased on dECM except for stem cell matrices to promote osteogenesis. Gene expression of an osteogenic-inhibitory/adipogenesis-promotive transcription factor, peroxisome proliferator-activated receptor γ (PPARG), was inhibited on stem cell matrices and osteogenic early-stage matrices (Hoshiba et al. 2012b) by the suppression of canonical Wnt signaling via the binding between chondroitin sulfate and Wnt ligands (Hoshiba et al. 2009, 2011b, 2012b). These results suggest that osteogenic early-stage matrices promote osteogenesis by increasing osteogenic transcription factor expression and suppressing unexpected apoptosis (Fig. 17.3c) (Hoshiba et al. 2009, 2012b). Similar results were obtained in the case of adipogenesis. PPARG expression levels were similar on the dECM. However, RUNX2 (suppressing adipogenesis) expression was suppressed on stem cell matrices and adipogenic early/late-stage matrices. Moreover, the expression of TAZ, an osteogenesis promotive and adipogenesis inhibitory factor, was suppressed on only adipogenic early-stage matrices. These results suggest that adipogenic early-stage matrices promoted adipogenesis by inhibiting osteogenesis (Fig. 17.3d) (Hoshiba et al. 2010, 2012b). Similar to the stepwise osteogenesis-/adipogenesis-mimicking matrices, stepwise myogenesis-mimicking matrices were prepared by differentiating myoblasts into myotubes (Hoshiba and Yokoyama 2020). Myotube formation was promoted on myogenic early-stage matrices by the suppression of inhibitor of DNA binding (ID) gene expression via the inhibition of bone morphogenetic protein (BMP) signaling activation. As demonstrated in these studies, cultured cell-derived dECM can be used as a suitable tool to examine the comprehensive roles of ECM in stem cell differentiation.
17.3.1.4 Future Problems in Stem Cell Differentiation Culture with dECM
As described above (Sect. 17.3.1.1), the cells exhibited opposite effects on stem cell differentiation on the dECM even though the dECM originated from the same source (Waele et al. 2015; Crapo et al. 2012). It is necessary to understand why the opposite effects were obtained; otherwise, unexpected side effects might occur for bioindustrial applications. However, the feasibility of dECM might be expanded to regulate stem cell differentiation if the effects of dECM can be changed by the preparation methods, cell types, and the provided form of dECM to the cells, differentiation culture conditions, etc.
There are many reports of decellularization methods, and their effects on the compositions and structures of dECM have been examined (Gilbert et al. 2006; Keane et al. 2015). The composition and structure impact stem cell differentiation. Therefore, the optimization and standardization of dECM preparation methods are required. In addition to the dECM preparation methods, the optimal methods for the preservation and sterilization of dECM should also be investigated. However, a limited number of studies have been performed on preservation (Fidalgo et al. 2018; Tsuchiya et al. 2014). Mechanistic analyses will be helpful for maximizing the effects of dECM. There are numerous reports of the mechanisms of stem cell differentiation by molecular biological methods. dECM can be modified to improve the functionalities of dECM by comparisons with these reports. In addition to molecular biological analyses, mass spectrometric analyses will improve the dECM.
17.3.2 Stemness Maintenance
Stemness is maintained in the stem cell niche in vivo (Mercier et al. 2002; Meran et al. 2017). Therefore, reconstruction of the ECM in the stem cell niche has been attempted by decellularization techniques. For this purpose, cultured cell-derived dECM has been mainly prepared to mimic native ECM in the stem cell niche.
17.3.2.1 Somatic Stem Cells
In this field, the maintenance of MSC stemness has been well investigated. Generally, MSCs spontaneously differentiate and lose their stemness during in vitro expansion culture. Spontaneous differentiation of MSCs is suppressed on undifferentiated MSC-derived dECM (Chen et al. 2007). Additionally, MSC differentiation is partially suppressed even under osteogenic and adipogenic differentiation conditions (Hoshiba et al. 2009, 2010). This suppression mechanism of MSC differentiation on the dECM is partially unveiled. BMP signaling is one of the triggers for MSC differentiation. However, activation of BMP signaling is inhibited by undifferentiated MSC-derived dECM (Chen et al. 2007; Hoshiba et al. 2009). It is speculated that BMP or BMP signaling inhibitory molecules (e.g., Chordin) are captured by ECM components, such as chondroitin sulfate, to regulate their accessibility to cells. In other words, BMP cannot activate intracellular signaling effectively when BMP is captured by the ECM and its accessibility to cells is suppressed. Alternatively, BMP signaling inhibitory molecules accumulate in the ECM via capture, leading to the effective suppression of BMP signaling.
