Abstract
To compare oxidative dissolution rates of chalcopyrite by different consortia of moderately thermophilic acidophiles, various defined mixed cultures of three bacteria Acidithiobacillus caldus s2, Leptospirillum ferriphilum YSK, and Sulfobacillus sp. LN and one archaeon Ferroplasma thermophilum L1 were studied in batch shake flask cultures incubated at 45 °C. Chalcopyrite dissolution was determined by measuring variations of soluble copper, ferric iron, and pH. Microbial population dynamics involved in bioleaching process were monitored using real-time quantitative polymerase chain reaction (PCR) technology. The complex consortia containing both chemoautotrophic (L. ferriphilum and At. caldus) and chemomixotrophic (Sulfobacillus LN and F. thermophilum) moderate thermophiles were found to be the most efficient in all of those tested. Mutualistic interactions between physiologically distinct moderately thermophilic acidophiles, involving transformations of iron and sulfur and transfer of organic compound, were considered to play a critical role in promoting chalcopyrite dissolution. The real-time PCR assay was reliable to analyze population dynamics of moderate thermophiles in bioleaching systems, and the analysis results were consistent with physiological characteristics of these strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The solubilization of metals from the mineral ores by some acidophilic microorganisms and the subsequent recovery of metals form solution are referred to as bioleaching (Rohwerder et al. 2003). Chalcopyrite (CuFeS2) is the most abundant and refractory of the copper sulfide, therefore bioleaching of chalcopyrite is the key industry target. The majority of researches were focused on the application of mesophilic microorganisms including Acidithiobacillus ferrooxidans, Leptospirillum ferrooxidans, and Acidithiobacillus thiobacillus that grow optimally at 25–40 °C, whereas at this temperature range bioleaching of chalcopyrite has very slow dissolution rate (Watling 2006). In recent years, some studies (Goebel and Stackebrandt 1994; Norris et al. 2000; Rawlings et al. 1999; Hawkes et al. 2006) have suggested that Leptospirillum spp., At. caldus, Sufobacillus spp., and Ferroplasma cupricumulans play significant roles in the dissolution of mineral ores at bioleaching systems operated at 45–50 °C. A number of studies also have shown that, at high temperature, the solubilization of copper from mineral ore proceeds much more efficiently (Dew et al. 2000; Plumb et al. 2002).
Nowadays, there has been an increasing interest in the application of mixed cultures leaching mineral sulfides at 40–50 °C and microbial community analysis (Okibe et al. 2003; Okibe and Johnson 2004). Okibe et al. (2003) analyzed the microflora of three in-line stirred tanks in which a polymetallic concentrate was oxidized at 45 °C, and the community consisted of three bacteria (At. caldus, Leptospirillum sp., and Sulfobacillus sp.) and one archaeon (Ferroplasma sp.). We also found that the moderate thermophilic enrichments from acid mine drainages have similar community structure. The above results suggest that these simple communities can be readily constructed. The oxidative dissolution of pyrite (FeS2) by various defined combinations of seven moderate thermophiles bacteria (Leptospirillum MT6, Acidimicrobium ferrooxidans, At. caldus, Alicyclobacillus Y004, and three Sulfobacillus spp.) and one archaeon (Ferroplasma MT17) are examined (Okibe and Johnson 2004). However, there are few reports on chalcopyrite bioleaching by defined consortia of moderately thermophilic acidophiles. Therefore, efforts should be focused on construction of effective consortia for chalcopyrite bioleaching with moderately thermophilic acidophiles. Furthermore, the microbial successions in chalcopyrite leaching systems, and how such successions influence the mineral dissolution process, are poorly understood and should be investigated. In order to optimize the operating conditions and enhance leaching efficiency, it is necessary to get the best combination of moderate thermophilic acidophiles and monitor the population dynamics of these microorganisms in response to temperature, pH, and other parameters.
Restriction fragment length polymorphism, single strand conformation polymorphism, and denaturing gradient gel electrophoresis have been used to reveal the structure of microbial communities in leaching environments (Battaglia-Brunet et al. 2002; Demergasso et al. 2005; Diaby et al. 2007). However, these methods have poor performance in quantitative analysis. Fluorescence in situ hybridization has been successfully applied to quantify members of acidophiles in bioleaching environment (González-Toril et al., 2003; Okibe and Johnson 2004). This method represents an important step towards quantitative population analysis of bioleaching microorganisms, but it is time consuming and laborious. Real-time quantitative PCR based on the online fluorescence detection of PCR products offers a rapid and simple method to quantify microbial populations in community and it has been successfully applied to quantify bacteria and archaea in mineral sulfide bioleaching systems (Liu et al. 2006).
