Abstract
With the rapid development of nanotechnology, nanomaterials have been widely applied to bone regeneration. Stem cells, scaffold, and growth factors are commonly regarded as three crucial factors contributing to successful bone tissue engineering. The application of nanomaterials significantly improves the physicochemical and biological properties of the scaffold, which could create biomimetic environment for the osteogenic differentiation of stem cells and sustained release of the growth factors. In this part, we focus on the discussion about the stem cells, nanomaterials, and growth factors which are applied in bone tissue engineering.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Bone tissue is the most important supportive tissue which could continuously remodel and rebuild throughout the lifetime. Bone defects or bone fracture is common diseases affecting the normal function of skeletal system. Although there are internal self-repair and remodel for the pathological injuries, severe bone defect caused by traumas, tumor, or infection still need extra medical intervention. Bone grafts are also alternative candidates for the treatment of bone defects, but the sources are also limited and autogenous bone grafts could be invasive. Currently, nanomaterials have been applied to bone tissue engineering because of their unique nanoscale properties such as specific surface area, porosity, and mechanical property [1,2,3]. Seed cells, scaffold, and growth factors are considered to be three crucial factors for tissue engineering [4]. As an important part, the 3D scaffold plays a vital role in bone regeneration. A suitable scaffold can mimic the microenvironment of cell growth and provide biomimetic structures with good biocompatibility for cell proliferation and new bone growth [5, 6]. Due to the good biological property, nano-biomaterial has become an ideal material for the development of 3D scaffold for bone regeneration. Various nano-biomaterials such as nanocomposite materials, nanofiber materials, nano-bioactive materials, and injectable nanomaterials have been synthesized and used in the research of bone tissue engineering, presenting broad application prospects [7, 8].
Natural bone tissue consists of apatite and polymer collagen fibers, which have hierarchical structures and excellent mechanical properties. In order to mimic the biological structures, various nanomaterials and scaffolds are supposed to be applied to create biomimetic environment for stem cell osteogenic differentiation and bone regeneration [9]. The interaction between the stem cells and nanomaterials is extremely complex, which could be affected by many factors. The physicochemical and mechanical properties of different nanomaterials directly determined the biological potentials for bone regeneration. Understanding different properties of these nanomaterials is crucial for better regeneration results. How to perfectly combine different nanomaterials with complementary properties and precisely manipulate the osteogenesis differentiation of stem cell play key roles in current researches.
1.2 Stem Cell Types Applied to Nanomaterial-Based Bone Regeneration
Cells, scaffold, and growth factors are three crucial factors for tissue engineering. As special cells with multilineage differentiation capacity, stem cells are crucial for tissue engineering, which has revolutionized tissues engineering area, especially for the bone regeneration. Nowadays, many kinds of stem cells have been identified, which could be generally concluded into two different types: embryonic stem cell (ESC) and adult stem cell. They have been widely studied for tissue engineering because of their self-renewal capacity and multilineage differentiation potentials. But the application of ESCs is limited by their limited sources and ethical requirement. Adult stem cells are more commonly studied and applied to bone tissue engineering, and we will concentrate on the discussion about adult stem cells.
1.2.1 Mesenchymal Stem Cells (MSCs)
MSCs are the most common stem cell types which have multilineage differentiation and self-renewal capacity. As a multipotent stem cell, MSC could transform to osteoblast, chondrocyte, and adipocyte (Fig. 1.1). MSCs are firstly identified in the bone marrow, and then many other tissues were proved the existence of MSCs such as the skin, dental pulps, blood vessel, and adipose tissues. Since the first isolation in the 1950s, MSCs have been proved with multipotent and self-renewable capacities, which could differentiate into bone, muscles, adipose tissue, cartilage, and neural cell.
The evolution process of osteoblasts and osteoclasts during the bone formation process. Reprinted with permission from ref. [10] Copyright (2015) Nature Publishing Group
1.2.1.1 Bone Marrow Stromal Cells (BMSCs)
Since they were firstly isolated in the 1960s, BMSCs have been widely applied to tissue engineering especially for bone tissue regeneration. Early in 1997, Komori et al. found BMSCs could express runt-related transcription factor 2 (Runx2), which was regarded as the key osteogenesis transcription factor [11]. In other words, BMSCs still retain the plasticity and stemness for potential osteogenesis differentiation [12,13,14].
BMSCs have been extensively studied for their osteogenic potentials since their isolation and identification. It is the earliest heterogeneous and primitive cell type found in the bone marrow, which are currently the most extensively applied cells for the bone tissue reconstruction and regeneration as a result of the easy obtain, culture, low immunogenicity, and easy transfection [15]. The identification of BMSCs is usually performed by flow cytometry and immunofluorescence staining. BMSCs mainly express surface markers such as CD29, CD44, and CD90 and don’t express hematopoietic cell surface markers CD34 and CD45, which is the main difference in comparison with hematopoietic cells [16]. BMSCs can differentiate into osteoblasts under certain conditions and contribute to the productions, secretions, and mineralization of bone-related matrix, thereby achieving bone regeneration. The in vitro osteogenesis differentiation of BMSC largely depends on the osteogenesis induction culture medium, which include dexamethasone, β-glycerol phosphate, and vitamin C. Dexamethasone enhances the osteogenesis of BMP-2 and stimulates RUNX2, ALP, OPN, and OCN expression; β-glycerol phosphate provides phosphorus ions and induces activation of ALP; vitamin C regulates the homeostasis of extracellular matrix collagen and promotes cell differentiation [17].
The combination treatment with BMSCs and biomaterials has been proved to be able to enhance the bone formation in vitro and in vivo. Many studies have proved that the local treatment of BMSC could accelerate the healing process of large-scale bone defect such as craniomaxillofacial defect. Although BMSCs have been widely applied to bone engineering and have great potentials for multilineage differentiation, limitations also exist. For example, the proliferation and differentiation abilities of BMSCs could possibly be declined after continuous culture and self-renewal ability could be limited. Moreover, the source from bone marrow is also limited. Furthermore, their differentiation potential could be also altered by different culture environment. More importantly, even if the BMSCs were purely isolated, only part of the BMSCs could be susceptible to osteogenesis [18]. In addition, the in vitro expansion of BMSCs could possibly cause immunological rejection responses after in vivo plantation.
1.2.1.2 Adipose-Derived Stem Cells (ASCs)
Besides BMSCs, the other abundant resource of mesenchymal stem cell is ASCs, which have also been applied to tissue engineering, especially for bone regeneration. Different from BMSCs, ASCs have advantages including easy access and isolation, less invasiveness, promising osteogenic ability, low immunogenicity, and immune regulation effects [19]. More importantly, ASCs are more abundant in sources by hundred folds [20]. ASCs were proved to have pluripotential ability of differentiating into other mesodermal lineages and ectodermal lineages. Despite the pluripotential ability, ADSCs lack the capacity to differentiate to the embryonic and extraembryonic tissue types like embryonic stem cells.
The surface markers expressed in ASCs include CD29, CD44, CD73, and CD90, but the hematopoietic-related surface markers CD34, CD45, and CD79 are negatively expressed, which are similar with the surface marker of BMSCs. Different from BMSCs, ASCs express CD36 and CD49d but do not express CD48f and CD104, which could be used to differentiate ASCs and BMSCs. In 2013, the International Fat Applied Technology Society defined the cell phenotype of ADSCs for uniform isolation and identification: (1) the phenotype of newly separated ADSCs is CD31 (−)/CD34 (+)/CD45 (−)/CD235a (−), and the phenotype of ADSCs cultured in vitro is CD31 (−)/CD44 (+)/CD45 (−)/CD73 (+)/CD90 (+)/CD105 (+) [21]. For biomedical application in bone regeneration, both the BMSC and ASC are very promising for osteogenic differentiation. However, the proliferation rate of ADSCs is faster than BMSCs. More importantly, ASCs could maintain their cellular activities in a good status including proliferation, differentiation, and metabolism under in vivo pathological environment [22]. Immunomodulatory effect is another specific characteristic of ADSCs, such as secretion of growth factors and inflammatory factors, promoting angiogenesis and so on. ASCs maintained their anti-inflammatory ability and play important role for microenvironmental regulation in the pathological environment.
Generally, ASCs have similar characteristics for bone tissue engineering with BMSCs, but ASCs have several special capacities including more abundant in sources, faster proliferation, and immunoregulation capacity, which might promise better bone regeneration outcomes. But limitation and challenges also exist; the phenotypes of ASCs are different in vivo and in vitro. The phenotype will also change following continuous proliferation and differentiation such as CD34 expression. Furthermore, ASCs isolated from fat in different tissues may have discrepant differentiation potentials. Therefore, the mechanisms of induced differentiation require further investigation for better biomedical application of ASCs.
1.2.1.3 Dental Pulp Stem Cells (DPSCs)
As one important type of MSC, DPSCs are isolated from dental pulp tissue, which also have multiple differentiation capabilities. DPSCs were firstly isolated and identified by Gronthos et al. [23] in 2000. These MSC-like cells in dental pulp tissues also express the MSC markers like CD29, CD105, CD146, CD166, and STRO-1 [24]. DPSCs could be isolated in human third molar and exfoliated deciduous teeth (SHED). Miura et al. [25] firstly isolated DPSCs from SHED and applied them to in vivo bone tissue engineering. According to the different sources from permanent teeth and exfoliated deciduous teeth, there are some differences between the hDPSCs and SHED. SHEDs are isolated from deciduous teeth and they could be more immature than hDPSCs. In other words, SHEDs have stronger capacity in terms of proliferation and differentiation. Meanwhile, obtaining SHED from deciduous teeth could be easier, which is advantageous for clinical application [26]. Compared with DPSCs from normal teeth, DPSCs isolated from supernumerary teeth have higher proliferation capacity and differentiation potential [27].
Since the potential differentiation ability and accessibility, DPSCs also have potentials in bone tissue engineering. Dental pulp tissues are accessible organs and have recently attracted much attention for MSC isolation and tissue engineering. DPSCs have excellent proliferation capacity and could retain the characteristics of stem cells after cultured by many generations. Besides the multilineage differentiation, undifferentiated DPSCs also have immunoregulation capacity. DPSCs could suppress the proliferation of T cell and B cell, increase the number of regulatory T cell, and produce TGF-β, IL-6, IL-10, nitric oxide (NO), and prostaglandin(PG)-E2 [28].
Although dental pulp seems an alternative tissue for stem cell isolation, the use of DPSCs is also limited due to the small quantity and longer culture for enough cells for tissue engineering. Furthermore, the in vivo application for bone regeneration of DPSCs could be also limited. For example, in a histological analysis for 3-year transplant of DPSCs in human mandibles, the regenerated bone was compact bone and lack of vasculatures [29]. Therefore, the manipulation for the uncertain differentiation still requires further study. Besides, biological activity of dental pulp tissue may be declined with the age increase, and the autologous sources of DPSCs could be limited. Meanwhile, it still needs long-term exploration about the immune rejection of allogeneic DPSCs after transplantation.
1.2.2 Other Types of Adult Stem Cells
Many tissues and organs have the capacity of repair and regeneration, in which many adult stem cells could be isolated and applied to regenerative medicine. These adult stem cells play their unique roles in regenerative medicine such as neural stem cells, periosteal stem cells, corneal stem cells, and so on.
1.3 Nanomaterials Applied to Stem Cell Osteogenic Differentiation
During the past decades, various types of nanomaterials have been exploited and applied to nanomedicine. Many nanomaterials have been proved to influence bio-response of stem cells like proliferation and differentiation. For bone regeneration, osteogenesis differentiation of stem cell is very crucial for new bone formation. Many researches have discussed the osteogenic effects of nanomaterials and their potentials for bone tissue engineering. Unique cellular responses could occur depending on different types of materials, which is summarized as follows.