Because the dECM can inhibit MSC differentiation, the dECM have been applied for in vitro MSC expansion culture. Chen and colleagues reported that undifferentiated MSC-derived dECM could suppress spontaneous differentiation and maintain the differentiation ability into osteoblasts and adipocytes after in vitro expansion culture (Chen et al. 2007). In particular, the differentiation ability of MSCs is maintained on undifferentiated MSC-derived dECM for a more extended period (i.e., higher passage number) than tissue culture polystyrene (TCPS). Similar to MSCs, other somatic stem cells, such as hematopoietic stem/progenitor cells (HSPCs) and umbilical cord blood-derived nonhematopoietic stem cells (UCB-NHSCs), have been cultured on the dECM. HSPCs were cultured on the bone marrow stromal cell line (MS-5)-derived dECM, and HSPCs showed effective expansion on the dECM with HSPC-specific surface markers (Tiwari et al. 2013). UCB-NHSCs were cultured on dECM derived from bone marrow stromal cells (containing MSCs) (Wu et al. 2016). UCB-NHSCs could be expanded on the dECM and exhibited differentiation ability into three germ layers, indicating the maintenance of pluripotency.
17.3.2.2 Pluripotent Stem Cells
Pluripotent stem cells, such as ES and iPS cells, have also been cultured on the dECM. Usually, these pluripotent stem cells are cultured on feeder cells (e.g., embryonic fibroblasts). Therefore, dECM have been prepared by the culture of these feeder cells. ES cells can be successfully grown on embryonic fibroblast-derived dECM without feeder cells (Klimanskaya et al. 2005). Moreover, the ES cells possessed the ability to differentiate into three germ layers after passage culture for more than 6 months. Additionally, ES cells can be newly established on the dECM without feeder cells.
Recently, it has been reported that laminin-511/521 is important for the maintenance of pluripotent stem cells (Nakagawa et al. 2014). Therefore, cells producing abundant laminins can be used for the preparation of dECM for pluripotent stem cells. Vuoristo et al. prepared dECM with a human choriocarcinoma cell line, JAR, which produces abundant laminins and applied this JAR cell-derived dECM for iPS cell culture (Vuoristo et al. 2013). Additionally, they reported that iPS cells can be grown on JAR cell-derived dECM with the expression of undifferentiation markers and that the cells possess pluripotency. Moreover, they successfully prepared new iPS cells from fibroblasts on the JAR cell-derived dECM. Fibroblasts transfected with retroviruses containing OCT4, KLF4, SOX2, and c-myc were seeded on both JAR cell-derived dECM and Matrigel. The induction efficacy of iPS cells was similar between JAR cell-derived dECM and Matrigel. Prepared iPS cells could also be subcultured on JAR cell-derived dECM.
17.3.2.3 Future Problems in Stem Cell Maintenance Culture with dECM
17.3.2.3.1 Feasibility to Other Stem Cells
dECM have been mainly used for in vitro expansion and culture of MSCs. Currently, undifferentiated MSC-derived dECM is commercially available from StemBioSys, Inc. Although it is evident that dECM is helpful for in vitro expansion culture of MSCs with the maintenance of their stemness, the feasibility of dECM is not confirmed for other stem cell cultures. In particular, the passage numbers at which somatic stem cells can be subcultured are limited, and their stemness is lost during their in vitro expansion. Therefore, it is important for bioindustrial applications with stem cells to investigate whether the cells can be subcultured for a long period and whether their stemness is maintained.
17.3.2.3.2 Cell Sources for dECM Preparation
Tissue-/organ-derived dECM may not be suitable for stem cell maintenance culture because the concept of dECM for this purpose is to reconstitute the stem cell niche. Therefore, almost all dECM for this purpose are derived from cultured cells. These cell sources are often the same as the cells that are cultured on the dECM. This is a major problem to resolve because the availability of stem cells (particularly somatic stem cells) is limited. To solve this problem, the establishment of new cell lines is one approach (Kusuma et al. 2017). The other approach is to generate cells producing extrinsic ECM molecules. The development of alternative cell sources is a major challenge for dECM research.
17.3.2.3.3 Mechanisms
For bioindustrial applications alone, it is not necessary to unveil the mechanisms to maintain stemness on the dECM. However, the mechanisms will help to improve the effectiveness of dECM. There are two approaches for the study of mechanisms: intracellular signaling analyses and ECM compositional analyses. Both are important. Intracellular signaling analyses, particularly upstream signaling that is linked with the ECM, will promote the understanding of how cells interact with the ECM. ECM compositional analyses will unveil important ECM components for stemness maintenance. If the ECM components are clarified, the development of alternative cell sources for dECM preparation will be accelerated.
