Abstract
Biohydrometallurgy is a modern, steadily developing alternative metal production technology based on the use of microorganisms and their metabolic products, such as ferric iron, sulfuric acid, etc. for the extraction of metals from ores. Microbiological processing of ores and concentrates has economic, technical and, most importantly, environmental advantages over traditional technologies. Heap leaching is successfully used for recovery of copper from a secondary mineral—chalcocite (Cu2S). However, the main world reserves of copper are found in the form of chalcopyrite (CuFeS2). Chalcopyrite is the most refractory mineral and undergoes chemical or biological oxidation at a very low rate. One of the most common ways to enhance copper extraction from chalcopyrite is the use of thermophiles. Besides, the intensity of biooxidation of sulfide minerals depends on the pH, redox potential, Fe2+/Fe3+ ratio, metals ion concentration and the microorganisms used. It was revealed that the mixed cultures and consortia of moderate thermophilic microorganisms were more efficient and stable in the oxidation of chalcopyrite than pure cultures. From this point of view, developing and optimizing microbial associations for use in commercial copper leaching systems remain an important challenge. In this paper bioleaching of chalcopyrite by pure and mixed cultures of moderate thermophilic bacteria S. thermosulfidooxidans and thermotolerant sulfur or iron oxidizing bacteria L. ferriphilum CC, as well as the influence of physicochemical factors on this process have been investigated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bioleaching of chalcopyrite
- Kinetics of copper recovery
- Moderate thermoacidophiles
- Associations of iron- and sulfur-oxidizing bacteria
9.1 Introduction
Biomining is a modern, steadily developing alternative metal production technology using biohydrometallurgy for the processing of mineral raw materials. Biohydrometallurgy is based on the use of microorganisms and their metabolic products, such as ferric iron, sulfuric acid, etc. for the extraction of metals from ores. Microbiological processing of ores and concentrates has economic, technical and, most importantly, environmental advantages over traditional roasting and autoclave oxidation at high pressures and temperatures (Rawlings 2002; Rawlings et al. 2003).
Biohydrometallurgical processes in the biomining industry are implemented in heap leaching and continuous tank leaching reactors. Leaching has been used to recover copper from ores since ancient times (Ehrlich 1999). However, the development of modern commercial bioleaching processes was associated with the discovery of the first sulfur and iron oxidizing bacteria Acidithiobacillus ferrooxidans in the middle of the nineteenth century (Temple and Colmer 1951).
Commercial heap bioleaching was first implemented at the Bingham Kanyon copper mine (Utah, USA) to recover copper from low-grade ores. Subsequently, since 1980, numerous heap bioleaching units of copper have been put into operation in many countries of the world and at the end of the last century, world copper production by biohydrometallurgy reached to 25% (Brombacher et al. 1997).
Tank leaching is widely used in the biomining industry for a number of advantages. Tank leaching is a highly controlled process and provides high bioleaching efficiency in terms of rate and recovery of metals. Constant control of aeration, pH and temperature allows optimizing growth and activity of microorganisms functioning in the tanks. In addition, tank leaching leads to long-term selection and domination of those microorganisms that can grow and function more efficiently under tank leaching conditions.
Tank reactors are used for both bioleaching and biooxidation processes. Bioleaching is the dissolution of insoluble metal sulfides into soluble compounds with further extraction of metals from leaching solutions. Tank bioleaching is used to recover base metals such as copper, zinc, and nickel from the corresponding sulfides.
During biooxidation, the target product (metal) passes into a solid phase. Biooxidation is often used to recover gold and silver. The process is based on the oxidation of pyrite and arsenypyrite using microorganisms in order to release gold in the crystal lattice of minerals, followed by its extraction by traditional cyanidation. The first biooxidation unit for the pretreatment of gold ores was commercialized in 1986 by Gencor at the Fairview mine in South Africa (van Aswegen et al. 1991). Due to the minimal ecological impact on the environment and a number of other advantages, tank biooxidation is successfully used to remove iron and arsenic from gold-bearing ores, gradually replacing the indicated physicochemical and pyrometallurgical technologies (Gahan et al. 2012).
Heap leaching of copper is widely used in the biomining industry, but all existing technologies are based on the extraction of copper from a secondary mineral—chalcocite (Cu2S) (Olson et al. 2003; Gahan et al. 2012). Chalcocite is readily leached under the action of protons to form covellite (CuS) (Eq. 9.1) and by Fe (III) (Eq. 9.2) formed as a result of bacterial oxidation of Fe (II).
Covellite is also subsequently leached with Fe (III) ions (Eq. 9.3).
Chalcopyrite (CuFeS2) is the most abundant sulfide mineral. It is currently estimated that 70% of the world copper reserves now occur in chalcopyrite deposits (Watling 2006). However, chalcopyrite is the most refractory mineral to chemical and biological leaching resulting in low dissolution rates. The main reason for low dissolution rate of chalcopyrite is the formation of passive layer on mineral surface. Reaction products of bioleaching such as elemental sulfur and iron-hydroxide (jarozite) precipitate on the mineral surface and hinder its further dissolution by limiting the flow of bacteria and reaction products to and from the mineral surface (Dopson et al. 2003; Fu et al. 2008; Johnson 2014; Rawlings et al. 1999).
One of the most common solutions to this problem suggested by researchers is to perform bioleaching of chalcopyrite at high temperature using thermophiles. However it worth mentioning that in many cases, instead of extreme thermophiles (70–80 °C) industry has preferred moderate thermophilic microorganisms because they are more resistant to higher pulp densities and higher heavy metal concentrations.
Another alternative for enhancement of chalcopyrite bioleaching is the use of silver as a chemical catalyst. Researchers reported that copper recovery from chalcopyrite was improved through the use of silver as a catalyst in both the presence and the absence of bacteria (Ballester et al. 1990; Gómez et al. 1999; Cancho et al. 2007). It is considered that as a result of a chemical reaction involving an interchange between the silver and the copper and iron from the chalcopyrite lattice silver sulfide is formed on the mineral surface (Eq. 9.4):
Silver sulfide dissolves in the presence of ferric ion and the catalyst is regenerated:
The silver effect is enhanced in the presence of iron and sulfur-oxidizing microorganisms. On the one hand, microorganisms contribute to regeneration of Fe3+ and, on the other hand, oxidize the elemental sulfur layer produced on the chalcopyrite surface preventing chalcopyrite passivation.
9.2 Bioleaching of Sulfide Minerals
9.2.1 Mechanisms of Oxidation of Sulfide Minerals
Currently, there are three main mechanisms in the bioleaching of sulfide minerals (Tributsch 1999) (Fig. 9.1):
The main mechanisms of bioleaching of sulfide minerals (according to Tributsch 1999): (a) Indirect leaching, when the activity of microorganisms is limited by the regeneration of the leaching agent—Fe (III), (b) Contact leaching, which assumes the attachment of microorganisms to the surface of the mineral, which creates an environment and facilitates the leaching of the mineral through electrochemical dissolution using Fe (III) ions contained in EPS. (c) Cooperative leaching, which assumes cooperation between microorganisms attached to the surface of mineral and free microorganisms in solution. Attached cells, using contact leaching, release and dissolve chemicals that serve as an energetic substrate for free microorganisms
Depending on the type of mineral, two different ways of indirect oxidation of minerals are distinguished (Sand et al. 1995, 2001; Schippers and Sand 1999; Suzuki 2001). Metal sulfides, the valence bonds of which are obtained exclusively from metal orbitals, are oxidized with Fe (III) and cannot be attacked by protons (FeS2, MoS2, and WS2). The dissolution of these minerals, according to the studies by Steudel (1996) proceeds through the formation of thiosulfate (Fig. 9.2).
Scheme of thiosulfate (a) and polysulfide (b) mechanisms of indirect oxidation of sulfide minerals (according to Schippers and Sand 1999)
Other sulfides, in which the orbitals of metals and sulfur participate in the formation of valence bonds, are soluble in acid and are attacked by both protons and Fe (III) ions (ZnS, CdS, NiS, CoS, CuS, and CuS2). The dissolution of these sulfides proceeds by another mechanism—through the formation of polysulfides (Fig. 9.2).
Polysulfide mechanism:
The resulting ferrous iron (Fe2+) and sulfur (S8) are further oxidized by chemolithotrophic iron and sulfur-oxidizing bacteria (Eqs. 9.8 and 9.9):
Consequently, the role of bacteria in the processes of indirect oxidation of minerals is to supply Fe3+ ions (for oxidative attack) and/or protons (for hydrolytic attack).
