Abstract
The goal of the present work was to study the bioleaching of chalcopyrite (CuFeS2), enargite (Cu3AsS4), and tennantite (Cu12As4S13) by pure and mixed cultures of moderately thermophilic microorganisms belonging to the groups predominant in technological processes (Acidithiobacillus caldus MBC-1, Sulfobacillus thermosulfidooxidans SH-1, and Acidiplasma sp. MBA-1) under different conditions (temperature of 40 to 60°C, presence of Fe2+ ions, pyrite (FeS2), and pyrrhotite (FeS)). Bioleaching of copper from chalcopyrite and enargite was shown to depend on temperature and to be almost independent of the composition of microbial culture. Under optimal conditions (50–55°C), after 30 days 25–27 and 14% of copper was leached from chalcopyrite and enargite, respectively. Increase in the temperature up to 60°C led to the inhibition of copper bioleaching from both minerals. The optimal temperature for tennantite bioleaching was 45°C, while the rate of bioleaching was significantly dependent on the composition of the microbial culture. In an experiment with a mixed culture of all three strains, 26% of copper was leached, while in experiments with pure cultures of A. caldus MBC-1, S. thermosulfidooxidans SH-1, and Acidiplasma sp. MBA-1, after 30 days 12, 21, and 18% of copper was leached, respectively. Addition to the medium of Fe2+ as ferrous sulfate resulted in an increased rate of copper leaching from enargite and tennantite, which may be explained by the fact that Fe3+ ions are generated during microbial Fe2+ biooxidation to Fe3+, which is a strong oxidizing agent and plays an important role in the leaching of sulfide minerals. In the presence of pyrite and pyrrhotite, the rates of enargite and tennantite leaching increased, probably due to the presence of Fe3+ ions in the medium, which were generated during the biooxidation of iron sulfide minerals. The results of the work demonstrated that different environmental factors affected the bioleaching of copper minerals in different ways, which is of practical importance, in particular, for planning the trials for bioleaching of mineral raw materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Most of the world’s copper reserves (about 90%) are contained in sulfide ores, which are mainly treated by producing copper concentrates and their pyrometallurgical processing (World Copper Factbook, 2017; Pietrzyk and Tora, 2018). Copper metallurgy presently encounters a problem caused by depletion of high-grade and easily processed mineral raw materials, which is especially urgent from the point of view of replenishment of the raw-material base of large operating plants in old mining areas (Watling, 2006; Mashkovtsev, 2007; Filippou et al., 2007; Gentina and Acevedo, 2013; Mudd et al., 2013; Northey et al., 2014; Natalenko et al., 2015; Ivanov et al., 2017; Singer, 2017; Polyanskaya et al., 2018; Mudd and Jowitt, 2018). Therefore, low-grade ores, including refractory ones, as well as the ores containing toxic impurities, such as arsenic, are presently being processed (Filippou et al., 2007). Their processing by pyrometallurgical methods presents a number of challenges, such as the loss of valuable products with ore dressing wastes and the need to use special gas purification systems, since conventional pyrometallurgical processing of such raw materials leads to the production of highly toxic volatile arsenic oxides As2O3 and As4O6 (Diaz et al., 2018).
Hydrometallurgical technologies based on acid leaching and bioleaching methods, which, according to different estimates, currently provide from 15 to 20% of world copper production, are an alternative for the pyrometallurgical processing of sulfide ores, including copper ores (Johnson, 2014; World Copper Factbook, 2017). While acid leaching is mainly used for processing of oxide ores, bioleaching is widely used for processing of sulfide ores, including low-grade and difficult-to-concentrate ones (Gentina and Acevedo, 2013; Johnson, 2014).
For processing of the low-grade sulfide ores of non-ferrous metals, heap and dump bioleaching are mainly used. In industrial scale, biohydrometallurgical technologies for copper heap leaching are mainly used in countries with a warm climate, for example, Chile and some other countries of South America (Gentina and Acevedo, 2013). Currently, about 5% of the world’s copper production is carried out by heap bioleaching (Johnson, 2014).
Bioleaching of non-ferrous metals is based on oxidation of sulfide minerals by acidophilic microorganisms that use ferrous iron and elemental sulfur or reduced sulfur species as an energy source. Acidophilic chemolithotrophic microorganisms used in biohydrometallurgy have various physiological properties that provide for their adaptation to a wide range of conditions (Johnson, 2014; Kondrat’eva et al., 2015). Thus, it is possible to increase the efficiency of biotechnological methods for processing sulfide ores by varying the bioleaching conditions. In industrial scale, pure cultures of microorganisms are not used and bioleaching processes are performed by mixed microbial populations. Succession in microbial populations associated with a change in conditions of technological processes during the treatment of mineral raw materials is often observed. Since oxidation of sulfide minerals is an exothermic process, an increase in temperature and replacement of mesophilic microorganisms by thermophiles occur during heap bioleaching (Brierley, 2003; Plumb et al., 2007; Riekkola-Vanhanen, 2007).