17.3.3 Rejuvenation of Somatic Stem Cells
Recently, increasing evidences have demonstrated the rejuvenation of somatic stem cells by dECM (Fig. 17.4).
17.3.3.1 Rejuvenation of Passaged Stem Cells
Many somatic stem cells lose their differentiation ability after in vitro expansion. There are several trials to recover their differentiation ability using dECM derived from cells with lower passage numbers (Pei et al. 2011; Lai et al. 2010). Pei et al. cultured passage 5 MSCs on the dECM derived from undifferentiated MSCs (< passage 5), and their MSC functions on the dECM were compared with those on TCPS (Pei et al. 2011). MSCs recovered their growth ability and the expression of stage-specific embryonic antigen-4 (SSEA-4) on the dECM (Lai et al. 2010). Moreover, the intracellular reactive oxygen species (ROS) level was lower on the dECM than on the TCPS. Differentiation ability was also checked. Chondrogenic and osteogenic capabilities of MSCs expanded on the dECM were at higher levels than those on TCPS. On the other hand, the adipogenic ability of MSCs expanded on the dECM was lower than that on TCPS. These results suggest the possibility of dECM rejuvenating or recovering stem cells whose abilities were lost.
17.3.3.2 Rejuvenation of Stem Cells Isolated from Aged Donors
The differentiation ability of somatic cells is also lost by individual aging; that is, the differentiation ability of stem cells isolated from aged donors is lower than that of stem cells isolated from younger donors. Stem cells isolated from aged donors have been cultured on dECM derived from more immature cells (Ng et al. 2014; Sun et al. 2011). Ng et al. cultured MSCs isolated from adult donors on the dECM derived from undifferentiated fetal and adult MSCs and fibroblasts (Ng et al. 2014). Adult MSC growth was promoted on the dECM derived from fetal MSCs (passages 3 and 4) compared with dECM derived from adult MSCs (passage 3) and fibroblasts and TCPS. Additionally, the cell size (increasing cell size is a cell senescence phenomenon) was smaller on the dECM derived from fetal MSCs (passage 3) than on the dECM derived from adult MSCs (passage 3) and fibroblasts and TCPS. Moreover, the differentiation ability of adult MSCs tended to be higher on dECM derived from fetal MSCs (passage 3) than on dECM derived from adult MSCs (passage 3) and fibroblasts and TCPS.
Sun et al. also prepared dECM derived from younger and aged donors (younger and aged dECM) and cultured MSCs isolated from younger and aged donors (younger and aged MSCs) on these dECM (Sun et al. 2011). Aged MSCs possessed osteogenic differentiation ability on younger and aged dECM even after in vivo expansion culture, although the aged MSCs lost osteogenic differentiation ability on TCPS. Comparing younger and aged dECM, both younger and aged MSCs on younger dECM exhibited higher osteogenic differentiation ability than on aged dECM. Additionally, they examined intracellular ROS levels. Intracellular ROS levels were lower on younger dECM compared with aged dECM and TCPS. Moreover, a younger dECM increased telomerase activity and ATP levels in both younger and aged MSCs. Finally, they checked the osteogenesis in vivo. The osteogenesis levels of aged MSCs were similar to those of younger MSCs when the cells were expanded on younger dECM. In contrast, aged MSCs exhibited lower osteogenic levels than younger MSCs when the cells were expanded on TCPS. These reports demonstrate that dECM derived from MSCs isolated from younger donors can rejuvenate the stemness of MSCs isolated from aged donors.
Similar to MSCs, some somatic stem cells have tried to rejuvenate with dECM. Aged tendon stem cells were cultured on the dECM derived from younger tendon stem cells (Jiang et al. 2018). Senescence-associated (SA) β-galactosidase activity was decreased in aged tendon stem cells on the dECM derived from younger stem cells to a level similar to that of younger tendon stem cells. Additionally, the gene expression levels of octamer-binding transcription factor 4 (Oct-4), stage-specific embryonic antigen-1 (SSEA-1), tenomodulin, and scleraxis recovered to levels similar to those of younger tendon stem cells on the dECM derived from younger tendon stem cells. Similar results were obtained for synovium-derived and urine stem cells (Li et al. 2014; Pei et al. 2014).