Contact leaching is based on the attachment of cells to the surface of minerals. The attachment is carried out using extracellular polymer compounds (EPS). Attachment occurs mainly as a result of electrostatic interactions between positively charged cells and a negatively charged mineral (pyrite) at pH 2.0 (Gehrke et al. 1998; Sampson et al. 2000). According to the literature data, the attachment of cells to the mineral does not occur randomly, but preferably to the defective areas of the sulfide mineral (Edwards et al. 1998, 1999; Ohmura et al. 1993; Sanhueza et al. 1999; Gehrke et al. 1998, 2001; Rohwerder et al. 2003). It has been shown that cultures of At. ferrooxidans and Leptospirillum ferrooxidans have a chemosensory system—chemotaxis, which presents a positive reaction to the Fe (II)/Fe (III) gradient, thiosulfate, etc. (Acuña et al. 1992; Meyer et al. 2002).
It was shown that the rate and intensity of pyrite leaching increase upon direct contact of bacterial cells with the mineral. This is explained by an increase in the concentration of Fe (III) ions in the space between bacterial cells and the surface of pyrite (Fig. 9.3).
Model of the contact indirect leaching mechanism At. ferrooxidans (Rohwerder et al. 2003)
This space serves as a reaction medium filled with VPS (Sand et al. 1995; Sand and Gehrke 2006; Rohwerder et al. 2003; Yu et al. 2008, 2011). IPN contain complex ions of Fe (III) in concentrations many times higher than its concentration in the environment. Gehrke et al. (1998) showed the important role of EPS in the pyrite leaching process, since At. ferrooxidans cells lacking them are unable to leach pyrite.
9.2.2 Microorganisms Involved in the Leaching of Sulfide Minerals in Technological Processes
Bioleaching by microorganisms is widely used for the production of copper and other non-ferrous and precious metals all over the world (Donati and Sand 2007; Rawlings and Johnson 2007). Stirred continuous leaching reactors are characterized by constant parameters—temperature, pH and aeration. The constancy of conditions in the reactors causes a small number of dominant microorganism species. In general, biodiversity in reactors is limited to 2–4 species (Johnson et al. 2008; Rawlings et al. 1999; Sand et al. 1992). At the same time, it has been found that the composition of microbial consortia largely depends on the nature of the minerals and concentrates subjected to leaching (Table 9.1).
In heap leaching, conditions are not the same in terms of irrigation intensity, temperature, pH, aeration, redox potential, nutrient availability, etc., suggesting a wide variety of mineral-oxidizing microorganisms. Therefore, unlike reactors, leach heaps are characterized by significantly greater biodiversity of microorganisms. In this case, the dominant species can vary randomly, as well as depending on the stage of functioning of the heap (Table 9.2).
Thus, the main leaching bacteria belong to the genus Acidithiobacillus (= Thiobacillus) (Kelly and Wood 2000, 2005). The first representatives of this genus are extremely acidophilic iron and/or sulfur-oxidizing mesophilic bacteria At. ferrooxidans, Acidithiobacillus thiooxidans, which with the recently isolated and described moderately thermophilic Acidithiobacillus caldus belong to gram-negative γ-proteobacteria. Other leaching proteobacteria belong to the genera Acidiphilium and Leptospirillum (Hippe 2000; Coram and Rawlings 2002; Sand et al. 1992; Vardanyan and Akopyan 2003). Representatives of the genera Acidimicrobium, Ferroomicrobium, and Sulfobacillus are moderately thermophilic gram-positive bacteria (Clark and Norris 1996; Johnson and Roberto 1997; Norris et al. 1996). Leaching archaebacteria are extreme thermophiles belonging to the genera Sulfolobus, Acidianus, Metallosphaera (Fuchs et al. 1995, 1996; Kurosawa et al. 1998; Norris et al. 2000) and recently isolated mesophilic representatives of the genus Ferroplasma—F. acidiphilum (Golyshina et al. 2000), F. acidarmanus (Edwards et al. 2000).
L. ferrooxidans, Leptospirillum ferriphilum, and Acidimicrobium ferrooxidans are characterized by narrow specialization- they are able to oxidize only Fe2+, while At. ferrooxidans and Sulfobacillus spp. bacteria can grow due to the oxidation of reduced compounds of sulfur, Fe2+ and other metal ions. F. acidophilus (Johnson and Roberto 1997), Sulfobacillus spp. bacteria, as well as thermophilic archaea, are mixotrophs and cannot grow autotrophically (Johnson 1998).
Thus, it can be concluded that the most important bacteria in commercial bioleaching processes are iron and sulfur oxidizing bacteria Acidithiobacillus ferrooxidans, sulfur oxidizing Acidithiobacillus thiooxidans or Acidithiobacillus caldus and iron oxidizing Leptospirillum spp. bacteria. (Coram and Rawlings 2002; Okibe et al. 2003). Moreover, the dominant bacteria in the bioleaching processes operated at 45–50 °C are Leptospirillum ferriphilum and At. caldus (Johnson 2014; Johnson et al. 2005; Rawlings and Johnson 2007; Sand et al. 1992; Schippers and Sand 1999). It has been confirmed that the permanent member of the associations, even under mesophilic conditions, is the species S. thermosulfidooxidans.
9.2.3 Factors Affecting the Intensity of Leaching of Sulfide Minerals in Technological Processes
9.2.3.1 Influence of the Composition of Microbial Consortia
The intensity of the process of biooxidation of sulfide minerals depends on the temperature, pH, redox potential, and the nature of the microorganisms used. Among these factors, microorganisms are the most important. A number of studies show that mixed cultures and consortia of microorganisms are more efficient and stable in the oxidation of sulfide minerals than pure cultures (Akcil et al. 2007; Falco et al. 2003; Fu et al. 2008; Baker and Banfield 2003; Johnson 2001).
From this point of view, developing and optimizing microbial consortia for use in commercial leaching systems remains an important challenge. Currently, two different “top-down” and “bottom-up” approaches have been proposed to develop the optimal microbial consortia for tank leaching (Rawlings and Johnson 2007).
The “top-down approach uses a highly diverse mixed culture as an inoculum for leaching sulfide ores. This approach is based on the assumption that a stable and efficient consortium of a limited number of bacteria can be formed during the leaching process, while others disappear. Using this approach, many researchers have succeeded in creating efficient and sustainable consortia for bioleaching at pulp densities no greater than 12% (d’Hugues et al. 2002; Zhou et al. 2009; Watling et al. 2013).
A study of the structures of communities formed during the leaching of chalcopyrite showed that their biodiversity was low (Rawlings and Johnson 2007). At the same time, it was noted that the physiological properties and functions of members of the community were complementary. Thus, it has been shown that this community can include not only iron-oxidizing bacteria (L. ferriphilum, Sulfobacillus acidophilus, F. thermoplasma) and RISCs oxidizing bacteria (At. caldus, S. acidophilus), but also mixotrophs (S. acidophilus, F. thermoplasma). Iron-oxidizing bacteria oxidize sulfide minerals using the ferric iron they produce. Sulfur-oxidizing bacteria accelerate the oxidation of minerals by removing the passivating layer of elemental sulfur. Mixotrophs can utilize organic matter contained in exudate or cell lysate, and thus reduce the toxic effect of organic matter on autotrophic bacteria such as L. ferriphilum. It is also possible that mixotrophs provide CO2 for autotrophs. These synergistic interactions between different species increase consortium stability and enhance metal extraction (Johnson 1998; Bacelar-Nicolau and Johnson 1999).
In contrast, a “bottom-up” approach is to create highly efficient, sustainable consortia to leach specific minerals. In such consortia, composite species complement each other in terms of physiological properties, such as the ability to oxidize sulfur and/or iron, to autotrophic or heterotrophic growth, etc. For the creation of such designed consortia, the decisive factors are temperature, pH, concentration of metals, toxic ions, etc. A number of researchers have shown that cultures obtained by this method are most effective in accelerating the oxidation of certain minerals (Johnson et al. 2008; Akcil et al. 2007; Okibe and Johnson 2004; d’Hugues et al. 2009; Bryan et al. 2011; Mejia et al. 2009).