The interaction between microorganisms in populations carrying out bioleaching processes significantly affects the rate and efficiency of sulfide ores biooxidation (Kondrat’eva et al., 2015; Mahmoud et al., 2017). Since bioleaching of sulfide minerals on industrial scale is accompanied by an increase in temperature and is carried out at temperatures of 40–50°C, in microbial populations performing industrial biooxidation processes, moderately thermophilic microorganisms usually predominate. It was shown that such microorganisms include bacteria of the genera Aci-dithiobacillus and Sulfobacillus, as well as archaea of the genera Acidiplasma and Ferroplasma belonging to the family Ferroplasmaceae (Rawlings et al., 1999; Okibe et al., 2003; Dopson and Lindstrom, 2004; Morin and d’Hugues, 2007; van Hille et al., 2011, 2013; Muravyov and Bulaev, 2013; Bulaev et al., 2020; Mahmoud et al., 2017) (Table S1, supplementary materials). Moderately thermophilic representatives of the genus Acidithiobacillus species (A. caldus) are autotrophic microorganisms that provide organic carbon sources for mixotrophic and heterotrophic representatives of the genera Sulfobacillus and Acidiplasma in microbial communities (Kondrat’eva et al., 2015).
Chalcopyrite (CuFeS2) is the most widespread copper mineral of greatest importance to industry (Baba et al., 2012). It should be noted that while this mineral is refractory to the process of biological leaching, chalcopyrite concentrates are successfully treated by pyrometallurgical methods (Watling, 2006; Baba et al., 2012). Arsenic-containing copper minerals are also widespread. Enargite (Cu3AsS4) is often found in epithermal copper–gold deposits, while tennantite (Cu12As4S13) may occur in mesothermal deposits (Filippou et al., 2007). It should be noted that processing of the ores containing enargite and tennantite is a problem due to toxic gas emissions (Filippou et al., 2007; Diaz et al., 2018). Therefore, different alternative methods, for example, autoclave leaching, are proposed for processing of arsenic-containing copper ores and concentrates (Yagudina, 2015).
Biooxidation of sulfide minerals is a multi-stage process, which is performed via different mechanisms, and is associated with formation of various intermediates that may inhibit bioleaching. For instance, a layer of elemental sulfur, which is relatively inert, is formed on the surface of some sulfide minerals, which leads to a decrease in the bioleaching rate (Schippers and Sand, 1999). Chalcopyrite bioleaching has been extensively studied for many years (Rodriguez et al., 2003; Ma et al., 2018). Thus, it was shown that the prevalence of sulfur-oxidizing microorganisms in a chalcopyrite-oxidizing mixed microbial culture makes it possible to accelerate its bioleaching (Ma et al., 2018). In contrast to chalcopyrite biooxidation, bioleaching of enargite and tennantite has been less thoroughly studied. In developing approaches for biotechnological processing of diverse mineral raw materials, information is required on dependence of the bioleaching of various sulfide minerals on such environmental factors as temperature, pH, and Eh, as well as on the composition of the microbial population that performs the process.
The goal of the present work was to study the bioleaching of chalcopyrite, enargite, and tennantite at different temperatures by pure and mixed cultures of moderately thermophilic microorganisms belonging to microbial groups predominant in biohydrometallurgical processes of tank biooxidation of sulfide concentrates in order to evaluate the effect of different factors on bioleaching of arsenic-containing copper sulfide minerals and compare it with the effect of the same factors on chalcopyrite bioleaching.
MATERIALS AND METHODS
Copper sulfide minerals chalcopyrite (CuFeS2), enargite (Cu3AsS4), and tennantite (Cu12As4S13), as well as pyrite (FeS2) and pyrrhotite (FeS), milled to a particle size of not more than 75 μm were the subjects of the study. Pyrite (Akchatau Mine, Karaganda region, Kazakhstan); chalcopyrite (Huanzala Mine, Peru) provided by the Mineral Store Kamnevedy (Russia); enargite (Bereznyakovskoye field, Chelyabinsk region, Russia) provided by the Collection Mineral Store (Russia); tennantite (Berezovsky field, Sverdlovsk region, Russia) provided by Mir Samotsvetov company (Russia), and pyrrhotite (Taezhnoye field, Republic of Sakha (Yakutia), Russia) provided by Museum of Geology of UE Sakhageoinform of the State Committee for Geology of the Republic of Sakha (Yakutia) (Russia) were used in the study. Minerals were identified according to their macroscopic properties. The contents of main elements (iron, copper, arsenic, and antimony) in mineral samples were determined.
Pure and mixed cultures of moderately thermophilic acidophilic microorganisms, including strains Acidithiobacillus caldus MBC-1, Sulfobacillus thermosulfidooxidans SH-1, and Acidiplasma sp. MBA-1 previously isolated from samples of ores and pulps of biooxidation reactors (Bulaev et al., 2012; Muravyov and Bulaev, 2013) were used. The properties of the strains are listed in Table 1. It should be noted that all studied strains are capable of oxidizing sulfur, although A. caldus MBC-1 is the most active sulfur oxidizer, as was shown in a model experiment (Fig. S1, supplementary materials). These strains were used for experiments, since they represent the groups of microorganisms predominant in microbial populations that carry out reactor biooxidation of sulfide concentrates with various compositions, including industrial-scale processes (Table S1, supplementary materials). Since understanding the role of individual microorganisms in the bioleaching of various sulfide minerals is important for understanding the regularities of the process, both pure and mixed cultures of microbial strains in various combinations were used for the experiments.
Cultivation conditions. The experiments were performed using liquid medium containing the following (g/L distilled water): (NH4)2SO4, 3.0; KCl, 0.2; MgSO4 · 7H2O, 0.5; K2HPO4, 0.5. The initial pH of 1.5 was adjusted by adding 1.5 mL/L of concentrated sulfuric acid to the medium. As two studied strains (S. thermosulfidooxidans SH-1 and Acidiplasma sp. MBA-1) require an organic carbon source for growth, 0.02% (wt/vol) yeast extract (YE) was added to the medium.