1.3.1 Polymeric Nanomaterials
Polymeric NPs have been extensively introduced into biomedicine area because of the good biocompatibility and drug-loading capacity. Meanwhile, surface modification imparts polymeric NPs unlimited possibilities for better osteogenic induction capabilities. Besides, good biodegradability also contributes the extensive application of polymeric nanomaterials. For example, PLGA and chitosan are commonly used for tissue engineering. Chitosan is well known as a biocompatible, biodegradable, and nontoxic biomaterial, which has great potentials for physicochemical modifications due to its porosity, tensile strength, and biocompatibility [30]. For example, Wu et al. [31] fabricated chitosan NPs as carrier to deliver microRNA to MSCs, and enhanced delivery efficiency was observed. As a result, osteogenic differentiation of MSCs obviously increased. More importantly, chitosan NPs showed good biocompatibility and no toxicity to the MSCs. Similarly, Chen et al. [32] also used chitosan NPs as nano-carrier to deliver the stable modified hsa-miR-199a-5p (agomir); this chitosan NPs/agomir complex significantly improved the in vivo bone regeneration. Besides drug carriers, polymeric nanomaterials could be employed as scaffolds for bone regeneration [33]. Generally speaking, polymeric nanomaterials could be used as promising candidate to regulate stem cell osteogenic differentiation and bone tissue regeneration.
1.3.2 Metal-Based Nanomaterials
As a common type of nanomaterials, metal-based nanomaterials also showed their potentials for bone regeneration. Due to the unique metallicity, metal-based nanomaterials could induce osteogenic differentiation by causing mechanical stress to the stem cells. Currently reported osteogenic metal-based nanomaterials include gold NPs (AuNPs), silver NPs (AgNPs), titanium NPs (TiNPs), and iron NPs (FeNPs), and their osteogenic potentials are discussed as follows.
AuNPs could be regarded as promising nanomaterial for tissue engineering because they have satisfying biocompatibility, easy modification, and antimicrobial ability [34]. Naturally, many studies have reported their potentials for bone regeneration as well. For example, Yi et al. [35] treated MSCs with AuNPs and studied the cellular responses. The results turned out to be that AuNPs induced MSC osteogenic differentiation toward osteoblast cell rather than adipocyte cell. The underlying mechanism was that AuNPs could interact with the cell membrane and cytoplasm, which caused mechanical stress to the MSCs and activated osteogenesis-related gene expressions. More than MSCs, AuNPs were also proved to have osteogenic induction effect for human periodontal ligament stem cells (hPDLSCs). Niu et al. [36] investigated the induction of AuNPs for the osteogenic differentiation of hPDLSCs and detected osteogenic transcriptional profile of hPDLSCs after treated with AuNPs; the analysis suggested that the expressions of ALP, osterix, collagen I, and RUNX2 were significantly enhanced, which was important for osteogenic differentiation. In addition to pure AuNPs, easy functionalization and modification contribute to more extensive application of gold nanomaterials. Modified AuNPs were reported to enhance osteogenic differentiations of stem cell in many studies [37, 38].
Besides AuNPs, AgNPs also contributed to regulate the fate of stem cells. AgNPs are well known for their antimicrobial/antiviral properties and are often integrated into bone grafts as antimicrobial agents. Although the antibacterial activity of nanoscale silver nanomaterials is widely confirmed, the osteogenic properties remain controversial. For different kinds of stem cells, the results might be different. For example, Qin et al. [39] suggested that AgNPs could induce urine-derived stem cells differentiated toward osteogenic profile when AgNPs were at proper concentrations (for instance, 4 ug/mL). However, when the seed cells came to hMSCs, the results might be different. Liu et al. [40] suggested that AgNPs caused cytotoxicity to hMSCs and AgNPs didn’t change the osteogenesis-related gene expression, which meant that AgNPs didn’t induce the osteogenic differentiation of hMSCs. Therefore, the osteogenic properties may vary according to different circumstances and seed cells as well. Although the antimicrobial effects may be advantageous for the use of AgNPs in bone regeneration, the cytotoxicity is also a nonnegligible problem for AgNPs [41].
Other types of metal-based nanomaterials also have positive influence for their osteogenic properties, which makes them play unique roles in bone regeneration and tissue engineering such as TiO2NPs [42], iron oxide NPs [43], and so on.
1.3.3 Silica-Based Nanomaterials
Silica is one of the important elements for skeletal system, and silica-based nanomaterials are promising biomaterials due to their good biocompatibility [44]. It was proved that silica NPs showed no negative effect to the cell viability and exhibit size- and dose-independent cytocompatibility on hMSCs [45]. Furthermore, the ALP activity and bone nodule production of hMSCs were obviously enhanced after treated by silica NPs, which demonstrated the osteogenic induction effect of silica NPs. The osteogenic effects may derive from the Si release from the silica NPs as a result of cellular lysosomal degradation. Besides biocompatibility, porousness is the other unique property for silica nanomaterials. Due to the chemical modification property, nanoporously structured silica NPs attracted much interests in bone tissue engineering. Chemical modification could enhance the osteoinductive effect of silica NPs. Christel et al. [46] modified the nanoporous silica materials with bone growth factor BMP-2; the complex showed obvious osteoinductive effects on ASCs. Same osteoinductive effects could be found in other studies with different modification and composites [47, 48].
1.3.4 Carbon-Based Nanomaterials
Carbon nanomaterials have drawn increasing interests in biomedical application because of the excellent physicochemical and biocompatible characteristics [49, 50]. Their uniquely manipulative spatial structures including 2D and 3D impart them more structural possibilities for scaffold fabrication in tissue engineering, which could simulate the structure of biological bone extracellular matrix. Graphene (GR), graphene oxide (GR), and carbon nanotube (CNT) are common forms of carbon-based nanomaterials, which could be applied to bone tissue engineering.
Since the first report in 2004 of graphene by Novoselov and Geim, GR has been extensively applied to biomedical area due to its extraordinary physicochemical properties [51]. As single-layer 2D nanosheets, many studies have reported their positive impacts on the stem cell regulation [52, 53]. GR could provide a biocompatible scaffold for hMSCs and promote the osteogenic differentiation [54]. CNT is a new type of nanomaterial which have special shape and morphology with a cylindrical architecture, which make CNT a promising candidate for biomedicine [55]. Many studies have proved the osteoinductive effect of CNTs, and the array of CNTs could affect the stem cell responses. It was suggested that only single-walled CNT without any other chemical/biochemical treatment could initiate osteogenic differentiation of hMSCs [56]. If hMSCs were cultivated on the multiwalled carbon nanotube (MWCNT) arrays, the cells showed different behaviors like well-spread and spiral-shaped cell colons, and osteocalcin (OCN) gene expression was enhanced in comparison with hMSCs cultured on dish [57]. Moreover, the combination of GR and CNT could also serve as osteoinductive hybrids. Yan et al. [49] fabricated GR/SWCNT complex and treated rat MSCs with these hybrids. After treatment by GR/SWCNT complex, osteogenic-related gene expressions and mineralized matrix nodule formation were enhanced. On the contrary, adipocyte-related genes were downregulated.
1.3.5 Nucleic Acid-Based Nanomaterials
As a novel type of nanomaterial, nucleic acid nanomaterials have drawn rising attention due to their excellent biocompatibility and editability. Nucleic acids (DNA, RNA) and nucleic acid analogs such as PNA and LNA play important roles in regulating gene and protein expression, which finally manipulate cell activities such as proliferation, migration, and differentiation [58]. DNA nanomaterials are more widely studied due to their self-assemble property according to the principle of Watson-Crick base pairing. As a result, various types of DNA origami have been reported with unique spatial structure and biological activities.
Our previous work has studied one of the DNA origamis, tetrahedral framework DNA nanostructures (TFNAs). Due to their tetrahedral nanostructure, cellular uptake of TFNAs could be more efficient than oligonucleotides. The multiple biological effects of TFNAs were extensively investigated including promoting cell migrations, proliferations, and differentiations, which suggested the great potentials of TFNAs in the tissue engineering area [59]. Zhou et al. [60] proved that TFNAs could promote the proliferation and osteogenic/odontogenic differentiations of DPSCs as the osteogenic-related gene and protein expressions were enhanced. Shao et al. [61] studied the effects of TFNAs on the osteogenic differentiations of ADSCs and found that TFNAs activated osteogenic potential of ADSCs via Wnt/β-catenin signaling pathway. TFNAs could also serve as novel drug carriers for functional nucleic acids like siRNA, microRNA, lncRNA, and oligonucleotides to achieve better bone regeneration results.
1.3.6 Hydroxyapatite
As basic components of biological bone tissue, hydroxyapatite has been widely applied to bone regeneration because of the satisfying biocompatibility and bioactivity. Natural bone tissue has hierarchical structures which mainly composed of periodically arranged inorganic nano-hydroxyapatites and organic collagen fibers. HA-based bioceramics have excellent osteoinductive and osteoconductive activity; the microporous structure of the material could lead to the high adsorption and accumulation of various endogenous bone growth factors, which will activate the differentiation of MSCs into osteoblasts and ultimately achieve osteogenesis induction. But the mechanical properties of HA prepared by the existing process are not good enough, which limits its wider application. Therefore, nano-HA/polymer composite biomaterials are more commonly applied for better mechanical properties which we will discuss in other parts. Although there are many types of HA/polymer composites, the standard of the properties requires to be unified; long-term follow-up is required to evaluate the clinical potentials.
1.4 Properties of Nanomaterials Affecting Osteogenic Differentiation and Bone Formation
The osteogenic differentiation of stem cells and bone formation process have intimate connection with the chemical, physical, mechanical, and biological properties of related nanomaterials as shown in Fig. 1.2.
The illustration of ideal properties and structures for nanomaterial-based bone regeneration. Reprinted with permission from ref. [1] Copyright (2017) The Royal Society of Chemistry
1.4.1 Mechanical Properties
Bone tissues have strong load-bearing ability which consists of HA nanocrystals and fiber-shaped collagen molecules. One of the goals for bone regeneration is to simulate the hierarchical structure of biological bone tissue. Optimal scaffolds are supposed to have similar mechanical property to the natural bone to provide biomimetic environment for osteogenic differentiation of stem cells. For severe bone defect area, scaffolds should provide structural support for the bone regeneration. The matrix stiffness also plays important roles in osteogenic differentiation of stem cells [62]. Therefore, mechanical property of scaffold materials is very crucial for successful bone regeneration results, and suitable mechanical property seems to be the most basic requirement for bone regeneration scaffold.
Stem cells are not only regulated by biological molecular signals such as growth factors but also regulated by mechanical properties of scaffolds [63]. The mechanical signals will induce cell differentiation to different subtypes. Polymers and bioceramics are common materials which could provide suitable mechanical and structural support for bone regeneration. Although polymers are reported to be useful in bone regeneration, single type of polymer seems not to satisfy the mechanical requirement. Therefore, the combination of polymers and inorganic materials is usually more common to improve the mechanical properties for better engineering. As essential component of natural bone tissues, HA is the most commonly used material to improve mechanical properties of the nanocomposite to better mimic the microstructures of biological bone [64]. Wei et al. [65] analyzed the structural effects of nano-HA/polymer composite scaffold for bone regeneration, they found that combination of nano-HA with the PLLA polymers enhanced the mechanical property by about two folds with suitable microarchitecture, which could be favorable for cell adherence and differentiation. Other types of materials could be also used to improve better mechanical property. Zhang et al. [66] incorporated octadecylamine-functionalized nanodiamond into PLLA polymers and studied the effect of mechanical properties changes on the bone formation process. The results demonstrated that incorporation of 10% wt nanodiamond obviously enhanced the tensile property of the composites. The increase in mechanical property increased the mineralization and bonelike apatite growth.
The hierarchical structures provide natural bone with excellent mechanical and biological properties. Therefore, the mechanical property of bone tissue scaffold should mimic the natural bone, which means that compression modulus should be 45–100 MPa. Different types of polymers and inorganic phases could be served to develop scaffolds with varied mechanical properties via adjusting ratio and conjugation manners of different components.
1.4.2 Porosity
Porosity is another crucial factor contributing to successful development of bone tissue scaffold. There are also requirements for void ratio and pore sizes to provide better environment for bone regeneration. Proper pore size and ratio are favorable for cell ingrowth and nutrition/waste exchanges. Too small pore size will prevent the cell ingrowth and may lead to cellular capsules around the scaffolds. Meanwhile, too large pore size could possibly reduce the surface area and mechanical strength of the scaffolds [9].