9.2.4 Bioleaching of Chalcopyrite
Studies have shown that mixed cultures of iron and sulfur oxidizing bacteria At. ferrooxidans and At. thiooxidans are more effective in leaching chalcopyrite (CuFeS2) than the corresponding pure cultures. The presence of sulfur-oxidizing bacteria At. thiooxidans increases the rate of dissolution of the mineral and the percentage of copper recovery. However, a mixed culture consisting of the moderately thermophilic bacteria L. ferrooxidans and At. caldus leaches chalcopyrite more efficiently than the mesophilic bacterium At. ferrooxidans in pure and mixed cultures (Fu et al. 2008). On the other hand, it has been noted that after 12–16 days of leaching, the dissolution rate of chalcopyrite At. ferrooxidans decreases, which coincides with the formation of jarosite as a passivating layer on the surface of the mineral during bioleaching. The use of a mixed culture consisting of L. ferriphilum and At. caldus leads to a sharp decrease in the pH of the medium as a result of intense sulfur oxidation, which in turn prevents the formation of jarosite and promotes chalcopyrite leaching (Fu et al. 2008). In addition, L. ferriphilum and At. caldus, being thermophilic bacteria, leach chalcopyrite more intensively than mesophiles, since the rate of oxidation reactions increases with increasing temperature.
It has been found that cultures containing autotrophic and mixotrophic bacterial species are more effective in stimulating bioleaching of chalcopyrite than mixed cultures containing three or four species, such as At. caldus, L. ferriphilum, Sulfobacillus sp. and Ferroplasma thermophilum (Wang et al. 2014).
9.2.4.1 Effect of Growth Conditions
It was previously found that the highest rate of oxidation of elemental sulfur and ferrous iron by moderate thermophilic bacteria S. thermosulfidooxidans occurred under mixotrophic conditions in the presence of 0.02% yeast extract (Vardanyan et al. 1990, Vardanyan et al. 2015). The data presented in Table 9.3, showed that the amount of iron and copper transferred into the medium under the conditions of mixotrophic growth of S. thermosulfidooxidans str. 69 and str. 86, respectively, were 2.9 and 1.2 times more compared with their autotrophic growth. It should be noted that under autotrophic conditions, the Fe2+ ion dominated in the medium, and weak growth of bacteria and oxidation of CuFeS2 were observed. In contrary to autotrophic conditions during the growth of strains on chalcopyrite under mixotrophic conditions in the presence of yeast extract, the Fe3+ ion dominated in the medium. Due to the better growth of the strains and oxidation of sulfide sulfur under mixotrophic conditions, a decrease in pH of the medium to pH 1.7 was observed.
9.2.4.2 Effect of Concentration of Substrate
Table 9.4 shows comparative activities of different strains of S. thermosulfidooxidans 86 in oxidation of CuFeS2 depending on its concentration. The studies have shown that the higher the concentration of chalcopyrite the more the amount of copper and iron is leached. It should be noted that at all concentrations tested, strains 86 and 69 showed significantly higher activity of CuFeS2 oxidation in comparison with strains VKM V1269 and 41. Thus, str. 86 exceeded strains 1269 and 41 by 4.4–6.3 and 3.2–4.2 times for copper and iron, respectively. In case of str. 69 these values were 1.8–1.9 and 1.2–1.7 times for copper and iron, respectively. Thus, in the oxidation of chalcopyrite among the studied strains, the str. 86 shows the highest activity. This fact can probably be explained by the pronounced high ability of the strain to oxidize sulfur.
The studies have shown that the dependence of the iron and copper leaching rates on chalcopyrite concentrations is described by a typical saturation curve (Fig. 9.4). Therefore, by analogy of the kinetics of enzymatic reactions, the bacterial oxidation rates of chalcopyrite can be described by the Michaelis-Menten equation:
The latter in reverse coordinates is known as Lineweaver-Burk the modification:
Based on the graph of the dependence of the iron and copper leaching rates on concentration of chalcopyrite in reverse coordinates 1/V and 1/S, the values of Km and Vmax for each strain of S. thermosulfidooxidans were determined (Fig. 9.5).
According to the data presented in Table 9.5, str. 86 possess with the lowest value of Km, consequently the highest affinity to the substrate—1.3% CuFeS2. The values of the maximum oxidation rate of chalcopyrite, determined by the leached copper and iron, differ slightly in the studied strains. Nevertheless, relatively high rate of leaching of copper and iron 250 mg/L per hour was observed in str. 86.
9.2.4.3 Effect of pH
The oxidation of chalcopyrite by S. thermosulfidooxidans str. 86 was studied in the pH range 1.0–3.0. At the beginning of experiment the increase of pH of the bioleaching solution was observed. According to the mechanism of bioleaching of chalcopyrite the increase in pH occurs due to the consumption of acid during the protonic attack of chalcopyrite (Eq. 9.12):
After 4–5 days pH started to decrease because of the oxidation of elemental sulfur by S. thermosulfidooxidans str. 86 (Eq. 9.13):
Simultaneously, with pH decrease the extraction of copper and iron from chalcopyrite increased. The data presented in Fig. 9.6 show that the largest amount of copper and iron was leached at pH 1.6. At pH values above 1.8 and below 1.3, the oxidation activity of chalcopyrite decreased, and the process stopped at pH 3.0, the lower pH limit was below 1.0.
9.2.4.4 Effect of Pulp Density
The effect of high concentrations of chalcopyrite on the composition of bacterial consortia during the adaptation of cultures was studied using clone libraries of the 16S rRNA gene and DGGE analyses. Analyses have shown that with increasing pulp density, the amount of L. ferriphilum in the consortium decreases and is not detected at all when the pulp density exceeds 4% (6 or 8%). At. caldus and S. acidophilus are found in all pulp densities and adaptation stages. The amount of F. thermoplasma changes dramatically during adaptation. So, in the initial stages, the bacterium is hardly detected, and at the end it makes up 30% of the consortium. The percentage of At. caldus in the consortium at 20% pulp density gradually decreases from 60% at the beginning to 16% at the end of the process. S. acidophilus becomes dominant in the middle stages (66%). Despite the fact that the percentage of F. thermoplasma in the initial and middle stages was very low, at the end this figure reached 66% (Wang et al. 2014).
In leaching systems functioning under moderately thermophilic conditions, F. thermoplasma is the dominant bacterium in the final stages (Zhou et al. 2008; Zhang et al. 2009; Hawkes et al. 2006). It is believed that an increase in pulp density causes high partial pressure, limits the transport of oxygen and carbon dioxide, which leads to inhibition of bacterial growth (Zhou et al. 2009).
An important stage in obtaining highly active cultures of leaching bacteria is their adaptation to high pulp densities. Cultivation under conditions of gradually increasing densities of pulp or metal ions is a well-known method for increasing the bioleaching properties of bacteria and is widely used by many researchers (Astudillo and Acevedo 2008; Zhou et al. 2009; Cameron et al. 2010; Haghshenas et al. 2009; Rawlings 2005).
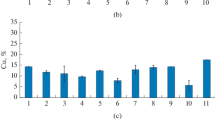
Studies carried out showed that bioleaching of copper and iron from chalcopyrite enhanced by increasing pulp density (PD) from 2 to 10%. However, at 15% of PD bioleaching of copper and iron by strain S. thermosulfidooxidans str. 86 decreased. Maximum extraction of copper and iron by S. thermosulfidooxidans str. 86 occurred at 10% of chalcopyrite (Fig. 9.7a, b).
9.2.4.5 Effect of Particle Size
As shown in Fig. 9.7 the rates of copper and iron dissolution increased with decreasing particle size from +125μm to size fraction of +45,—63μm. Maximum yield of copper (0.7 g/L) and total iron (1.23 g/L) reached the size range of +45,—63μm after 13 days of bioleaching of chalcopyrite by S. thermosulfidooxidans str. 86 (Fig. 9.8). The explanation for this increase is that smaller particle size has more surface area, enhancing metal dissolution.
9.2.4.6 Effect of Oxidative-Reductive Potential
It has been shown that Fe2+ and Fe (III) ions, depending on the concentration, have different effects on the growth and activity of iron-oxidizing bacteria. Das et al. (1999) report that Fe3+ ions at low concentrations stimulate oxygen consumption by bacteria, but at high concentrations they inhibit Fe2+ oxidation. A decrease in the rate of leaching of chalcopyrite at high concentrations of Fe3+ has also been reported by other authors (Howard and Crundwell 1999; Third et al. 2002; Hiroyoshi et al. 2007; Petersen and Dixon 2006; Rodrıguez et al. 2003).
Córdoba et al. (2008b) studied the effect of iron ions on the dissolution of chalcopyrite at low and high potentials and found that, despite the fact that Fe3+ ions were responsible for the oxidation of chalcopyrite, Fe2+ played an important role in controlling the formation and deposition of jarosiate.
