Abstract
One of the biggest challenges to the developing societies is metal pollution, especially in the regions of mining and plating that is affecting people worldwide. The use of conventional strategies in removing the waste is expensive and generates a large amount of toxic wastes, thereby affecting the environment adversely. This has resulted in the drift from the normal strategies to the use of eco-friendly strategies for the removal of metallic wastes being present within the soil. This technique of remediation uses the microbial organisms or microbial biomass that helps in detoxifying the soil from the toxic effects of inorganic metallic salts and heavy metals. Microbial biomass mainly comprises of extracellular polymeric substances (EPS) which increases the efficiency of metal sequestering for the purpose of field bioremediation. The EPS is released by the microbial cells for the purpose of self-defense which mainly occurs during various environmental stresses such as starvation, temperature, pH, and other physiological or rheological stress conditions. It contains a large amount of anionic charge hence it causes large sequestering of metallic ions. Thus this chapter will focus on the biofilm-associated bioremediation of heavy metals, and the mechanism which is helping the process to occur.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
In order to maintain the standards of the environmental regulatory bodies, significant strategies are to be undertaken to reduce the organic and inorganic pollutants that contaminate ecosystems (Trapani et al. 2010). Heavy metals are natural high density elements that are required in various biological processes in trace amounts. Recalcitrant and toxic compounds containing heavy metals are a result of numerous urban and enhanced industrial activities. Accounting to its ease in solubility, products from pharmaceuticals, pesticides, plastics, rubbers, tanneries, and organic chemical industries lead to the accumulation of metals like Pb2+, Cr2+/ Cr3+, Cd2+, Ni2+/Ni4+, Zn2+, Cu2+, Hg2+, etc. that further triggers bio-magnifications (Lakherwal 2014). Metals tend to be toxic on accumulation, as they are nondegradable and can only be altered to different valance states and transformed by sorption, methylation etc. So this nonbiodegradability and the rising accumulation of free forms of heavy metals can lead to irreversible and indigenous health issues that affect bones, kidney, heart, and even brain. They can pose mutagenic, cytotoxic, or even carcinogenic effects (Ray and Ray 2009). The common physicochemical practices to reduce the load of these heavy metal ions include coagulation, reverse osmosis, chemical precipitation, nanofiltration, ultrafiltration, etc. But these procedures are not very favorable due to high reagent demand, costing, operational effectiveness, high energy demand, etc. Thus, for sustainable environmental detoxification, to reduce heavy metal ions to a safer level of concentration, there is a need to undertake environment friendly methods and strategies (Prasad and Prasad 2012). Here, microbial communities of bacteria, microalgae, fungi, and yeast play a vital role in degradation, neutralization, and mineralization as processes of remediation in order to remove organic and inorganic terrestrial and aquatic pollution of heavy metal ions. Efficient utilization of microbial metabolism in place of physicochemical procedures is a safe and efficient approach (Pandey and Jain 2002). Sorption of metal ions is an electrostatic or covalent interaction of the metal with peripherally available functional group on the sorbent. When such sorbents are of biological origin of eukaryotic or prokaryotic microbial biomass, such phenomenon is called biosorption (Comte et al. 2008; Lau et al. 2005). An environment-friendly approach is thus biofilm-mediated bioremediation. In order to thrive in stringent environmental conditions like high temperature, pH, salinity, and heavy metal-contaminated site, the microbial community produces extracellular polymeric substances (EPS) (de Carvalho 2018). EPS are biosynthetic polymers made up of mainly polysaccharides, proteins, and uronic acid that are produced in light of self-defense against environmental stress. The EPS composition hinders the metal ion penetration into the cellular surface and thereby helps in sequestration of the metals. Biofilms are formed by the association of single or various species of bacteria, algae, or fungi, and its subsequent structure and composition of EPS produced can alter on the basis of environmental conditions (Yin et al. 2019).
As biofilms are capable to absorb, sequester, and immobilize various environmental pollutants (namely heavy metal ions), hence biofilm-mediated bioremediation is an effective approach. Thus, the attempt of this chapter is to focus on survival strategy of the microorganisms in the impacted (heavy metal contaminated) environment, the protective and beneficial approach of biofilms for the producing community, the biosynthesis of the polymer, i.e., the biofilm’s life cycle and thereby metal ion to the biofilm interaction. Finally, in order to understand the mechanisms involved in metal ion uptake by the EPS, the interaction mechanism is also studied. Lastly, the scope and miscellaneous uses of biofilm and possible adverse environmental impacts have also been discussed.
8.2 Heavy Metals and Their Toxicity
As an important part of the global ecobiological system and vital component of metabolic pathways, heavy metals are sufficient even in trace amount. Such are the metals with atomic numbers more than 20 and of metallic density more than 5 g/cm3 with distinct chemical characteristics. Majority are present in all ecosystems in varying concentrations along with trace quantities of essential metals like Mn, Ni, Zn, Mg, Cu, etc. They tend to have prolonged half-lives and resist biodegradation, thus are potent toxic to the ecosystem (Hussain et al. 2013). Environmental accumulation of these metals as a result of overloaded byproducts and toxic sludges from industries over long period of time is a serious matter of concern now.
When human body gets exposed to elevated amounts of these metals, they bind to various biomolecules and exert toxicity via different mechanisms like disrupting enzymatic functions, hinder in process of DNA formation and production of reactive oxygen species (ROS) (Yu 2001; Gauthier et al. 2014). Metals like lead, cadmium, and mercury are able to interact with cell membrane ligands, disrupt channels, and confer metabolic disturbances and cytotoxicity (Tavares and Carvalho 1992). Methylation or harmful thiol formation by chromium and arsenic, binding of cadmium to certain protein to render it dysfunctional, saturation and lipid peroxidation due to iron, lead causing free radical imbalance are few of the toxicological manifestations of heavy metal ions (Jaishankar et al. 2014).
Various plants and animal diseases have been reported due to metal toxicity. A prominent and burning example is the Minamata Bay (Japan) tragedy, where due to the Minamata disease, several people lost lives on consumption of shellfish that were heavily mercury contaminated. Zinc plays a vital role in plant growth and its defense mechanism. Upon accumulation, it reacts with oxygen and acids to produce potential toxic compounds harming the plants (Fosmire 1990). According to WHO recommended guidelines, arsenic concentration above 10μg/L might cause bronchitis, liver cirrhosis, or even cancer. Dietary intake of cobalt above 6μg may cause chronic asthma or pneumonia. Renal and hepatic systems, and even the immune system are affected on overexposure to lead or copper ions (Flora et al. 2012).
