Abstract
The accumulation of Cd(II), Cu(II), Pb(II) and Zn(II) at mg L−1 concentration levels by inactive freeze-dried biomass of Pseudomonas Putida has been investigated. These metals could be efficiently removed from diluted aqueous solutions. A contact time of 10 min was sufficient to reach equilibrium. The pH has a strong effect on metal biosorption and the optimal pH values were 6.0, 5.0–6.0, 6.0–6.5 and 7.0–7.5 for Cd(II), Cu(II), Pb(II) and Zn(II) respectively. Under these conditions there was 80% removal for all metals studied. The process of biosorption can be described by a Langmuir-type adsorption model. This model accounts for 98% of the data variance. The K A and q max parameters for each metal are strongly correlated (at confidence levels greater than 98%) with the metal acidity, quantified by the constant of the corresponding M(OH)+ complex, thus confirming previous assertions by other authors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The industrial use of metals leads to an alarming increase of metal concentrations in the environment. Metals are among main environmental concerns because of their unique characteristics: unlike organic pollutants, they are non-biodegradable and hence are accumulated by living organisms. The problem is aggravated by their high dispersion; they are widely spread in aquatic systems [1] from where they enter the food chain.
In general, chemical pollutants at low concentrations are difficult to remove from aqueous solutions. The available technologies for metal removal include chemical precipitation, complexation or extraction with organic solvents, but they become inefficient when applied to metals present at trace concentrations. Adsorption is one of the few alternatives available for such situations and some inorganic materials such as alumina [2] and activated carbon [3] have been tested. The possibility of using biological solids has been also investigated: algae [4, 5, 6, 7], yeast [8], fungi [9] and bacteria [10, 11, 12, 13, 14] have been proposed to accumulate metal ions under the general term of biosorption [15]. The biosorption of metals can be produced by two main mechanisms [16]: active (dependant upon metabolism) and passive (independent of metabolism). The former may only take place in the restricted range of conditions (pH, temperature, nutrients) that will allow the cells to maintain their life functions. Passive processes, on the other hand, take place in living and dead cells and occur in a broader range of environmental conditions. The efficiency of biosorption is usually higher in the passive process [17, 18] and it seems to occur via an ion-exchange process, in which metal ions compete with hydrogen ions for negatively charged binding sites on the cell wall [19, 20, 21]. To inactivate these microorganisms, different physical and chemical treatments have been proposed [22]. Although these treatments could change the structural properties of the biological solids and therefore their ability for biosorption, freeze-drying does not seem to modify the structure of the wall cells [23].
In this paper, biosorption of cadmium, copper, lead an zinc by freeze-dried Pseudomonas Putida has been investigated. Cadmium and lead are toxic at very low concentrations, whereas copper and zinc are essential but recognized as potentially toxic at higher concentrations. Pseudomonas Putida is widely distributed in nature and its basic habitat is wastewaters and soils.
Experimental
Reagents
Stock solutions of Cd(II), Cu(II), Pb(II) and Zn(II) were prepared daily by dilution of Carlo Erba standards (1,000 mg L−1). Acetate, succinate and borate buffer solutions (0.1 M) were prepared from the appropriate reagent (analytical-grade acetic acid, succinic acid and boric acid, respectively) and sodium hydroxide and used to cover the pH range 4.0–9.5. Glassware, sample bottles and reagent containers were decontaminated before use by soaking in nitric acid 2 M for 2 days and then rinsed several times with water. Deionized water was used throughout the experimental study.
Instruments
The biosorption experiments were performed with shaking and the centrifugation was carried out at 5,500 rpm. Values of pH were determined by using standardized procedures. Mineralization of samples was carried out in a homemade UV digestor equipped with a 150-W mercury lamp [24].
Heavy metals were measured by differential pulse anodic stripping voltammetry (DPASV) with a Metrohm 663 VA Stand connected to a central control unit Metrohm E506 Polarecord. A conventional three-electrode system was utilized, consisting of a Metrohm multimode electrode used in the hanging mercury drop electrode (HMDE) mode, an Ag/saturated AgCl/3 M KCl reference electrode and a Pt rod as counter electrode. A medium drop size of the HMDE was selected and the mercury used was triply distilled.