The redox potential (ORP) of the leaching solution depends on the Fe3+/Fe2+ ratio and is determined by the Nernst equation (Eq. 9.14):
The ORP in leaching systems increases as a result of the activity of iron-oxidizing microorganisms. The increase in ORP promotes the decomposition of minerals such as chalcocite (Cu2S), covellite (CuS) and pyrite (FeS2). However, in the case of chalcopyrite, according to some authors, the maximum dissolution rate of the mineral occurs at low ORP (Hiroyoshi et al. 1999, 2000, 2007; Third et al. 2002; Sandström et al. 2005; Córdoba et al. 2008a; Gericke et al. 2010; Ahmadi et al. 2010, 2011).
Thus, ORP is one of the most important environmental parameters affecting the leaching of chalcopyrite and copper concentrates.
The oxidation of chalcopyrite by the mesophilic bacterium At. ferrooxidans becomes more difficult with time due to the passivation of the mineral surface. Studies have shown that slowdown of copper leaching is often accompanied by a decrease in the pH of the medium. Based on this, it was assumed that the reason for the decrease in copper leaching was most likely the formation of jarosite. Iron ions, depending on the reaction of the leaching medium, can precipitate in the form of jarosite (Eq. 9.11).
Jarosite forms a passivating layer on the surface of the mineral, which prevents diffusion and hence reduces the rate of chalcopyrite leaching (Stott et al. 2000; Okamoto et al. 2005; Yu et al. 2011).
According to other researchers, the reason for the passivation of chalcopyrite is the high ORP of the leaching medium, which is determined by the ratio of Fe3+/Fe2+ ions (Third et al. 2002; Gericke et al. 2010). Therefore, one of the approaches to overcome this effect is to maintain a low level of redox potential in the leach solution. The effect of low ORP on chalcopyrite bioleaching is reported in the early studies of Ahonen and Tuovinen (1993). In order to reduce the redox potential of the solution, the authors propose to suppress aeration and thereby inhibit iron oxidation.
It has been shown that the dissolution of copper from chalcopyrite linearly increases at the ORP from 320 to 370 mV. Under these conditions, oxidation of F2+ by bacteria is inhibited (Bevilaqua et al. 2014). Córdoba et al. (2008b) studied the effect of ORP on the chemical leaching of chalcopyrite at 68 °C. It was shown that with the ORP of 300–400 mV, almost 90% of copper was leached in 6 days, and with the ORP of 500–600 mV, only 30% in 13 days. Based on the data obtained, the authors concluded that the ORP values from 400 to 450 mV were threshold or limiting, above which the oxidative dissolution of chalcopyrite was gradually slowed down due to the passivating effect (Córdoba et al. 2008a). The release of ferrous iron from chalcopyrite and the precipitation of ferric iron resulted in a low ORP. The same authors report that the ORP rises sharply to 650 mV in bacterial cultures and improves the dissolution of copper, while these conditions do not affect chemical leaching.
Other researchers report that in the presence of 40 mM Fe2+, twice as much copper is leached as when using At. ferrooxidans, due to the low ORP in the absence of At. ferrooxidans (Hiraishi et al. 2000). The authors proposed a model of chalcopyrite leaching, leading to the formation of chalcosite (Cu2S), which was significantly rapidly and easily oxidized by oxygen and Fe3+. SO2 can be used as a chemical reducing agent to suppress ORP.
The leaching rate of chalcopyrite increases with an increase in the ORP of the solution and reaches a maximum value at the optimal ORP. At higher ORPs, the leaching rate decreases.
Similar results were obtained (Third et al. 2000), who studied the effect of stimulating or inhibiting the oxidative activity of iron-oxidizing bacteria in chalcopyrite leaching. The conducted studies allowed them to conclude that the formation of Fe3+ as a result of bacterial oxidation of Fe2+ inhibited the dissolution of chalcopyrite. The authors proposed to control ORP using oxygen limitation. With controlled ORP, the bacterial oxidation of Fe2+ was limited, but at the same time, a sufficient amount of Fe3+ was supplied to oxidize chalcopyrite, and as a result, copper extraction increased by 2 times. At the same time, passivation was delayed, but not completely eliminated (Third et al. 2002).
9.2.4.7 Influence of Ferric Iron (Fe3+)
Fe3+ is a permanent component of chalcopyrite leaching medium. Therefore, the study of the effect of Fe3+ ion on its oxidation is of particular importance. Figure 9.9 shows the leaching dynamics of copper and iron during oxidation of chalcopyrite without Fe3+ and when Fe3+ ion added to the medium.
As can be seen from the data presented in Fig. 9.9, Fe3+ ions, at the studied concentrations, stimulate the oxidation of chalcopyrite by str. 86.
Thus, Fe3+ ion, at concentrations of 1.0 and 1.9 g/L stimulates the oxidation of chalcopyrite in str. 86 by 1.3–1.4 and 1.9 times, respectively. The stimulation effect of Fe3+ can be explained by its direct involvement in the oxidation of chalcopyrite, known as the indirect route of its leaching. The Fe3+ ion interacts with CuFeS2 to form Fe2+ and elemental sulfur, which are then subjected to bacterial oxidation and react again with chalcopyrite. The stimulating effect of Fe3+ has also been confirmed in At. ferrooxidans (Keller and Murr 1982; Lizama and Suzuki 1989).
Chalcopyrite is an acid-soluble metal sulfide and consequently is dissolved by both the ferric ions and proton attack (Rawlings et al. 1999; Watling 2006).
Ferric iron oxidizes chalcopyrite to copper and ferrous ions in solution and elemental sulfur (Eq. 9.18). The activity of L. ferriphilum CC in mixed cultures resulted in regeneration of oxidative agent—Fe(III). However, the presence of sulfur-oxidizing At. albertensis SO-2 in the mixed culture, by which the sulfur is oxidized to sulfuric acid prevents the formation of jarosite on the chalcopyrite surface.
9.2.4.8 Tolerance of Microorganisms to Copper Ion
Acidophilic leaching microorganisms occupy the most metal-rich natural and anthropogenic ecosystems, such as acidic drainage water, tailings and other waste products from concentrators. The study of the resistance and adaptation of these microorganisms to high concentrations of metals is of great scientific and practical interest. This interest is primarily caused by the problem of obtaining strains of leaching microorganisms resistant to high concentrations of copper for use in biogeotechnological processes.
Studies have shown that when the concentration of copper inside a bacterial cell exceeds the permissible level, the mechanisms of bacterial resistance are activated (Rensing and Grass 2003; Magnani and Solioz 2007). Thus, in gram-negative bacteria, this is an active excretion of copper from the cytoplasm into the periplasmic space by means of p-type ATP-ases localized in the inner membrane (Rensing and Grass 2003). Certain microorganisms can pump or expel copper from the cytoplasm directly into the extracellular space using the resistant nodule division (RND) system. This type of detoxification is well known and described in E. coli (Outten et al. 2001). The ability of some bacteria to bind copper in the periplasmic space using copper chaperones has also been reported (Dopson et al. 2003; Puig and Thiele 2002).
The study of the resistance of acidophilic bacteria to copper is limited only to the gram-negative bacterium At. ferrooxidans. At. ferrooxidans is resistant to high concentrations of copper (up to 800 mM CuSO4) and other metals (Dew et al. 1999; Orell et al. 2010). L. ferrooxidans is able to grow in the presence of 5 mM copper in the medium (Johnson et al. 1992). The resistance of bacteria to copper is very important from the point of view of their application in biotechnological processes, where the concentration of copper ions can vary in the range from 15 to 100 mM CuSO4 (Watling 2006).
It has been shown that At. ferrooxidans ATCC 23270 can function at high copper concentrations due to about 10 genes on the chromosome that are directly related to its copper resistance. They include three genes encoding type p ATPases related to copper transport (copA1Af, copA2Af, and copBAf), three genes related to RND, responsible for the removal of copper from the cell (cusAAf, cusBAf, cusCAf) and two genes, coding periplasmic chaperones for copper (cusFAf and copCAf) (Navarro et al. 2009). Recently, it has been found that the high resistance of some At. ferrooxidans strains is due to the presence of additional copper-resistant genes in their genome in the form of genetic islands (GI) (Orellana and Jerez 2011).
Expression of most of these genes was established in At. ferrooxidans grown in the presence of high copper concentrations using real-time RT-PCR.