The objectives of this work were to compare the dissolution efficiencies of chalcopyrite by various mixed cultures of moderate thermophilic acidophiles and monitor the population dynamics during chalcopyrite bioleaching using real-time quantitative PCR technique. The results of the study may provide information to the ‘logical design’ of artificial consortia of moderate thermophilic acidophiles that could be used to bioleaching of particular mineral sulfides.
Materials and methods
Microorganisms and culture conditions
The moderately thermophilic acidophiles selected in this study were At. caldus strain s2 (Qiu et al. 2007), L. ferriphilum strain YSK (Gao et al. 2006), F. thermophilum strain L1 (Zhou et al. 2008), and Sulfobacillus sp. strain LN based on their known properties. All strains were isolated by our laboratory and were maintained as active cultures. Microorganisms were grown in appropriate liquid media in pure culture at 45 °C and were used as inocula in mixed culture bioleaching experiments.
L. ferriphilum YSK was grown in medium 9K with initial pH of 1.6, and Sulfobacillus sp. LN was grown in medium 9K with initial pH of 1.5 supplemented with 0.02% (w/v) yeast extract. At. caldus s2 was maintained in Starky basal salt medium with sulfur as the energy source, the initial pH was 2.0. 9K medium (in g l−1): (NH4)2SO4, 3; KCl, 0.1; K2HPO4, 0.5; MgSO4·7H2O, 0.5; Ca(NO3)2, 0.01; FeSO4·7H2O, 44.7. Starky-S medium (in g l−1): (NH4)2SO4, 3; KH2PO4, 3; MgSO4·7H2O, 0.5; CaCl2·2H2O, 0.25; S, 10. F. thermophilum L1 was inoculated into basal nutrient medium containing (in g l−1): (NH4)2SO4, 1.5; MgSO4·7H2O, 0.25; KH2PO4, 0.25; Na2SO4, 0.25; FeSO4·7H2O, 30; yeast extract, 0.2.
Bioleaching experiments
X-ray diffraction (XRD) analysis of the mineral sample showed chalcopyrite was the major component (62.2%) and PbS (25.8%) was the minor one together with small amounts of bornite (Cu5FeS4) and sphalerite (ZnS). The chemical composition of the mineral sample was 20.13% iron, 28.49% copper, and 27.36% sulfur. The mineral was crushed and then passed through a sieve with a pore size of 75 μm.
Bioleaching experiments were carried out in 500-ml shake flasks containing 200 ml medium at 45 °C, 170 rpm with initial pH 1.3. 9K basal salts medium without ferrous sulfate was used in chalcopyrite bioleaching experiments. The pulp density was 2% (w/v). The leaching experiments were carried out in triplicate.
Pure cultures of At. caldus s2, L. ferriphilum YSK, F. thermophilum L1, and Sulfobacillus sp. LN were harvested by centrifugation and washed twice in sterilized water adjusted to pH 2.0 with sulfuric acid. Then the cells were suspended in 9K medium without ferrous sulfate and were used as inocula. According to the design, equal number of cells (approx. 1 × 107 cells ml−1) for each strain was inoculated into shake flasks, respectively. Samples were withdrawn at regular intervals and analyzed for dissolved copper, total dissolved iron, ferrous iron, pH, and community structure.
Bioleaching experiments were performed using the following five consortia: 1 At. caldus s2 and L. ferriphilum YSK; 2 At. caldus s2 and F. thermophilum L1; 3 At. caldus s2 and F. thermophilum L1 with 0.02% (w/v) yeast extract; 4 At. caldus s2, L. ferriphilum YSK and F. thermophilum L1; 5 At. caldus s2, L. ferriphilum YSK, F. thermophilum L1 and Sulfobacillus sp. LN. The abiotic controls were also designed. The leaching experiments lasted for 28 days.
Physicochemical analysis
Copper and total iron concentrations in solution were measured by atomic absorption spectrometry. The ferrous iron concentration was ascertained by titration with potassium dichromate (K2Cr2O7). The pH of the leaching systems was measured with a pH meter. The leached residues were filtered, washed, and dried using a freeze drier and finally analyzed by XRD. The cell densities of cultures in the flasks were determined by a cell counting chamber.
