Abstract
The emergence of precision medicine and our latest understanding of the biological characteristics of ovarian cancer (OC) have led to the discovery of drug targets, novel anticancer agents, and their predictive biomarkers. The genetics of OC is an evolving biomarker for predicting outcomes. Several completed and ongoing clinical trials used this concept for better patients’ selection and stratification. The exploitation of specific molecular vulnerabilities in OC for drug development such as BRCA and BRCAness is a milestone in the current management of this women’s cancer. Without a doubt, OC is one of the solid cancers that have benefited from genetic biomarkers for the implementation of targeted agents such as PARP inhibitors in clinical practice. This progress is discussed in this chapter based on recent studies and clinical trials.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Few therapeutic advances were achieved in improving survival outcomes in the first-line therapy of ovarian cancer (OC). However, predictive and prognostic biomarkers have considerably changed outcomes in some settings in women with this aggressive cancer (Le Page et al. 2020a; b; El Bairi et al. 2017a, b; Madariaga et al. 2020). An illustrative example is the important number of clinical trials, prospective studies, and retrospective real-world cohorts that have demonstrated the favorable impact of BRCA mutations on therapy response and prognosis in OC (Madariaga et al. 2019; Lorusso et al. 2020). Moreover, BRCA mutations and other variants in homologous recombination repair (HRR) genes are now used for OC patients’ selection for poly-ADP-ribose polymerase inhibitors (PARPi). BRCA, BRCAness, and HRR are associated with genomic instability and synthetic lethality in OC and are potential predictors of pharmacological sensitivity to platinum agents and PARPi (Konstantinopoulos and Matulonis 2018). Remarkably, as a result of the relevant success of cancer genetics in the field of translational oncology, there is an increasing number of clinical trials in OC that use genetic alterations as biomarkers for patient’s selection, stratification, and prediction of drug response; particularly using umbrella and basket trial designs (Tsimberidou et al. 2020). As described in the other chapters of this book, some of their results provided considerable information for clinical use and it is not surprising to see other starting and ongoing trials in this highly active research area of OC. The current chapter focuses on the impact of genetic variants on outcomes in OC.
4.2 Ovarian Cancer Genetics as a Biomarker of Response to Chemotherapy and Survival Outcomes
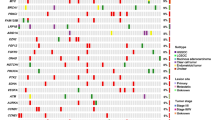
Platinum-based chemotherapy is currently considered the backbone of OC therapy. Carboplatin and cisplatin bind to DNA and induce structural adducts which in turn cause considerable damages to cancer cells, and therefore driving cell cycle arrest and mitochondrial apoptosis (Galluzzi et al. 2012). Enhanced response to these anticancer drugs is observed in patients with mutated BReast Cancer 1 and 2 genes (BRCA1/2) which confer impairment of DNA repair mechanisms (Quinn et al. 2009; Madariaga et al. 2019). Several preclinical reports have shown that cells harboring BRCA variants have superior sensitivity to platinum-based chemotherapy (Madariaga et al. 2019). This loss of function is considered the key driver of responsiveness to these agents and is a well-established predictive biomarker in OC. Clinically, women with both germline and somatic mutated BRCA were found to have increased response to platinum-based chemotherapy (Alsop et al. 2012; Gorodnova et al. 2015; Vencken et al. 2011; Pennington et al. 2014; Leunen et al. 2009) (for detailed review, see: Le Page et al. 2020a, b). During a relapse, these improved outcomes were also observed in platinum-resistant OC with BRCA mutations (Alsop et al. 2012). Thus, platinum re-challenge is an approach for recurrent OC patients with germline mutated BRCA carriers (Madariaga et al. 2019). In addition to high immune infiltrates, increased mutational burden, and loss of heterozygosity, BRCA mutations are considered as key determinants of exceptional long-term OC survival (Yang et al. 2018; Hoppenot et al. 2018). This was further confirmed by several meta-analyses of survival outcomes in OC (summarized in Table 4.1). Remarkably, a large study that enrolled 316 high-grade serous OC patients found that BRCA2, but not BRCA1, was associated with superior chemotherapy response and also improved survival outcomes (Yang et al. 2011). Mechanistically, both BRCA1 and BRCA2 are important complementary members of the genes involved in DNA damage repair. However, accumulating evidence suggests that the principal function of BRCA2 is the regulation of RAD51 that has a pivotal role in double-strand break repair (Davies et al. 2001) rather than tumor suppression ensured particularly by BRCA1. Functions of BRCA1 encompass cell cycle arrest checkpoint control (Yarden et al. 2002; Sharma et al. 2018), mitotic spindle assembly (Joukov et al. 2006; Xiong et al. 2008), and centrosome duplication (Mullee and Morrison 2016; Kais et al. 2012; Sankaran et al. 2007; Hsu and White 1998) and their failure can predispose to cancer initiation rather than conferring sensitivity to platinum DNA-crosslink agents. Therefore, these fundamental data may explain this difference in survival and drug response in this previous study. Importantly, the “mutator phenotype” hypothesis in OC patients with mutations beyond BRCA1 is a potential driver of chemotherapy response in this setting as well. Despite these important observations, the acquisition of reversion mutations in BRCA genes