Taking into consideration the cost, effectiveness, and end products produced after the physical treatments like flocculation, ion exchange, electrodialysis, etc. to reduce these metal ion concentrations to safer limits in the environment, it urges for imperative need to find environment-friendly and economic strategies (Alluri et al. 2007). Many microbes can thrive in metal contaminated sites and potentially accumulate soluble or particulate metal ions. Microbial biomasses of fungi, yeasts, and bacteria act as bioabsorbents of these heavy metal ions (Das et al. 2008). To survive in stringent environment, these eukaryotic and prokaryotic biomasses produce biosynthetic polymers that act as a shield and help in sequestering the metal ions via bio-immobilization or biotransformation (Fig. 8.1).
8.3 Biofilm: Composition and Structure
8.3.1 Composition
Biofilm is a cluster of microorganisms of same or different species that organize to attach themselves on living or nonliving surfaces while shielding themselves with a protective EPS layer from antibiotic agents, predators, or environmental stress. Mostly composed of water, the EPS is made of mostly homo or hetero polysaccharides, proteins, nucleic acids, humic acid, lipids, as well as low molecular weight non-polymeric substances. FTIR spectroscopic analysis shows predominantly polysaccharides and proteins as the components of biofilm (Mosharaf et al. 2018). This microbial mucoid polysaccharide helps in cell aggregation, maturation of microcolonies, attachment to surface, and water-holding capacity and protects the cell cluster from stringent environment (Czaczyk and Myszka 2007). They are best differentiated not on the basis of origin but on the basis of separation, as soluble or bound EPS (Pal and Paul 2008). Ionic bridges that interlink the polysaccharide chains are due to uronic acid and other divalent metallic cations, which also entrusts the acidic character of EPS (Majumdar et al. 2013; Dogan et al. 2015) .The exopolysaccharide composition differs with diverse microbial aggregates, phase of growth, and its extraction method, like production of capsular EPS during the log phase of growth, whereas slime is formed during stationary phase (Wingender et al. 1999). EPS with polysaccharide backbone of repetitive units of same or different monosaccharides are differentiated on the basis of monomer units and linkages. The heteropolysaccharides are composed of units of N-acetylglucosamine or N-acetylgalactoseamine, d-glucose, l-rhamnose with intermittent presence of glycerol, phosphate, or acetyl groups, whereas homopolysaccharides are classified as Fructan, polygalactan, α-d-glucan, and β-d-glucan (Sharon 1966). Variety of EPS are produced upon alteration of the polysaccharide backbone by change in polymeric length due to variations in side chains and functional groups (Whitfield 1988). Those EPS with abundant anionic functional groups are reported to be better heavy metal remediator and a suitable biosorber. Moreover, as a nonliving adsorbent, EPS is considered more useful than living microorganisms to avoid pathogenecity concerns (Gavrilescu 2004). Some of the anionic bacterial EPS reported are xanthan (Xanthomonas campestris), galactopol (Pseudomonas oleovorans), hyaluronan (Pseudomonas aeruginosa), gellan (Sphingomonas paucimobilis), alginate (Azotobacter vinelandii) (Freitas et al. 2009, 2011). PsI polysaccharide produced by Pseudomonas aeruginosa is reported to maintain biofilm architecture and is responsible for intracellular and cell surface adhesion (Ma et al. 2012).
eDNA or extracellular DNA is a constituent of EPS that are formed from lysed cells within the biofilm. Recent studies show that these eDNA are important constituents of the EPS as they maintain the structural integrity of the biofilm, enhance cell–cell communication, and act as nanofilaments for electron transfer (Martins et al. 2010). These eDNA ranges in length of 1000–10,000 bp (Romero et al. 2018).
8.3.2 EPS Synthesis
The EPS is synthesized intra- or extracellularly depending upon the type of polysaccharide it produces. Precisely considering bacterial EPS biosynthesis, it is mostly seen homopolysaccharides are produced extracellularly when responsible precursors are transferred from substrate to the growing end of polysaccharide chain by suitable enzymes. The polysaccharide then matures and assembles itself with varying branches (Boels et al. 2001).
Comparatively a complex sequence occurs during the intracellular synthesis and transport of the polysaccharides. Regulatory molecules of interconnected metabolic pathways and various enzymes are involved during such synthesis process. It involves active or passive passage of substrate sugar into the cell which then gets catabolized by phosphorylation or periplasmic oxidation (Freitas et al. 2011). Intracellular synthesis involves the assembly of the high molecular weight, hydrophilic polymer in the cytoplasm and its transport via the cell membrane. The Gram-negative bacteria is studied to biosynthesize via the following pathways (Fig. 8.2).
-
(a)
ABC transporter-dependent pathway: polymerization occurs at the inner cell membrane, in the cytoplasm side (Cuthbertson et al. 2009) (i) the Wzx–Wzy-dependent pathway, wherein the polymer monomeric units are accumulated at the inner face of the cytoplasmic membrane and polymerized at the periplasm.
-
(b)
Wzx–Wzy-dependent pathway: The monomer units are assembled in the inner face of the cell membrane, whereas polymerization takes place in the cytoplasm.
-
(c)
Synthase-dependent pathway: Here the stand is secreted across the cell wall membrane, where the homopolymers are translocated and polymerization takes place with a single precursor of sugar and single synthase protein (Table 8.1).
Biosynthesis of EPS in Gram-positive and Gram-negative bacteria. Initiating with substrate diffusion, conversion within the cytoplasm, and thereby transport via polysaccharide assembly for the growing chain in the cytoplasmic membrane, elongation of the assembled polysaccharide in the peptidoglycan, maturation and transportation by ABC-dependent or Wzx–Wzy pathway to the extracellular environment
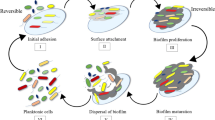
8.3.3 Biofilm Structure and Its Formation
Unlike the planktonic growth, the development of biofilm has distinct and significant mechanism. The biofilm formation and growth are ubiquitous to bacterial cells and occur sometimes under diverse stringency. The biofilm formation, its attachment, and maturation mostly requires a moist environment. Attachment surface may be biotic or abiotic, and their interaction interface can be of any form of solid-liquid, liquid-liquid, or solid-air. Different stages of microbial life cycle, is reported to produce various forms of biofilm.
The overall process of formation involves four distinct steps initiating with the attachment of the planktonic forms of microbes to a moist surface by Van der Waals or electrostatic forces of attraction. Following it is the procedure of microcolony formation, maturation, and development of biofilm and finally detachment from the surfaces (Jamal et al. 2018).