Genomic DNA extraction
DNA was extracted from pure cultures of microorganisms grown in shake flasks using a TIANamp genomic DNA purification kit (Tiangen Biotech, Co., Ltd., Beijing, China).
DNA extraction was carried out on the bioleaching samples using the protocol modified from Liu et al. (2006). Solids (concentrate and free cells) were harvested from the 5 ml samples by centrifugation at 12,000 ×g for 10 min and extracted genomic DNA was dissolved in 60 μl TE (10 mM Tris–HCl pH 8.0, 0.1 mM EDTA). DNA samples were visualized by staining with ethidium bromide after electrophoresis through a 1% (w/v) agarose gel. For PCR purposes, the purified genomic DNA concentration was measured spectrophotometrically using a NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA) and adjusted to final concentration of 25 ng μl−1.
Design and specificity of PCR primers
The primers used in this study are listed in Table 1. Primers NR-R2, Acaldus-P1, and Lfer-P1 were referenced by Liu et al. (2006) and primers Fer-P1, Sacid-F, and Sacid-R were designed by using Primer Premier 5.0. All primers were synthesized by Sangon (Sangon Biological Engineering Technology & Services, Co., Ltd., Shanghai, China). The specific fragments were amplified and then checked by 1.5% agarose gel electrophoresis and ethidium bromide staining to ensure the specificity of primers and the size of PCR products. DNA sequencing was carried out by Sangon and BLAST analysis in GenBank to check the products. These primers were also tested for the requirements imposed by real-time quantitative PCR (see below).
PCR and real-time PCR
Conventional PCR was performed with a T-Gradient Thermoblock (Biometra, Göttingen, Germany). The PCR program was initial 5 min at 95 °C, followed by 35 cycles of 15 s at 95 °C, 30 s at 55 °C, 30 s at 72 °C, and a final cycle of 72 °C for 7 min. PCR products were analyzed by agarose gel electrophoresis and, where necessary, purified using a QIAquick-spin PCR purification kit (Qiagen, Hilden, Germany). The DNA concentrations of PCR products were measured spectrophotometrically using a NanoDrop® ND-1000 spectrophotometer. As the fragment lengths of all 16S rRNA genes were known, the numbers of DNA were directly calculated from the concentration of the PCR products. The PCR products were diluted serially from 103 to 108 copies μl−1 and amplified by real-time PCR to construct standard curves.
The real-time PCR was carried out with iCycler iQ Real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, USA). The reaction mixture contained 25 μl of SYBR® Green Real-time PCR Master Mix (Toyobo Co., Ltd., Osaka, Japan) which contains Taq DNA polymerase, dNTP, MgCl2, and SYBR Green I dye, 2 μl of a 10 mM solution of sense/anti-sense primer, 5 μl of template DNA, and H2O added to a total of 50 μl. The negative controls were also designed. The real-time PCR program consisted of one cycle of 95 °C for 5 min, and then 40 cycles of 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. At the completion of each run, melting curves for the amplicons were measured by raising the temperature 0.5 °C from 55 to 95 °C while monitoring fluorescence. The specificity of the PCR amplification was checked by examining the melting curve for T m, its symmetry, and the lack of non-specific peaks. All tests were conducted in triplicate.
Results
Comparison of chalcopyrite leaching by defined mixed culture of moderately thermophilic acidophiles
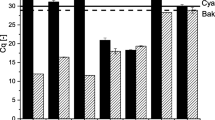
Results of bioleaching of chalcopyrite by mixed culture of moderately thermophilic acidophiles are shown in Fig. 1a. The percentage of released copper in the bioleaching experiment was much greater than that observed in the abiotic control experiment (13.3%). The results showed that different mixed cultures of moderate thermophiles had different percentage recovery of copper after 28 days. The copper extraction from chalcopyrite was greatly enhanced in the flasks with consortia 3 (62.3%) compared with consortia 2 (43.6%) without addition of yeast extract. Also, 68.9% and 72.9% copper were leached from chalcopyrite with consortia 1 and consortia 4, respectively. Consortia 5 showed the strongest ability of leaching chalcopyrite (78.9%) among all the defined consortia.