Murphy et al. [67] investigated the impact of pore size on the cellular adherence, proliferation, and migration on the porous scaffolds with 85–325 μm pore sizes. Although the final number of osteoblasts was the most abundant after 7-day observation for the biggest pore size, there was a suddenly increased peak for the 120 μm pore size scaffold. This might suggest that pore size was related to the surface area, which plays important roles in inducing initial cell attachment, because scaffold with large pore size has smaller surface area. The results also suggested that the cell adherence could not always be explained by surface area; if the range of pore sizes was 85–325 um, the surface area theory couldn’t explain. After the cell attachment, bigger pore size could provide more space for the cell proliferation and migration; finally they suggested that 325 um pore size was suitable for bone tissue engineering.
Since decades ago, discussion about the impact of pore sizes on the bone regeneration has been emerging. Pore sizes from tens to thousands microns have been reported for bone regeneration. An early study suggested that the ideal pore size for optimal bone in-growth rates was 100–135 um [68]. There were also studies suggesting that bone formation and vascularization required the pore size bigger than 300 μm. If the pore sizes are <300 μm, the scaffold tended to induce osteochondral ossification rather than osteogenesis [69, 70]. However, there were also study investigating the osteoinductive ability of nanoporous titanium with pore size of 30 nm and 100 nm; the results demonstrated that only substrates with 30 nm pores induced osteogenic differentiation of human neural crest-derived stem cells and substrates with 100 nm pore size didn’t induce osteogenic differentiation [71]. There are evidences suggesting that macropores (>100 μm) are favorable for bone ingrowth and angiopoiesis, but microporosity (pore size <20 um) is also regarded as important way to improve the osteoinductive ability of scaffold. Microporosity could provide the scaffold with larger surface area and better permeability, which could enhance protein adsorption on the scaffold and improve cell-scaffold interaction [72]. Besides surface area, micropore-induced capillarity could also enhance the cell adherence, bone growth, and distribution in the scaffold [73, 74]. Besides the effect of pore size, porosity ratio is also a crucial factor for the bone formation and mechanical property of scaffold. Chen et al. [75] developed porous titanium scaffold for bone regeneration; the 30–50% porosity samples were similar with the structure of natural bone. hMSCs easily adhered and proliferated on the surface and grow into the porosity structures also indicated osseointegration potentials.
Porosity contributes to the regulation of bone tissue ingrowth and is an essential factor for successful bone regeneration results. Adequate pore size contributed to high surface area, osteogenic protein adsorption, and cell adherence and ingrowth. It has been preferably considered that if the pore size is between 90 and 200 um, it could induce better bone formation. But for different materials and stem cells, the porosity could be different for the optimal osteoinductive outcomes.
1.4.3 Hydrophilicity
The hydrophilicity of the material surface is an important factor affecting the cell behaviors like adhesion and morphology. The hydrophilicity decrease of scaffold could lead to poor cell adherence [76]. There are many factors that affect the hydrophilicity of the material such as surface roughness, surface topology, and surface physicochemical conditions, which all could cause contact angle and wettability changes.
Many physicochemical methods could improve the hydrophilicity but vary from different materials. Chemical methods include surface oxidation, grafting modification, copolymerization, and surfactant modification. Physical methods include blending modification, high-energy radiation, and so on. For example, surface modification with collagen could be a feasible way to improve the hydrophilicity, and the incorporation of collagen on the polymer surface significantly enhanced the hydrophilicity and furtherly improved the attachment of fibroblasts [77]. Chemical modification to introduce diethylaminoethyl groups onto the polymer could also improve surface hydrophilicity and roughness, which subsequently enhanced the cell attachment and proliferation [78].
In summary, the methods for hydrophilicity improvement include two general ways: (1) surface roughness changes via physicochemical modification, which mainly changed the contact angle and wettability changes of the material surface, and (2) incorporation and coupling of hydrophilic components such as biological polymers and surfactants. The improvement of hydrophilicity will enhance cell/protein attachment, cell proliferation, and spreading on the scaffold surface, which promise better bone regeneration results.
1.4.4 Biodegradability
As we mentioned before, mechanical properties of scaffold play important roles for the structural support for bone formation. Although these polymers and inorganic components could optimize the mechanical structure for better bone regeneration results, the non-absorbable components such as metal or carbon could possibly cause cytotoxicity after long-term existence. Ideal scaffold should have proper biodegradability, and the absorption rate should be consistent with the bone formation rate [79]. After enough ECM are produced to provide structural support, the scaffold should be resorbed to prevent adverse effects.
After planted in vivo, the scaffold degradation suffered from biological degradation such as free radicals, enzymes, and cellular phagocytosis. Biodegradability materials which could be applied for bone regeneration include bioceramic, natural, and synthetic polymers. Synthetic polymers could also have good absorbability, but some degradation components have toxic and side effects. For example, the degradation products of PLGA are acidic components, which could increase tissue acidity and cause inflammatory responses. For bioceramics, they could be poor in toughness and flexibility, but the degradation products such as Ca2+ and PO43− could deposit and promote bone formation [80, 81]. Natural polymers have excellent absorbability such as collagens, gelatins, and chitosan, but they usually have poor mechanical and processing performance.
Therefore, it’s important to choose proper materials for scaffold design. Meanwhile, the degradation rate could be manipulated via changing the structures and composition of the polymers such as crystallinity and hydrophobicity. More importantly, the key point is to control the absorption rates and ensure that the scaffolds can withstand the appropriate external force before the new bone completely replaces the scaffold.
1.4.5 Biocompatibility
Biocompatibility is the most basic requirement for scaffold materials. It directly determined whether the nanomaterial could be applied to bone regeneration or not. It depends on the interaction between the materials and biological tissues, which includes two aspects: the host response and material reaction. For the host response, the most direct one is immunoreaction; the original components or subordinate degradation product may cause cytotoxicity or inflammatory reaction. Other negative responses for the host are mutagenicity and teratogenicity. For the material responses, the living system could have negative effects on the material including abnormal degeneration, corrosion, degradation, and absorption. The interactions between living cell and scaffolds is extremely complex. The biocompatibility reflected in the interaction between biological system and the materials, which could be affected by material components and their physicochemical properties. These factors will significantly affect cell adhesion, proliferation, spreading, biochemical activity and differentiation orientation, etc. The cell growth mode in turn directly affects the biocompatibility of the materials. Therefore, material modification and functionalization are the common ways to improve the biocompatibility of most materials.
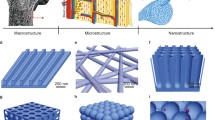
1.5 Nanostructures and Scaffolds Applied to Bone Tissue Engineering
The design strategies of the scaffolds for bone tissue engineering should be biomimetic and simulate the biological environment of the biological bone matrix. As one crucial part of the strategy, optimal scaffold should be osteoconductive, osteogenic, and osteoinductive. Good scaffolds could incorporate and release growth factors to initiate and manipulate cellular activities and provide a suitable environment to stimulate bone repair and regeneration [82]. Since nanomaterials are applied to tissue engineering, nanoscale scaffolds significantly changed the tissue regeneration profile. With unique physicochemical properties, nano-sized materials have special biological properties to regulate cell responses like proliferation, migration, and differentiation. The strategy for bone tissue engineering scaffold design is to fabricate 3D structures with nanoscale and microscale effects, which is advantageous for cell attachment and differentiation. A variety of nanostructured scaffolds have been reported for bone regeneration. The major nanostructures are nanopattern [83], nanopores [84], nanospheres [85], nanofibers [86], nanotubes [87] and nanocomposites [88, 89]. Their fabrication, properties, interaction with stem cells, and osteogenic potentials are discussed in this part.
1.5.1 Nanopatterns
Nanopattern is one type of scaffold regulating cell responses via manipulating the surface nanotopography of the scaffold. The cellular behaviors on the scaffold could be different according to different surfaces [90]. Therefore, the architectural design and surface topography are very important for bone regeneration. Since researches have reported the guidance and regulation effect of surface morphology on the cell attachment, it’s important to fully understand the nanopattern design.
Understanding how the nanotopography influence the cell attachment, morphology, and gene expressions is helpful to optimize the surface design of the nanopatterns. Tsimbouri et al. [91] investigated the role of the nanotopography in regulating the morphology and phenotype of MSC; the results demonstrated that the cell attachment, nucleus, and lamin morphology varied according to different nanotopographies. The interaction between stem cell and ECM could possibly directly or indirectly change the cell responses, which is called mechano-transduction. To furtherly understand the effect of mechano-transduction caused by surface topography, they used two nanotopographies, high intracellular tensions and osteogenic surface (near square 50, NSQ50) and self-renewal enhancing surface (square, SQ); the main differences between these two surfaces are the size of nanopits on the surface. The SQ nanotopography caused less phenotypical change, while NSQ50 nanotopography regulated osteogenic differentiation of MSCs.
In natural bone tissues, especially the bone during the healing process, nanodot-like topography with different intensity could be observed [92], which suggested that nanodot-like topography may be a feasible way for the scaffold to simulate the biological bone ECM. Kim et al. [93] fabricated nanopatterned substratum with different nanopillar intensity to design biomimetic bone tissue engineering scaffold. Among three different nanopillar pattern arrays (width to spacing ratio 1:1, 1:3, 1:5), nanopatterned substratum with 1:3 ratio showed the best bone mineralization results. The nanopillar pattern density also influences the attachment of osteoblast-like cells, which is a crucial step for bone regeneration on the scaffold. Besides, the results also demonstrated that attached cells spread more on the sparser nanopatterns. All these findings suggested that nanotopographical density could be regarded as a potential strategy for scaffold design. In conclusion, the nanopattern of the scaffold surface could regulate stem cell responses as a result of mechano-transduction. Cellular cytoskeleton contractility of the stem cell contributes to the mechanosensitivity of stem cell. Therefore, manipulating the nanopattern surface such as texture and nanopit intensity could be an efficient strategy for bone tissue engineering scaffold design.
1.5.2 Microspheres/Nanospheres
Nanospheres are characterized by their porous structures and controlled drug release. As we know, growth factors are crucial parts, and the sustained release of growth factors are encouraged for better bone formation outcomes. For conventional bulk scaffold, the initial burst releases of growth factors couldn’t satisfy the requirements of long-duration release for bone formation. Therefore, nanospheres are expected to achieve controlled delivery of growth factor and extend their functional durations. Meanwhile, nanospheres have a large specific surface area, and cells could quickly attach and proliferate on the nanosphere surface in a short time.
The advantages of nanospheres in bone tissue engineering include sustained release for bioactive molecules, porosity optimization of bulk scaffolds, and injectable formulation scaffold design [85]. Nanosphere materials include polymer, ceramic, and composites. Polymeric microsphere/nanosphere is a common type of drug delivery systems since the 1970s. Natural polymeric nanospheres are favorable for bone regeneration because of natural biocompatibility and biodegradability. Common natural polymeric nanospheres include collagen, gelatin, chitosan, alginate, and so on. Natural polymer has cell recognition part, which is favorable for cell attachment. But mechanical strength might be a challenge for these natural polymeric nanospheres such as collagen. Synthetic polymeric nanospheres could provide proper mechanical properties such as PLA and PLGA. The biological activities of these synthetic materials could be well-controlled such as drug loading capacity and drug release kinetics. For example, Jeon et al. [94] fabricated heparin-incorporated PLGA nanospheres for fibroblast growth factor release profile investigation, the result demonstrated that fibroblast growth factor release from PLGA nanospheres remained for 3 weeks, and an initial burst release was observed. Although scaffold design could take advantage of the controlled growth factor release, most of these polymers have poor osteo-conductivity and osteo-inductivity. Inorganic microspheres/nanospheres could be alternative candidates for scaffolds with better mechanical properties such as CaP, bioglass, and other bioactive ceramics [95, 96]. However, the poor control of drug release restricted the practical application, and combination with other types of polymers will be favored for bone tissue engineering. For instance, Leeuwenburgh et al. [97] incorporated CaP nanocrystal with gelatin microspheres; these nanocomposites reduced the drug release rates and enhanced calcifying capacity, which combines the drug sustainability of gelatin and osteoinductive ability of bioactive CaP.
In summary, nanospheres have been extensively studied due to the potential drug delivery ability. They could be used as dispersed phase and building blocks. The most crucial roles for nanosphere in bone tissue engineering are vehicle for sustained drug release and enhancing the porosity of bulk scaffolds.