Some of these genes associated with copper resistance were also found in Leptospirillum spp. bacteria using metagenomic analyses of nucleotide sequences (Simmons et al. 2008). Two genes were also isolated from L. ferriphilum cells, which were responsible for bacterial resistance to arsenic ions. One of them is identical to the gene previously identified in the cells of arsenic-resistant strains At. caldus (Tuffin et al. 2006). It has been hypothesized about horizontal gene transfer between leaching and other bacteria, which is an important factor for the transmission of resistance to metals, as well as adaptive and other advantageous properties of these acidophiles (Dopson et al. 2003).
An example of a high tolerance to Fe (III) is the competition between iron-oxidizing bacteria in leaching solutions, which according to Rawlings et al. (1999) flows in favor of L. ferrooxidans and S. themosulfidooxidans (Vardanyan et al. 1990; Boon et al. 1999). It was shown that Fe (III) ions competitively inhibit the oxidation of Fe (II) in At. ferrooxidans, L. ferrooxidans, and S. themosulfidooxidans (Vardanyan et al. 1990; Boon et al. 1999).
It should be noted that the growth of bacteria in the form of a biofilm significantly increases the resistance of bacteria to metals (Costerton et al. 1995; Harneit et al. 2006; Sand and Gehrke 2006; Sutherland 2001).
The influence of copper on oxidation of iron by S. thermosulfidooxidans 86 and L. ferriphilum CC was studied in the range of concentration from 10 to 250 mM. It was shown that iron oxidation by L. ferriphilum CC is inhibited about 50% in the presence of copper in concentration of 10 mM, while inhibition of iron oxidation by S. thermosulfidooxidans 86 reached 45% only at 100 mM copper (Table 9.6). Thus, tolerance of S. thermosulfidooxidans 86 to copper was much more higher than that of L. ferriphilum CC.
Resistance of microorganisms to metals is of great scientific and practical interest which is caused by the problem of obtaining strains of metal leaching bacteria resistant to high concentrations of metals for application in biogeotechnological processes. In this paper the influence of copper and zinc on the oxidation of iron by L. ferriphilum CC and S. thermosulfidooxidans 86 depending on substrate concentration was studied. It was shown that the tolerance of S. thermosulfidooxidans 86 to copper and zinc was much higher than that of L. ferriphilum CC. It was revealed that the increase in the concentration of the substrate led to the decrease in the inhibitory effect of copper and zinc on the oxidation of Fe2+ by L. ferriphilum CC and S. thermosulfidooxidans 86. Thus, the increase of substrate concentration will allow in some ways to overcome the inhibitory effect of copper and zinc. It was established that with the growth of bacteria the decrease of inhibitory effect of metal ions was observed. It is assumed that during their growth bacterial cells form biofilm consisting of extracellular polymeric substances (EPS) and create accordingly a less toxic and more favorable environment for the growth of cells in the presence of copper and other ions (Fig. 9.10).
9.2.4.9 Oxidation of CuFeS2 by Mixed Cultures
It is well-known that the most important bacteria in commercial bioleaching processes are iron and sulfur oxidizing bacteria Acidithiobacillus ferrooxidans, sulfur oxidizing Acidithiobacillus thiooxidans or Acidithiobacillus caldus and iron oxidizing Leptospirillum spp. bacteria. (Coram and Rawlings 2002; Okibe et al. 2003). Moreover, the dominant bacteria in the bioleaching processes operated at 45–50 °C are Leptospirillum ferriphilum, At. caldus, and S. thermosulfidooxidans (Johnson 2014; Johnson et al. 2005; Rawlings and Johnson 2007; Sand et al. 1992; Schippers and Sand 1999).
It is well-known that in natural biocenoses, there are complex relationships between bacteria, including different forms of reciprocal feeding (Dopson and Lindstrom 1999; Bacelar-Nicolau and Johnson 1999; Johnson 1998; Tuovinen et al. 1994). Taking into consideration the above-mentioned associations of sulfobacilli and other iron and/or sulfur oxidizing bacteria have been developed to significantly increase the activity of sulfobacilli in the oxidation of pyrite and chalcopyrite.
The dynamics of oxidation of chalcopyrite by S. thermosulfidooxidans str.86 and thermotolerant sulfur or iron oxidizing bacteria and their association is shown in Fig. 9.11. According to the data presented, the highest oxidation activity of CuFeS2 showed S. thermosulfidooxidans str.86 growing under mixotrophic conditions in the presence of 0.02% yeast extract. The studies have shown that neither iron oxidizing L. ferrooxidans str.72, nor sulfur oxidizing bacteria At. tandzuti str.5 in monoculture are capable of performing the oxidation of chalcopyrite. However, when L. ferrooxidans str. 72 was grown in association with sulfur oxidizing bacteria str. 5 an increase of oxidation of chalcopyrite was observed (Fig. 9.11).
The same poor oxidation of CuFeS2 was observed during autotrophic growth of S. thermosulfidooxidans str. 86 (Fig. 9.12). The studies carried out showed that the activity of autotrophically growing str. 86 significantly increased when grew together with At. tandzuti str. 5. Thus, for 8 days of cultivation by the amount of leached copper (0.96 g/L), this association reached the efficiency observed by mixotrophically growing str. 86 (0.94 g/L). During 18 days of growth, the mentioned association by the amount of leached iron (2.16 g/L) significantly exceeds str. 86 grown under mixotrophic conditions in the presence of yeast extract (1.6 g/L). It should be noted that no significant oxidation of CuFeS2 was observed by S. thermosulfidooxidans growing together with L. ferrooxidans str. 72 in the absence of yeast extract with str. 72 (Fig. 9.12).
It can be concluded that the co-cultivation of sulfobacilli with thermotolerant sulfur oxidizing bacteria allows to achieve the efficiency of oxidation of CuFeS2 without adding an organic carbon source to the medium, which is necessary for the growth of sulfobacilli extract (Vardanyan 1998, 2003;Vardanyan and Vardanyan 2016).
Thus, the use of thermotolerant sulfur-oxidizing bacteria with sulfobacilli allows to perform the oxidation of chalcopyrite without the addition of organic substances by intensity, observed during the growth of moderate thermophiles under mixotrophic conditions in the presence of yeast extract. Syntrophic relationships are thought to arise between the strains studied during chalcopyrite oxidation. Probably, strain 86 of moderately thermophilic bacteria provides sulfur oxidizing bacteria with elemental sulfur and its reduced compounds.
9.3 Conclusion
It has been shown that the dependence of the copper and iron leaching rates on chalcopyrite concentrations is described by a typical saturation curve. Therefore, by analogy of the kinetics of enzymatic reactions, the bacterial oxidation rates of chalcopyrite can be described by the Michaelis-Menten equation: S. thermosulfidooxidans str. 86 has shown the lowest Km value, consequently the highest affinity to the substrate-CuFeS2 and highest maximal rate of copper leaching. Decrease of particle size leads to the increase of mineral surface area enhancing copper dissolution. Results obtained have shown that ferric iron (Fe3+) stimulates the oxidation of chalcopyrite in moderate thermophilic sulfobacilli that can be explained by direct involvement of Fe3+ in the oxidation of chalcopyrite, known as the indirect route of its leaching. Chalcopyrite is an acid-soluble metal sulfide and consequently is dissolved by both the proton attack and ferric iron (Rawlings et al. 1999; Watling 2006). The Fe3+ ion interacts with CuFeS2 to form elemental sulfur and ferrous iron (Fe2+) (Eq. 9.18), which are then subjected to bacterial oxidation and react again with chalcopyrite.
The activity of iron oxidizing bacteria L. ferriphilum CC in association resulted in regeneration of oxidizing agent—Fe3+. However, the presence of sulfur-oxidizing At. tandzuti in association, by which the sulfur is oxidized to sulfuric acid prevents the formation of passivating sulfur layer and jarosite on the chalcopyrite surface. It can be concluded that the co-cultivation of sulfobacilli with thermotolerant sulfur oxidizing bacteria allows considerably increasing the efficiency of oxidation of CuFeS2 without adding an organic carbon source to the medium, which is necessary for the growth of sulfobacilli extract (Vardanyan 1998, 2003; Vardanyan and Vardanyan 2016). Thus, the use of thermotolerant sulfur-oxidizing bacteria in association with sulfobacilli allows to perform the oxidation of chalcopyrite under autotrophic conditions by intensity, observed during their growth under mixotrophic conditions in the presence of 0.02% yeast extract. Syntrophic relationships are thought to arise between the strains studied during chalcopyrite oxidation. Probably, S. thermosulfidooxidans str. 86 provides sulfur oxidizing bacteria with elemental sulfur and its reduced compounds.