Variations of copper concentration (a), pH (b), and ferric iron concentration (c) during bioleaching of chalcopyrite by defined mixed cultures of moderately thermophilic acidophiles. ▪ Abiotic control, ● At. caldus/L. ferriphilum, ▴ At. caldus/Ferroplasma sp., ▾ At. caldus/L1 with 0.02% yeast extract, ◂ At. caldus/L. ferriphilum/F. thermophilum, ▸ At. caldus/L. ferriphilum/F. thermophilum/Sulfobacillus LN
It can be seen from Fig. 1b that the pH of bioleaching systems increased initially and subsequently decreased. However, the pH of abiotic control increased gradually at all times.
Variations of soluble ferric iron concentration in leachates are shown in Fig. 1c. Initial ferric iron concentrations were low in all leaching systems. Ferric iron concentration in leachates of consortia 1, 3, 4, and 5 started increasing from the fifth day, 10 to 15 days later, the ferric iron concentration decreased. No obvious variations of ferric iron concentration were observed in leachates of abiotic control and consortia 2.
The inoculated cell densities of consortia 4 were about 3 × 107 cells ml−1. It was detected that the cell densities decreased from 1.33 × 108 cells ml−1 in the middle stage to about 7.86 × 107 cells ml−1 in the latter stage.
In bioleaching system 5, the initial cell densities were about 4 × 107 cells ml−1, and then the cell densities of total prokaryotes were 1.39 × 108 cells ml−1 in the middle stage. In the latter stage, cell densities were 8.91 × 107 cells ml−1.
The XRD analysis results indicated that the leached residues of consortia 1, 4, and 5 primarily comprised chalcopyrite, jarosite, anglesite, and lead dioxide. However, jarosite was not detected in the leached residues of abiotic control, consortia 2 and 3. The leached residue of abiotic control mainly contained chalcopyrite, anglesite, and lead dioxide, while leached residue of consortia 2 and 3 mainly contained chalcopyrite, pyrite, anglesite, and lead dioxide.
Verification of specificity of PCR products
The quality of the amplified conventional PCR products was checked by 1.5% (w/v) agarose gel electrophoresis and ethidium bromide staining. Each PCR product contained a single fragment of the expected size (Fig. 2) and the result of BLAST analysis (data not shown) revealed that the consistency of PCR products sequence alignment were all 100%, which proved amplified DNA fragments were correct.
Melting curve analyses showed that the fluorescent signal obtained in a simultaneous real-time PCR assay originated from specific PCR products and not from artifacts like primer dimers. Analyses of real-time PCR data showed that a single melting peak corresponding to the standard DNA was observed for all samples (data not shown).
Verification of real-time PCR assay
All standard curves had a high correlation coefficient, similar amplification efficiency, and similar slope (Table 2). PCR amplification of specific 16S rRNA was tested in the presence of non-specific, competing 16S rRNA samples of the other species in this study. The experiment that tested the amplification of 16S rRNA-specific DNA in the presence and absence of 1.17 × 105 copies of all other species in this study was carried out. No apparent difference was observed in the presence and absence of the competing non-specific DNA with all primers tested based on the patterns of exponential fluorogenic amplifications (data not shown). The results demonstrated that the assay could be used to detect and quantify target DNA extracted from samples containing mixed microbial species.
To further validate the real-time PCR assay, individual standard 16S rRNA amplicons (each at 3.9 × 104 copies/test) from four different species including At. caldus s2, L. ferriphilum YSK, F. thermophilum L1, and Sulfobacillus sp. LN were amplified using specific primers and their copy numbers were calculated by reference to their respective standard curves. Results showed that the copy numbers estimated by the assay matched the actual copies within a factor of 0.8–1.1 (Table 2), demonstrating that the real-time PCR assay could be used to quantify individual 16S rRNA copies in mixed DNA samples.
Real-time PCR quantification of moderately thermophilic acidophiles in chalcopyrite leaching systems
The real-time PCR assay was used to track the microbial succession of consortia 4 and 5 in bioleaching environments. Leachate samples were withdrawn at 15th day and 28th day.
The analysis results indicated that community structure of consortia 4 varied dramatically during the bioleaching process (Fig. 3). Although the three strains had the same cell numbers in consortia 4 at the beginning, At. caldus s2 and L. ferriphilum YSK together accounted for more than 99% of total prokaryotes in the bioleaching system in the middle stage. F. thermophilum L1 was detected only in the latter stage and the 16S rRNA copy number accounted for 22% of the total copies detected.