Organism growing conditions
Pseudomonas Putida cells were grown in flasks at 30 °C with shaking at 200 rpm for 24 h. The growth media for the experiments were Tryptic Soy Agar (TSA) from Merck, prepared by dissolving 40 g in 1 L deionised water (pH 7.3 at 37 °C), and Tryptic Soy Broth (TSB) from Biolife prepared by dissolving 30 g in deionized water to total dissolution (pH 7.3 at 37 °C). These growing media were sterilized in an autoclave at 121 °C for 20 min before inoculation. The bacterial cells were harvested by centrifugation at 12,400 g (9,500 rpm) and then washed three times with distilled water.
Cell inactivation
Freeze-drying of the cells was performed at a temperature ranging from −40 °C to −50 °C and a pressure of 10 mbar. When the process was finished, bacterial cells were reduced to powder and stored at −3 °C in the darkness until use.
Analytical methods
The biosorption experiments were performed by preparing suspensions with 1 mg mL−1 of inactive cells in different media containing a given amount of the studied metal. After the contact and centrifugation, the metal present in the supernatant solutions was determined by DPASV, and the retained metal was calculated by difference. DPASV requires the destruction of the dissolved organic matter, so the solutions were previously mineralised by UV-digestion [24]. The experimental procedure, optimised for our solutions by using Taguchi experimental designs [25], was as follows: 20 mL of the supernatant solution was transferred into a quartz tube with 60 μL of 40% hydrogen peroxide and 25 μL of concentrated sulfuric acid and the mixture was irradiated for 45 min.
The digested sample was then transferred to the voltammetric cell, and its pH adjusted to around 4.5 by addition of 2 M sodium acetate. The solution was deoxygenated by purging with water-saturated nitrogen for 10 min. A new mercury drop was extruded and the plating potential was set at −1.0 V for 60 s whilst the solution was stirred at 1,920 rpm. Then the stirrer was stopped and after 30 s, the electrode potential was scanned towards more positive potential values at a scan rate of 20 mV s−1 and using a superimposed differential pulse of 50-mV amplitude, a pulse duration of 60 ms and an interval between pulses of 400 ms. Stripping peaks were registered at different potentials for each metal: Zn(II) around −1.00 V, Cd(II) around −0.60 V, Pb(II) around −0.40 V and Cu(II) around 0.00 V. The standard additions method was used to quantify the metal concentration and avoid matrix effects.
Results and discussion
Centrifugation time
The biosorption studies imply the contact between the biomass and the solution containing the metal. In order to accurately determine the amount of metal retained by the biomass it becomes necessary to separate the liquid and the solid phases. In this case, the separation was accomplished by centrifugation, so a study to determine the optimal centrifugation time was carried out. A set of suspensions containing different amounts (2–34 mg) of biomass in 25 mL of water was prepared. The suspensions were centrifuged and the absorbance at 660 nm of the supernatant solutions was measured at different times. The time for constant absorbance (t c) was taken as an indicator parameter of the end of centrifugation. A plot of centrifugation time (t c) versus the amount of biomass in mg showed a linear relationship (see Fig. 1). To simplify later calculations, the t c value corresponding to 25 mg (giving 1 mg mL−1 "concentrations" of biomass), that is 25 min, was selected as centrifugation time.
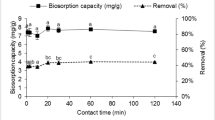
Reaction time
Suspensions containing 100 μg L−1 of the metal ion and 1 mg L−1 of biomass were prepared and, after different reaction times, the metal remaining in the supernatant solution was determined by DPASV. The measured concentrations were plotted as a function of time, as shown in Fig. 2 which displays the results for Cu(II) and Pb(II) (similar results were obtained for Cd(II) and Zn(II)). As shown, metal concentrations reach an approximately constant value for reaction times of about 5 min. This short time required for biosorption is in accordance with the results given by other researchers such as Jackson et al. [26], Tsezos and Volesky [27, 28] or Huang et al. [23] who reported contact times for complete biosorption in the same order of magnitude for similar biomass. In our case, a reaction time of 10 min was selected to ensure an optimum metal uptake.
Effects of pH on biosorption
The pH of the metal solution plays a crucial role in passive microbial biosorption [29]. It has been shown that the affinity of cationic species for the functional groups present in the cellular surface is strongly dependent on the pH [30, 31]. Figure 3 summarizes the results of the adsorption of metal ions Cd(II), Cu(II), Pb(II) and Zn(II) by Pseudomonas Putida as a function of pH.