17.3.3.3 Future Problems for Stem Cell Rejuvenation with dECM
Studies of somatic stem cell rejuvenation by dECM have just begun. Therefore, there are many unclarified points. For example, there are few somatic stem cells which were proved for rejuvenation with dECM, although various types of somatic stem cells exist in the body. It is unclear whether dECM can lead to the rejuvenation of other somatic stem cells. The feasibility of rejuvenation by dECM should be examined for other stem cells. Additionally, it is not clear which types of dECM can rejuvenate stem cells. There are various points to prepare cultured cell-derived dECM: donor age (including fetal donors), passage number, and cell types (e.g., stem cells or non-stem cells). At this point, it seems that younger stem cells with lower passage numbers are suitable to prepare dECM for rejuvenation. Additionally, it appears that aged or multiple-passaged stem and non-stem cells are not ideal for this purpose (Ng et al. 2014). Mechanism analyses have almost never been performed. Several studies have focused on intracellular ROS levels, ATP levels, and telomerase activity (Sun et al. 2011; Pei et al. 2011). However, it is unclear how these parameters are modulated by the dECM.
17.4 Future Perspectives of dECM for Stem Cell-Based Bioindustrial Applications
It has become clear that the dECM can regulate more functions of stem cells than previously thought. Therefore, there seem to be many possible applications of dECM.
17.4.1 Research Use
The dECM can be used as in vitro ECM models for comprehensive studies of ECM roles in the regulation of stem cell functions (Hoshiba et al. 2016). Single isolated ECM molecules are examined to clarify the roles of the ECM in the regulation of stem cell functions, although the ECM is composed of many molecules, and these molecules are orchestrated to activate intracellular signaling pathways to regulate stem cell functions. However, it is difficult to understand how each effect given by each ECM molecule is orchestrated and how the total effects influence stem cell functions by studies with only single ECM molecules. dECM can be helpful as an experimental control to understand how the ECM influences stem cell functions.
17.4.2 In Vitro Applications
As described above, stem cells show unique functions in the dECM. Therefore, the dECM are useful substrates for stem cell culture. Indeed, undifferentiated MSC-derived dECM is now commercially available from StemBioSys as CELLvo™. Additionally, tissue-/organ-derived dECM is commercially available from several companies (such as Xylyx Bio). They claimed that these dECM could be used for the 3D culture of stem cells. However, reports of subsequent dECM applications are very limited because in vitro applications of dECM in bioindustries have just started. Further trials and application reports are required for their broader applications.
17.4.3 In Vivo Clinical Applications
Today, dECM have been used in the clinic, and many dECM products for clinical use are commercially available (Nakamura et al. 2017; Crapo et al. 2011). However, clinical applications of dECM with stem cells seem to remain under research. This seems to be due to the lack of dECM sources, particularly tissues and organs, and the difficulty of preparation without a large batch-to-batch difference. The solutions for these problems are required. Moreover, a few studies have been performed from the viewpoints of clinical applications. As described above, dECM showed unique possibilities, for example, site-specific differentiation and the suppression of unexpected differentiation (including hypertrophy during chondrogenesis). Additionally, clinical applications with stem cells are still developing. Therefore, it is expected that clinical applications with stem cells and dECM will be expanded in the future.
17.5 Conclusions
Almost all dECM for stem cell culture are now at the research level. In the past decade, researches on dECM for stem cell culture focused on the effects of dECM on stem cell functions as described above. These studies have unveiled the unique effects of dECM on stem cell functions, particularly differentiation, stemness maintenance, and rejuvenation. On the other hand, a few applications of these dECM are commercially available, although stem cells exhibit unique functions on the dECM. This is due to the difficulty of preparation and small dECM source supplementation. These problems should be solved to expand the feasibility of dECM for stem cell-based bioindustrial applications. Moreover, more application studies of dECM are required as well as basic researches in the next decade. Then, methods will open for stem cell-based bioindustrial applications in the future.