-
Initial surface attachment: Intricate study on biofilm formation reports the initial attachment of the microbes on a moist surface. Their arrival instigates a formation of conditioning layer with the organic matters within a short span of time (Qi and Christopher 2019). The microbial population attaches itself to the surface with its pili, fimbriae, by van der Waals force or via electrostatic forces of attraction. The adhesion to the surface brings about cohesive forces among the cells to expand the film formation.
-
Colony formation: The intercellular signaling calls for rapid cell multiplication and thus microcolony formation. The spatial arrangement of the clustered microcolonies determine the architecture of the biofilm. The side chains of the formed EPS by these colonies further strengthens the biofilm’s anchoring to the surface. This confirms the further maturation at that surface (Randrianjatovo-Gbalou et al. 2017).
-
Architectural maturation: Intracellular signaling, replication, and nutrient transfer increase the microbial population to the desired density (Vasudevan 2014). Maturation-specific genes are expressed during this stage of development that is facilitated by the phenomenon of Quorum Sensing. This three-dimensional biofilm development can produce as thick as few inches of film by some bacteria.
-
Microbial detachment: Biofilm detachment may be caused by physical forces of shear strain like natural abrasion or hydrodynamic forces which erodes off biofilm layers (Webb 2009). This passive dispersion of sloughing might occur due to aged biofilm, deprivation of nutrients, natural erosion, etc. But regardless of the detachment process, the conditioning layer tends to remain attached to the substratum, chopping off the outer layer with saccharolytic enzymes produced by the microorganisms (Fig. 8.3).
8.4 Biofilm-Producing Microbiota
Biofilm producing microbial biomass have been widely reported for heavy metal bioremediation. The microbial population predominantly includes bacteria along with fungi, microalgae, and yeast. Mostly the metal sequestration takes place by adsorption or bioaccumulation. These metal-resistant strains are efficient producers of biosynthetic polymers (EPS), and their immobilized forms are better-off with metal interaction and its reaction kinetics as reports suggest that production of EPS enhances due to attachment to a substrate (Vandevivere and Kirchman 1993).
8.4.1 Bacteria in Bioremediation of Heavy Metals
The lab-scale efficiency of microbial biomass varies with the onsite physicochemical and other environmental factors (Ayangbenro and Babalola 2017). Almost all bacterial cells, due to varying size, cellular composition like teichoic acid, N-acetylglucosamine, or N-acetyl muramic acid, their robust adaptivity, and metabolic secretion are able to produce biofilms and thrive in harsh environments. This helps the bacterial biomass to accomplish efficient removal of the nonbiodegradable heavy metals (Hassan et al. 2010; Özer and Özer 2003). Numerous species have been reported as important biosorbents like Listeria monocytogenes (Colagiorgi et al. 2017), Pseudomonas fluorescens (Lopez et al. 2000), Pseudomonas putida, Sphingomonas sp. (Douterelo et al. 2018), Micrococcus luteus (Puyen et al. 2012), Staphylococcus xylosus (Aryal et al. 2010), and metabolically superior consortium of Acinetobacter sp. and Arthrobacter sp. (De et al. 2008), Acinetobacter sp. IrC1 and Cupriavidus sp. IrC4 (Irawaiti et al. 2018). The teichoic acid on the bacterial cell wall is the potential site for bacterial chemisorptions (Mosa et al. 2016). Upon bacterial exposure to contaminants, enzymatic induction calls for remediation by metal chelation or electrostatic interaction. Reports suggests that zeolite-immobilized Desulfovibrio desulfuricans used in a batch reactor show metal removal efficiency of 90.1% 98.2%, and 99.8%, of Ni, Cu, and Cr6+, respectively (Kim et al. 2015). Also efficient Pb reduction by Bacillus megaterium (2.13–0.03 mg/L), Cr reduction (1.38–0.08 mg/L) by Aspergillus niger, and Cd reduction (0.4–0.03 mg/L) by Bacillus subtilis has been reported by Abioye et al. (2018).
8.4.2 Fungi in Bioremediation of Heavy Metals: Mycoremediation
Fungi are the decomposers that are involved in vital nutrient cycling in the nature and adapt to harsh conditions similar to that of bacteria. But the bacterial and fungal glycan and other biosynthetic enzymes differ in sequence homology (Sheppard and Howell 2016). Yet these organisms are capable of biofilm formation by clustering and attaching to a surface or entrapping itself inside the EPS to shield from environmental stringency. Reports suggest that in the course of biofilm formation, the fungal ability of surface attachment is due to the presence of hydrophobin protein. This protein signals the adhesion of fungal spore on hydrophobic surfaces (Pérez-Mendoza et al. 2011). Various members those are able to form biofilm are species of Neocosmopora and Acremonium (Douterelo et al. 2018), Candida albicans (Rajendran et al. 2016), and those with prominent metal removal efficacy are Coprinopsis atramentaria reported to bioaccumulate 94.7% of 800 mg/L of Pb2+ (Luna et al. 2016), Candida sphaerica has removal efficiency of Pb, Zn, Fe of 79%, 90%, and 95%, respectively. Reduction of Cr (VI) to Cr (III) by yeast strains of Rhodotorula mucilage, S. cerevisiae, Pichia guilliermondii, and Yarrowia lipolytica has also been reported (Chatterjee et al. 2012). Fungi are studied to biosorp and accumulate heavy metals by means of ion-exchange or complexation mainly in their fruit bodies (Ogbo and Okhuoya 2011). Fructification, age of mycelia, and metal exposure time-span determine the degree of removal efficacy (Floudas et al. 2012).
8.4.3 Algae in Bioremediation of Heavy Metals: Phycoremediation
Algae are the organisms that produce a large quantity of biomass, able to survive in toxic environments with low nutrient availability. The most prominent members with effective heavy metal bioremediative ability are those of microalgae. Their various cell surface chemical moieties are the metal binding sites like with amide, carboxyl, hydroxyl groups (Abbas et al. 2014). Algae like bacteria and fungi, bioremediate heavy metals by adsorption, cellular integration of the toxicants or by degradation (Chabukdhara et al. 2017). Among the various algal groups, heavy metal phycoremediation by biosorption is best reported by Phaeophyta (brown algae) (Brinza et al. 2007; Oyedepo 2011). Table 8.1 enlists the heavy metal remediating bacteria, fungi, and algae (Table 8.2).