1.5.3 Nanotubes
Since firstly discovered by Japanese scientist Iijima in 1991 [98], this type of nanometer-sized hollow tube has been widely studied and applied to optoelectronic devices, nanosensors, nanocomposite materials, and biomedical area [99]. Since then, carbon nanotubes have always been a research hotspot because of their high stability and good mechanical and electrical properties. Besides, carbon nanotube material can increase the cellular adsorption rate and promote bone regeneration, so it has been extensively applied to bone tissue engineering [100]. Carbon nanotubes can be simply regarded as hollow tubes rolled up with graphite sheets, and they are separated into single-walled carbon nanotubes (SWNTs) and multiwalled carbon nanotubes (MWNTs). Generally, the diameters of SWNTs range from 0.5 nm to 10 nm, and the diameters of MWNTs range from 10 nm to 50 nm.
Carbon nanotubes are regarded as good scaffold material for the high strength, low density, and good biocompatibility. Unique tubular structure imparts carbon nanotubes special regulation effects on the cellular responses [101]. In 2006, Zanello et al. [102] studied the potential role of carbon nanotubes for scaffold materials, and this was the first time to prove that osteoblasts could grow and proliferate on carbon nanotubes. The cell morphology of osteoblasts was significantly changed, and obvious cell growth was observed. To figure out the potential mechanisms how carbon nanotubes influence the different cellular responses of the attached cells, Lin et al. [103] compared carbon nanotubes with GP; they reported that a large amount of protein adsorption on the surface of carbon nanotubes might be one of the mechanisms to promote the functional development of osteoblasts and predicted that carbon nanotubes are an osteoconductive material. The same evidence could be found in the study of Aoki et al. [104]: SaOS2 cells were cultured on the carbon nanotubes, and polycarbonate membranes (PC) coated carbon nanotubes and graphite. Carbon nanotubes showed better affinity for proteins and cells on the carbon nanotubes and showed better cell spreading, cell proliferation, and ALP activities. In all, high protein affinity of carbon nanotubes could be regarded as the reason of the enhanced cellular responses.
When we are deigning the materials for bone tissue scaffold, mechanical properties will be a very crucial factor. Carbon nanotubes have low specific gravity and high aspect ratio and can be repeatedly bent and twisted without damaging the structure. Therefore, carbon nanotubes are the best load-bearing reinforcing materials for the fabrication of composite materials with satisfying strength, light weight, and good performance. Compared with ceramic-based and metal-based materials, carbon nanotubes have a lower density, so it is easier to form high-strength, lightweight, and flexible scaffold materials. From the research of microtubule structure, it is found that the typical shape of single-walled carbon nanotubes is 0.5–1.5 nm in diameter and about 100–300 nm in length, which is very similar to natural bone, so it can mimic the collagen skeleton in geometric form that is beneficial to the deposition of inorganic substances such as calcium and phosphorus and then induces the nucleation and growth of hydroxyapatite.
Li et al. [105] investigated the osteoinductive effects of MWNTs on hMSCs; the adherence, proliferation, osteogenesis-related gene expression, and mineralization of hMSCs were significantly enhanced, and carbon nanotubes also enhanced the ectopic bone formation in vivo. But what are the different impacts on the cell behaviors between the single-walled nanotubes and multiwalled nanotubes? Hideki et al. [106] coated glass disks with SWNTs and MWNTs and treated MSCs with differently coated glasses. During the first 2 weeks, both SWNTs- and MWNTs-coated glasses promoted the early differentiation of MSCs to osteoblast. However, at the later stages of differentiation, higher osteocalcin expressions, mineralization, and calcium phosphate deposition were observed on SWNTs-coated glasses. Therefore, SWNTs might have better osteoinductive abilities than MWNTs in the late stage. The reasons for this difference might be the surface nanotopography and density of CNT; higher intensity promoted the osteogenic differentiation of MSCs. For the specially topological CNTs, the cell proliferation and osteogenic differentiation of MSCs could be enhanced. Specially patterned and aligned CNTs will enhance the expression of osteogenesis-related genes, which is a result of cytoskeletal tension in the aligned hMSCs [107]. Some other possible mechanism might be electrical stimulation from electrically conductive property CNT [108, 109].
Although CNTs exhibit potentially encouraging ability for osteogenesis, limitation also exists. Potential toxicity is one of the major nonnegligible problems for the application of CNTs in biomedical area [110]. After years of study, the cytotoxicity of CNTs is gradually discovered. The hydrophobicity, nonbiodegradability, and insolubility all contributed to the cytotoxicity of CNTs, which largely limited the biomedical application [111]. The existing chronic toxicity arise concerns for the long-term biocompatibility of CNTs after CNTs are applied to in vivo scaffolds. Evidence showed that CNTs might induce cellular DNA damages and apoptosis; the mutation frequency was twofold enhanced in comparison with the normal mutation frequency [112]. Some other cytotoxicity of CNTs include membrane damages, oxidative stress, and mitochondrial dysfunction [113]. After in vivo application, CNTs might cause organs damages such as oral, dermal, pulmonary, and systemic toxicities (immune responses) in a time- and dose-dependent manner [114,115,116]. Therefore, there is rare biomedical applications of pure CNTs due to their potential toxicities. Surface modification and functionalization were used to reduce the toxicity and increase the biocompatibility of CNTs [117]. Functionalization increased the solubility, hydrophilia, and solubility and subsequently changed their biological properties. The functionalization methods and components include surfactants, biomolecules, nucleic acids, and natural and synthetic polymers. Adsorption of serum proteins largely decreased the cytotoxicity of CNTs in comparison with pristine CNTs and change the cell interaction manner [118, 119]. Polyethylene glycol (PEG) was also used as surface modifications for many nanomaterials due to their excellent biocompatibility. Song et al. [120] studied the toxicity of PEG-coated CNTs on BMSCs, and the PEG imparts favorable biocompatibility to the CNTs. Natural polymers could also be used to functionalize CNTs. Sibel et al. [121] prepared nanotube-chitosan scaffolds, and the chitosan-MWCNT nanocomposites didn’t cause significant cytotoxicity to the chondrocyte cells. In all, surface modification of CNTs increased the dispersibility, biostability, and biocompatibility, which will improve the properties for wider biological applications.
1.5.4 Nanofibers
Like we mentioned before, the ideal scaffolds should be able to mimic the biological bone structures. As porous and hierarchical structures, nanofiber scaffold has been extensively studied for bone regeneration. The nanofiber scaffolds have similar morphological structures to the biological bone matrix and promote cell attachment and stem cell differentiation, which could be regarded as ideal scaffolds to provide structural supports. In terms of manufacturing techniques, nanofibers could be fabricated via several processes such as electrospinning, thermally induced phase separation (TIPS), self-assembling peptide nanofiber scaffold (SAPNS) [122], and bacterial cellulose (BC).
Electrospinning is a common technique for nanofiber scaffold fabrication. Polymer solutions are spun in the strong electric fields, the droplets at the needle will be transformed from spherical shapes to conical shapes and are continuously extended, finally forming fiber filaments. Under different conditions, manufactured polymer fibers could be different in diameters ranging from nanometers to microns. Due to the simple manufacturing equipment, low spinning cost, and abundant polymer sauces, electrospinning has become one of the main ways for effectively manufacture the nanofiber materials. A wide variety of nanofibers have been fabricated via electrospinning including organic, organic/inorganic composite, and inorganic nanofibers. Many factors could influence the spinning process including polymer property, shape of spinneret needle, needle-collector distance, and environmental parameters.
Materials used for electrospinning include natural materials (gelatin, hyaluronic acid, chitosan, collagen, etc.) and synthetic materials (polylactic acid (PLA), polyglycolic acid (PGA), polycaprolactone (PCL), etc.). The nanofibrous forms of these materials are polyporous with biomimetic structures. Over the past decades, the great potentials of electrospinning for bone tissue engineering have been demonstrated. For instance, Yoshimoto et al. [123] reported PCL nanofiber scaffold fabricated by electrostatic fiber spinning technique; PCL have good biodegradability, biocompatibility, and mechanical properties. Rat MSCs penetrated through the nanofibers, and much ECM was found after 1-week culture. Furthermore, the polymer fibers were covered by multiple layers of cells at 4 weeks, and mineralization and type I collagen could be found, which suggested great potentials of PCL nanofibers for bone regeneration. Other types of nanofiber scaffold also encourage the application of electrospinning nanofibers in bone tissue engineering [124].
Nanofibers fabricated with mono-component materials may not totally satisfy the requirement bone scaffold. Both natural materials and synthetic materials have some disadvantages. For example, natural materials might have insufficient mechanical strength, and synthetic polymer materials might lack bioactivity and biocompatibility. Therefore, composite materials are more commonly used for electrospinning nanofiber design and fabrication. The combination of different types of materials could optimize physicochemical properties of nanofiber scaffolds. For instance, Yang et al. [125] incorporated chitosan into the PCL nanofibers; the chitosan-containing PCL nanofibers significantly enhanced the cell adhesion of MC 3T3-E1 cells. This kind of incorporation not only solved the insufficient mechanical properties of pure electrospun chitosan; it also changed the poor cell adhesion of pure PCL nanofibers. Linh et al. [126] fabricated polyvinyl alcohol/gelatin (PVA/GE) polymer composite nanofibers. PVA and GE are commonly used in biomedical area due to their biodegradable and biocompatible properties, but the PVA/GE scaffolds could be possibly dissolved in aqueous phases because of their hydrophilic and solubility. But after two components were cross-linked by methanol, the dissolution of the nanofibers in aqueous phases was significantly reduced. Meanwhile, the biological biocompatibility of the scaffold was promoted via GE incorporation.
Nanofiber scaffolds provided a good opportunity to optimize the scaffold design, but challenges still exist for clinical application of nanofiber scaffold. Further researches are required to manipulate the interactions between scaffolds and biological system, the pore size, mechanical properties, toxicity, etc. Furthermore, more researches and evidences are required to furtherly explore the clinical application.
1.5.5 Nanocomposites
Natural bone tissues themselves could be regarded as nanocomposite structures, which are consisted of inorganic HA and organic collagen fiber matrix ranging from nanoscale to microscale [127]. Single type of material couldn’t totally simulate the biostructures and component of the biological bone. So nanocomposites could be regarded as potential candidates, which could mimic the bone matrix environment and biological properties [128]. As we discussed before, various types of materials have been proved to have osteoinductive properties, but polymeric composite materials are more extensively applied in bone tissue engineering because their physicochemical properties are more similar with the hierarchical and nanostructures of the natural bone [64].
Unlimited possibilities exist in the components for nanocomposite synthesis, but more common combination way for nanocomposites for bone regeneration is biocompatible polymer and bioactive inorganic nanomaterials [129, 130]. The polymeric polymers have many advantages such as good biocompatibility, easy modification, structural supporting, moldability, etc., which could play the role of organic collagen fiber matrix of natural bone. The inorganic bioactive materials could arise special bioactivity of the attached cells and optimize the biophysical and biochemical reactions, such as HA, tricalcium phosphate (TCP), calcium carbonate, and bioactive ceramic [128]. This kind of combination attracted much attention for biomimetic synthesis of bone-like nanocomposites, which combine the strength, stiffness, and osteoconductive properties of inorganic components with the flexibilities, toughness, and biodegradability of organic phases [131]. Xin et al. [132] incorporated HA nanoparticles into the PMMA scaffolds to form HA/PMMA nanocomposites and found that the adherence and proliferation of osteoblasts are enhanced compared to single PMMA scaffolds. Similarly, Sharifi et al. [133] prepared nanocomposites composed of polyhexamethylene carbonate fumarate (PHMCF) and nano-sized HA; the addition of nano-sized HA improved the mechanical property of the nanocomposites and enhanced cell proliferation. There are many other studies that reported the HA-polymer nanocomposites, which changed the biological activities of the nanocomposites. Besides HA nanocomposites, other bioceramics such as TCP and calcium phosphates could also be incorporated in nanocomposites as bioactive components to optimize the mechanical properties [134, 135].