References
Aguado BA, Caffe JR, Nanavati D et al (2016) Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche. Acta Biomater 33:13–24
Bai S-M, Wang Q, Yu X-L et al (2018) Grafted neural stem cells show lesion-specific migration in radiation-injured rat brains. RSC Adv 8:5797–5805
Baiguera S, Gaudio CD, Lucatelli E et al (2014) Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials 35:1205–1214
Batchelder CA, Martinez ML, Tarantal AF (2015) Natural scaffolds for renal differentiation of human embryonic stem cells for kidney tissue engineering. PLoS One 10:e0143849. https://doi.org/10.1371/journal.pone.0143849
Bruno RD, Fleming JM, George AL et al (2017) Mammary extracellular matrix directs differentiation of testicular and embryonic stem cells to form functional mammary glands in vivo. Sci Rep 7:40196. https://doi.org/10.1038/srep40196
Cai R, Nakamoto T, Kawazoe N et al (2015) Influence of stepwise chondrogenesis-mimicking 3D extracellular matrix on chondrogenic differentiation of mesenchymal stem cells. Biomaterials 52:199–207
Castelló-Cros R, Khan DR, Simons J et al (2009) Staged stromal extracellular 3D matrices differentially regulate breast cancer cell responses through PI3K and beta1-integrins. BMC Cancer 9:94. https://doi.org/10.1186/1471-2407-9-94
Chaturvedi V, Dye DE, Kinnear BF et al (2015) Interactions between skeletal muscle myoblasts and their extracellular matrix revealed by a serum free culture system. PLoS One 10:e0127675. https://doi.org/10.1371/journal.pone.0127675
Chen X-D, Dusevich V, Feng JQ et al (2007) Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res 22:1943–1956
Chen G, Gulbranson DR, Hou Z et al (2011) Chemically defined conditions for human iPS cell derivation and culture. Nat Methods 8:424–429
Cheng H-W, Tsui Y-K, Cheung KMC et al (2009) Decellularization of chondrocyte-encapsulated collagen microspheres: a three-dimensional model to study the effects of acellular matrix on stem cell fate. Tissue Eng Part C 15:697–706
Choi K-H, Choi BH, Park SR et al (2010) The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials 31:5355–5365
Cortiella J, Niles J, Cantu A et al (2010) Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 16:2565–2580
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243
Crapo PM, Medberry CJ, Reing JE et al (2012) Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 33:3539–3547
Datta N, Pham QP, Sharma U et al (2005) Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials 26:971–977
Datta N, Pham QP, Sharma U et al (2006) In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A 103:2488–2493
Duffy CRE, Zhang R, How S-E et al (2014) Long term mesenchymal stem cell culture on a defined synthetic substrate with enzyme free passaging. Biomaterials 35:5998–6005
Dzobo K, Motaung KSC, Adesida A (2019) Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review. Int J Mol Sci 20:4628. https://doi.org/10.3390/ijms20184628
Fidalgo C, Iop L, Sciro M et al (2018) A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater 67:282–294
Freytes DO, Martin J, Velankar SS et al (2008) Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 29:1630–1637
Furuyama A, Mochitate K (2000) Assembly of the exogenous extracellular matrix during basement membrane formation by alveolar epithelial cells in vitro. J Cell Sci 113:859–868
Furuyama A, Iwata M, Hayashi T et al (1999) Transforming growth factor-beta1 regulates basement membrane formation by alveolar epithelial cells in vitro. Eur J Cell Biol 78:867–875
Ganjibakhsh M, Mehraein F, Koruji M et al (2019) Three-dimensional decellularized amnion membrane scaffold promotes the efficiency of make germ cells generation from human induced pluripotent stem cells. Exp Cell Res 384:111544. https://doi.org/10.1016/j.yexcr.2019.111544
Gao S, Guo W, Chen M et al (2017) Fabrication and characterization of electrospun nanofibers composed of decellularized meniscus extracellular matrix and polycaprolactone for meniscus tissue engineering. J Mater Chem B 5:2273–2285
Geiger B, Yamada KM (2011) Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3:a005033. https://doi.org/10.1101/cshperspect.a005033
Ghaedi M, Calle EA, Mendez JJ et al (2013) Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest 123:4950–4962
Gilbert TW, Sellaro TL, Badylak SF (2006) Decellularization of tissues and organs. Biomaterials 27:3675–3683
Goh S-K, Olsen P, Banerjee I (2013) Extracellular matrix aggregates from differentiating embryoid bodies as a scaffold to support ESC proliferation and differentiation. PLoS One 8:e61856. https://doi.org/10.1371/journal.pone.0061856