8.5 Metal–Microbe Interaction and EPS-Mediated Strategies for Remediation
The physical methods involved to clean up the environment from metal accumulation involve high-cost techniques and production of toxic byproducts. This calls for cost-effective and environment-friendly biological methods. Exploiting the microbial metabolism is a gentle approach to combat heavy metal toxicity. But not all the time these metals pose to be toxic to the microbes. The concentration of metal and microbes, mode of interaction with the metals as active or passive uptake and subsequently develop metal tolerance or resistance. (Alluri et al. 2007). This microbial metal tolerance can be specific or nonspecific. Mercury methylation is an example of specific tolerance that produces volatile toxic methyl mercury, whereas nonspecific tolerance may be constitutive or inducible. Production of cysteine-rich metallothionine peptides by Pseudomonas putida or E. coli is an example of nonspecific inducible resistance (Rajendran et al. 2003). On the other hand, exopolysaccharide production is a nonspecific tolerance. Adsorption, bioleaching, bioaccumulation, and redox transformations are the modes of interaction among the microorganisms to uptake metal ions.
8.5.1 EPS-Mediated Metal Biosorption: Mechanism, Advantages, and Disadvantages
Among various biotransformation procedures, bioadsorption has been seen to be more feasible due to its inherent advantages and applicability. Unlike bioaccumulation or redox transformations, biosorption is a metabolism-independent procedure which can be achieved by both live and dead biomass via different physicochemical procedures (Vijayaraghavan and Yun 2008). While it is a challenge for the live cells beyond lethal concentration of metals or in restricted nutrient availability, to survive metabolically actively, dead cells on the other hand are independent of such constrain to sequester metal by adsorption and its storage (Aksu 2005). Moreover, since the dead biomass only accumulate the metals within, without involving it in metabolic processes, so they can be profitably desorbed and the metal ions can be utilized, acting as reversible ion exchanger. Even though these microbial biomass (live or dead) are efficient and economical solution for metal removal, but its efficiency of application on industrial scale is still a constraint which needs to be worked upon further (Wang and Chen 2009).
8.5.2 Strategies of Heavy-Metal and EPS Interaction and Its Remediation
The exopolysaccharides are made up of repetitive sugar units that are responsible for metal chelation by interacting with their functional groups via ion exchange, physical sorption, or precipitation mechanisms. The charged metal ions interact with anionic EPS for the presence of ionizable carboxylate, phosphate, and amine groups on the EPS backbone (Liu and Fang 2002). The hydroxyl, carboxyl, and amino functional groups aid in metal binding and stabilize the metal–polymer interaction by forming co-ordination bonds (Cozzi et al. 1969).
8.5.3 Types of EPS and Its Remediation Strategies
8.5.3.1 Dead Biomass EPS
EPS of a dead biomass and favorable environmental condition gives maximum metal uptake capacity as a biosorbent. An acidic pH is mostly favorable for metal uptake as an overall positive charge is created due to lowered pH that protonates the functional group which interacts well with the negatively charged EPS (Sultan et al. 2012). However, the adsorption capacity of immobilized EPS is head over dead biomass EPS. Freundlich–Langmuir adsorption model in a research report suggested that in acidic pH of 2, copper ions were maximally chelated by dead biomass EPS with a concentration of 26 mg/g of EPS where the metal load was 91.66 ppm (Ozdemir et al. 2005).
8.5.3.2 Homogeneous EPS
Many single species microbiota are reported to thrive in multi-metal contaminated sites, bind to or sequestrate metal specifically or nonspecifically, by either tolerance or resistance. Cells along with their produced polysaccharide together are seen to be more efficient in adsorption of heavy metals. Various microbes and specially bacteria have been widely reported for such bioremediating activity like Methylobacterium organophilum (copper and lead removal) (Kim et al. 1996), Rhizobium radiobacter (biosorption of zinc and lead) (Wang et al. 2013), microalgae Halomonas sp. (calcium, iron, magnesium, aluminum) (Gutierrez et al. 2012), cyanobacteria Anabaena spiroids (manganese) (Freire-Nordi et al. 2005), etc.
8.5.3.3 Immobilized EPS
Studies on specificity and reaction kinetics have shown the excellency of the immobilization techniques. Attachment of microbial cells on a surface enhances not only EPS production but also metal binding ability without elevating the growth rate (Vandevivere and Kirchman 1993). The EPS secreted by Paenibacillus polymyxa immobilized on agar beads (Hassiba et al. 2014) and Chryseomonas luteola immobilized on alginate beads (Ozdemir et al. 2005) are able to efficiently adsorb various metals like cadmium, cobalt, nickel cobalt, etc.
The test microorganism and the metal concentration are more of a concern than other factors of colony size, procedure of metal–microbe interaction, etc. (Cruz-Vega et al. 2008). Hence for isolating a powerful bioremediating microbe, such criteria must be essentially considered.
8.6 Challenges with Biofilm and Future Prospects
The aggregation of microbial population called biofilms, able to attach to various surfaces, are explicitly studied for environmental pollution remediation. An economical as well as sensitive application of the ubiquitous exopolysaccharide (EPS) produced by the microbial biomass is an efficient approach to metal ion bioremediation. Along with the various environmental factors, the EPS matrix composed of various polysaccharides, nucleic acids, humic acid, and proteins helps in formation and stabilization of the biofilm, defining its 3D structure. These biofilm-forming microbial population adapt to thrive in harsh conditions; hence, several dynamics governs the EPS efficacy of metal removal. As discussed before, the charge composition of EPS, types, quantity, and charge distribution of the metal pollutants, environmental factors of temperature, pH, and salinity are all, in combination, determining factors of the onsite sorption efficacy of the biopolymer. Moreover, the lab-based efficacy must also be replicated at the actual site of pollution which needs to be monitored and checked for proper desorption of the metals from the polymer for sustainable remediation. Desired modification of the polymer for immobilization and improved selectivity for metals can be made of EPS hybrids with ceramic, porous silica, etc. (Kariminiaae-Hamedaani et al. 2003; Shi et al. 2011). Selection of anionic EPS producers and mixed culture EPS gives better results in bioremediation, but scaling up the technology, out from laboratory as field trials, needs more research for successful application (Kiliç et al. 2015).
Microbial biofilms are also utilized in several promising ways as in production of microbial fuel cell (MFC) that oxidizes organic matter to produce electricity, as bioemulsifier and biocapsule, as biofilm traps and microbial canaries (Moreno-García et al. 2018). As an emerging scope, Biofilm-Integrated Nanofiber Display (BIND) is a nano-biotechnological approach to develop programmable biomaterials like adhesive biofilm that are able to bind various surfaces (Nguyen et al. 2014).