References
Acuña J, Rojas J, Amaro A, Toledo H, Jerez C (1992) Chemotaxis of Leptospirillum ferrooxidans and other acidophilic chemolithotrophs: comparison with the Escherichia coli chemosensory system. FEMS Microbiol Lett 75:37–42
Ahmadi A, Schaffie M, Manafi Z, Ranjbar M (2010) Electrochemical bioleaching of high grade chalcopyrite flotation concentrate in a stirred tank reactor. Hydrometallurgy 104:99–105
Ahmadi A, Schaffie M, Petersen J, Schippers A, Ranjbar M (2011) Conventional and electrochemical bioleaching of chalcopyrite concentrates by moderately thermophilic bacteria at high pulp density. Hydrometallurgy 106:84–92
Ahonen L, Tuovinen O (1993) Redox potential-controlled bacterial leaching of chalcopyrite ores. In: Torma A, Wey J, Lakshmanan V (eds) Biohydrometallurgical technologies, Bioleaching Processes. The Minerals, Metals and Materials Society, Warrendale, PA, pp 571–578
Akcil A, Ciftci H, Deveci H (2007) Role and contribution of pure and mixed cultures of mesophiles in bioleaching of a pyritic chalcopyrite concentrate. Miner Eng 20:310–318
Astudillo C, Acevedo F (2008) Adaptation of Sulfolobus metallicus to high pulp densities in the biooxidation of a flotation gold concentrate. Hydrometallurgy 92:11–15
Bacelar-Nicolau P, Johnson D (1999) Leaching of pyrite by acidophilic heterotrophic iron-oxidizing bacteria in pure and mixed cultures. Appl Environ Microbiol 65:585–590
Baker B, Banfield J (2003) Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44:139–152
Ballester A, González F, Blázquez M, Gómez C, Mier J (1990) The influence of several ions in the bioleaching of metal sulphides. Hydrometallurgy 23:221–235
Bevilaqua D, Lahti-Tommila H, Garcia O Jr, Puhakka J, Tuovinen O (2014) Bacterial and chemical leaching of chalcopyrite concentrates as affected by the redox potential and ferric/ferrous iron ratio at 22oC. Int J Miner Process 132:1–7
Boon M, Ras C, Heijnen J (1999) The ferrous iron oxidation kinetics of Thiobacillus ferrooxidans in batch cultures. Appl Microbiol Biotechnol 51:813–819
Brombacher C, Bachofen R, Brandl H (1997) Biohydrometallurgical processing of solids: a patent review. Appl Microbiol Biotechnol 48:577–587
Bruhn D, Thompson D, Naoh K (1999) Microbial ecology assessment of a mixed copper oxide/sulfide dump leach operation. In: Amils R, Ballester A (eds) Biohydrometallurgy and the environment. Toward the mining of the 21st century, process metallurgy. Elsevier, Amsterdam, pp 799–808
Bryan C, Joulian C, Spolaore P, El Achbouni H, Challan-Belval S, Morin D, d’Hugues P (2011) The efficiency of indigenous and designed consortia in bioleaching stirred tank reactors. Miner Eng 24:1149–1156
Cameron R, Yeung C, Greer C, Gould W, Mortazavi S, Bédard P, Morin L, Lortie L, Dinardo O, Kennedy K (2010) The bacterial community structure during bioleaching of a low-grade nickel sulphide ore in stirred—tank reactors at different combinations of temperature and pH. Hydrometallurgy 104:207–215
Cancho L, Blázquez M, Ballester A, González F, Muñoz J (2007) Bioleaching of a chalcopyrite concentrate with moderate thermophilic microorganisms in a continuous reactor system. Hydrometallurgy 87:100–111
Clark D, Norris P (1996) Acidomicrobium ferrooxidans gen. Nov., sp. nov.: mixed-culture ferrous iron oxidation with Sulfobacillus species. Microbiology (UK) 142:785–790
Coram N, Rawlings D (2002) Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates south African commercial biooxidation tanks that operate at 40°C. Appl Environ Microbiol 68:838–845
Córdoba E, Muñoz J, Blázquez M, González F, Ballester A (2008a) Leaching of chalcopyrite with ferric ion. Part I: general aspects. Hydrometallurgy 93:81–87
Córdoba E, Muñoz J, Blázquez M, González F, Ballester A (2008b) Leaching of chalcopyrite with ferric ion. Part II: effect of redox potential. Hydrometallurgy 93:88–96
Costerton J, Lewandowski Z, Caldwell D, Korber D, Lappin-Scott H (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745
Das T, Ayyappan S, Chaudhury GR (1999) Factors affecting bioleaching kinetics of sulfide ores using acidophilic microоrganisms. Biometals 12:1–10
d’Hugues P, Foucher S, Galle-Cavalloni P, Morin D (2002) Continuous bioleaching of chalcopyrite using a novel extremely thermophilic mixed culture. Int J Miner Process 66:107–119
d’Hugues P, Joulian C, Spolaore P, Morin D, Bryan C, Challan-Belval S, El Achbouni H (2009) Adaptation and evolution of microbial consortia in a stirred tank reactor bioleaching system: indigenous population versus a defined consortium. Adv Mat Res 71:79–82
Demergasso C, Galeguillos P, Escudero L, Zepeda V, Castillo D, Casamayor E (2005) Molecular characterization of microbial population in a low-grade copper ore bioleaching test heap. Hydrometallurgy 80:241–253
Dew D, Lawson E, Broadhurst J (1997) The BIOX process for biooxidation of gold-bearing ores or concentrates. In: Rawlings D (ed) Biomining: theory, microbes and industrial processes. Springer, Berlin, pp 45–80
Dew D, Muhlbauer R, van Buuren C (1999) Bioleaching of copper sulphide concentrates with mesophiles and thermophiles. Alta copper 99 Brisbane, Australia
Donati E, Sand W (2007) Microbial processing of metal sulphides. Springer, Berlin, pp 1–314
Dopson M, Lindstrom EB (1999) Potential role of Thiobacillus caldus in Arsenopyrite Leaching. Appl Environ Microbiol 65:36–40
Dopson M, Lindstrom E (2004) Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite and chalcopyrite. Microb Ecol 48:19–28
Dopson M, Baker-Austin C, Koppineedi P, Bond P (2003) Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic micro-organisms. Microbiology 149:1959–1970
Edwards K, Schrenk M, Hamers R, Banfield J (1998) Microbial oxidation of pyrite: experiments using microorganisms from an extreme acidic environment. Am Mineral 83:1444–1453
Edwards K, Goebel B, Rodgers T, Schrenk M, Gihring T, Cardona M, Mcguire M, Hamers R, Pace N, Banfield J (1999) Geomicrobiology of pyrite (FeS2) dissolution: case study at Iron Mountain, California. Geomicrobiol J 16:155–179
Edwards K, Bond P, Gihring T, Banfield J (2000) An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 279:1796–1799
Ehrlich H (1999) Past, present and future of biohydrometallurgy. In: Amils R, Ballester A (eds) Biohydrometallurgy and the environment toward the mining of the 21st century, IBS99. Elsevier, Amsterdam, pp 3–12
Falco L, Pogliani C, Curutchet G, Donati E (2003) A comparison of bioleaching of covellite using pure cultures of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans or a mixed culture of Leptospirillum ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 71:31–36
Fu B, Zhou H, Zhang R, Qiu G (2008) Bioleaching of chalcopyrite by pure and mixed cultures of Acidithiobacillus spp. and Leptospirillum ferriphilum. Int Biodet Biodegrad 62:109–115
Fuchs T, Huber H, Teiner K, Burggraf S, Stetter K (1995) Metallosphaera prunae, sp. nov., a novel metal-mobilizing, thermoacidophilic archaeum, isolated from a uranium mine in Germany. Syst Appl Microbiol 18:560–566
Fuchs T, Huber H, Burggraf S, Stetter K (1996) 16S rDNA-based phylogeny of the archaeal order Sulfolobales and reclassification of Desulfurolobus ambivalens as Acidianus ambivalens comb. nov. Syst. Appl Microbiol 19:56–60
Gahan C, Srichandan H, Kim D, Akci A (2012) Biohydrometallurgy and biomineral processing technology: a review on its past, present and future. Res J Recent Sci 10:85–99
Gehrke T, Telegdi J, Thierry D, Sand W (1998) Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl Environ Microbiol 64:2743–2747
Gehrke T, Hallmann R, Kinzler K, Sand W (2001) The EPS of Acidithiobacillus ferrooxidans - a model for structure-function relationships of attached bacteria and their physiology. Water Sci Technol 43:159–167