Experiments were performed in flasks with 100 mL of liquid medium and 2 g of the relevant mineral on a rotary shaker (200 rpm) for 30 days. Experiments to determine the effect of the culture composition on the rate of mineral bioleaching were carried out at 45°C, since all the strains studied grow at this temperature. The following combinations of the strains were used in the experiments: pure culture S. thermosulfidooxidans SH-1; pure culture Acidiplasma sp. MBA-1; pure culture Acidithiobacillus caldus MBC-1; mixed culture S. thermosulfidooxidans SH-1 and A. caldus MBC-1; mixed culture Acidiplasma sp. MBA-1 and A. caldus MBC-1; and mixed culture S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1. Dependence of the mineral bioleaching rate on temperature was also studied in the range of 40 to 60°C, with increments of 5°C. A mixed culture containing all three strains was used in these experiments. In all experiments, microorganisms were inoculated so that the initial cell number of each strain was ~1 × 107 cells/mL.
Bioleaching experiments with this mixed culture were also carried out at 50°C in the presence of 35 mM (~2 g/L) Fe2+ ions, which were added to the medium in the form of FeSO4 · 7H2O. Microbial biooxidation of Fe2+ ions led to the generation of Fe3+ ions, which are an oxidizing agent and can affect the biooxidation of sulfide minerals.
An experiment to determine the effect of the presence of iron sulfide minerals, pyrite (FeS2) and pyrrhotite (FeS), on the bioleaching of tennantite and enargite was also conducted. Experiments were performed in flasks with 100 mL of liquid medium supplemented with 1 g enargite or tennantite and 1 g of pyrite or pyrrhotite on a rotary shaker (200 rpm) at 50°C for 30 days using mixed culture of S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1 strains.
Sample analysis. To perform the analysis, samples of the liquid phase were collected after 10, 20 and 30 days of cultivation. The paremeters measured in the samples collected were pH, redox potential (Eh), and concentrations of ferrous and ferric iron, copper, and arsenic. Iron ions concentrations were determined spectrophotometrically using the rhodanide method (Reznikov et al., 1970). The arsenic content in the medium was measured using inductively coupled plasma atomic emission spectroscopy. The copper concentration was measured using a Perkin Elmer 3100 atomic absorption spectrometer (United States). The rate of copper leaching from the minerals was calculated by the concentration of copper ions in the medium.
RESULTS AND DISCUSSION
The results obtained in experiments on biooxidation of the minerals are summarized in Tables 2–4 and Figs. 1–4, as well as in Figs. S2–S4 in supplementary materials.
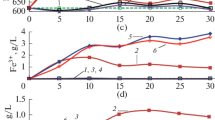
Copper extraction from chalcopyrite (a), enargite (b), and tennantite (c) after 30 days of bioleaching. Designations: 1— pure culture S. thermosulfidooxidans SH-1, 45°C; 2—pure culture A. caldus MBC-1, 45°C; 3—pure culture Acidiplasma sp. MBA-1, 45°C; 4—S. thermosulfidooxidans SH-1 and A. caldus MBC-1, 45°C; 5—Acidiplasma sp. MBA-1 and A. caldus MBC‑1, 45°C; 6—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1, 40°C; 7—S. thermosulfidooxidans SH‑1, Acidiplasma sp. MBA-1, and A. caldus MBC-1, 45°C; 8—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1 and A. caldus MBC-1, 50°C; 9—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1, 55°C; 10—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1, 60°C; 11—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1 and A. caldus MBC-1, 50°C, medium with 35 mM Fe2+.
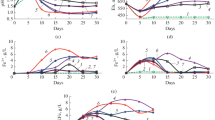
Arsenic concentration in the medium after 30 days of enargite (a) and tennantite (b) bioleaching. Designations: 1—pure culture S. thermosulfidooxidans SH-1, 45°C; 2— pure culture A. caldus MBC-1, 45°C; 3— pure culture Acidiplasma sp. MBA-1, 45°C; 4—S. thermosulfidooxidans SH-1 and A. caldus MBC-1, 45°C; 5—Acidiplasma sp. MBA-1 and A. caldus MBC-1, 45°C; 6—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1, 40°C; 7—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1, 45°C; 8—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1 and A. caldus MBC-1, 50°C; 9—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1, 55°C; 10—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1, 60°C; 11—S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1 and A. caldus MBC-1, 50°C, medium with 35 mM Fe2+.
Changes in medium parameters during bioleaching and arsenic concentrations after 30 days of enargite and tennantite bioleaching in the presence of pyrite and pyrrhotite: a, pH; b, Eh; c, Fe3+ ion concentration (g/L); d, Fe2+ ion concentration (g/L); e, arsenic concentration. Designations: 1—enargite; 2—enargite and pyrite; 3—enargite and pyrrhotite; 4—tennantite; 5— tennantite and pyrite; 6—tennantite and pyrrhotite.
During oxidation of chalcopyrite under all conditions, except bioleaching at temperatures of 50, 55, and 60°C, led to a gradual increase in pH (up to 1.91–1.95) (Figs. S2a and S2b, curves 1–7). At 50 and 55°C, the pH values increased to 1.92 and 1.82, respectively, after 20 days, and then decreased to 1.63 and 1.72, respectively, after 30 days of the bioleaching (Fig. S2b, curves 8 and 9). At 60°C, the pH value gradually increased and reached 2.27 after 30 days (Fig. S2b, curve 10).