At. caldus s2 and Sulfobacillus sp. LN were all through the dominant microorganisms in bioleaching system 5. The 16S rRNA copy numbers of L. ferriphilum YSK accounting for the total copies decreased from 20% in the middle stage to about 2% in the latter stage. In contrast, the 16S rRNA copy numbers of F. thermophilum L1 accounted for 41% of the total copies detected in the final stage, though the proportion of F. thermophilum L1 in the community was small at middle stage (Fig. 4).
Discussion
In real-time PCR analysis, all standard curves had high correlation coefficients and similar slopes; moreover, the PCR amplification efficiencies of designed specific primers were appropriate (see Table 2). Therefore, the use of the curves as standards was allowed. The single melting peak shows that there were no artifacts like primer dimers. Furthermore, the moderately thermophilic acidophiles used in the study contain only one to two copies of 16S rRNA per genome (Liu et al. 2006). The variations of 16S rRNA copies of each strain should roughly represent the population dynamics. Therefore, the real-time PCR assay can be applied for rapid detection and quantification of the variation of moderate thermophiles involved in bioleaching of chalcopyrite.
At. caldus, Leptospirillum spp., Sufobacillus spp., and Ferroplasma spp. used in the present study have been detected or isolated previously (Okibe et al. 2003) and are often present in significant numbers in mineral leaching operations. They are also often selected to construct ‘logically designed’ consortia at 40–60 °C (Rawlings and Johnson 2007). L. ferriphilum YSK is an obligately autotrophic iron-oxidizing moderate thermophile. It has been suggested that At. caldus s2 might contribute, indirectly, to sulfide mineral oxidation by generating acidity, and also by removing elemental sulfur that might otherwise impair the dissolution process by forming passivation layers (Dopson and Lindström, 1999). Ferroplasma spp. are iron-oxidizing acidophilic archaea and appear to be a very diverse group of microorganisms (Dopson et al. 2004; Golyshina et al. 2000; Hawkes et al. 2006). F. thermophilum L1 is a mixotrophic and moderate thermophilic archaeon. Sulfobacillus sp. LN is mixotrophic iron- and sulfur-oxidizing moderate thermophile.
There are two basically mechanisms involved in the bioleaching of sulfide. In direct mechanism, it is supposed that the sulfur moiety of the mineral is biologically oxidized to sulfate without any detectable intermediate. In contrast, in the so-called indirect mechanism, it basically comprises the oxidizing reaction of iron (III) ions during metal sulfide dissolution. In this chemical reaction, iron (II) ions and elemental sulfur (S8) shall be generated. Then, these compounds are biologically oxidized to iron (III) ions and sulfate. The production of sulfate also results in the decrease of pH value in leachate (Sand et al. 2001).
During the leaching process, a passivation layer formed by the jarosite precipitation on the mineral surface is thought as the cause of slow dissolution, and the passivation layer hinders greater copper extraction by restricting the flow of bacteria, nutrients, oxidants, and reaction products to and from the mineral surface (Stott et al. 2000; Watling 2006). In our experiments, the dissolution of chalcopyrite also experienced similar changes. An initial pH increase in the bioleaching systems was due to the acid dissolution and ferrous iron oxidation. Because of the oxidation of elemental sulfur by sulfur-oxidizing bacteria and the formation of jarosite, the pH started to decrease in bioleaching environments after 5 days. The soluble ferric iron concentration decreased after 20 days in the bioleaching systems 1, 4, and 5 because of jarosite precipitation.
The consortia 2 were less effective than consortia 3. A little yeast extract is necessary for F. thermophilum L1 growth in the laboratory and may provide growth factors for F. thermophilum L1, but in its natural environments, other microorganisms like fungi may provide this growth factor (Dopson et al. 2004). Compared with consortia containing L. ferriphilum, consortia 2 and 3 seemed to be less effective to leach copper from chalcopyrite. L. ferriphilum had higher specific growth rate and ferrous iron oxidation rate than F. thermophilum, which resulted in the higher leaching efficiency. The consortia 4 had higher leaching efficiency than consortia 1, which indicated that F. thermophilum also contributed to mineral dissolution. Compared with the consortia containing two or three species of physiologically distinct moderately thermophilic acidophiles, the more complex consortia 5 had the highest leaching efficiency.