Gericke M, Govender Y, Pinches A (2010) Tank bioleaching of low-grade chalcopyrite concentrates using redox control. Hydrometallurgy 104:414–419
Goebel B, Stackebrandt E (1994) Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl Environ Microbiol 60:1614–1621
Golyshina O, Pivovarova T, Karavaiko G, Kondrateva T, Moore E, Abraham W, Lunsdorf H, Timmis K, Yakimov M, Golyshin P (2000) Ferroplasma acidiphilum gen. Nov., sp. nov., an acidophilic, autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. Nov., comprising a distinct lineage of the archaea. Int J Syst Evol Microbiol 50:997–1006
Gómez E, Ballester A, Blázquez M, González F (1999) Silvercatalysed bioleaching of a chalcopyrite concentrate with mixed cultures of moderately thermophilic microorganisms. Hydrometallurgy 51:37–46
Haghshenas D, Alamdari E, Torkmahalleh M, Bonakdarpour B, Nasernejad B (2009) Adaptation of Acidithiobacillus ferrooxidans to high grade sphalerite concentrate. Miner Eng 22:1299–1306
Harneit K, Goksel A, Kock D, Klock J, Gehrke T, Sand W (2006) Adhesion to metal sulfide surfaces by cells of Acidiothiobacillus ferrooxidans, Acidiothiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 83:245–254
Hawkes R, Franzmann P, Plumb J (2006) Moderate thermophiles including “Ferroplasma cuprexacervatum” sp. Nov., dominate an industrial scale chalcocite heap bioleaching operation. Hydrometallurgy 83:229–236
Hippe H (2000) Leptospirillum gen. Nov. (ex Markosyan 1972), nom. Rev., including Leptospirillum ferrooxidans sp. nov. (ex Markosyan 1972), nom. Rev. and Leptospirillum thermoferrooxidans sp. nov. (Golovacheva et al. 1992). Int J Syst Evol Microbiol 50:501–503
Hiraishi A, Matsuzawa Y, Kanbe T, Wakao N (2000) Acidisphaera rubrifaciens gen. Nov., sp. nov., an aerobic bacteriophyllcontaining bacterium isolated from acidic environments. Int J Syst Evol Microbiol 50:1539–1546
Hiroyoshi N, Hirota M, Hirajima T, Tsunekawa M (1999) Inhibitory effect of ironoxidizing bacteria on ferrous-promoted chalcopyrite leaching. Biotechnol Bioeng 64:478–483
Hiroyoshi N, Miki H, Hirajima T, Tsunekawa M (2000) A model for ferrous-promoted chalcopyrite leaching. Hydrometallurgy 57:31–38
Hiroyoshi N, Kuroiwa S, Miki H, Tsunekawa M, Hirajima T (2007) Effects of coexisting metal ions on the redox potential dependence of chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 87:1–10
Howard D, Crundwell F (1999) A kinetic study of the leaching of chalcopyrite with Sulfolobus metallicus. In: Biohydrometallurgy and the environment toward the mining of the 21st century. Elsevier, Amsterdam, pp 209–217
Johnson D (1998) Biodiversity and ecology of acidophilic microorganisms. FEMS Microbiol Ecol 27:307–317
Johnson D (2001) Importance of microbial ecology in the development of new mineral technologies. Hydrometallurgy 59:147–157
Johnson D (2014) Biomining—biotechnologies for extracting and recovering metals from ores and waste materials. Curr Opin Biotechnol 30:24–31
Johnson D, Roberto F (1997) Biodiversity of acidophilic bacteria in mineral leaching and related environments. In: Proceedings of IBS Biomine’ 97 Conf., Australian Mineral Foundation, Glenside, Australia, Part 3, pp 1–10
Johnson D, Ghauri M, Said M (1992) Isolation and characterization of an acidophilic, heterotrophic bacterium capable of oxidizing ferrous iron. Appl Environ Microbiol 58:1423–1428
Johnson D, Okibe N, Hallberg K (2005) Differentiation and identification of iron-oxidizing acidophilic bacteria using cultivation techniques and amplified ribosomal DNA restriction enzyme analysis. J Microbiol Methods 60:299–313
Johnson D, Okibe N, Wakeman K, Yajie L (2008) Effect of temperature on the bioleaching of chalcopyrite concentrates containing different concentrations of silver. Hydrometallurgy 94:42–47
Keller L, Murr L (1982) Acid-bacterial and ferric sulfate leaching of pyrite single crystals. Biotechnol Bioeng 24:83–96
Kelly D, Wood A (2000) Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. Nov., Halothiobacillus gen. Nov., and Thermithiobacillus gen. Nov. Int J Syst Evol Microbiol 50:511–516
Kelly D, Wood A (2005) Genus I. Acidothiobacillus. In: Brener D, Krieg N, Staley R (eds) Bergey’s manual of systematic bacteriology, 2nd edn. Springer, New York, pp 60–62
Kurosawa N, Itoh Y, Iwai T, Sugai A, Uda I, Kimura N, Horiuchi T, Itoh T (1998) Sulfurisphaera ohwakuensis gen. Nov., sp. nov., a novel extremely thermophilic acidophile of the orderSulfolobales. Int J Syst Bacteriol 48:451–456
Lizama H, Suzuki I (1989) Synergistic competitive inhibition of ferrous iron oxidation by thiobacillus ferrooxidans by increasing concentrations of ferric iron and cells. Appl Environ Microbiol 55:2588–2591
Magnani D, Solioz M (2007) How bacteria handle copper. In: Nies D, Silver S (eds) Bacterial transition metal homeostasis. Springer, Heidelberg, pp 259–285
Mejia E, Ospina J, Marquez M, Morales A (2009) Oxidation of chalcopyrite (CuFeS2) by Acidithiobacillus ferrooxidans and a mixed culture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans like bacterium in shake flasks. Adv Mat Res 71:385–388
Meyer G, Schneider-Merck T, Böhme S, Sand W (2002) A simple method for investigations on the chemotaxis of a. ferrooxidans and D. vulgaris. Acta Biotechnol 22:391–399
Mikkelsen D, Kappler U, McEwan A, Sly L (2006) Archaeal diversity in two thermophilic chalcopyrite bioleaching reactors. Environ Microbiol 8:2050–2055
Navarro C, Orellana L, Mauriaca C, Jerez C (2009) Transcriptional and functional studies of Acidithiobacillus ferrooxidans genes related to survival in the presence of copper. Appl Environ Microbiol 75:6102–6109
Norris P, Clark D, Owen J, Waterhouse S (1996) Characteristics of Sulfobacillus acidophilus sp. nov. and other moderately thermophilic mineral-sulphide-oxidizing bacteria. Microbiology 142:775–783
Norris P, Burton N, Foulis N (2000) Acidophiles in bioreactor mineral processing. Extremophiles 4:71–76
Ohmura N, Kitamura K, Saiki H (1993) Selective adhesion of Thiobacillus ferrooxidans to pyrite. Appl Environ Microbiol 59:4044–4050
Okamoto H, Nakayama R, Kuroiwa S, Hiroyoshi N, Tsunekawa M (2005) Normalized redox potential used to assess chalcopyrite column leaching. J MMIJ 121:246–254
Okibe N, Johnson D (2004) Biooxidation of pyrite by defined mixed cultures of moderately thermophilic acidophiles in pH-controlled bioreactors: significance of microbial interactions. Biotechnol Bioeng 87:574–583
Okibe N, Gericke M, Hallberg K, Johnson D (2003) Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation. Appl Environ Microbiol 69:1936–1943
Olson G, Brierley J, Brierley C (2003) Bioleaching review part B: progress in bioleaching: applications of microbial processes by the minerals industries. Appl Microbiol Biotechnol 63:249–257
Orell A, Navarro C, Arancibia R, Mobarec J, Jerez C (2010) Life in blue: copper resistance mechanisms of bacteria and archaea used in industrial biomining of minerals. Biotechnol Adv 28:839–848
Orellana L, Jerez C (2011) A genomic island provides Acidithiobacillus ferrooxidans ATCC 53993 additional copper resistance: a possible competitive advantage. Appl Microbiol Biotechnol 92:761–767
Outten F, Huffman D, Hale J, Ó Halloran T (2001) The independent cue and cus system confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J Biol Chem 276:30670–30677
Petersen J, Dixon D (2006) Competitive bioleaching of pyrite and chalcopyrite. Hydrometallurgy 83:40–49
Puig S, Thiele D (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6:171–180
Rawlings D (2002) Heavy metal mining using microbes. Annu Rev Microbiol 56:65–91