In most variants of the experiment with chalcopyrite, Eh values corresponded to the values observed during the active oxidation of sulfide minerals (>750 mV) (Figs. S2c and S2d), while at 60°C, the observed value of Eh was significantly lower than those in other variants of the experiment, and amounted to approximately 600 mV (Fig. S2d, curve 10). In the experiment with Acidiplasma sp. MBA-1, Eh values were relatively low, reaching 692 mV after 30 days (Fig. S2c, curve 3).
In experiments with pure and mixed cultures at 40 and 45°C, the total concentration of iron ions (Fe3+ and Fe2+) amounted to 0.9–1.1 g/L after 30 days of bioleaching (Figs. S2e and S2f, curves 1–7). At 50°C, it amounted to 0.7 g/L (Fig. S2f, curve 8), while at 55°C, it was significantly lower, approximately 0.4 g/L (Fig. S2f, curve 9). At 60°C, the total concentration of iron ions was 0.8 g/L (Fig. S2f, curve 10). It should be noted that in all variants of the experiment, with the exception of bioleaching at 60°C, almost all of the iron was represented by Fe3+ ions, while concentrations of Fe2+ ions were residual (Figs. S2f – S2h, curves 1–9). At 60°C, after 30 days of bioleaching, Fe3+ and Fe2+ concentrations were 0.1 and 0.7 g/L, respectively (Figs. S2f and S2h, curve 10).
At a higher temperature, the rate of formation of jarosite in solutions of ferric sulfate increased according to the reaction (Das et al., 1996):
where Me are K+, Na+, \({\text{NH}}_{{\text{4}}}^{ + },\) and H3O+ ions.
This leads to a decrease in the concentration of Fe3+ ions in the medium and may explain the relatively low concentration of iron ions at 55°C (Figs. S2f and S2h, curve 9). At 60°C, iron was mainly represented by Fe2+ ions; therefore, a significant decrease in the concentration of iron ions in the medium due to formation of jarosite precipitates did not occur (Figs. S2f and S2h, curve 10). Based on the data on the concentration of iron ions in the medium under various experimental conditions, it can be argued that under almost all conditions, at 60°C biooxidation was inhibited, which led to a high ratio of Fe2+/Fe3+ ions concentrations (Fig. S2f and S2h, curve 10).
Data on changes in the copper concentrations in the liquid phase during chalcopyrite bioleaching are shown in Table 2. It should be noted that in experiments with different pure and mixed cultures of microorganisms, the concentration of copper ions in the medium and rate of copper bioleaching (Fig. 1a, bars 1–5 and 7) did not differ much (16–18%). In the same time, the rate of bioleaching depended on temperature and reached a maximum at 50 and 55°C (25 and 27%, respectively) (Fig. 1a, bars 8 and 9). At 60°C, the leaching rate was slightly lower (17%) (Fig. 1a, bar 10), which was probably due to a decrease in the activity of microorganisms at high temperature.
During enargite bioleaching, the pH in different variants did not differ significantly, and after 30 days it amounted to 1.6–1.7 (Figs. S3a and S3b, curves 1–10). In the variant where the medium was supplemented with 35 mM Fe2+, pH value after 30 days of bioleaching was slightly lower than in other variants (1.55) (Fig. S3b, curve 11).
In most variants of enargite oxidation, Eh value after 30 days was in the range of 750–760 mV (Figs. S3c and S3d, curves 1–8), but when bioleaching was performed at 60° C it was lower and comprised 593 mV (Fig. S3d, curves 10). This indicated that at 60°C the activity of the strains decreased compared to lower temperatures. In the variant where the medium was supplemented with 35 mM Fe2+, Eh values were significantly higher (835 mV) (Fig. S3d, curve 11) than under other conditions, which may be explained by a significantly higher concentration of Fe3+ ions, which were formed during the Fe2+ oxidation, in comparison with other variants.
Arsenic concentrations in the medium after 30 days of enargite bioleaching are shown on Fig. 2a. At 45°C, the arsenic concentration did not differ significantly and amounted to 0.33–0.39 g/L (Fig. 2a, bars 2–5 and 7). An exception was an experiment with a pure culture of S. thermosulfidooxidans SH-1, in which the arsenic concentration was 0.47 g/L (Fig. 2a, bar 1). Arsenic concentration was also relatively high at 50°C (0.46 g/L) (Fig. 2a, bar 8). At the same time, arsenic concentrations were relatively low (0.19 and 0.17 g/L, respectively) at 40 and 60°C (Fig. 2a, bars 6 and 10). In the variant where the medium was supplemented with 35 mM Fe2+, arsenic concentration was also relatively low (0.20 g/L) (Fig. 2a, bar 11).
The results of copper bioleaching from enargite are shown in Table 3 and on Fig. 1b. Copper extraction from enargite at 45°C by different cultures did not differ significantly (10–12%) (Fig. 2b, bars 2–5). The leaching rate was slightly higher in the variants with a pure culture of S. thermosulfidooxidans SH-1 and mixed culture of three strains (14 and 13%, respectively) (Fig. 2b, bars 1 and 7). The rates of enargite bioleaching by the mixed culture of three strains at temperatures of 45 to 55°C were almost equal (13–14%) (Fig. 2b, bars 7–9); however, the rates of copper leaching at 40 and 60°C were lower (8 and 6%, respectively) (Fig. 2b, bars 6 and 10). Thus, the copper leaching rate obviously depended on temperature, but bioleaching activity decreased at 60°C.