Uncontrolled growth of biofilms on suitable surfaces are nowadays a potent matter of concern in the health sector. The growth of biofilm on medical instruments, surface of catheters, and medical implants may cause nosocomial infections, on teeth (plaque), it is also implicated in the otitis media (an acute ear infection). Even avid growth of these biofilms are seen over household items like cutting board, toilet sink, wash basin, etc., which need to be checked and sanitized. Biofilm formation is a major concern in the food industry as they cause havoc product spoilage with growth of pathogenic bacteria, mechanical blockage, and post processing contamination. Hence, to combat with such biofilm problems, effective and systematic antibiofilm strategies are to be undertaken for large-scale application.
The great versatility of the microflora helps in with proficient method of metal ion sequestration and restoration of contaminated ecobiological systems. Thus, in order to effectively use EPS for bioremediation, it must be studied properly from its formation, maturation, EPS–metal binding, and therefore sustainably remove heavy metals from the environment.
References
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Technol 3(4):74–102
Abioye OP, Oyewole OA, Oyeleke SB, Adeyemi MO, Orukotan AA (2018) Biosorption of lead, chromium and cadmium in tannery effluent using indigenous microorganisms. Braz J Biol Sci 5(9):25–32
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026
Alluri HK, Ronda SR, Settalluri VS, Bondili JS, Suryanarayana V, Venkateshwar P (2007) Biosorption: an eco-friendly alternative for heavy metal removal. Afr J Biotechnol 6
Apiratikul R, Pavasant P (2008) Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour Technol 99(8):2766–2777
Arbanah M, Najwa MR, Halim KH (2012) Biosorption of Cr(III), Fe(II), cu(II), Zn(II) ions from liquid laboratory chemical waste by Pleurotus ostreatus. Int J Biotechnol Wellness Ind 1(3):152–162
Aryal M, Ziagova M, Liakopoulou-Kyriakides M (2010) Study on arsenic biosorption using Fe(III)-treated biomass of Staphylococcus xylosus. Chem Eng J 162(1):178–185
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1):94
Bahobil A, Bayoumi RA, Atta HM, El-Sehrawey MM (2017) Fungal biosorption for cadmium and mercury heavy metal ions isolated from some polluted localities in KSA. Int J Curr Microbiol Appl Sci 6(6):2138–2154
Bano A, Hussain J, Akbar A, Mehmood K, Anwar M, Hasni MS, Ullah S, Sajid S, Ali I (2018) Biosorption of heavy metals by obligate halophilic fungi. Chemosphere 199:218–222
Bayramoğlu G, Yakup Arıca M (2009) Construction a hybrid biosorbent using Scenedesmus quadricauda and Ca-alginate for biosorption of Cu(II), Zn(II) and Ni(II): kinetics and equilibrium studies. Bioresour Technol 100(1):186–193. https://doi.org/10.1016/j.biortech.2008.05.050
Bhagat N, Vermani M, Bajwa HS (2016) Characterization of heavy metal (cadmium and nickle) tolerant gram negative enteric bacteria from polluted Yamuna River, Delhi. Afr J Microbiol Res 10:127–137
Bharagava RN, Mishra S (2018) Hexavalent chromium reduction potential of Cellulosimicrobium sp., isolated from common effluent treatment plant of tannery industries. Ecotoxicol Environ Saf 147:102–109
Boels IC, Kranenburg RV, Hugenholtz J, Kleerebezem M, Vos WM (2001) Sugar catabolism and its impact on the biosynthesis and engineering of exopolysaccharide production in lactic acid bacteria. Int Dairy J 11:723–732
Brinza L, Dring M, Gavrilescu M (2007) Marine micro and macro algal species as biosorbents for heavy metals. Environ Eng Manag J 6(3):237–251
Cardenas-Gonzalez JF, Acosta-Rodriguez I, Teran-Figueroa Y, Rodriguez-Perez AS (2017) Bioremoval of arsenic (V) from aqueous solutions by chemically modified fungal biomass. 3 Biotech 7(3):article 226
Chabukdhara M, Gupta SK, Gogoi M (2017) Phycoremediation of heavy metals coupled with generation of bioenergy. In: Algal biofuels. Springer, Berlin, pp 163–188
Chatterjee S, Chatterjee NC, Dutta S (2012) Bioreduction of chromium (VI) to chromium (III) by a novel yeast strain Rhodotorula mucilaginosa (MTCC 9315). Afr J Biotechnol 11(83):14920–14929
Chaturvedi MK (2011) Studies on chromate removal by chromium-resistant Bacillus sp. isolated from tannery effluent. J Environ Prot Sci 2(1):76
Chen XC, Wang YP, Lin Q, Shi JY, Wu WX, Chen YX (2005) Biosorption of copper(II) and zinc(II) from aqueous solution by Pseudomonas putida CZ1. Colloids Surf B Biointerfaces 46(2):101–107
Cheng KC, Demirci A, Catchmark JM (2011) Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol 92(1):29
Colagiorgi A, Bruini I, Di Ciccio PA, Zanardi E, Ghidini S, Ianieri A (2017) Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens 6:41
Coleman RJ, Patel YN, Harding NE (2008) Identification and organization of genes for diutan polysaccharide synthesis from Sphingomonas sp. ATCC 53159. J Ind Microbiol Biotechnol 35:263–274
Comte S, Guibaud G, Baudu M (2008) Biosorption properties of extracellular polymeric substances (EPS) towards cd: cu and Pb for different pH values. J Hazard Mater 151:185–193
Congeevaram S, Dhanarani S, Park J, Dexilin M, Thamaraiselvi K (2007) Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater 146(1–2):270–277
Cozzi D, Desideri PG, Lepri L (1969) The mechanism of ion exchange with algenic acid. J Chromatogr A 40:130–137
Cruz-Vega D, Cervantes-González E, Ammons D, Rojas-Avelizapa LI, García-Mena J, Pless RC, Rojasavelizapa NG (2008) Tolerance and removal of metals by microorganisms isolated from a pitch lake. In: Proceedings of the 1st international conference on hazardous waste management, pp 117–118
Cuthbertson L, Mainprize IL, Naismith JH, Whitfield C (2009) Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram-negative bacteria. Microbiol Mol Biol 73(1):155–177
Czaczyk K, Myszka K (2007) Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Polish J Environ Stud 16(6):799–806