can restore BRCA proteins expression and induce resistance to platinum-based therapy and also PARPi (Milanesio et al. 2020). Therapeutically, a recent meta-analysis documented that pharmacological blockade of DNA end-joining repair signaling may improve the stability of drug response by preventing the acquisition of reversion BRCA mutations (Tobalina et al. 2021). Promisingly, detection of these reversion mutations can be performed using real-time liquid biopsy approaches. Based on massively parallel targeted sequencing, Weigelt et al. showed recently that prospective evaluation of circulating-free DNA has the potential to non-invasively identify putative BRCA1 or BRCA2 reversion mutations with restored functions in women with OC and breast cancer (Weigelt et al. 2017). Similarly, two other recent reports confirmed these findings and showed that detected BRCA mutations using liquid biopsy in OC patients are associated with acquired resistance to treatments (Christie et al. 2017; Lin et al. 2019). Methylation phenomena in BRCA1 promoter were also suggested as a biomarker of chemosensitivity in OC (Ignatov et al. 2014). However, a meta-analysis of individual data (n = 2636) demonstrated that patients with BRCA1-methylated OC had similar survival outcomes as compared to those with non-BRCA1-methylated tumors (Kalachand et al. 2020). Other mutated genes outside the BRCA family (Table 4.2) such as members of the HRR pathway particularly RAD51, which are found in approximately 50% of high-grade serous OC, were also found to predict chemosensitivity (Fuh et al. 2020; da Costa et al. 2019). Moreover, this HRR deficiency has also a value for prognostic stratification of OC patients (Takaya et al. 2020; Morse et al. 2019). Patients with this fundamental vulnerability had high infiltration of immune cells particularly tumor-infiltrating lymphocytes (TILs) which correlate with better survival and may make these women highly responsive to immune-checkpoint blockade (Ledermann 2019; Morse et al. 2019; Konstantinopoulos et al. 2015) (see Chap. 3 for details). Currently, this biomarker is used for predicting response to PARPi rather than platinum-based chemotherapeutics. The European Society for Medical Oncology (ESMO) stated that assays for clinical evaluation of HRR deficiency are useful in predicting the likely magnitude of benefit from PARP inhibition but additional biomarkers with improved accuracy are needed to better stratify patients (Miller et al. 2020).
Research in this area of biomarkers discovery has also provided other perspectives for non-platinum chemotherapy such as the natural compound trabectedin and pegylated liposomal doxorubicin (PLD) (Madariaga et al. 2019; El Bairi et al. 2019). Trabectedin (known as Yondelis®) is a marine compound isolated from the colonial tunicate Ecteinascidia turbinate that acts as a cytotoxic alkylating agent and also as a vascular disruptor (El Bairi et al. 2019). It was approved in several countries of the European Union for the treatment of OC as a late-line therapy in combination with PLD for recurrent platinum-sensitive disease. The efficacy of trabectedin was found associated with deficient HRR systems in various clinical trials (El Bairi et al. 2018; Ventriglia et al. 2018). Previously, an exploratory analysis of the randomized phase 3 OVA-301 study that compared the efficacy of trabectedin and PLD versus PLD alone in women with recurrent OC showed that germline BRCA1 mutant tumors had improved median PFS (13.5 vs. 5.5 months, p = 0.0002), OS (23.8 versus 12.5 months, p = 0.0086), and higher response rates (49 vs. 28%) (Monk et al. 2015). Moreover, women with BRCA wild-type OC had no improvements in median OS (19.1 versus 19.3 months; p = 0.9377) (Monk et al. 2015). BRCA status and BRCAness were also used for patients’ selection in the MITO-15 phase II study that investigated trabectedin in women with recurrent OC (Lorusso et al. 2016). BRCA status was not associated with response to trabectedin nor with survival (Lorusso et al. 2016). However, the recent findings of another randomized phase III trial that compared the efficacy of trabectedin combined with PLD in the same previous setting showed significant overall survival (OS) benefits for patients harboring BRCA mutations (34.2 vs. 20.9 months; HR: 0.54, 95% CI: 0.33–0.90; p = 0.016) (Monk et al. 2020). Similarly, improved outcomes for median PFS were also noticed for patients with BRCA mutant tumors (HR: 0.72, 95% CI: 0.48–1.08; p = 0.039) (Monk et al. 2020). The DNA damaging agent PLD used in the recurrent setting was also found to be more effective in tumors with BRCA mutations. Two previous retrospective studies demonstrated that BRCA-associated OC women had improved sensitivity to PLD, greater PFS (Adams et al. 2011), and also OS (Safra et al. 2014). Regarding taxane chemotherapy which is used in combination with carboplatin in the first-line setting as a standard of care and as a single agent for recurrent platinum-resistant disease; data on BRCA as a predictor of response are sparse. In prostate cancer, the correlation between mutated BRCA and poor response to docetaxel was noticed (Nientiedt et al. 2017). In addition, mutated BRCA1-associated breast cancer was found less sensitive to taxane chemotherapy (Kriege et al. 2012). In OC, the inhibition of endogenous BRCA1 expression was reported to be associated with decreased sensitivity to antimicrotubule agents (Quinn et al. 2007). Moreover, median OS in patients with higher BRCA1-expression was found improved after treatment with taxanes (23 vs. 18.2 months; HR: 0.53; p = 0.12) (Quinn et al. 2007). Other emerging genes that might impact drug response and prognosis in OC can be found in Tables 4.2 and 4.3.