In all cases, metal uptake by the biomass increases with increasing pH and reaches a maximum after which the metal uptake decreases. The optimal pH values for Cd(II), Cu(II), Pb(II) and Zn(II) were, respectively, 6.0, 5.0–6.0, 6.0–6.5 and 7.0–7.5. These results suggest that the adsorption of metals onto the biomass could be ruled by ionic attraction. At low pH values the inactivated cell surface becomes more positively charged, reducing the attraction between metal ions and functional groups on the cell wall. In contrast, when the pH increases the cell surface is more negatively charged and the process of retention is favoured [10, 11, 32, 33] until a maximum is reached around pH 7. However, for values of pH higher than the optimum, the formation of hydroxylated complexes of the metal will also compete with the active sites and as a consequence, the retention will decrease again. Table 1 shows a comparative study between the results reached in this work and the results published by other researchers using different active and inactive biomass. We can see that in general, the state of the material (i.e. active or inactive) has a greater effect on the values than its type: the pH range of freeze-dried Pseudomonas Putida coincides with that found for inactive Pseudomonas aeruginosa, whereas it differs from the pH range found for active Pseudomonas Putida.
The maximum retention percentages were about 80% for all the metal ions. As above, the obtained percentages are higher than those found for active materials [17] and are in accordance with the results reported by Huang et al. [22], who describe an increase of the retained metal when the material is inactivated.
Biosorption models
The two most widely accepted surface adsorption models for single-solute systems used in the literature are the Langmuir and Freundlich models [34, 35].
The general Langmuir sorption model is expressed by:
where q is the uptake of the metal ion by the biomass (mol g−1), q max is the maximum uptake (mol g−1), C f is the final concentration of metal in the solution (mol L−1) and K L is the Langmuir adsorption equilibrium constant (mol L−1). This model implies the possibility of saturation of the adsorptive surface, so q eventually can reach a maximum value (q max).
The Langmuir general model has been adapted by Nelson et al. [38] to the case of metal adsorption by biomass, by considering biosorption as a simple metal complex formation:
whose conditional formation constant becomes:
where M represents the free metal, S the free surface sites and MS the bonded sites. The symbols [] and {} refer, respectively, to concentrations in mol L−1 and g L−1. In the suspension formed by the solid biomass and the metal solution, the following mass-balance can be applied:
where S T is the total biomass (g L−1) and X m the number of complexing sites (mol g−1) that coincides with the q max parameter of the general Langmuir model. By rearranging Eqs. (2) and (3), the following equation is obtained:
Equation (4) is identical to Eq. (1) of the general Langmuir model, by making X M/K A equal to K L. K A is a conditional constant whose actual value will depend on the conditions of the reaction medium, and particularly on pH. By rearranging Eq. (4) the model can be easily linearized:
As 1/q and [M] can be experimentally determined, the values of q max and K A can be obtained by least-squares fitting.
On the other hand, the general form of the Freundlich model is:
where q and C f have the same meaning as in Eq. (1), and k and n relate to the capacity and intensity of adsorption, respectively. The Freundlich model does not imply a saturation of the adsorptive surface and can be directly adapted to the biosorption of metals:
and linearized by taking logarithms:
where the parameters k and n are determined as in the Langmuir case.
To ensure the higher metal adsorption capacity, the pHs of the solutions were adjusted to their respective optimum values (i.e. 6.0 for Cd and Cu, 6.5 for Pb and 7.0 for Zn). A comparative study of the four metal ions at the same pH was also performed at the arbitrarily chosen pH value of 4.5.
A plot of q (mg g−1) versus [M] (mg L−1) showed a similar behaviour for the four metals (Fig. 4): q increases up to a maximum value that remains constant.
Only the Langmuir model showed a good fit to the experimental data, as can be seen in Fig. 5 that shows the linearized plots. The Freundlich model did not fit the data with the exception of the first data points, so this model is only suitable at low metal concentrations. The linearized Freundlich plots are not shown here.
Linearized Langmuir data as applied to the results given in Fig. 4 for each metal ion a at their respective optimum pH and b at pH 4.5
Table 2 summarizes the numerical results of the Langmuir parameters K A and q max and the r 2 coefficient. The r 2 coefficient gives the amount of variance explained by the model, so it can be used to evaluate the goodness of fit. In all occasions, the variance not explained by the model (100(1−r 2)) was lower than 3%, so the Langmuir model is adequate to describe the behaviour of the biosorption of metals on inactive Pseudomonas Putida. In this model, the metal ions retained by the biomass increase up to a constant value, due to the progressive diminution of the free binding sites on the cell wall.