Das N, Vimala R, Karthika P (2008) Biosorption of heavy metals—an overview. Indian J Biotechnol 7:159–169
de Carvalho CCJ (2018) Marine biofilms: a successful microbial strategy with economic implications. Front Mar Sci 5:126
De J, Ramaiah N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Marine Biotechnol 10(4):471–477
Dogan NM, Doganli GA, Dogan G, Bozkaya O (2015) Characterization of extracellular polysaccharides (EPS) produced by thermal Bacillus and determination of environmental conditions affecting exopolysaccharide production. Int J Environ Res 9(3):1107–1116
Douterelo I, Fish K, Boxall JJ (2018) Succession of bacterial and fungal communities within biofilms of a chlorinated drinking water distribution system. Water Res 141:74–85
Elshafie A, Joshi SJ, Al-Wahaibi YM, Al-Bahry SN, Al-Bemani AS, Al-Hashmi A, Al-Mandhari MS (2017) Isolation and characterization of biopolymer producing Omani Aureobasidium pullulans strains and its potential applications in microbial enhanced oil recovery. In: SPE Oil and Gas India Conference and Exhibition. Society of Petroleum Engineers, SPE-185326-MS
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martinez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Gorecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kues U, Kumar TK, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Duenas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, St John F, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS (2012) The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336(6089):1715–1719
Fosmire GJ (1990) Zinc toxicity. Am J Clin Nutr 51(2):225–227
Freire-Nordi CS, Vieira AAH, Nascimento OR (2005) The metal binding capacity of Anabaena spiroides extracellular polysaccharide: an EPR study. Process Biochem 40(6):2215–2224. https://doi.org/10.1016/j.procbio.2004.09.003
Freitas F, Alves VD, Pais J, Costa N, Oliveira C, Mafra L, Hilliou L, Oliveira R, Reis MA (2009) Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour Technol 100:859–865
Freitas F, Alves VD, Reis MA (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol 29:388–398
Frutos I, Garcia-Delgado C, Garate A, Eymar E (2016) Biosorption of heavy metals by organic carbon from spent mushroom substrates and their raw materials. Int J Environ Sci Technol 13(11):2713–2720
Gauthier PT, Norwood WP, Prepas EE, Pyle GG (2014) Metal–PAH mixtures in the aquatic environment: a review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat Toxicol 154:253–269
Gavrilescu M (2004) Removal of heavy metals from the environment by biosorption. Eng Life Sci 4:219–232
Gutierrez T, Biller DV, Shimmield T, Green DH (2012) Metal binding properties of the EPS produced by Halomonas sp: TG39 and its potential in enhancing trace element bioavailability to eukaryotic phytoplankton. Biometals 25:1185–1194
Hassan SHA, Awad YM, Kabir MH, Oh SE, Joo JH (2010) Bacterial biosorption of heavy metals. Chapter 4. In: Malik CP, Verma A (eds) Biotechnology: cracking new pastures. MD Publications, New Delhi., ISBN-13: 978–8175332331, pp 79–110
Hassiba M, Naima A, Yahia K, Zahra S (2014) Study of lead adsorption from aqueous solutions on agar beads with EPS produced from Paenibacillus polymyxa. Chem Eng Trans 38:31–36
Horvathova H, Kadukova J, Stofko M (2009) Biosorption of Cu2+ and Zn2+ by immobilized algae biomass of Chlorella kessleri. Acta Metall Slovaca 15(4):255–263
Hussain A, Alamzeb S, Begum S (2013) Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, district Mardan, Pakistan. Food Chem 136(3–4):1515–1523
Ibrahim WM (2011) Biosorption of heavy metal ions from aqueous solution by red macroalgae. J Hazard Mater 192(3):1827–1835
Irawaiti W, Yuwono T, Ompusunggu NP (2018) Growth characteristics and copper accumulation of bacterial consortium Acinetobacter sp. and Cupriavidus sp. isolated from a wastewater treatment plant. Biodivers J 19(5):1884–1890
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity: mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72
Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Rafiq M, Kamil MA (2018) Bacterial biofilm and associated infections. J Chin Med Assoc 81:7–11
Javaid A, Bajwa R (2008) Biosorption of electroplating heavy metals by some basidiomycetes. Mycopathologia 6(1&2):1–6
Joshi SJ, Al-Wahaibi YM, Al-Bahry S, Elshafie A, Al-Bemani AS, Al-Hashmi A, Samuel P, Sassi M, Al-Farsi H, Al-Mandhari MS (2016) Production and application of schizophyllan in microbial enhanced heavy oil recovery. In: SPE EOR conference at oil and gas West Asia. Society of Petroleum Engineers, SPE-179775-MS
Kadimpati KK, Mondithoka KP, Bheemaraju S, Challa VR (2013) Entrapment of marine microalga, Isochrysis galbana, for biosorption of Cr(III) from aqueous solution: isotherms and spectroscopic characterization. Appl Water Sci 3(1):85–92
Kafilzadeh FS, Abolahrar M, Kargar M, Ghodsi M, Sch J (2013) Isolation and identification of cadmium-resistant bacteria in Soltan Abad river sediments and determination of tolerance of bacteria through MIC and MBC. Eur J Exp Biol 3(5):268–273
Kariminiaae-Hamedaani HR, Kanda K, Kato F (2003) Wastewater treatment with bacteria immobilized onto a ceramic carrier in an aerated system. J Biosci Bioeng 95:128–132
Kiliç NK, Kürkçü G, Kumruoğlu D, Dönmez G (2015) EPS production and bioremoval of heavy metals by mixed and pure bacterial cultures isolated from Ankara stream. Water Sci Technol 72:1488–1494
Kim SY, Kim JH, Kim CJ, Oh DK (1996) Metal adsorption of the polysaccharide produced from Methylobacterium organophilum. Biotechnol Lett 18:1161–1164
Kim IH, Choi JH, Joo JO, Kim YK, Choi JW, Oh BK (2015) Development of a microbe-zeolite carrier for the effective elimination of heavy metals from seawater. J Microbiol Biotechnol 25(9):1542–1546
Klimmek S, Stan HJ, Wilke A, Bunke G, Buchholz R (2001) Comparative analysis of the biosorption of cadmium, lead, nickel, and zinc by algae. Environ Sci Technol 35(21):4283–4288