In all, nanocomposite scaffolds incorporate the advantages of different types of materials and are helpful to synthesize biomimetic scaffolds with structural and mechanical advantages similar with the real bone tissues. A wide range of combinations provide great opportunities to simulate the structure and morphology of native bones, but controllable bone regeneration and complex interactions between nanocomposites and bone tissue still require further studies.
1.6 Growth Factors and Molecular Pathways Involved in Osteogenic Differentiation and Bone Tissue Engineering
Over the past decades, it has been proved that nanomaterials could regulate cell response and facilitate cell migrations, proliferations, and differentiations. Besides stem cells and nanomaterial-based scaffolds, growth factors are also crucial in osteogenic differentiation induction of stem cells. As biological molecules, the growth factor usually has short half-life in living system and could be easily degraded. Meanwhile, the systematic application or sudden release of growth factors would cause side effects including edema, ectopic bone formations, delayed bone formations, or even carcinogenesis. Therefore, the scaffold achieves the sustained releases of growth factors and effective regulation of stem cells. The underlying molecular mechanism requires further exploration and understanding. The complete osteogenic differentiation includes the following process: bone progenitor cells differentiate into pre-osteoblasts and then form mature osteoblasts, and osteoblasts are mineralized in the extracellular matrix and become mature osteoblasts. Osteogenic differentiations of stem cells could be affected by physical, chemical, and biological factors and mediated by many regulatory factors and proteins. Therefore, research on relevant signaling pathways is essential for the development of bone regeneration scaffolds [27]. The participation of important signaling pathways in bone development has been confirmed by various studies. The role of some important signaling pathways in osteogenic differentiation of stem cells and bone regeneration, such as the Wnt/β-catenin pathway, Notch signaling pathway, BMP/TGF-β pathway, and PI3K/Akt/mTOR pathway, which will be explained as follows.
1.6.1 Bone Morphogenetic Protein (BMP)
BMPs are the most widely used osteogenic growth factors, which could regulate stem cell proliferation and differentiation to osteoblast, thereby inducing new bone formation. Furthermore, BMP is also the only growth factor with ectopic osteogenesis ability. It is also the main factor that induces bone and cartilage formation and is expressed during body growth, endochondral ossification, and early repair of fractures and is also crucial in embryo growth and regeneration of the skeletal system. The two ways of bone formation, intra-membrane osteogenesis and endochondral osteogenesis, are directly induced by BMP. More than 40 subtypes of BMP have been identified and more commonly studied for bone regeneration which include BMP-2, BMP-4, BMP-6, BMP-7, BMP-9, and BMP-15. But the most studied is BMP-2, which has been approved by FDA for bone regeneration and has great potential in bone regeneration [136]. The regulation effects of BMP rely on two major signal pathways: Smad pathway and p38-MAPK pathway, which could induce osteogenic differentiation alone but also could collaborate with other growth factors to promote osteogenesis and bone formation. Take BMP-Smad signaling pathway, for example; endogenous or exogenous BMP signals bind with BMP receptor I and BMP-II on the cell membrane to induce phosphorylation of BMP-I and then interact with BMPs-specific Smad proteins. Phosphorylation of Smad proteins enter the nucleus and upregulate the expression of Runx2 and Osterix, which are two key factors regulating the osteogenesis process, thereby inducing bone formation [137].
BMP2 is currently the most studied and strongest osteogenic member of the BMP family. It’s not only involved in osteogenesis but also in the key stages of embryo development and differentiation. It could also promote MSC to differentiate into osteoblast and has high osteogenic induction activity. BMP-2 also participate in bone healing process, Vivianne et al. [138] found that BMP-2 was mainly located in the periosteal layer and the endogenic expression of BMP-2 was essential for promoting fracture healing. For osteogenesis ability, the target cells of BMP-2 are undifferentiated mesenchymal cells and induce specific periosteum progenitor cells such as mesenchymal cells in muscles and around blood vessels, to irreversibly differentiate into cartilage and osteocytes. The application of BMP-2 in the therapy of bone fracture, trauma, and defects has achieved encouraging results in experimental research and clinical applications. The incorporation of BMP-2 into scaffolds promise good bone regeneration results. For example, Sun et al. [139] developed fibroin/nano-HA scaffold and conjugated BMP-2 into the scaffold through chemical combination; the controlled release of BMP-2 obviously improved the attachment and osteogenic differentiations of BMSCs. Besides, it should be noticed that BMP-2 could also stimulate the proliferation of osteoclasts while promoting osteogenesis. In the later stage of bone healing, BMP-2 regulates osteoclast to directly or indirectly stimulate osteoclast differentiation and participate in the bone reconstruction [140, 141].
BMP-9 was firstly identified in the cDNA library of mouse liver [142]. It’s involved in regulating cell proliferation, differentiation, and apoptosis, which cannot only regulate cell endothelial function and promote angiogenesis but also induce bone formation. BMP-9 is considered as one of the BMPs with powerful osteoinductive differentiation ability which is even better than BMP-2 [143,144,145]. It was also a major regulator of angiogenesis and chondrogenesis [146]. Since it has powerful osteogenic ability, BMP-9 could be used for bone regeneration. Zhang et al. [147] developed nano-HA-collagen-MWCNT composite scaffold carrying BMP-9 and found that BMP-9 scaffold could promote BMSCs to differentiate into osteoblast in vitro and induce more bone in vivo formation. Studies have revealed several regulatory pathways related to BMP-9 and osteoblast differentiation such as the classic WNT signaling pathways, Notch signaling pathways, mitogen-activated protein kinases (MAPKs) signaling pathways, the insulin growth factor 2 (IGF2)/PI3K/AKT signaling pathway, etc. For example, Cao et al. [50] suggested that Notch signal enhances early osteogenesis of MSCs induced by BMP-9 both in vitro and in vivo. The enhancement of Notch signaling pathway obviously enhanced the osteogenic differentiation induction ability of BMP-9 [148]. Tang et al. [149] investigated the roles of Wnt/β-catenin pathways in the BMP-9-mediated osteogenic differentiations of MSCs; they reported that Wnt3A and BMP-9 could significantly enhance the ALP activities in MSCs and they have synergistic effects on each other to regulate the osteogenic differentiations of MSCs. Downregulation of β-catenin expressions resulted in sharp decreases in osteocalcin expression stimulated by BMP-9. Li et al. [150] investigated the interaction between TGF-Smad and BMP-MAPK pathway; they found that BMP-9 induced osteogenic differentiations of MSC differentiation through the MAKP pathway and enhanced p38 and c-JNK. Besides these classical signaling pathways, other pathways also contribute to the osteogenic differentiations of MSCs regulated by BMP-9, such as insulin growth factor 2/PI3K/AKT signaling pathway and retinoid A (RAs) signaling pathways.
Other subtypes of BMP family such as BMP-4, BMP-6, and BMP-7 also participate in osteogenic differentiations of stem cells and bone formation. For example, study has demonstrated that if BMP-4 signaling was inhibited, obvious osteoporosis could occur, which suggested that BMP-4 signaling could be involved in regeneration and bone therapy [151]. The regulation effects of the BMP proteins incorporate with each other to synergistically promote the osteogenesis and bone formation.
1.6.2 Vascular Endothelial Growth Factor (VEGF)
VEGF is special in bone tissue engineering for their ability to induce neovascularization/angiogenesis. It is a type mitotic regulator of vascular endothelial cell, which participates in biological vascularization process, vascular permeability, and tissue inflammation. Besides angiogenesis regulation, it also participates in bone development, fracture repair, and promoting the proliferation and differentiation of bone-derived osteoblast [152]. There are two VEGF receptors Fltl and Flk in BMSCs; Fltl exists in the cytoplasm and nucleus, while Flkl is mainly found in the nucleus. After the Flkl or Fltl gene is deleted, the number of osteoblasts can be reduced, which indicated that both receptors are crucial for the differentiations of osteoblasts [153]. It could increase the osteogenic activity of osteoblasts and reduce osteoclast activity to promote bone formation and reconstruction. VEGF can directly regulate osteoblasts and increase the expression of osteoblasts ALP activity and promote their proliferation and differentiation and the formation of calcium nodules [154].
It was proved that exogenous VEGF can effectively promote the expression of early markers of osteoblasts [155]. After the BMSCs transfected with the VEGF gene, the levels of ALP, collagen I, and osteocalcin and the number of new blood vessels increased significantly [156]. It’s proved that if the receptor of VEGF was blocked, the osteogenesis-related gene expressions and mineralization of MSCs would be reduced [157]. Generally, VEGF play important roles in bone regeneration at two aspects: (1) promote the angiogenesis and increase the microcirculation number to provide better blood supply for the bone tissue and (2) regulate bioactivities of BMSCs, osteoblast, and osteoclast to improve microstructures of new bone.
1.6.3 Basic Fibroblast Growth Factor (bFGF)
FGF is a group of homologous polypeptide family, and more than 20 subtypes have been discovered, which could be generally concluded into basic FGF and acid FGF, in which bFGF is more commonly studied. bFGF belongs to the heparin-binding growth factor family and could promote mitosis, cell growth, migrations, vascularization, wound healings, and tissue repairs. bFGF could promote the capillary to grow into bone grafts and accelerates the ossification of cartilage that requires blood supply, thereby increasing osteogenesis. Meanwhile, bFGF could promote the bone matrix synthesis of osteoblasts.
Zhang et al. [158] reported the acceleration of fracture healings by overexpression of bFGF; the acceleration effect was a result of the increase of VEGF expression and differentiation of MSCs to osteoblasts, which promoted angiogenesis and bone matrix production. Similarly, bFGF could also be used for tissue engineering scaffolds to achieve better bone regeneration results. Nakamura et al. [159] incorporated bFGF into collagen scaffolds and applied the scaffold in the bone defect area; the controlled releases of bFGF significantly increased the bone volume and mineral content. However, the osteogenic effects of bFGF could act in time-dependent manners. Qian et al. [160] reported the time-dependent mechanism of bFGF on osteogenic differentiation of DPSCs; bFGF promoted osteogenic differentiation of DPSCs at the first week and inhibited osteogenesis in vitro and in vivo when it came to the second week. In recent years, the role of bFGF in osteogenesis and bone regeneration has attracted more and more attention and has broad prospects in the treatment of fractures and bone defects. But limitations also exist such as short half-life, which is the common limitation for most of the growth factors.
1.6.4 Insulin-like Growth Factor-1 (IGF-1)
IGF-1 is one type of growth factor rich in skeletal system and able to induce the osteogenic differentiation of MSCs [161]. It could also regulate bone growth through endocrine, paracrine, and autocrine including mediation of growth hormone and PTH-regulated skeletal activity. IGF-1 could regulate bone metabolism and stimulate osteoblasts to produce ECM proteins such as osteocalcin and collagen I, thereby promoting the bone matrix production and fracture healing. Under pathological conditions, MSCs expressing IGF-1 could promote the bone mineralization, thereby promoting fracture healing and improving the mechanical strength of fracture healing sites.
The loss of osteogenic potentials in the aging BMSCs was regarded as a critical issue for the bone deficit. Chen et al. [162] treated the aging BMSCs with high dose of IGF-1, and they found that the proliferation rates and osteogenic potentials of these aging cells were enhanced. The results suggested that IGF-1 could largely enhance osteogenic capability. Yuan et al. [163] investigated the gene expressions of MC3T3-E1 osteoblasts after the induction of IGF-1, the results of osteogenesis-related gene expressions (DMP1, PHEX, SOST, BMP2, RUNX2, OPN, and OCN) were obviously upregulated, and IGF-1 enhanced organic matrix production and bone mineralization. Several pathways are reported to participate in the IGF-1-induced osteogenesis such as ERK, JNK, and MAPK pathways [164].
IGF-1 could also enhance the osteogenesis via cooperation with other growth factors such as BMP. For example, Gustavo et al. [165] reported the synergistic effect of IGF-1 and BMP; they found that IGF-1 significantly enhances BMP-induced osteogenic differentiations of murine preosteoblasts and the ALP activity is higher than that of BMP-after combining with BMP-6. Bruno et al. [166] compared the osteoinductive potentials of IGF-1 and BMP-7 on MSCs; they found that BMSCs are more sensitive to the induction of IGF-1 and suggested the great potentials of IGF-1 to improve osteogenic differentiation of MSC.