The microorganism dynamics in bioleaching process should be consistent with the bioleaching environments. The intermediates including elemental sulfur and many polythionates are readily oxidized by sulfur-oxidizing microorganisms such as At. caldus. That is why these prokaryotes also thrive in chalcopyrite-oxidizing environments. Leptospirillum spp. are known to be commonly detected iron-oxidizing microorganisms in acid mine drainage and in bioleaching systems (Olson et al. 2003). However, as an obligate autotroph, L. ferriphilum YSK was sensitive to organic materials. Therefore, the numbers of L. ferriphilum YSK decreased in the latter stages of bioleaching with the accumulation of organic compounds derived from moderate thermophiles (exudates and cell lysates). Moreover, there was not enough ferrous iron to be utilized by L. ferriphilum YSK in the latter stages (data not shown). F. thermophilum L1 was detected and accounted for the large proportion only in the latter stage of chalcopyrite leaching. It is possible that the growth of F. thermophilum L1 was stimulated by the exudates and dead biomass. Furthermore, the leachates pH of consortia 4 and 5 decreased to about 1.0 in the later stages and the copper concentration increased up to about 4.5 g l−1, which means the low pH value and the high metal ion concentration may result in the shift of the iron-oxidizing microorganisms L. ferriphilum YSK and F. thermophilum L1. Similar results were also obtained by other researchers (Okibe et al. 2003, Okibe and Johnson 2004). In this experiment, the proportion of Sulfobacillus sp. LN all through accounted for a considerable number in total prokaryotes, which was also consistent with other reports (Demergasso et al. 2005). The reason is possible that Sulfobacillus sp. LN can both oxidize ferrous iron and sulfur.
This study indicates that consortia including autotrophic and mixotrophic acidophiles are superior to those including only autotrophs in bioleaching systems, and interactions between acidophiles are capable of promoting chalcopyrite oxidation and copper extraction. Some iron- and sulfur-oxidizing mixotrophs are capable of utilizing organic compounds and may accelerate mineral oxidation (or at least more effectively) (Johnson 1998; Hallberg and Johnson 2001; Watling 2006). The real-time PCR assay was proved to be an effective and rapid method to monitor the population dynamics in bioleaching systems, especially for the simple microbial community.
References
Battaglia-Brunet F, Clarens M, d’Hugues P, Godon JJ, Foucher S, Morin D (2002) Monitoring of a pyrite-oxidising bacterial population using DNA single-strand conformation polymorphism and microscopic techniques. Appl Microbiol Biotechnol 60:206–211
Demergasso CS, Galleguillos PA, Escudero LV, Zepeda VJ, Castillo D, Casamayor EO (2005) Molecular characterization of microbial populations in a low-grade copper ore bioleaching test heap. Hydrometallurgy 80:241–253
Dew DW, Van Buuren C, McEwan K, Bowker C (2000) Bioleaching of base metal sulphide concentrates: a comparison of high and low temperature bioleaching. J S Afr I Min Metall 100:409–413
Diaby N, Dold B, Pfeifer HR, Holliger C, Johnson DB, Hallberg KB (2007) Microbial communities in a porphyry copper tailings impoundment and their impact on the geochemical dynamics of the mine waste. Environ Microbiol 9:298–307
Dopson M, Lindström EB (1999) Potential role of Thiobacillus caldus in arsenopyrite bioleaching. Appl Environ Microb 65:36–40
Dopson M, Baker-Austin C, Hind A, Bowman JP, Bond PL (2004) Characterization of Ferroplasma isolates and Ferroplasma acidarmanus sp. Nov., extreme acidophiles from acid mine drainage and industrial bioleaching environments. Appl Environ Microb 70:2079–2088
Gao J, Xie J, Ding J, Kang J, Cheng H, Qiu G (2006) Extraction and purification of magnetic nanoparticles from strain of Leptospirillum ferriphilum. T Nonferr Metal Soc 16:1417–1420
Goebel BM, Stackebrandt E (1994) Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl Environ Microb 60:1614–1621