Kralj S, van Geel-Schutten IGH, Rahaoui H, Leer RJ, Faber EJ, van der Maarel MJEC, Dijkhuizen L (2002) Molecular characterization of a novel glucosyltransferase from Lactobacillus reuteri strain 121 synthesizing a unique, highly branched glucan with α-(1 4) and α-(1 6) glucosidic bonds. Appl Environ Microbiol 68:4283–4291
Kumar JI, Oommen C (2012) Removal of heavy metals by biosorption using freshwater alga Spirogyra hyalina. J Environ Biol 33(1):27–31
Kumaran NS, Sundaramanicam A, Bragadeeswaran S (2011) Adsorption studies on heavy metals by isolated cyano bacterial strain (Nostoc sp.) from Uppanar estuarine water, southeast coast of India. Res J Appl Sci 7(11):1609–1615
Kwarciak-Kozlowska A, Slawik-Dembiczak L, Banka B (2014) Phycoremediation of wastewater: heavy metal and nutrient removal processes. Environ Prot Nat Resour 25(4):51–54
Lakherwal D (2014) Adsorption of heavy metals: a review. Int J Environ Res Dev 4:41–48
Lau T, Wu X, Chua H, Qian P, Wong P (2005) Effect of exopolysaccharides on the adsorption of metal ions by Pseudomonas sp. CU-1. Water Sci Technol 52:63–68
Liu H, Fang HH (2002) Characterization of electrostatic binding sites of extracellular polymers by linear programming analysis of titration data. Biotechnol Bioeng 80(7):806–811
Lopez A, Lazaro N, Priego J, Marques A (2000) Effect of pH on the biosorption of nickel and other heavy metals by Pseudomonas fluorescens 4F39. J Ind Microbiol Biotechnol 24(2):146–151
Luna JM, Rufino RD, Sarubbo LA (2016) Biosurfactant from Candida sphaerica UCP0995 exhibiting heavy metal remediation properties. Process Saf Environ 102:558–566
Ma L, Wang S, Wang D, Parsek MR, Wozniak DJ (2012) The roles of biofilm matrix polysaccharide Psl in mucoid Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol 65:377–380
Majumdar I, D'souza F, Bhosle NB (2013) Microbial exopolysaccharides: effect on corrosion and partial chemical characterization. J Indian Inst Sci 79(6):539
Martins M, Uppuluri P, Thomas DP, Cleary IA, Henriques M, Lopez-Ribot JL, Oliveira R (2010) Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia 169:323–331
McIntosh M, Stone BA, Stanisich VA (2005) Curdlan and other bacterial (1, 3)-β-D-glucans. Appl Microbiol Biotechnol 68:163–173
Moreno-García J, García-Martinez T, Moreno J, Mauricio JC, Ogawa M, Luong P (2018) Impact of yeast flocculation and biofilm formation on yeast-fungus coadhesion in a novel immobilization system. Am J Enol Vitic 69:278–288
Mosa KA, Saadoun I, Kumar K, Helmy M, Dhankher OP (2016) Potential biotechnological strategies for the cleanup of heavy metals and metalloids front. Plant Sci 7:303
Mosharaf MK, Tanvir MZH, Haque MM, Haque MA, Khan MAA, Molla AH, Mohammad ZA, Islam MS, Talukder MR (2018) Metal-adapted bacteria isolated from wastewaters produce biofilms by expressing proteinaceous Curli fimbriae and cellulose nanofibers. Front Microbiol 9:1334
Nayak AK, Panda SS, Basu A, Dhal NK (2018) Enhancement of toxic Cr (VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides L. Int J Phytoremediation 20(7):682–691
Nguyen PQ, Botyanszki Z, Tay PKR, Joshi NS (2014) Programmable biofilm-based materials from engineered curli nanofibres. Nat Commun 5(1)
Nishimura J (2014) Exopolysaccharides produced from Lactobacillus delbrueckii subsp. bulgaricus. Adv Microbiol 4:1017
Ogbo EM, Okhuoya JA (2011) Bio-absorption of some heavy metals by Pleurotus tuber-regium Fr. Singer (an edible mushroom) from crude oil polluted soils amended with fertilizers and cellulosic wastes. Int J Soil 6(1):34–48
Oyedepo TA (2011) Biosorption of lead (II) and copper (II) metal ions on Calotropis procera (Ait.). J Pure Appl Chem:1–7
Özcan E, Sargın S, Göksungur Y (2014) Comparison of pullulan production performances of air-lift and bubble column bioreactors and optimization of process parameters in air-lift bioreactor. Biochem Eng J 92:9–15
Ozdemir G, Ceyhan N, Manav E (2005) Utilization of an exopolysaccharide produced by Chryseomonas luteola TEM05 in alginate beads for adsorption of cadmium and cobalt ions. Bioresour Technol 96(15):1677–1682. https://doi.org/10.1016/j.biortech.2004.12.031
Ozdemir G, Ceyhan N, Manav E (2005) Utilization of an exopolysaccharide produced by Chryseomonas luteola TEM05 in alginate beads for adsorption of cadmium and cobalt ions. Bioresour Technol 96:1677–1682
Özer A, Özer D (2003) Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto S. cerevisiae: determination of biosorption heats. J Hazard Mater 100(1–3):219–229
Pal A, Paul AK (2008) Microbial extracellular polymeric substances: central elements in heavy metal bioremediation. Indian J Microbiol 48(1):49
Pandey G, Jain GRK (2002) Appl Environ Microbiol 68:5789–5795
Pardo R, Herguedas M, Barrado E, Vega M (2003) Biosorption of cadmium, copper, lead and zinc by inactive biomass of Pseudomonas putida. Anal Bioanal Chem 376(1):26–32
Pérez-Mendoza D, Coulthurst SJ, Sanjuán J, Salmond GP (2011) N-Acetylglucosamine-dependent biofilm formation in Pectobacterium atrosepticum is cryptic and activated by elevated c-di-GMP levels. Microbiology 157:3340–3348
Pollock TJ (1993) Gellan-related polysaccharides and the genus Sphingomonas. Microbiology 139(8):1939–1945
Prasad MNV, Prasad R (2012) Nature’s cure for cleanup of contaminated environment_a review of bioremediation strategies. Rev Environ Health 27(4):181–189
Prasad AS, Varatharaju G, Anushri C, Dhivyasree S (2013) Biosorption of lead by Pleurotus florida and Trichoderma viride. Br Biotechnol J 3(1):66–78
Puyen ZM, Villagrasa E, Maldonado J, Diestra E, Esteve I, Sole A (2012) Biosorption of lead and copper by heavy-metal tolerant Micrococcus luteus DE2008. Bioresour Technol 126:233–237
Qi L, Christopher GF (2019) Role of flagella, type IV pili, biosurfactants, and extracellular polymeric substance polysaccharides on the formation of pellicles by Pseudomonas aeruginosa. Langmuir 35:5294–5304