Although K A allows us to make a comparison of the affinity of the biomass towards the metal ions, it must be taken into account that it is a conditional constant. Its value will depend on the experimental conditions, particularly pH, which remains a major factor in biosorption. None of the studied metal ions has significant side reactions at the pH values studied [39]; therefore, we can directly compare the K A values at constant pH. The K A values at pH 4.5 follow the same order as that at optimum pH values: Pb(II) >Cu(II) >Cd(II) ≈Zn(II).
Different mechanisms have been proposed to explain the biosorption of metals. The first one is based on ionic exchange equilibrium [40]. In this case the charge density of the metal ions will be the governing factor. For the four metal ions studied, Pb(II) has the lowest radius (450 pm), followed by Cd(II) (500 pm) and Cu(II) and Zn(II) with a similar value of 600 pm. Therefore ionic attraction and exchange do not fully explain the biosorption, so other phenomena must be also taken into account.
Huang et al. [23] and Pagnanelli et al. [41] have pointed out that the acidity of cations can be an important factor influencing biosorption. We have found a strong correlation between K A and metal acidity, represented by β M(OH) [42] the stability constant of M(OH)+ which is the first hydroxy-metal complex (Table 2).
At pH 4.5 the regression equation is: K A=1.69+8.34×10−6 β M(OH). This linear model explains a 95.1% of the data variance and the regression is significant with an a posteriori confidence level p=0.025. Therefore, the effect of the acidity of the cations is extremely important and cannot be disregarded.
The q max is another main parameter when considering the use of biomass for removal of metal ions. The q max (mg g−1 at pH 4.5) follow a sequence identical to that for K A: Pb(II) >Cu(II) >Cd(II) ≈ Zn(II). If we consider the values at the optimum pH, the order becomes Pb(II) >Cu(II) ≈Zn(II) >Cd(II). These results agree with those reported in ref. [23] for Aspergillus oryzae (Pb(II) >Cu(II) >Cd(II) ≈Zn(II)), in ref. [41] for Sphaerotilus natans (Pb(II) >Cu(II) >Zn(II) >Cd(II)) and in ref. [33] for Nocardia sp. (Pb(II) >Cu(II) >Cd(II)). Some authors have also made a comparison of q max with other materials, including active sludge [43] and active carbon [44] and reported similar sequences.
A significant correlation was also found between q max and β M(OH). The regression equation at pH 4.5 is q max=25.00+9.24×10−5 β M(OH). The amount of explained variance is 96.2% and the regression is statistically significant at an a posteriori confidence level p=0.019, so the effect of the acidity of cations as a prime ruling factor is again highlighted.
As the pH is an easily controllable experimental parameter, the strong dependence of the retention on it opens many possibilities for the use of the freeze-dried Pseudomonas Putida biomass in the separation of the studied metals. On an analytical scale, the biomass could be used to preconcentrate trace metals prior to their analysis, whereas on an industrial scale, biomass could be used for depuration of polluted effluents.
Conclusions
-
Inactive freeze-dried Pseudomonas Putida has been shown to be able to accumulate Cd(II), Cu(II), Pb(II) and Zn(II) at mg L−1 concentration level, with considerably high capacities.
-
More than 80% of metal ions were rapidly removed in less than 5 min contact time.
-
Results also demonstrate that pH is a factor with a main implication in these processes. For the four heavy metals studied, the maximum retention was achieved at pH values ranging from 5.0 to 7.5.
-
The Langmuir model can adequately represent the distribution of the metals between the solution and the solid biomass. The conditional constants, K A and q max, derived from least-squares fits can be used to quantify metal adsorption.
-
There is a strong correlation between acidity of the metals (quantified by β M(OH)) and the Langmuir parameters K A and q max. The regression equations could be used to predict values for K A and q max for other metal ions and the biomass studied.
-
Finally, the results also suggest that inactive Pseudomonas Putida could be used as a basis for the preconcentration and/or isolation of the studied metals on analytical or industrial scales.