Golyshina OV, Pivovarova TA, Karavaiko GL, Kondrat’eva TF, Moore ERB, Abraham WR, Lunsdorf H, Timmis KN, Yakimov MM, Golyshin PN (2000) Ferroplasma acidiphilum gen. nov., sp. nov., an acidophilic autotrophic, ferrous-iron-oxidizing, cell-wall-lacking, mesophilic member of the Ferroplasmaceae fam. Nov., comprising a distinct lineage of the Archaea. Int J Syst Evol Micr 50:997–1006
González-Toril E, Llobet-Brossa E, Casamayor EO, Amann R, Amils R (2003) Microbial ecology of extreme acidic environment, the Tinto River. Appl Environ Microb 69:4853–4865
Hallberg KB, Johnson DB (2001) Biodiversity of acidophilic prokaryotes. Adv Appl Microbiol 49:37–84
Hawkes RB, Franzmann PD, O’hara G, Plumb JJ (2006) Ferroplasma cupricumulans sp. nov., a novel moderately thermophilic, acidophilic archaea isolated from an industrial-scale chalcocite bioleach heap. Extremophiles 10:525–530
Johnson DB (1998) Biodiversity and ecology of acidophilic microorganisms. FEMS Microbiol Ecol 27:307–317
Liu C, Plumb J, Hendry P (2006) Rapid specific detection and quantification of bacteria and archaea involved in mineral sulfide bioleaching using real-time PCR. Biotechnol Bioeng 94:330–336
Norris PR, Burton NP, Foulis NAM (2000) Acidophiles in bioreactor mineral processing. Extremophiles 4:71–76
Okibe N, Gericke M, Hallberg KB, Johnson DB (2003) Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation. Appl Environ Microb 69:1936–1943
Okibe N, Johnson DB (2004) Biooxidation of pyrite by defined mixed culture of moderately thermophilic acidophiles in pH-controlled bioreactor: significance of microbial interaction. Biotechnol Bioeng 87:574–583
Olson GJ, Brierley JA, Brierley CL (2003) Bioleaching review part B: Progress in bioleaching: application of microbial processes by the minerals industries. Appl Microbiol Biotechnol 63:249–257
Plumb JJ, Gibbs B, Stott MB, Robertson WJ, Gibson JAE, Nichols PD, Watling HR, Franzmann PD (2002) Enrichment and characterisation of thermophilic acidophiles for the bioleaching of mineral sulphides. Miner Eng 15:787–794
Qiu G, Fu B, Zhou H, Liu X, Gao J, Liu F, Chen X (2007) Isolation of a strain of Acidithiobacillus caldus and its role in bioleaching of chalcopyrite. World J Microbiol Biotechnol 23:1217–1225
Rawlings DE, Johnson DB (2007) The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology 153:315–324
Rawlings DE, Tributsch H, Hansford GS (1999) Reasons why ‘Leptospirillum’-like species rather than Thiobacillus ferrooxidans are the dominant iron-oxidizing bacteria in many commercial processes for the biooxidation of pyrite and related ores. Microbiology 145:5–13
Rohwerder T, Gehrke T, Kinzler K, Sand W (2003) Bioleaching review part A: Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl Microbiol Biotechnol 63:239–248
Sand W, Gehrke T, Jozsa PG, Schippers A (2001) (Bio)chemistry of bacterial leaching—direct vs. indirect bioleaching. Hydrometallurgy 59:159–175
Stott MB, Watling HR, Franzmann PD, Sutton D (2000) The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching. Miner Eng 13:1117–1127
Watling HR (2006) The bioleaching of sulphide minerals with emphasis on copper sulphides—a review. Hydrometallurgy 84:81–108
Zhou H, Zhang R, Hu P, Zeng W, Xie Y, Wu C, Qiu G (2008) Isolation and characterization of Ferroplasma thermophilum sp. nov., a novel extremely acidophilic, moderately thermophilic archaeon and its role in bioleaching of chalcopyrite. J Appl Microbiol 105:591–601
Acknowledgements
This research was supported by the National Nature Science Foundation of China (no. 50621063), the National Basic Research Program of China (“973” Program, 2004CB619204), the China Ocean Mineral Resources Research and Development Association (no. DYXM-115-02-2-07), and the State Oceanic Administration of People’s Republic of China (no. 200805032).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Rb., Wei, Mm., Ji, Hg. et al. Application of real-time PCR to monitor population dynamics of defined mixed cultures of moderate thermophiles involved in bioleaching of chalcopyrite. Appl Microbiol Biotechnol 81, 1161–1168 (2009). https://doi.org/10.1007/s00253-008-1792-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1792-8