Rajendran P, Muthukrishnan J, Gunasekaran P (2003) Microbes in heavy metal remediation, Indian J. Exp Biol 41:935–944
Rajendran R, Sherry L, Nile CJ, Sherriff A, Johnson EM, Hanson MF, William C, Munro CA, Jones BJ, Ramage G (2016) Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection—Scotland, 2012–2013. Clin Microbiol Infect 22:87–93
Ramrakhiani L, Majumder R, Khowala S (2011) Removal of hexavalent chromium by heat inactivated fungal biomass of Termitomyces clypeatus: surface characterization and mechanism of biosorption. Chem Eng J 171(3):1060–1068
Randrianjatovo-Gbalou I, Rouquette P, Lefebvre D, Girbal-Neuhauser E, Marcato-Romain CE (2017) In situ analysis of Bacillus licheniformis biofilms: amyloid-like polymers and eDNA are involved in the adherence and aggregation of the extracellular matrix. J Appl Microbiol 122:1262–1274
Ray S, Ray MK (2009) Al Ameen J Med Sci 2:57–63
Romero CM, Martorell P, López AG, Peñalver CN, Chaves S, Mechetti M (2018) Architecture and physicochemical characterization of Bacillus biofilm as a potential enzyme immobilization factory. Colloids Surf B Biointerfaces 162:246–255
Salehizadeh H, Shojaosadati SA (2003) Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res 37(17):4231–4235
Sarada B, Prasad MK, Kumar KK, Murthy CV (2014) Cadmium removal by macro algae Caulerpa fastigiata: characterization, kinetic, isotherm and thermodynamic studies. J Environ Chem Eng 2(3):1533–1542
Sari A, Tuzen M (2008) Biosorption of cadmium(II) from aqueous solution by red algae (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. J Hazard Mater 157(2–3):448–454
Schatschneider S, Persicke M, Watt SA, Hublik G, Pühler A, Niehaus K, Vorhölter FJ (2013) Establishment, in silico analysis, and experimental verification of a large-scale metabolic network of the xanthan producing Xanthomonas campestris pv. Campestris strain B100. J Biotechnol 167(2):123–134
Senthilkumar R, Vijayaraghavan K, Jegan J, Velan M (2010) Batch and column removal of total chromium from aqueous solution using Sargassum polycystum. Environ Prog Sustain Energy 29(3):334–341
Shah J, Brown RM (2005) Towards electronic paper displays made from microbial cellulose. Appl Microbiol Biotechnol 66(4):352–355
Sharon N (1966) Polysaccharides. Annu Rev Biochem 35(1):485–520
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275(1):131–141. https://doi.org/10.1016/j.jcis.2004.01.036
Sheppard DC, Howell PLJ (2016) Biofilm exopolysaccharides of pathogenic fungi: lessons from bacteria. J Biol Chem 291:12529–12537
Shi W, Tao S, Yu Y, Wang Y, Ma W (2011) High performance adsorbents based on hierarchically porous silica for purifying multicomponent wastewater. J Mater Chem 21:15567–15574
Shoaib A, Aslam N, Aslam N (2013) Trichoderma harzianum: adsorption, desorption, isotherm and FTIR studies. J Anim Plant Sci 23:1460–1465
Sima F, Mutlu EC, Eroglu MS, Sima LE, Serban N, Ristoscu C, Petrescu SM, Oner ET, Mihailescu IU (2011) Levan nanostructured thin films by MAPLE assembling. Biomacromolecules 12(6):2251–2256
Simpson CL, Cheetham NWH, Giffard PM, Jacques NA (1995) Four glucosyltransferases, GTFJ, GTFK, GTFL and GTFM, from Streptococcus salivarius ATCC 25975. Microbiology 141:1451–1460
Sultan S, Mubashar K, Faisal M (2012) Uptake of toxic Cr (VI) by biomass of exo- polysaccharides producing bacterial strains. Afr J Microbiol Res 6:3329–3336
Sun D, Yang J, Wang X (2010) Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale 2(2):287–292
Taştan BE, Ertuğrul S, Dönmez G (2010) Effective bioremoval of reactive dye and heavy metals by Aspergillus versicolor. Bioresour Technol 101:870–876
Tavares TM, Carvalho FM (1992) Avaliação de exposição de populaces humanas a metais pesados no ambiente: Exemplos do Recôncavo Baiano. Revista Química Nova 15(2):147–154
Trapani DD, Mannina G, Torregrossa M, Viviani G (2010) Quantification of kinetic parameters for heterotrophic bacteria via respirometry in a hybrid reactor. Water Sci Technol 61:1757–1766
Vandevivere P, Kirchman DL (1993) Attachment stimulates exopolysaccharide synthesis by a bacterium. Appl Environ Microbiol 59:3280–3286
Vasudevan R (2014) Biofilms: microbial cities of scientific significance. J Microbiol Exp 1:00014
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wang L, Yang J, Chen Z, Liu X, Ma F (2013) Biosorption of Pb (II) and Zn (II) by extracellular polymeric substance (eps) of Rhizobium Radiobacter: equilibrium, kinetics and reuse studies. Arch Environ Prot 39:129–140
Webb JS (2009) Differentiation and dispersal in biofilms. http://citeseerx.Ist.Psu.Edu/viewdoc/download
Whitfield C (1988) Bacterial extracellular polysaccharides. Can J Microbiol 34:415–420
Wingender J, Neu TR, Flemming HC (1999) What are bacterial extracellular polymeric substances? In: Microbial extracellular polymeric substances. Springer, Berlin, Heidelberg, pp 1–19
Yin W, Wang Y, Liu L, He J (2019) Biofilms: the microbial “protective clothing” in extreme environments. Int J Mol Sci 20:3423
Yu MH (2001) Impacts of environmental toxicants on living systems. In: Environmental Toxicology. CRC Press LLC, Boca Raton, FL
Zouboulis AI, Loukidou MX, Matis KA (2004) Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem 39(8):909–916
Conflict of Interest
A mutual declaration by all the authors states that there is no conflict of interest.
Moreover, this chapter does not contain any study conducted upon human or animals, by any authors.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jasu, A., Lahiri, D., Nag, M., Ray, R.R. (2021). Biofilm-Associated Metal Bioremediation. In: Joshi, S.J., Deshmukh, A., Sarma, H. (eds) Biotechnology for Sustainable Environment. Springer, Singapore. https://doi.org/10.1007/978-981-16-1955-7_8
Download citation
DOI: https://doi.org/10.1007/978-981-16-1955-7_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1954-0
Online ISBN: 978-981-16-1955-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)