It should be noted that the concentrations of iron ions were residual in all variants, apart from the experiment with the medium supplemented with ferrous sulfate, since enargite may contain iron only as impurities. In the variant with the medium containing 35 mM Fe2+, the total iron concentration decreased to 0.89 g/L after 10 days and was 0.71 g/L by the end of the experiment (Fig. S3e). After 10 days of bioleaching, iron in the medium was completely oxidized to Fe3+, which indicated the active oxidation of iron by microorganisms. It is probable that concentration of iron ions decreased more than twice compared with the initial one due to the formation of jarosite. It should be noted that the rate of copper leaching from enargite was the highest in the variant with addition of ferrous sulfate to the medium (17%) (Fig. 1b, bar 11). The relatively low concentration of arsenic in the medium in this experiment (Fig. 2b, bar 11) may be explained by the formation of scorodite in the presence of relatively high concentrations of Fe3+ ions (Das, 2019):
Thus, the presence of iron ions in the medium led to an increase in the rate of copper leaching from enargite, but, at the same time, caused arsenic reprecipitation.
During tennantite bioleaching, the pH value in most experiments did not differ significantly. It gradually increased during the leaching and after 30 days of the experiment amounted to 1.99–2.13 (Fig. S4a and S4b, curves 2–10). In the experiment with a pure culture of S. thermosulfidooxidans SH-1 (Fig. S4a, curve 1) and in the variant where the medium was supplemented with 35 mM Fe2+ (Fig. S4b, curve 11), the pH values after 30 days were higher than in other variants (2.36 and 2.67, respectively).
The Eh values in most variants of tennantite oxidation after 30 days were on average lower than during the oxidation of the mixture of chalcopyrite and tennantite, and amounted to 589–700 mV in different variants of the experiment (Figs. S4a and S4b, curves 1–4 and 8–11). In experiments at 40 and 45°C, Eh values was slightly higher in the variants with mixed cultures of the strains S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1 (Figs. S4a and S4b, curves 6 and 7) (723 and 753 mV, respectively). Eh value was also slightly higher in the experiment with a mixed culture of Acidiplasma sp. MBA-1 and A. caldus MBC-1 than in other variants (Fig. S4a, curve 5). It should be noted that in the variant with the medium supplemented with 35 mM Fe2+, Eh was relatively low and amounted to 608 mV after 30 days of the experiment (Fig. S4b, curve 11).
Arsenic concentrations in the medium after 30 days of tennantite bioleaching are shown on Fig. 2b. During tennantite bioleaching at 45°C, arsenic concentrations in experiments with different cultures differed significantly and ranged from 0.27 to 0.49 g/L in contrast to experiments with enargite (Fig. 2b, bars 1–5 and 7). It was the lowest in an experiment with pure culture of A. caldus MBC-1 (Fig. 2b, bar 2), while in experiments with mixed cultures it reached a highest values (Fig. 2b, bars 4, 5, and 7). Arsenic concentration was the lowest in an experiment in which the medium was supplemented with ferrous sulfate, which was obviously due to precipitation of scorodite, as in the experiment with enargite (Fig. 2b, bar 11).
The results of copper bioleaching from tennantite are shown in Table 4 and on Fig. 1c. In contrast to chalcopyrite and enargite, the rates of copper extraction from tennantite at 45°C by different cultures of microorganisms differed significantly and ranged from 12 to 27% (Fig. 1c, bars 1–5 and 7). The rate of copper leaching was the lowest in the experiment with a pure culture of A. caldus MBC-1 (Fig. 1c, bar 2), while in the experiments with mixed cultures Acidiplasma sp. MBA-1 and A. caldus MBC-1 (Fig. 1c, bar 5) and S. thermosulfidooxidans SH-1, Acidiplasma sp. MBA-1, and A. caldus MBC-1, rates of leaching were the highest (Fig. 1c, bar 7) (27 and 26%, respectively). Thus, it can be argued that tennantite bioleaching was more dependent on the composition of the microbial culture than bioleaching of chalcopyrite and enargite. The optimum temperature for tennantite bioleaching was 45°C (26%) (Fig. 1c, bar 7), whereas at lower and higher temperatures the rates of copper leaching were of 13 to 18% (Fig. 1c, bars 6 and 8–10).
Similar to the experiments with enargite, the concentrations of iron ions in the medium were residual, with the exception of the variant in which iron sulfate was added to the medium. The patterns of changes in the concentration of Fe3+ and Fe2+ ions in experiments with tennantite (Fig. S4e) differed from those obtained in the experiments with enargite (Fig. S3e). After 10 days of bioleaching, the total concentration of iron decreased to 0.22 g/L, and at the end of the experiment it was only 0.12 g/L. At the same time, iron in the medium was almost completely oxidized to Fe3+ after 10 days of the bioleaching, which indicated active iron oxidation by microorganisms. The almost complete oxidation of iron may probably lead to its sedimentation. After 20 days of bioleaching, Fe3+ ions concentration decreased to 0.03 g/L, and after 30 days, it was 0.01 g/L. After 20 and 30 days of bioleaching, Fe2+ ions concentrations were 0.12 and 0.11 g/L, respectively. This was also confirmed by the low Eh value at the end of the experiment. Although the rate of copper bioleaching in this variant was higher than under similar conditions in the medium without ferrous sulfate, copper concentration stopped growing after 20 days of bioleaching (Table 4).