Hamano S, Tomokiyo A, Hasegawa D et al (2018) Extracellular matrix from periodontal ligament cells could induce the differentiation of induced pluripotent stem cells to periodontal ligament stem cell-like cells. Stem Cells Dev 27:100–111
Harburger DS, Calderwood DA (2009) Integrin signalling at a glance. J Cell Sci 122:159–163
Harris GM, Raitman I, Schwarzbauer JE (2018) Cell-derived decellularized extracellular matrices. Methods Cell Biol 143:97–114
He H, Liu X, Peng L et al (2013) Promotion of hepatic differentiation of bone marrow mesenchymal stem cells on decellularized cell-deposited extracellular matrix. Biomed Res Int 2013:406871. https://doi.org/10.1155/2013/406871
Higuchi Y, Shiraki N, Yamane K et al (2010) Synthesized basement membranes direct the differentiation of mouse embryonic stem cells into pancreatic lineages. J Cell Sci 123:2733–2742
Higuchi S, Lin Q, Wang J et al (2013) Heart extracellular matrix supports cardiomyocyte differentiation of mouse embryonic stem cells. J Biosci Bioeng 115:320–325
Hirata M, Yamaoka T (2017) Hepatocytic differentiation of iPS cells on decellularized liver tissue. J Artif Organs 20:318–325
Hong X, Yuan Y, Sun X et al (2018) Skeletal extracellular matrix supports cardiac differentiation of embryonic stem cells: a potential scaffold for engineered cardiac tissue. Cell Physiol Biochem 45:319–331
Hoshiba T (2017) Cultured cell-derived decellularized matrices: a review towards the next decade. J Mater Chem B 5:4322–4331
Hoshiba T (2018) An extracellular matrix (ECM) model at high malignant colorectal tumor increases chondroitin sulfate chains to promote epithelial-mesenchymal transition and chemoresistance acquisition. Exp Cell Res 370:571–578
Hoshiba T (2019) Decellularized extracellular matrix for cancer research. Materials 12:1311. https://doi.org/10.3390/ma12081311
Hoshiba T, Gong J (2018) Fabrication of cell-derived decellularized matrices on three-dimensional (3D)-printed biodegradable polymer scaffolds. Microsyst Technol 24:613–617
Hoshiba T, Tanaka M (2015) Optimization of the tissue source, malignancy, and initial substrate of tumor cell-derived matrices to increase cancer cell chemoresistance against 5-fluorouracil. Biochem Biophys Res Commun 457:353–357
Hoshiba T, Tanaka M (2016) Decellularized matrices as in vitro models of extracellular matrix in tumor tissues at different malignant levels: mechanism of 5-fluorouracil resistance in colorectal tumor cells. Biochim Biophys Acta Mol Cell Res 1863:2749–2757
Hoshiba T, Yokoyama N (2020) Decellularized extracellular matrices derived from cultured cells at stepwise myogenic stages for the regulation of myotube formation. Biochim Biophys Acta Mol Cell Res 1867:118658. https://doi.org/10.1016/j.bbamcr.2020.118658
Hoshiba T, Kawazoe N, Tateishi T et al (2009) Development of stepwise osteogenesis-mimicking matrices for the regulation of mesenchymal stem cell functions. J Biol Chem 284:31164–31173
Hoshiba T, Kawazoe N, Tateishi T et al (2010) Development of extracellular matrices mimicking stepwise adipogenesis of mesenchymal stem cells. Adv Mater 22:3042–3047
Hoshiba T, Lu H, Yamada T et al (2011a) Effects of extracellular matrices derived from different cell sources on chondrocyte functions. Biotechnol Prog 27:788–795
Hoshiba T, Kawazoe N, Chen G (2011b) Mechanism of regulation of PPARG expression of mesenchymal stem cells by osteogenesis-mimicking extracellular matrices. Biosci Biotechnol Biochem 75:2099–2104
Hoshiba T, Yamada T, Lu H et al (2012a) Maintenance of cartilaginous gene expression on extracellular matrix derived from serially passaged chondrocytes during in vitro chondrocyte expansion. J Biomed Mater Res A 100:694–702
Hoshiba T, Kawazoe N, Chen G (2012b) The balance of osteogenic and adipogenic differentiation in human mesenchymal stem cells by matrices that mimic stepwise tissue development. Biomaterials 33:2025–2031
Hoshiba T, Chen G, Endo C (2016) Decellularized extracellular matrix (ECM) as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int 2016:6397820. https://doi.org/10.1155/2016/6397820
Hosokawa T, Betsuyaku T, Nishimura M et al (2007) Differentiation of tracheal basal cells to ciliated cells and tissue reconstruction on the synthesized basement membrane substratum in vitro. Connect Tissue Res 48:9–18
Hwang J, Huang Y, Burwell TJ et al (2017) In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS Nano 11:9825–9835
Hynes RO, Naba A (2012) Overview of the matrisome-an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 4:a004903. https://doi.org/10.1101/cshperspect.a004903
Jaramillo M, Yeh H, Yarmush ML et al (2018) Decellularized human liver extracellular matrix (hDLM)-mediated hepatic differentiation of human induced pluripotent stem cells (hIPSCs). J Tissue Eng Regen Med 12:e1962–e1973. https://doi.org/10.1002/term.2627