1.6.5 Other Growth Factors Related to Bone Regeneration
There are some other growth factors which could possibly participate in the bone regeneration process such as transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), tumor necrosis factor-α (TNF-α), epidermal growth factor (EGF), and so on. TGF-β family is involved in regulating embryonic development, tissue regeneration, and immune system functions, which mainly consist of TGF-β1, TGF-β2, and TGF-β3. After binding with receptors, TGF-β could regulate cell growth, proliferation, differentiation, apoptosis, invasion, extracellular matrix synthesis, angiogenesis, and other biological responses. In terms of bone formation, TGF could accumulate MSCs to the bone resorption site and promote them to differentiate into mature osteoblasts via activating MAPK and Smad signals. For example, Yokota et al. [167] used TGF-β to induce MSCs and found that TGF-β could obviously enhance the expression of ALP in MSCs and induce osteogenic differentiations of MSCs in dosage-dependent manners. Manal et al. [168] studied the osteogenesis capacity of TGF-β1 with chitosan scaffolds, as the increase of ALP activities, mineralization, and osteogenesis gene expressions demonstrated that the combination of TGF-β1 and scaffold exhibits their potentials in bone tissue engineering.
PDGF could also contribute the bone formation and regeneration. It’s a peptide found in platelets, which participate in neovascularization and stabilization. Currently, five subtypes have been found, among which PDGF-BB could enhance the proliferations and differentiations of osteoblasts and inhibit that of osteoclasts. The role of PDGF in osteogenic differentiation could be possibly controversial because it was reported that the inhibition of the PDGF receptors didn’t significantly affect the osteogenic differentiation of hMSCs [169]. But many studies still suggested the positive effects of PDGF in the bone formation and regeneration. As an early inflammatory factor, the role of TNF-α in bone regeneration is enhancing proliferations, chemotactic migrations, and differentiations and influences bone formation [170]. As a type of co-growth factor, EGF could activate multiple downstream signaling pathways, which could regulate the biological activities of chondrocytes, osteoblasts, and osteoclasts [171].
The osteogenic differentiations of stem cell induced by various growth factors has been gradually clarified, but due to difference between artificial delivery and biological regulation in living system, more researches are needed to mimic the biological regulation effects of different growth factors, and much work are needed to achieve the precise control of these growth factors in bone tissue engineering such as time, concentration, the combination and ratio of different factors, and the order of priority of the growth factors.
References
Li Y, Liu C. Nanomaterial-based bone regeneration. Nanoscale. 2017;9:4862–74.
Wang Q, Yan J, Yang J, Li B. Nanomaterials promise better bone repair. Mater Today. 2016;19:451–63.
Zhao F, Wang J, Guo H, Liu S, He W. The effects of surface properties of nanostructured bone repair materials on their performances. J Nanomater. 2015;2015:1–11.
Shi S, Jiang W, Zhao T, Aifantis KE, Wang H, Lin L, Fan Y, Feng Q, Cui FZ, Li X. The application of nanomaterials in controlled drug delivery for bone regeneration. J Biomed Mater Res A. 2015;103:3978–92.
Smith LA, Ma PX. Nano-fibrous scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2004;39:125–31.
Kim HD, Amirthalingam S, Kim SL, Lee SS, Rangasamy J, Hwang NS. Biomimetic materials and fabrication approaches for bone tissue engineering. Adv Healthc Mater. 2017;6:1700612.
Gong T, Xie J, Liao J, Zhang T, Lin S, Lin Y. Nanomaterials and bone regeneration. Bone Res. 2015;3:15029.
McMahon RE, Wang L, Skoracki R, Mathur AB. Development of nanomaterials for bone repair and regeneration. J Biomed Mater Res B Appl Biomater. 2013;101:387–97.
Tian T, Guo B, Liao J, Zhang T, Ma Q, Zhang Q, Cai X. Characterization, specific demand and application of nanomaterials in bone regeneration. J Nanosci Nanotechnol. 2016;16:9381–92.
Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005.
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64.
Galotto M, Campanile G, Robino G, Cancedda FD, Bianco P, Cancedda R. Hypertrophic chondrocytes undergo further differentiation to osteoblast-like cells and participate in the initial bone formation in developing chick embryo. J Bone Miner Res Off J Am Soc Bone Miner Res. 1994;9:1239–49.
Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102(Pt 2):341–51.
Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–8.
Wu J, Zhang W, Ran Q, Xiang Y, Zhong JF, Li SC, Li Z. The differentiation balance of bone marrow mesenchymal stem cells is crucial to hematopoiesis. Stem Cells Int. 2018;2018:1540148.
Hall SR, Jiang Y, Leary E, Yavanian G, Eminli S, O'Neill DW, Marasco WA. Identification and isolation of small CD44-negative mesenchymal stem/progenitor cells from human bone marrow using elutriation and polychromatic flow cytometry. Stem Cells Transl Med. 2013;2:567–78.
Lu L, Gao Y, Xu M, Ge RC, Lu L. Gene expression profiles associated with osteoblasts differentiated from bone marrow stromal cells. Asian Pac J Trop Med. 2014;7:344–51.
Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113:2806–12.
Ivanova-Todorova E, Bochev I, Dimitrov R, Belemezova K, Mourdjeva M, Kyurkchiev S, Kinov P, Altankova I, Kyurkchiev D. Conditioned medium from adipose tissue-derived mesenchymal stem cells induces CD4+FOXP3+ cells and increases IL-10 secretion. J Biomed Biotechnol. 2012;2012:295167.
Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–4.
Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641–8.
Zheng CX, Sui BD, Liu N, Hu CH, He T, Zhang XY, Zhao P, Chen J, Xuan K, Jin Y. Adipose mesenchymal stem cells from osteoporotic donors preserve functionality and modulate systemic inflammatory microenvironment in osteoporotic cytotherapy. Sci Rep. 2018;8:5215.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30.
Malekfar A, Valli KS, Kanafi MM, Bhonde RR. Isolation and characterization of human dental pulp stem cells from cryopreserved pulp tissues obtained from teeth with irreversible pulpitis. J Endod. 2016;42:76–81.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12.
Leyendecker Junior A, Gomes Pinheiro CC, Lazzaretti Fernandes T, Franco BD. The use of human dental pulp stem cells for in vivo bone tissue engineering: a systematic review. J Tissue Eng. 2018;9:2041731417752766.
Lu X, Liu SF, Wang HH, Yu F, Liu JJ, Zhao YM, Zhao SL. A biological study of supernumerary teeth derived dental pulp stem cells based on RNA-seq analysis. Int Endod J. 2019;52:819–28.
Hossein-Khannazer N, Hashemi SM, Namaki S, Ghanbarian H, Sattari M, Khojasteh A. Study of the immunomodulatory effects of osteogenic differentiated human dental pulp stem cells. Life Sci. 2019;216:111–8.
Giuliani A, Manescu A, Langer M, Rustichelli F, Desiderio V, Paino F, De Rosa A, Laino L, d'Aquino R, Tirino V, Papaccio G. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: biological and clinical implications. Stem Cells Transl Med. 2013;2:316–24.
Shukla SK, Mishra AK, Arotiba OA, Mamba BB. Chitosan-based nanomaterials: a state-of-the-art review. Int J Biol Macromol. 2013;59:46–58.
Wu G, Feng C, Hui G, Wang Z, Tan J, Luo L, Xue P, Wang Q, Chen X. Improving the osteogenesis of rat mesenchymal stem cells by chitosan-based-microRNA nanoparticles. Carbohydr Polym. 2016;138:49–58.
Chen X, Gu S, Chen B-F, Shen W-L, Yin Z, Xu G-W, Hu J-J, Zhu T, Li G, Wan C, Ouyang H-W, Lee T-L, Chan W-Y. Nanoparticle delivery of stable miR-199a-5p agomir improves the osteogenesis of human mesenchymal stem cells via the HIF1a pathway. Biomaterials. 2015;53:239–50.
Ji J, Tong X, Huang X, Wang T, Lin Z, Cao Y, Zhang J, Dong L, Qin H, Hu Q. Sphere-shaped nano-hydroxyapatite/chitosan/gelatin 3D porous scaffolds increase proliferation and osteogenic differentiation of human induced pluripotent stem cells from gingival fibroblasts. Biomed Mater (Bristol, Engl). 2015;10:045005.
Xiang Z, Wang K, Zhang W, Teh SW, Peli A, Mok PL, Higuchi A, Suresh KS. Gold nanoparticles inducing osteogenic differentiation of stem cells: a review. J Clust Sci. 2018;29:1–7.
Yi C, Liu D, Fong C-C, Zhang J, Yang M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano. 2010;4:6439–48.
Niu C, Yuan K, Ma R, Gao L, Jiang W, Hu X, Lin W, Zhang X, Huang Z. Gold nanoparticles promote osteogenic differentiation of human periodontal ligament stem cells via the p38 MAPK signaling pathway. Mol Med Rep. 2017;16:4879–86.
Nah H, Lee D, Heo M, Lee J, Lee S, Heo DN, Seong J, Lim H-N, Lee Y-H, Moon H-J, Hwang Y-S, Kwon IK. Vitamin D-conjugated gold nanoparticles as functional carriers to enhancing osteogenic differentiation. Sci Technol Adv Mater. 2019;20:826–36.
Deng J, Zheng H, Zheng X, Yao M, Li Z, Gao C. Gold nanoparticles with surface-anchored chiral poly(acryloyl-L(D)-valine) induce differential response on mesenchymal stem cell osteogenesis. Nano Res. 2016;9:3683–94.
Qin H, Zhu C, An Z, Jiang Y, Zhao Y, Wang J, Liu X, Hui B, Zhang X, Wang Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int J Nanomedicine. 2014;9:2469–78.
Liu X, He W, Fang Z, Kienzle A, Feng Q. Influence of silver nanoparticles on osteogenic differentiation of human mesenchymal stem cells. J Biomed Nanotechnol. 2014;10:1277–85.
Sengstock C, Diendorf J, Epple M, Schildhauer T, Köller M. Effect of silver nanoparticles on human mesenchymal stem cell differentiation. Beilstein J Nanotechnol. 2014;5:2058–69.
Lv L, Liu Y, Zhang P, Zhang X, Liu J, Chen T, Su P, Li H, Zhou Y. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials. 2015;39:193–205.
Wang Q, Chen B, Cao M, Sun J, Wu H, Zhao P, Xing J, Yang Y, Zhang X, Ji M, Gu N. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials. 2016;86:11–20.
Shadjou N, Hasanzadeh M. Silica-based mesoporous nanobiomaterials as promoter of bone regeneration process. J Biomed Mater Res A. 2015;103:3703–16.
Yang X, Li Y, Liu X, Huang Q, He W, Zhang R, Feng Q, Benayahu D. The stimulatory effect of silica nanoparticles on osteogenic differentiation of human mesenchymal stem cells. Biomed Mater. 2016;12:015001.
Christel A, Neumann A, Kasper C, Behrens P. BMP2-loaded nanoporous silica nanoparticles promote osteogenic differentiation of human mesenchymal stem cells. RSC Adv. 2013;3:24222.
Castro A, Diba M, Kersten M, Jansen J, van den Beucken J, Yang F. Development of a PCL-silica nanoparticles composite membrane for guided bone regeneration. Mater Sci Eng C. 2017;85:154–61.
Yang X, Li Y, Liu X, Huang Q, Zhang R, Feng Q. Incorporation of silica nanoparticles to PLGA electrospun fibers for osteogenic differentiation of human osteoblast-like cells. Regen Biomater. 2018;5:229–38.
Yan X, Yang W, Shao Z, Yang S, Liu X. Graphene/single-walled carbon nanotube hybrids promoting osteogenic differentiation of mesenchymal stem cells by activating p38 signaling pathway. Int J Nanomed. 2016;11:5473–84.
Kang E-S, Kim D-S, Suhito I, Choo S-S, Kim S-J, Song I, Kim T-H. Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Converg. 2017;4:1.
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Electric field effect in atomically thin carbon films. Science. 2004;306:666–9.
Yang Y, Asiri AM, Tang Z, Du D, Lin Y. Graphene based materials for biomedical applications. Mater Today. 2013;16:365–73.