The results of enargite and tennantite bioleaching in the presence of pyrite and pyrrhotite are shown on Figs. 3 and 4. It was shown that the rate of copper leaching from both enargite and tennantite was higher in the presence of both pyrite and pyrrhotite than in experiments where enargite and tennantite were leached without the addition of other minerals (Figs. 3c and 4). Concentrations of arsenic in the medium in the presence of pyrite and pyrrhotite were lower (Fig. 3f) due to precipitation of arsenic in the presence of iron ions, the source of which was pyrite and pyrrhotite.
Although similar patterns of copper leaching were observed during enargite and tennantite bioleaching in the presence of pyrite and pyrrhotite, patterns of the changes in other parameters of the liquid phase were different.
The pH values during the bioleaching of enargite in all variants did not differ significantly and after 30 days of the experiment it amounted to 1.54–1.61 (Fig. 3a, curves 1–3). The Eh values in all variants of the experiments on enargite bioleaching were high (775–831 mV) (Fig. 3b, curves 1–3). In this case, the concentration of Fe3+ ions during enargite bioleaching in the presence of pyrite and pyrrhotite gradually increased and reached 0.38 and 0.43 g/L, respectively, after 30 days (Fig. 3d, curves 2 and 3). The concentrations of Fe2+ ions were an order of magnitude lower, increased for 20 days and then decreased (Fig. 3e, curves 2 and 3). Concentrations of iron ions in the medium during the bioleaching of enargite without pyrite and pyrrhotite were residual (Figs. 3d and 3e, curve 1).
During the bioleaching of tennantite, the pH values increased significantly and differed in different experimental variants (Fig. 3a, curves 4–6). After 30 days of tennantite bioleaching, the pH value was 1.87 (Fig. 3a, curve 4), while during bioleaching of tennantite in the presence of pyrite and pyrrhotite, it was 2.34 and 3.13, respectively (Fig. 3a, curves 5 and 6). Such an increase in pH led to a gradual decrease in the Fe3+ ion concentration (Fig. 3d, curves 5 and 6), since at relatively high pH values the rate of formation of insoluble Fe3+-containing compounds increased (Das et al., 1996). In this case, the concentration of Fe2+ ions increased during bioleaching and after 30 days amounted to 0.075 and 0.056 g/L in the presence of pyrite and pyrrhotite, respectively (Fig. 3e, curves 5 and 6).
Thus, it was shown that in the presence of pyrite and pyrrhotite, copper bioleaching from enargite and tennantite was accelerated (Fig. 3c), but data on the parameters of the liquid phase showed that the interaction of minerals with pyrite and pyrrhotite, as well as with microorganisms and the oxidizing agent Fe3+ probably differed, and the mechanisms of their oxidation may have their own peculiarities.
In this work, the study of copper sulfide minerals bioleaching was carried out in the temperature range that is usually maintained in industrial bioleaching reactors, as well as using the cultures of microorganisms that belong to the groups predominant in communities of industrial and laboratory scale reactors (Mahmoud et al., 2017) (Table S1). Thus, it was possible to evaluate the effect of temperature on leaching of the studied minerals, as well as the contribution of various groups of microorganisms to the oxidation of certain minerals. It should be noted that bioleaching of various sulfide minerals has been carried out by different authors using different experimental methods, which does not always make it possible to correctly compare the data obtained in different works. In this work, experiments with different sulfide minerals were carried out under the same methodological conditions, which makes it possible to compare the effect of different factors on leaching of three copper sulfide minerals.
It was shown that different environmental factors had different effects on the bioleaching of chalcopyrite, enargite, and tennantite. Thus, the rate of copper leaching from chalcopyrite and enargite depended to a greater extent on temperature, while the rate of copper leaching from tennantite was determined by the composition of the microbial culture. An increase in chalcopyrite bioleaching rate with increasing temperature is known and has been demonstrated in the works of various authors (Rodriguez et al., 2003; Liu et al., 2017). A similar dependence was also shown for the bioleaching of enargite, which was more actively leached by thermophilic archaea of the genus Sulfolobus at 68°C than by the mesophilic bacterium Acidithiobacillus ferrooxidans at 35°C (Munoz et al., 2006). The results of the present work demonstrate that the rate of chalcopyrite leaching was more dependent on temperature, increasing within the range of 40–55°C, and practically did not depend on the composition of the microbial culture. The rate of enargite bioleaching was less dependent on temperature and practically did not differ at the temperatures of 45 to 55°C. The rate of tennantite bioleaching did not increase at temperatures above 45°C, but decreased in the range of 45 to 55°C, but was largely determined by the composition of the microbial culture. Thus, it was shown that the same factors have a multidirectional effect on the bioleaching of copper sulfide minerals. It should be noted that in our previous work it was shown that chloride ion, which accelerates the process of chalcopyrite bioleaching, inhibits enargite and tennantite bioleaching (Bevilaqua et al., 2013; Bulaev et al., 2019). Thus, in this work, we obtained data that may be of interest both for further research on the processing of complex mineral raw materials and for basic scientific research on the mechanisms of interaction of bioleaching-performing microorganisms and sulfide minerals.