Jian R, Yixu Y, Sheyu L (2015) Repair of spinal cord injury by chitosan scaffold with glioma ECM and SB216763 implantation in adult rats. J Biomed Mater Res Part A 103A:3259–3272
Jiang D, Xu B, Gao P (2018) Effects of young extracellular matrix on the biological characteristics of aged tendon stem cells. Adv Clin Exp Med 27:1625–1630
Kaitsuka T, Noguchi H, Shiraki N et al (2014) Generation of functional insulin-producing cells from mouse embryonic stem cells through 804G cell-derived extracellular matrix and protein transduction of transcription factors. Stem Cells Transl Med 3:114–127
Keane TJ, Swinehart IT, Badylak SF (2015) Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84:25–34
Kim BS, Kwon YW, Kong J-S et al (2018a) 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials 168:38–53
Kim IG, Gil C-H, Seo J et al (2018b) Mechanotransduction of human pluripotent stem cells cultivated on tunable cell-derived extracellular matrix. Biomaterials 150:100–111
Klimanskaya I, Chung Y, Meisner L et al (2005) Human embryonic stem cells derived without feeder cells. Lancet 365:1636–1641
Kundu J, Michaelson A, Talbot K et al (2016) Decellularized retinal matrix: natural platforms for human retinal progenitor cell culture. Acta Biomater 31:61–70
Kusuma GD, Brennecke SP, O’Connor AJ et al (2017) Decellularized extracellular matrices produced from immortal cell lines derived from different parts of placental support primary mesenchymal stem cell expansion. PLoS One 12:e0171488. https://doi.org/10.1371/journal.pone.0171488
Lai Y, Sun Y, Skinner CM et al (2010) Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev 19:1095–1107
Lee DJ, Diachina S, Lee YT et al (2016) Decellularized bone matrix grafts for calvaria regeneration. J Tissue Eng 7:2041731416680306. https://doi.org/10.1177/2041731416680306
Leirós GJ, Kusinsky AG, Drago H et al (2014) Dermal papilla cells improve the wound healing process and generate hair bud-like structures in grafted skin substitutes using hair follicle stem cells. Stem Cells Transl Med 3:1209–1219
Li Y, Foss CA, Summerfield DD et al (2012) Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci U S A 109:14767–14772
Li J, Hansen KC, Zhang Y et al (2014) Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials 35:642–653
Liu G, David BT, Trawczynski M et al (2020) Advances in pluripotent stem cells: history, mechanisms, technologies, and applications. Stem Cell Rev Rep 16:3–32
Lu P, Takai K, Weaver VM et al (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3:a005058. https://doi.org/10.1101/cshperspect.a005058
Lu H, Hoshiba T, Kawazoe N (2011a) Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials 32:2489–2499
Lu H, Hoshiba T, Kawazoe N (2011b) Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 32:9658–9666
Mahara A, Sakuma T, Mihashi N et al (2019) Accelerated endothelialization and suppressed thrombus formation of acellular vascular grafts by modifying with neointima-inducing peptide: a time-dependent analysis of graft patency in rat-abdominal transplantation model. Colloids Surf B: Biointerfaces 181:806–813
Manabe R, Tsutsui K, Yamada T et al (2008) Transcriptome-based systematic identification of extracellular matrix proteins. Proc Natl Acad Sci U S A 105:12849–12854
McLenachan S, Hao E, Zhang D (2017) Bioengineered Bruch’s-like extracellular matrix promotes retinal pigment epithelial differentiation. Biochem Biophys Rep 10:178–185
Meran L, Baulies A, Li VSW (2017) Intestinal stem cell niche: the extracellular matrix and cellular components. Stem Cells Int 2017:7970385. https://doi.org/10.1155/2017/7970385
Mercier F, Kitasato JT, Hatton GI (2002) Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol 451:170–188
Mochitate K, Nagano R, Toya-Nakajima Y (2020) Bared basement membrane substrata: design, cellular assembly, decelularization and application to tissue regeneration and stem cell differentiation. In: Yamaoka T, Hoshiba T (eds) Decellularized extracellular matrix-characterization, fabrication and applications. Royal Society of Chemistry, Cambridge, pp 51–74
Mohammadian F (2018) Recent advances in stem cell and tissue engineering. In: Kaoud H (ed) Tissue regeneration. IntechOpen, London, pp 123–137
Naba A, Clauser KR, Hoersch S et al (2012) The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 11:M111.014647. https://doi.org/10.1074/mcp.M111.014647
Nakagawa M, Taniguchi Y, Senda S et al (2014) A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep 4:3594. https://doi.org/10.1038/srep03594
Nakamura N, Kimura T, Kishida A (2017) Overview of the development, applications, and future perspectives of decellularized tissues and organs. ACS Biomater Sci Eng 3:1236–1244