Reina G, Gonzalez-Dominguez JM, Criado A, Vazquez E, Bianco A, Prato M. Promises, facts and challenges for graphene in biomedical applications. Chem Soc Rev. 2017;46:4400–16.
Nayak T, Andersen H, Makam V, Khaw C, Bae S, Xu X, Ee P-L, Ahn J-H, Hong B, Pastorin G, Özyilmaz B. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano. 2011;5:4670–8.
Saliev T. The advances in biomedical applications of carbon nanotubes. Carbon. 2019;5:29.
Baik K, Park SY, Heo K, Lee K-B, Hong S. Carbon nanotube monolayer cues for osteogenesis of mesenchymal stem cells. Small (Weinheim an der Bergstrasse, Germany). 2011;7:741–5.
Xu B, Ju Y, Cui Y, Song G. Carbon nanotube array inducing osteogenic differentiation of human mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2015;51:182–8.
Yuxin Z, Wenjuan M, Yuxi Z, Chenchen M, Xiaoru S. Nucleic acids and analogs for bone regeneration. Bone Res. 2018;6:37.
Li S, Tian T, Zhang T, Cai X, Lin Y. Advances in biological applications of self-assembled DNA tetrahedral nanostructures. Mater Today. 2019;24:57–68.
Zhou M, Liu N-X, Shi S-R, Li Y, Zhang Q, Ma Q-Q, Tian T-R, Ma W-J, Cai X-X, Lin Y-F. Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway. Nanomedicine. 2018;14:1227–36.
Shao XR, Lin SY, Peng Q, Shi SR, Li XL, Zhang T, Lin YF. Effect of tetrahedral DNA nanostructures on osteogenic differentiation of mesenchymal stem cells via activation of the Wnt/β-catenin signaling pathway. Nanomedicine. 2017;13:1809–19.
Lv H, Li L, Zhang Y, Chen Z, Sun M, Xu T, Tian L, Lu M, Ren M, Liu Y, Li Y. Union is strength: matrix elasticity and microenvironmental factors codetermine stem cell differentiation fate. Cell Tissue Res. 2015;361:657–68.
Steward AJ, Kelly DJ. Mechanical regulation of mesenchymal stem cell differentiation. J Anat. 2015;227:717–31.
Swetha M, Sahithi K, Moorthi A, Srinivasan N, Ramasamy K, Selvamurugan N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int J Biol Macromol. 2010;47:1–4.
Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25:4749–57.
Zhang Q, Mochalin VN, Neitzel I, Hazeli K, Niu J, Kontsos A, Zhou JG, Lelkes PI, Gogotsi Y. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials. 2012;33:5067–75.
Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–6.
Klawitter JJ, Bagwell JG, Weinstein AM, Sauer BW. An evaluation of bone growth into porous high density polyethylene. J Biomed Mater Res. 1976;10:311–23.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91.
Kuboki Y, Jin Q, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S105–15.
Schurmann M, Wolff A, Widera D, Hauser S, Heimann P, Hutten A, Kaltschmidt C, Kaltschmidt B. Interaction of adult human neural crest-derived stem cells with a nanoporous titanium surface is sufficient to induce their osteogenic differentiation. Stem Cell Res. 2014;13:98–110.
Zhang K, Fan Y, Dunne N, Li X. Effect of microporosity on scaffolds for bone tissue engineering. Regen Biomater. 2018;5:115–24.
Polak SJ, Rustom LE, Genin GM, Talcott M, Wagoner Johnson AJ. A mechanism for effective cell-seeding in rigid, microporous substrates. Acta Biomater. 2013;9:7977–86.
Rustom LE, Boudou T, Lou S, Pignot-Paintrand I, Nemke BW, Lu Y, Markel MD, Picart C, Wagoner Johnson AJ. Micropore-induced capillarity enhances bone distribution in vivo in biphasic calcium phosphate scaffolds. Acta Biomater. 2016;44:144–54.
Chen Y, Frith JE, Dehghan-Manshadi A, Attar H, Kent D, Soro NDM, Bermingham MJ, Dargusch MS. Mechanical properties and biocompatibility of porous titanium scaffolds for bone tissue engineering. J Mech Behav Biomed Mater. 2017;75:169–74.
Chiang Z-C, Li H-Y, Chao A-C, Su Y-R. Characterization of the morphology and hydrophilicity of chitosan/caffeic acid hybrid scaffolds. J Polym Res. 2011;18:2205–12.
Sousa I, Mendes A, Pereira RF, Bártolo PJ. Collagen surface modified poly(ε-caprolactone) scaffolds with improved hydrophilicity and cell adhesion properties. Mater Lett. 2014;134:263–7.
Zhang W, Yi X, Sun X, Zhang Y. Surface modification of non-woven poly (ethylene terephthalate) fibrous scaffold for improving cell attachment in animal cell culture. J Chem Technol Biotechnol. 2008;83:904–11.
Adachi T, Osako Y, Tanaka M, Hojo M, Hollister SJ. Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration. Biomaterials. 2006;27:3964–72.
Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25:1539–60.
Yu NY, Schindeler A, Little DG, Ruys AJ. Biodegradable poly(alpha-hydroxy acid) polymer scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;93:285–95.
Reichert JC, Klein TJ, Kuaha K, Rijckaert B, Nöth U, Hutmacher DW. Bone tissue engineering. In: Umbilical cord blood. Singapore: World Scientific; 2011. p. 105–43.
Kim J, Bae W-G, Lim KT, Jang K-J, Oh S, Jang K-J, Jeon N, Suh K-Y, Chung JH. Density of nanopatterned surfaces for designing bone tissue engineering scaffolds. Mater Lett. 2014;130:227–31.
Greiner JF, Gottschalk M, Fokin N, Buker B, Kaltschmidt BP, Dreyer A, Vordemvenne T, Kaltschmidt C, Hutten A, Kaltschmidt B. Natural and synthetic nanopores directing osteogenic differentiation of human stem cells. Nanomedicine. 2019;17:319–28.
Wang H, Leeuwenburgh SC, Li Y, Jansen JA. The use of micro- and nanospheres as functional components for bone tissue regeneration. Tissue Eng Part B Rev. 2012;18:24–39.
Khajavi R, Abbasipour M, Bahador A. Electrospun biodegradable nanofibers scaffolds for bone tissue engineering. J Appl Polym Sci. 2015;133:42883.
Shi X, Hudson J, Spicer P, Tour J, Krishnamoorti R, Mikos A. Injectable nanocomposites of single-walled carbon nanotubes and biodegradable polymers for bone tissue engineering. Biomacromolecules. 2006;7:2237–42.
Sitharaman B, Shi X, Tran L, Spicer P, Rusakova I, Wilson L, Mikos A. Injectable in situ cross-linkable nanocomposites of biodegradable polymers and carbon nanostructures for bone tissue engineering. J Biomater Sci Polym Ed. 2007;18:655–71.
Katti K, Katti D, Ambre A. Nanocomposites for bone tissue engineering. Hoboken, NJ: Wiley; 2012.
Xiao Q-R, Zhang N, Wang X, Man X-Y, Yang K, Lü L-X, Huang N-P. Oriented surface nanotopography promotes the osteogenesis of mesenchymal stem cells. Adv Mater. 2017;4:1600652.
Tsimbouri P, Gadegaard N, Burgess K, White K, Reynolds P, Herzyk P, Oreffo R, Dalby MJ. Nanotopographical effects on mesenchymal stem cell morphology and phenotype. J Cell Biochem. 2014;115:380–90.
McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, Murawski K, Kingham E, Oreffo ROC, Dalby MJ. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10:637–44.
Kim J, Bae W-G, Lim K-T, Jang K-J, Oh S, Jang K-J, Li Jeon N, Suh K-Y, Hoon CJ. Density of nanopatterned surfaces for designing bone tissue engineering scaffolds. Mater Lett. 2014;130:227–31.
Jeon O, Kang SW, Lim HW, Hyung Chung J, Kim BS. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly(L-lactide-co-glycolide) nanospheres and fibrin gel. Biomaterials. 2006;27:1598–607.
Wu C, Zreiqat H. Porous bioactive diopside (CaMgSi(2)O(6)) ceramic microspheres for drug delivery. Acta Biomater. 2010;6:820–9.
Luz GM, Mano JF. Nanoengineering of bioactive glasses: hollow and dense nanospheres. J Nanopart Res. 2013;15:1457.
Leeuwenburgh SC, Jo J, Wang H, Yamamoto M, Jansen JA, Tabata Y. Mineralization, biodegradation, and drug release behavior of gelatin/apatite composite microspheres for bone regeneration. Biomacromolecules. 2010;11:2653–9.
Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–8.
Vardharajula S, Ali SZ, Tiwari PM, Eroglu E, Vig K, Dennis VA, Singh SR. Functionalized carbon nanotubes: biomedical applications. Int J Nanomedicine. 2012;7:5361–74.
Pei B, Wang W, Dunne N, Li X. Applications of carbon nanotubes in bone tissue regeneration and engineering: superiority, concerns, current advancements, and prospects. Nanomaterials (Basel). 2019;9:1501.
Lee J-R, Ryu S, Kim S, Kim B-S. Behaviors of stem cells on carbon nanotube. Biomater Res. 2015;19:3–3.
Zanello LP, Zhao B, Hu H, Haddon RC. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006;6:562–7.
Li X, Gao H, Uo M, Sato Y, Akasaka T, Feng Q, Cui F, Liu X, Watari F. Effect of carbon nanotubes on cellular functions in vitro. J Biomed Mater Res A. 2009;91:132–9.
Aoki N, Akasaka T, Watari F, Yokoyama A. Carbon nanotubes as scaffolds for cell culture and effect on cellular functions. Dent Mater J. 2007;26:178–85.
Li X, Liu H, Niu X, Yu B, Fan Y, Feng Q, Cui FZ, Watari F. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials. 2012;33:4818–27.
Mori H, Ogura Y, Enomoto K, Hara M, Maurstad G, Stokke BT, Kitamura S. Dense carbon-nanotube coating scaffolds stimulate osteogenic differentiation of mesenchymal stem cells. PLoS One. 2020;15:e0225589.
Namgung S, Baik KY, Park J, Hong S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano. 2011;5:7383–90.
Mooney E, Mackle JN, Blond DJ, O'Cearbhaill E, Shaw G, Blau WJ, Barry FP, Barron V, Murphy JM. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33:6132–9.
Saberi A, Jabbari F, Zarrintaj P, Saeb MR, Mozafari M. Electrically conductive materials: opportunities and challenges in tissue engineering. Biomol Ther. 2019;9:448.
Mishra V, Kesharwani P, Jain NK. Biomedical applications and toxicological aspects of functionalized carbon nanotubes. Crit Rev Ther Drug Carrier Syst. 2018;35:293–330.
Yan L, Zhao F, Li S, Hu Z, Zhao Y. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale. 2011;3:362–82.
Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007;7:3592–7.
Ong LC, Chung FF, Tan YF, Leong CO. Toxicity of single-walled carbon nanotubes. Arch Toxicol. 2016;90:103–18.
Firme CP 3rd, Bandaru PR. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine. 2010;6:245–56.
Johnston HJ, Hutchison GR, Christensen FM, Peters S, Hankin S, Aschberger K, Stone V. A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: the contribution of physico-chemical characteristics. Nanotoxicology. 2010;4:207–46.
Sharma P, Mehra NK, Jain K, Jain NK. Biomedical applications of carbon nanotubes: a critical review. Curr Drug Deliv. 2016;13:796–817.
Vardharajula S, Ali SZ, Tiwari PM, Eroğlu E, Vig K, Dennis VA, Singh SR. Functionalized carbon nanotubes: biomedical applications. Int J Nanomedicine. 2012;7:5361–74.
Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, Yang Y, Zhou R, Zhao Y, Chai Z, Chen C. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc Natl Acad Sci U S A. 2011;108:16968–73.
Lu N, Sui Y, Ding Y, Tian R, Li L, Liu F. Adsorption of human serum albumin on functionalized single-walled carbon nanotubes reduced cytotoxicity. Chem Biol Interact. 2018;295:64–72.