References
Fergusson JE (1990) (ed) The heavy elements: chemistry, environmental impact and health effects. Pergamon, Exeter
Huang CP, Rhoads EJ (1989) J Colloid Interf Sci 131:289–306
Cocero MJ, Soria JL, Fernández-Polanco F, Salvador F, Sánchez C, Merchán MD (1997) In: Baeyens J, Dolesj P, Taylor N, Waller G (eds) Current R&D efforts in physicochemical water treatment. Academic Press, Leuven, pp 95–115
Brady D, Letebele B, Duncan JR, Rose PD (1994) Water SA 20(3):213–218
Leusch A, Holan ZR, Volesky B (1995) J Chem Technol Biot 62:279–288
Pairat K (2002) Chemosphere 47:1081–1085
Nuhoglu Y, Malkoc E, Gürses A, Canpolat N (2002) Bioresource Technol 85:331–333
Wilhelmi BS, Duncan JR (1995) Biotechnol Lett 17(9):1007–1012
Wang J, Martínez T, Darnall D (1989) J Electroanal Chem 259:295
Volesky B, Holan ZR (1995) Biotechnol Prog 11:235–250
Wong PK, Lam KC, So CM (1993) Appl Microbiol Biotechnol 39:127–131
Chang JS, Law R, Chang CC (1997) Water Res 31(7):1651–1658
Humble AV, Gadd GM, Codd GA (1997) Water Res 31(7):679–1686
Wang L, Chua H, Zhou Q, Wong PK, Sin SN, Lo WL, Yu PH (2003) Water Res 37:561–568
Solari P, Zouboulis AI, Matis KA, Stalidis GA (1996) Sep Sci Technol 31(8):1075–1092
Gadd GM (1988) In: Rehm HJ, Reed G (eds) Biotechnology 6b: special microbial processes, VCH Verlagsgesellschaft, Weinheim, pp 401–433
Agraz R, van der Wal A, van Leeuwen HP (1994) Bioelectroch Bioener 34:53–59
Goddard PA, Bull AT (1989) Appl Microbiol Biot 31:314–319
Beveridge TJ (1981) Int Rev Cytol 72:229–317
Daughney J, Fein JB, Yee N (1998) Chem Geol 144:161–176
Fowle DA, Fein JB (1999) Geochim Cosmochim Acta 63:3059–3067
Huang CP, Huang CP, Morehart A (1990) Water Res 24(4):433–439
Huang JP, Huang CP, Morehart AL (1991) In: Vernel JP (ed) Heavy metals in the environment. Elsevier, Amsterdam, pp 329–349
Vega M, Pardo R, Barrado E, de la Fuente MA, del Valle JL (1994) Fresenius J Anal Chem 350:139–144
Taguchi G (1991) (ed) System of experimental designs, vol I and II, quality resources. Kraus and American Supplier Institute, USA
Jackson PJ, Anderson WL, DeWitt JG, Huei-Yang DK, Kuske CR, Moncrief RM, Rayson GD (1993) Vitro Cell Dev Biol 29:220–226
Tsezos M, Volesky B (1982) Biotechnol Bioeng 24:385–401
Tsezos M, Volesky B (1982) Biotechnol Bioeng 24:955–969
Pradhan AA, Levine AD (1995) Sci Total Environ 170(3):209–220
Schiewer S, Volesky B (1995) Environ Sci Technol 29:3049- 3058
Fein JB, Daughney CJ, Yee N, Davis TA (1997) Geochim Cosmochim Acta 61:3319–3328
Gardea-Torresdey JL, Becker-Hapak MK, Hosea JM, Darnall DW (1990) Environ Sci Technol 24:1372–1378
Sadowski Z (2001) Mineral Eng 14:547–552
Pagnanelli F, Trifoni M, Beolchini F, Esposito A, Toro L, Vegliò F (2001) Process Biochem 37:115–124
Özer A, Özer D, Ekiz HI (1999) Process Biochem 34:919–927
Fourest E, Roux JC (1992) Appl Microbiol Biot 37:399–403
Niu H, Xu XS, Wang JH, Volesky B (1993) Biotechnol Bioeng 42:785–787
Nelson PO, Chung AK, Hudson MC (1981) J WPCF 53(8):1323–1333
Ringbom A (1963) (ed) Complexation in analytical chemistry. Wiley, New York
Ferris FG, Beveridge TJ (1986) Can J Microbiol 32:594–601
Pagnanelli F, Esposito A, Toro L, Vegliò F (2003) Water Res 37:627–633
Smith RM, Martell E (1976) (eds) Critical stability constants, vol 4, inorganic complexes. Plenum, New York
Tien CT, Huang CP (1987) J Environ Eng-ASCE 113:285–299
Huang CP, Hsieh YS, Park SW, Corapcioglu OM, Bowers AR, Elliot HA (1986) In: Patterson JW, Passino R (eds) Metal speciation, separation and recovery. Ann Arbor Sci Publ, Ann Arbor, pp 437–465
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pardo, R., Herguedas, M., Barrado, E. et al. Biosorption of cadmium, copper, lead and zinc by inactive biomass of Pseudomonas Putida . Anal Bioanal Chem 376, 26–32 (2003). https://doi.org/10.1007/s00216-003-1843-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-1843-z