Narayanan K, Lim VY, Shen J et al (2014) Extracellular matrix-mediated differentiation of human embryonic stem cells: differentiation to insulin-secreting beta cells. Tissue Eng Part A 20:424–433
Navarro-Tableros V, Sanchez MBH, Figliolini F et al (2015) Recellularization of rat liver scaffolds by human liver stem cells. Tissue Eng Part A 21:1929–1939
Ng ES, Davis R, Stanley EG et al (2008) A protocol describing the use of a recombinant protein-based, anima product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc 3:768–776
Ng CP, Sharif ARM, Heath DE et al (2014) Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials 35:4046–4057
Olivares-Navarrete R, Lee EM, Smith K et al (2017) Substrate stiffness control osteoblastic and chondrocytic differentiation of mesenchymal stem cells without exogenous stimuli. PLoS One 12:e0170312. https://doi.org/10.1371/journal.pone.0170312
Ott HC, Matthiesen TS, Goh S-K et al (2008) Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 14:213–221
Pei M, He F, Kish VL (2011) Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A 17:3067–3076
Pei M, Li J, Zhang Y (2014) Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res 356:391–403
Piccoli M, D’Angelo E, Crotti S et al (2018) Decellularized colorectal cancer matrix as bioactive microenvironment for in vitro 3D cancer research. J Cell Physiol 233:5937–5948
Prewitz MC, Seib FP, von Bonin M et al (2013) Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat Methods 10:788–794
Satyam A, Kumar P, Fan X et al (2014) Macromolecular crowding meets tissue engineering by self-assembly: a paradigm shift in regenerative medicine. Adv Mater 26:3024–3034
Sellaro TL, Ravindra AK, Stolz DB et al (2007) Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng 13:2301–2310
Singelyn JM, Sundaramurthy P, Johnson TD et al (2012) Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol 59:751–763
Stern MM, Myers RL, Hammam N et al (2009) The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials 30:2393–2399
Sun Y, Li W, Lu Z et al (2011) Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J 25:1474–1485
Takeishi K, de I’Hortet AC, Wang Y et al (2020) Assembly and function of a bioengineered human liver for transplantation generated solely from induced pluripotent stem cells. Cell Rep 31:107711. https://doi.org/10.1016/j.celrep.2020.107711
Tiwari A, Tursky MI, Mushahary D et al (2013) Ex vivo expansion of haematopoietic stem/progenitor cells from human umbilical cord blood on acellular scaffolds prepared from MS-5 stromal cell line. J Tissue Eng Regen Med 7:871–883
Tsuchiya T, Balestrini JL, Mendez J et al (2014) Influence of pH on extracellular matrix preservation during lung decellularization. Tissue Eng Part C 20:1028–1036
Uygun BE, Soto-Gutierrez A, Yagi H et al (2010) Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 16:814–820
Vuoristo S, Toivonen S, Weltner J et al (2013) A novel feeder-free culture system for human pluripotent stem cell culture and induced pluripotent stem cell derivation. PLoS One 8:e76205. https://doi.org/10.1371/journal.pone.0076205
Waele JD, Reekmans K, Daans J et al (2015) 3D culture of murine neural stem cells on decellularized mouse brain sections. Biomaterials 41:122–131
Wu J, Sun Y, Block TJ et al (2016) Umbilical cord blood-derived non-hematopoietic stem cells retrieved and expanded on bone marrow-derived extracellular matrix display pluripotent characteristics. Stem Cell Res Ther 7:176. https://doi.org/10.1186/s13287-016-0437-6
Xiang J, Zheng X, Liu P et al (2016) Decellularized spleen matrix for reengineering functional hepatic-like tissue based on bone marrow mesenchymal stem cells. Organogenesis 12:128–142
Yan Y, Martin LM, Bosco DB et al (2015) Differential effects of acellular embryonic matrices on pluripotent stem cell expansion and neural differentiation. Biomaterials 73:231–242
Yang G, Rothrauff BB, Lin H et al (2013) Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 34:9295–9306
Yang L, Jiang Z, Zhou L et al (2017) Hydrophilic cell-derived extracellular matrix as a niche to promote adhesion and differentiation of neural progenitor cells. RSC Adv 7:45587–45594
Yin H, Wang Y, Sun Z et al (2016) Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomater 33:96–109
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Hoshiba, T. (2021). Decellularized Extracellular Matrix for Stem Cell Culture. In: Sheikh, F.A. (eds) Engineering Materials for Stem Cell Regeneration. Springer, Singapore. https://doi.org/10.1007/978-981-16-4420-7_17
Download citation
DOI: https://doi.org/10.1007/978-981-16-4420-7_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4419-1
Online ISBN: 978-981-16-4420-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)