Song G, Guo X, Zong X, Du L, Zhao J, Lai C, Jin X. Toxicity of functionalized multi-walled carbon nanotubes on bone mesenchymal stem cell in rats. Dent Mater J. 2019;38:127–35.
Ilbasmis-Tamer S, Ciftci H, Turk M, Degim T, Tamer U. Multiwalled carbon nanotube-chitosan scaffold: cytotoxic, apoptoti c, and necrotic effects on chondrocyte cell lines. Curr Pharm Biotechnol. 2017;18:327–35.
He B, Yuan X, Wu J, Bai Y, Jiang D. Self-assembling peptide nanofiber scaffolds for bone tissue engineering. Sci Adv Mater. 2015;7:1221–32.
Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–82.
Zaiss S, Brown T, Reichert J, Berner A. Poly(ε-caprolactone) scaffolds fabricated by melt electrospinning for bone tissue engineering. Materials. 2016;9:232.
Yang X, Chen X, Wang H. Acceleration of osteogenic differentiation of preosteoblastic cells by chitosan containing nanofibrous scaffolds. Biomacromolecules. 2009;10:2772–8.
Linh NT, Lee BT. Electrospinning of polyvinyl alcohol/gelatin nanofiber composites and cross-linking for bone tissue engineering application. J Biomater Appl. 2012;27:255–66.
Rogel M, Qiu H, Ameer G. The role of nanocomposites in bone regeneration. J Mater Chem. 2008;18:4223.
Sahoo NG, Pan YZ, Li L, He CB. Nanocomposites for bone tissue regeneration. Nanomedicine (Lond). 2013;8:639–53.
Pielichowska K, Blazewicz S. Bioactive polymer/hydroxyapatite (nano) composites for bone tissue regeneration. Biopolymers. 2010;232:97–207.
Rezwan K, Chen Q, Blaker J, Boccaccini A. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–31.
James R, Deng M, Laurencin C. Nanocomposites and bone regeneration. Front Mater Sci. 2011;5:342–57.
Xing Z, Han S-J, Shin Y-S, Koo T-H, Moon S, Jeong Y, Kang I-K. Enhanced osteoblast responses to poly(methyl methacrylate)/hydroxyapatite electrospun nanocomposites for bone tissue engineering. J Biomater Sci Polym Ed. 2013;24:61–76.
Sharifi S, Kamali M, Khadem Mohtaram N, Shokrgozar M, Rabiee SM, Atai M, Imani M. Preparation, mechanical properties, and in vitro biocompatibility of novel nanocomposites based on polyhexamethylene carbonate fumarate and nanohydroxyapatite. Polym Adv Technol. 2011;22:605–11.
Bakhtiari L, Rezaie HR, Hosseinalipour SM, Shokrgozar MA. Investigation of biphasic calcium phosphate/gelatin nanocomposite scaffolds as a bone tissue engineering. Ceram Int. 2010;36:2421–6.
Stodolak E, Szumera M, Blazewicz M. Osteoconductive nanocomposite materials for bone regeneration. Mater Sci Forum. 2012;730–732:38–43.
Hunziker EB, Enggist L, Kuffer A, Buser D, Liu Y. Osseointegration: the slow delivery of BMP-2 enhances osteoinductivity. Bone. 2012;51:98–106.
Tachi K, Takami M, Sato H, Mochizuki A, Zhao B, Miyamoto Y, Tsukasaki H, Inoue T, Shintani S, Koike T, Honda Y, Suzuki O, Baba K, Kamijo R. Enhancement of bone morphogenetic protein-2-induced ectopic bone formation by transforming growth factor-beta1. Tissue Eng Part A. 2011;17:597–606.
Chappuis V, Gamer L, Cox K, Lowery JW, Bosshardt DD, Rosen V. Periosteal BMP2 activity drives bone graft healing. Bone. 2012;51:800–9.
Sun J, Zhang Y, Li B, Gu Y, Chen L. Controlled release of BMP-2 from a collagen-mimetic peptide-modified silk fibroin–nanohydroxyapatite scaffold for bone regeneration. J Mater Chem B. 2017;5:8770–9.
Kanatani M, Sugimoto T, Kaji H, Kobayashi T, Nishiyama K, Fukase M, Kumegawa M, Chihara K. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J Bone Miner Res. 1995;10:1681–90.
James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, Soo C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev. 2016;22:284–97.
Song JJ, Celeste AJ, Kong FM, Jirtle RL, Rosen V, Thies RS. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136:4293–7.
Lamplot JD, Qin J, Nan G, Wang J, Liu X, Yin L, Tomal J, Li R, Shui W, Zhang H, Kim SH, Zhang W, Zhang J, Kong Y, Denduluri S, Rogers MR, Pratt A, Haydon RC, Luu HH, Angeles J, Shi LL, He TC. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells. 2013;2:1–21.
Khorsand B, Elangovan S, Hong L, Dewerth A, Kormann MS, Salem AK. A comparative study of the bone regenerative effect of chemically modified RNA encoding BMP-2 or BMP-9. AAPS J. 2017;19:438–46.
Nakamura T, Shirakata Y, Shinohara Y, Miron RJ, Hasegawa-Nakamura K, Fujioka-Kobayashi M, Noguchi K. Comparison of the effects of recombinant human bone morphogenetic protein-2 and -9 on bone formation in rat calvarial critical-size defects. Clin Oral Investig. 2017;21:2671–9.
Mostafa S, Pakvasa M, Coalson E, Zhu A, Alverdy A, Castillo H, Fan J, Li A, Feng Y, Wu D, Bishop E, Du S, Spezia M, Li A, Hagag O, Deng A, Liu W, Li M, Ho SS, Athiviraham A, Lee MJ, Wolf JM, Ameer GA, Luu HH, Haydon RC, Strelzow J, Hynes K, He TC, Reid RR. The wonders of BMP9: from mesenchymal stem cell differentiation, angiogenesis, neurogenesis, tumorigenesis, and metabolism to regenerative medicine. Genes Dis. 2019;6:201–23.
Zhang R, Li X, Liu Y, Gao X, Zhu T, Lu L. Acceleration of bone regeneration in critical-size defect using BMP-9-loaded nHA/ColI/MWCNTs scaffolds seeded with bone marrow mesenchymal stem cells. Biomed Res Int. 2019;2019:7343957.
Cao J, Wei Y, Lian J, Yang L, Zhang X, Xie J, Liu Q, Luo J, He B, Tang M. Notch signaling pathway promotes osteogenic differentiation of mesenchymal stem cells by enhancing BMP9/Smad signaling. Int J Mol Med. 2017;40:378–88.
Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, Su YX, Jiang W, Tang M, He Y, Wang Y, Chen L, Zuo GW, Shen J, Pan X, Reid RR, Luu HH, Haydon RC, He TC. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med. 2009;13:2448–64.
Li XL, Liu YB, Ma EG, Shen WX, Li H, Zhang YN. Synergistic effect of BMP9 and TGF-beta in the proliferation and differentiation of osteoblasts. Genet Mol Res. 2015;14:7605–15.
Chen S, Jia L, Zhang S, Zheng Y, Zhou Y. DEPTOR regulates osteogenic differentiation via inhibiting MEG3-mediated activation of BMP4 signaling and is involved in osteoporosis. Stem Cell Res Ther. 2018;9:185.
Hu K, Olsen BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–8.
Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–13.
Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. 2016;126:509–26.
Berendsen AD, Olsen BR. Regulation of adipogenesis and osteogenesis in mesenchymal stem cells by vascular endothelial growth factor a. J Intern Med. 2015;277:674–80.
Feng L, Wu H, Lingling E, Wang D, Feng F, Dong Y, Liu H, Wang L. Effects of vascular endothelial growth factor 165 on bone tissue engineering. PLoS One. 2013;8:e82945.
Murakami J, Ishii M, Suehiro F, Ishihata K, Nakamura N, Nishimura M. Vascular endothelial growth factor-C induces osteogenic differentiation of human mesenchymal stem cells through the ERK and RUNX2 pathway. Biochem Biophys Res Commun. 2017;484:710–8.
Zhang H, Kot A, Lay YE, Fierro FA, Chen H, Lane NE, Yao W. Acceleration of fracture healing by overexpression of basic fibroblast growth factor in the mesenchymal stromal cells. Stem Cells Transl Med. 2017;6:1880–93.
Nakamura S, Ito T, Okamoto K, Mima T, Uchida K, Siddiqui YD, Ito M, Tai M, Okubo K, Yamashiro K, Omori K, Yamamoto T, Matsushita O, Takashiba S. Acceleration of bone regeneration of horizontal bone defect in rats using collagen-binding basic fibroblast growth factor combined with collagen scaffolds. J Periodontol. 2019;90:1043–52.
Qian J, Jiayuan W, Wenkai J, Peina W, Ansheng Z, Shukai S, Shafei Z, Jun L, Longxing N. Basic fibroblastic growth factor affects the osteogenic differentiation of dental pulp stem cells in a treatment-dependent manner. Int Endod J. 2015;48:690–700.
Xue P, Wu X, Zhou L, Ma H, Wang Y, Liu Y, Ma J, Li Y. IGF1 promotes osteogenic differentiation of mesenchymal stem cells derived from rat bone marrow by increasing TAZ expression. Biochem Biophys Res Commun. 2013;433:226–31.
Chen CY, Tseng KY, Lai YL, Chen YS, Lin FH, Lin S. Overexpression of insulin-like growth factor 1 enhanced the osteogenic capability of aging bone marrow mesenchymal stem cells. Theranostics. 2017;7:1598–611.
Yuan Y, Duan R, Wu B, Huang W, Zhang X, Qu M, Liu T, Yu X. Gene expression profiles and bioinformatics analysis of insulin-like growth factor-1 promotion of osteogenic differentiation. Mol Genet Genomic Med. 2019;7:e00921.
Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S, Tang C, Wang Z, Yu J, Zhang G. Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol. 2012;137:513–25.
Rico-Llanos GA, Becerra J, Visser R. Insulin-like growth factor-1 (IGF-1) enhances the osteogenic activity of bone morphogenetic protein-6 (BMP-6) in vitro and in vivo, and together have a stronger osteogenic effect than when IGF-1 is combined with BMP-2. J Biomed Mater Res A. 2017;105:1867–75.
Reible B, Schmidmaier G, Moghaddam A, Westhauser F. Insulin-like growth factor-1 as a possible alternative to bone morphogenetic protein-7 to induce osteogenic differentiation of human mesenchymal stem cells in vitro. Int J Mol Sci. 2018;19:1674.
Yokota J, Chosa N, Sawada S, Okubo N, Takahashi N, Hasegawa T, Kondo H, Ishisaki A. PDGF-induced PI3K-mediated signaling enhances the TGF-beta-induced osteogenic differentiation of human mesenchymal stem cells in a TGF-beta-activated MEK-dependent manner. Int J Mol Med. 2014;33:534–42.
Farea M, Husein A, Halim AS, Abdullah NA, Mokhtar KI, Lim CK, Berahim Z, Mokhtar K. Synergistic effects of chitosan scaffold and TGFβ1 on the proliferation and osteogenic differentiation of dental pulp stem cells derived from human exfoliated deciduous teeth. Arch Oral Biol. 2014;59:1400–11.
Kumar A, Salimath BP, Stark GB, Finkenzeller G. Platelet-derived growth factor receptor signaling is not involved in osteogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2010;16:983–93.
Panagakos FS, Hinojosa LP, Kumar S. Formation and mineralization of extracellular matrix secreted by an immortal human osteoblastic cell line: modulation by tumor necrosis factor-alpha. Inflammation. 1994;18:267–84.
Bernier SM, Goltzman D. Effect of protein and steroidal osteotropic agents on differentiation and epidermal growth factor-mediated growth of the CFK1 osseous cell line. J Cell Physiol. 1992;152:317–27.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zhang, T., Zhou, R. (2021). Nanomaterials and Stem Cells for Bone Tissue Engineering. In: Lin, Y., Zhou, R. (eds) Advances in Nanomaterials-based Cell Biology Research. Springer, Singapore. https://doi.org/10.1007/978-981-16-2666-1_1
Download citation
DOI: https://doi.org/10.1007/978-981-16-2666-1_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2665-4
Online ISBN: 978-981-16-2666-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)