The results obtained indicate the need for in-depth studies of bioleaching processes of different types of mineral raw materials, in particular, the influence of various environmental factors on the biooxidation of different sulfide minerals that may be promising for further development of biohydrometallurgical technologies. It was shown that different copper sulfide minerals, often present in sulfide ores and concentrates, are most actively leached under different conditions (salinity, temperature) and by different groups of microorganisms. Data on the effect of environmental factors and the role of different acidophilic microorganisms in the oxidation of certain minerals allow us to develop approaches to regulate the technological parameters of the processes based on the specific composition of mineral raw materials, for example, to develop multistage bioleaching schemes that will include bioleaching stages at various temperatures that provide optimal conditions for the oxidation of various components of the concentrate.
REFERENCES
Baba, A.A., Ayinla, K.I., Adekola, F.A., Ghosh, M.K., Ayanda, O.S., Bale, R.B., Sheik, A.R., and Pradhan, S.R., A review on novel techniques for chalcopyrite ore processing, Int. J. Mining Engin. Min. Process, 2012, vol. 1, pp. 1–16.
Bevilaqua, D., Lahti, H., Suegama, P.H., Garcia, O., Benedetti, A.V., Puhakka, J.A., and Tuovinen, O.H., Effect of Na-chloride on the bioleaching of a chalcopyrite concentrate in shake flasks and stirred tank bioreactors, Hydrometallurgy, 2013, vol. 138, pp. 1–13.
Brierley, J.A., Response of microbial systems to thermal stress in heap-biooxidation pretreatment of refractory gold ores, Hydrometallurgy, 2003, vol. 71, pp. 13–19.
Bulaev, A., Elkina, Yu., Melnikova, E., and Melamud, V., Effect of sodium chloride on copper bioleaching from sulfide minerals and concentrates, SGEM, 2019, vol. 19, no. 1.3, pp. 799–804.
Bulaev, A.G., Pivovarova, T.A., Kuznetsov, B.B., Kolganova, T.V., and Kondrat’eva, T.F., Rates of sulfide mineral oxidation by acidophilic chemolithotrophic microbial communities from various sources, Microbiology (Moscow), 2012, vol. 81, pp. 397–404.
Bulaev, A., Melamud, V., and Boduen, A., Bioleaching of non-ferrous metals from arsenic-bearing sulfide concentrate, Solid State Phenomena, 2020, vol. 299, pp. 1064–1068.
Das, B., Theoretical study of formation of secondary arsenic minerals: scorodite and pharmacosiderite, ACS Earth Space Chem., 2019, vol. 3, pp. 192–201.
Das, G.K., Acharya, S., Anand, S., and Das, R.P., J-arosites: a review, mineral processing and extractive, Metallurgy Rev., 1996, vol. 16, pp. 185–210.
Diaz, J.A., Serrano, J., and Leiva, E., Bioleaching of arsenic-bearing copper ores, Minerals, 2018, vol. 8, article 215. https://doi.org/10.3390/min8050215
Dopson, M. and Lindstrom, E.B., Analysis of community composition during moderately thermophilic bioleaching of pyrite, arsenical pyrite, and chalcopyrite, Microb. Ecol., 2004, vol. 48, pp. 19–28.
Filippou, D., St-Germain, P., and Grammatikopoulos, T., Recovery of metal values from copper—arsenic minerals and other related resources, Miner. Process. Extr. Metall. Rev., 2007, vol. 28, pp. 247–298.
Gentina, J.C. and Acevedo, F., Application of bioleaching to copper mining in Chile, Electron. J. Biotechnol., 2013, vol. 16, p. 16. https://doi.org/10.2225/vol16-issue3-fulltext-12
Ivanov, A.I., Vartanyan, S.S., Chernykh, A.I., Volchkov, A.G., Konkina, O.M., Korchagina, D.A., Kuznetsov, V.V., Baryshev, A.N., Donets, A.I., and Likhachev, A.P., Current state of the mineral base of nonferrous metals (lead, zinc, copper, nickel, cobalt), Mineral Resources of Russia. Economics and Management, 2017, no. 4, pp. 11–18.
Johnson, D.B., Biomining—biotechnologies for extracting and recovering metals from ores and waste materials, Curr. Opin. Biotechnol., 2014, vol. 30, pp. 24‒31.
Kondrat’eva, T.F., Bulaev, A.G., and Muravyov, M.I., Mikroorganizmy v biogeotekhnologiyakh pererabotki sul’fidnykh rud (Microorganisms in Biotechnologies of Sulfide Ores Processing), Moscow: Nauka, 2015.
Liu, H., Xia, J., Nie, Z., Liu, L., Wang, L., Ma, C., Zheng,L., Zhao, Y., and Wen, W., Comparative study of S, Fe and Cu speciation transformation during chalcopyrite bioleaching by mixed mesophiles and mixed thermophiles, Miner. Eng., 2017, vol. 106, pp. 22‒32.
Mahmoud, A., Cezac, P., Hoadley, A.F.A., Contaminea, F., and D’Hugues, P., A review of sulfide minerals microbially assisted leaching in stirred tank reactors, Int. Biodeteriorat. Biodegradat., 2017, vol. 119, pp. 118‒146.
Ma, L., Wang, X., Liud, X., Wang, S., and Wang, H., Intensified bioleaching of chalcopyrite by communities with enriched ferrous or sulfur oxidizers, Bioresour. Technol., 2018, vol. 268, pp. 415‒423.