Rawlings D (2005) Characteristics and adaptability of iron- and sulfuroxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb Cell Fact 4:13
Rawlings DE, Johnson DB (2007) The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology 153:315–324
Rawlings D, Coram N, Gardner M, Deane S (1999) Thiobacillus caldus and Leptospirillum ferrooxians are widely distributed in continuous-flow biooxidation tanks used to treat a variety of metal-containing ores and concentrates. In: Biohydrometrallurgy and the environment toward the mining of the 21st century, part A. Elsevier, Amsterdam, pp 773–778
Rawlings D, Dew D, du Plessis C (2003) Biomineralization of metal-containing ores and concentrates. Trends Biotechnol 21:38–44
Rensing C, Grass G (2003) Escherichia coli mechanism of copper homeostasis in a changing environment. FEMS Microbiol Rev 27:197–213
Rodrıguez Y, Ballester A, Bla’zquez M, Gonza’lez F, Muñoz J (2003) New information on the chalcopyrite bioleaching mechanism at low and high temperature. Hydrometallurgy 71:47–56
Rohwerder T, Gehrke T, Kinzler K, Sand W (2003) Bioleaching review part A: progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl Microbiol Biotechnol 63:239–248
Sampson M, Phillips C, Blake R II (2000) Influence of the attachment of acidophilic bacteria during the oxidation of mineral sulfides. Miner Eng 13:373–389
Sand W, Gehrke T (2006) Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron (III) ions and acidophilic bacteria. Res Microbiol 157:49–56
Sand W, Rohde K, Sobotke B, Zenneck C (1992) Evaluation of Leptospirillum ferrooxidans for leaching. Appl Environ Microbiol 58:85–92
Sand W, Gehrke T, Hellmann R, Schippers A (1995) Sulfur chemistry, biofilm and the indirect attack mechanism a critical evaluation of bacterial leaching. Appl Microbiol Biotechnol 43:961–966
Sand W, Gehrke T, Jozsa P, Schippers A (2001) Biochemistry of bacterial leaching- direct vs. indirect bioleaching. Hydrometallurgy 59:159–175
Sandström A, Shchukarev A, Paul J (2005) XPS characterisation of chalcopyrite chemically and bioleached at high and low redox potential. Miner Eng 18:505–515
Sanhueza A, Ferrer I, Vargas T, Amils R, Jerez C (1999) Attachment of Thiobacillus ferrooxidans on synthetic pyrite of varying structural and electronic properties. Hydrometallurgy 51:115–129
Schippers A, Sand W (1999) Bacreial leaching of metal sulfides proceeds by two indirect mechanisms with thiosulfate or via polysulfides and sulfur. Appl Environ Microbiol 65:319–321
Simmons S, DiBartolo G, Denef V, Goltsman D, Thelen M, Banfield J (2008) Population genomic analysis of strain variation in Leptospirillum group II bacteria involved in acid mine drainage formation. PLoS Biol 6:1427–1442
Steudel R (1996) Mechanism for the formation of elemental sulfur from aqueous sulfide in chemical and microbiological desulfurization processes. Ind Eng Chem Res 35:1417–1423
Stott M, Walting H, Fransmann P, Sutton D (2000) The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching. Miner Eng 13:1117–1127
Sutherland I (2001) The biofilm matrix-an immobilized but dynamic microbial environment. Trends Microbiol 9:222–227
Suzuki I (2001) Microbial leaching of metals from sulfide minerals. Biotechnol Adv 19:119–132
Temple K, Colmer A (1951) The autotrophic oxidation of iron by a new bacterium, Thiobacillus ferrooxidans. J Bacteriol 62:605–611
Third K, Cord-Ruwisch R, Watling H (2000) The role of iron-oxidizing bacteria in stimulation or inhibition of chalcopyrite bioleaching. Hydrometallurgy 57:225–233
Third K, Cord-Ruwisch R, Watling H (2002) Control of the redox potential by oxygen limitation improves bacterial leaching of chalcopyrite. Biotechnol Bioeng 78:433–441
Tributsch H (1999) Direct versus indirect bioleaching. In: Proceedings of the International biohydrometallurgy symposium IBS’99. Elsevier, Amsterdam, pp 51–60
Tuffin I, Hector S, Deane S, Rawlings D (2006) Resistance determinants of highly arsenic-resistant strain of Leptospirilum ferriphilum isolated from a commercial biooxidation tank. Appl Environ Microbiol 72:2247–2253
Tuovinen O, Bhatti T, Bigham J, Hallberg K, Garcia O, Lindstrom E (1994) Oxidative dissolution of arsenopyrite by mesophilic and moderately thermophilic acidophiles. Appl Environ Microbiol 60:3268–3274
van Aswegen P, Godfrey M, Miller D, Haines A (1991) Developments and innovations in bacterial oxidation of refractory ores. Miner Metall Process:188–191
Vardanyan N (1998) The effect of external factors on pyrite oxidation by Sulfobacillus thermosulfidooxidans subsp. Asporogenes. Biotechnology 6:48–55
Vardanyan N (2003) Oxidation of pyrite and chalcopyrite by mixed cultures of Sulfobacilli and other Sulphur and Iron oxidizing bacteria. Biotechnology 6:79–83
Vardanyan N, Akopyan V (2003) Leptospirillum-like bacteria and evaluation of their role in pyrite oxidation. Microbiology 72:438–442
Vardanyan A, Vardanyan N (2016) Bioleaching of pyrite and chalcopyrite by new isolated thermotolerate sulfur-oxidizing bacteria Acidithiobacillus tandzuti sp.nov. Int J Sci Eng Res 7:203–207
Vardanyan N, Karavaiko G, Pivovarova T (1990) The effect of organic substances on the growth and oxidation of inorganic substrates by Sulfobacillus thermosulfidooxidanssub sp. asporogenes. Microbiology 59:411–417
Vardanyan A, Stepanyan S, Vardanyan N, Markosyan L, Sand W, Vera V, Zhang R (2015) Study and assessment of microbial communities in natural and commercial bioleaching systems. Miner Eng 81:167–172
Wang Y, Zeng W, Qiu G, Chen X, Zhou H (2014) A moderately thermophilic mixed microbial culture for bioleaching of chalcopyrite at high pulp density. Appl Environ Microbiol 80:741–750
Watling H (2006) The bioleaching of sulphide minerals with emphasis on copper sulphids—a review. Hydrometallurgy 84:81–108
Watling H, Collinson D, Shiers D, Bryan C, Watkin E (2013) Effects of pH, temperature and solids loading on microbial community structure during batch culture on a polymetallic ore. Miner Eng 48:68–76
Yu R, Tan J, Yang P, Sun J, Ouyang X, Dai Y (2008) EPS-contact-leaching mechanism of chalcopyrite concentrates by a. ferrooxidans. Trans Nonferrous Met Soc Chin 18:1427–1432
Yu R, Zhong D, Miao L, Wu F, Qiu G, Gu G (2011) Relationship and effect of redox potential, jarosites and extracellular polymeric substances in bioleaching chalcopyrite by acidithiobacillus ferrooxidans. Trans Nonferrous Met Soc Chin 21:1634–1640
Zhang R, Wei M, Ji H, Chen X, Qiu G, Zhou H (2009) Application of real-time PCR to monitor population dynamics of defined mixed cultures of moderate thermophiles involved in bioleaching of chalcopyrite. Appl Microbiol Biotechnol 81:1161–1168
Zhou H, Zhang R, Hu P, Zeng W, Xie Y, Wu C, Qiu G (2008) Isolation and characterization of Ferroplasma thermophilum sp. nov., a novel extremely acidophilic, moderately thermophilic archaeon and its role in bioleaching of chalcopyrite. J Appl Microbiol 105:591–601
Zhou H, Zeng W, Yang Z, Xie Y, Qiu G (2009) Bioleaching of chalcopyrite concentrate by a moderately thermophilic culture in a stirred tank reactor. Bioresour Technol 100:515–520
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Vardanyan, N., Vardanyan, A. (2021). Thermoacidophiles for Bioleaching of Copper. In: Egamberdieva, D., Birkeland, NK., Li, WJ., Panosyan, H. (eds) Microbial Communities and their Interactions in the Extreme Environment. Microorganisms for Sustainability, vol 32. Springer, Singapore. https://doi.org/10.1007/978-981-16-3731-5_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-3731-5_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-3730-8
Online ISBN: 978-981-16-3731-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)