Mashkovtsev, G.A., The current state of the mineral resource base of the national metallurgy industry, Mineral Resources of Russia. Economics and Management, 2007, no. 5, pp. 16–25.
Morin, D.H.R. and d’Hugues, P., Bioleaching of a cobalt containing pyrite in stirred reactors: a case study from laboratory scale to industrial application, in Biomining, Rawlings D.E., and Johnson, B.D., Eds., Berlin: Springer, 2007, pp. 35–55.
Mudd, G. and Jowitt, S., Growing global copper resources, reserves and production: discovery is not the only control on supply, Econ. Geol., 2018, vol. 113, pp. 1235‒1267.
Mudd, G.M., Weng, Z., and Jowitt, S.M., A detailed assessment of global cu resource trends and endowments, Econ. Geol., 2013, vol. 108, pp. 1163–1183.
Munoz, J.A., Blazquez, M.L., Gonzalez, F., Ballester, A., Acevedo, F., Gentina, J.C., and Gonzalez, P., Electrochemical study of enargite bioleaching by mesophilic and thermophilic microorganisms, Hydrometallurgy, 2006, vol. 84, pp. 175–186.
Muravyov, M.I. and Bulaev, A.G., Two-step oxidation of a refractory gold-bearing sulfidic concentrate and the effect of organic nutrients on its biooxidation, Minerals Engin., 2013, vol. 45, pp. 108‒114.
Natalenko, A.E., Pak, V.A., and Stavsky, A.P., Main trends in the development of the mineral resource base in the Russian Federation, Mineral Resources of Russia. Economics and Management, 2015, no. 1, pp. 2–8.
Northey, S., Mohr, S., Mudd, G., Weng, Z., and Giurco, D., Modelling future copper ore grade decline based on a detailed assessment of copper resources and mining, Resour. Conserv. Recycl., 2014, vol. 83, pp. 190‒201.
Okibe, N., Gericke, M., Hallberg, K.B., and Johnson, D.B., Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation, Appl. Environ. Microbiol., 2003, vol. 69, pp. 1936‒1943.
Pietrzyk, S. and Tora, B., Trends in global copper mining – a review, IOP Conference Series: Materials Science and Engineering, 2018, vol. 427, 012002.
Plumb, J.J., Hawkes, R.B., and Franzmann, P.D., The microbiology of moderately thermophilic and transiently thermophilic ore heaps, in Biomining, Berlin: Springer, 2007, pp. 217–235.
Polyanskaya, I.G., Ryl’kov, S.A., Yurak, V.V., and Maslennikov, V.V., Mineral and raw materials base of the Ural Federal District: on the way to a sustainable subsoil use (using solid commercial minerals as an example), Bull. Ural State Mining Univ., 2018, no. 3 (51), pp. 131–141.
Rawlings, D.E., Coram, N.J., Gardner, M.N., and Deane, S.M., Thiobacillus caldus and Leptospirillum ferrooxidans are widely distributed in continuous flow biooxidation tanks used to treat a variety of metal containing ores and concentrates, Process Metall., 1999, vol. 9, pp. 777‒786.
Reznikov, A.A., Mulikovskaya, E.P., and Sokolov, I.Yu., Metody analiza prirodnykh vod (Methods for Analysis of Natural Waters), Moscow: Nedra, 1970.
Riekkola-Vanhanen, M., Talvivaara black schist bioheapleaching demonstration plant, Adv. Mater. Res., 2007, vol. 20, pp. 30–33.
Rodriguez, Y., Ballester, B., Blazquez, M.L., Gonzalez, F., and Munoz, J.A., New information on the chalcopyrite bioleaching mechanism at low and high temperature, Hydrometallurgy, 2003, vol. 71, nos. 1–2, pp. 47‒56.
Singer, D.A., Future copper resources, Ore Geol. Rev., 2017, vol. 86, pp. 271–279.
van Hille, R.P., van Wyk, N., and Harrison, S.T.L., Review of the microbial ecology of BIOX® reactors illustrate the dominance of the genus Ferroplasma in many commercial reactors, in Biohydrometallurgy: Biotech Key to Unlock Minerals Resources Value, Changsha: Central South Univ. Press, 2011, p. 1021.
van Hille, R.P., van Wyk, N., Froneman, T., and Harrison, S.T.L., Dynamic evolution of the microbial community in BIOX leaching tanks, Adv. Mater. Res., 2013, vol. 825, pp. 331–334.
Watling, H.R., The bioleaching of sulphide minerals with emphasis on copper sulphides—a review, Hydrometallurgy, 2006, vol. 84, nos. 1‒2, pp. 81‒108.
World Copper Factbook 2017, Lisbon: International Copper Study Group, 2017.
Yagudina, Yu.R., Development and substantiation of the parameters of combined technology for processing of tennanite-containing ores of the Ural copper pyrite deposites, Extended Abstract Cand. Sci. (Tech.) Dissertation, Magnitopgorsk, 2015.
Funding
The work was supported by the President Grant of the Russian Federation, grant no. MK-6639.2018.8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Bulaev
Supplementary material
Rights and permissions
About this article
Cite this article
Elkina, Y.A., Melnikova, E.A., Melamud, V.S. et al. Bioleaching of Enargite and Tennantite by Moderately Thermophilic Acidophilic Microorganisms. Microbiology 89, 413–424 (2020). https://doi.org/10.1134/S0026261720040050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261720040050