4.3 Ovarian Cancer Genetics and Response to PARP Inhibitors
DNA damage response pathway is one of the invested targets in drug discovery for OC. PARP 1 and PARP2 are the principal enzymes of this pathway and are recruited during DNA lesions to orchestrate repair effectors activity (Lord and Ashworth 2017). PARP bound to damaged DNA and transfer poly-ADP-ribose units to various target proteins (PARylation process) required for DNA breaks repair such as topoisomerase and DNA ligase (for review, see: Franzese et al. 2019). Inhibition of PARP mediated DNA repair appeared to be a potential strategy that is widely known as synthetic lethality (Lord et al. 2015; Lord and Ashworth 2017) and has moved successfully into clinical trials several PARPi including rucaparib (Rubraca®), olaparib (Lynparza®), veliparib (ABT-888), niraparib (Zejula®) as well as the next-generation of this category such as talazoparib (Talzenna®). In 2005, two preclinical reports were published in Nature by Farmer et al. and Bryant et al. showed that mutant cancer cells with BRCA dysfunction are highly sensitive to PARP inhibition (Farmer et al. 2005; Bryant et al. 2005). Based on these substantial findings, this new concept was used as a rationale for developing trial designs of several PARPi for various cancers harboring this signature. In OC, many clinical studies that investigated oral PARPi have achieved their primary objectives and showed positive results from phase II-III trials in the front-line, for recurrent disease, or maintenance settings following platinum-based chemotherapy (Table 4.4).
4.3.1 Olaparib
Olaparib was the first-in-class developed PARPi and approved by the FDA and EMA in 2014 for treating OC (Franzese et al. 2019). Early trials (NCT00516373 and NCT00494442) showed favorable safety and tolerability profile which were represented mainly by reversible fatigue, anemia, and mild gastrointestinal symptoms (Fong et al. 2009, 2010; Audeh et al. 2010). Interestingly, these dose-finding trials demonstrated significant antitumor response in OC patients with BRCA mutations (Fong et al. 2010; Audeh et al. 2010). In a second interim analysis of OS and a preplanned analysis of data by BRCA mutation status of a randomized and double-blind phase II study (NCT00753545) that used olaparib as maintenance treatment for recurrent platinum-sensitive OC, Ledermann et al. found that patients with mutated BRCA had significantly longer PFS as compared with wild-type subjects (11.2 vs. 7.4 months) (Ledermann et al. 2014). However, in terms of OS, no significant difference was seen between the two groups (HR: 0.73; 95% CI: 0.45–1.17; p = 0.19 for BRCA mutated status and (HR: 0.99; 95% CI: 0.63–1.55; p = 0.96) for wild-type BRCA) (Ledermann et al. 2014). Moving from this immature evidence, the greatest clinical benefit was observed in BRCA-mutated recurrent and platinum-sensitive OC patients in another randomized phase II trial (NCT01081951) combining olaparib with standard chemotherapy (Oza et al. 2015). PFS in patients with mutated BRCA was significantly improved (HR: 0.21; 95% CI: 0.08–0.55; p = 0.0015) (Oza et al. 2015). These data were supported by an updated analysis of OS of NCT00753545 trial and showed that BRCA-mutated platinum-sensitive recurrent OC patients appear to have longer OS despite it did not achieve the planned level for statistical significance (p < 0.0095) (Ledermann et al. 2016). Confirmatory results from two randomized phase III trials (SOLO-1 and SOLO-2/ENGOT-Ov21) using olaparib as maintenance therapy for OC were reported recently. Pujade-Lauraine et al. conducted a phase III randomized, double-blind and placebo-controlled and multicenter trial to evaluate the efficacy of olaparib as maintenance treatment for platinum-sensitive, relapsed and BRCA mutated OC (Pujade-Lauraine et al. 2017). This study (NCT01874353; SOLO-2/ENGOT-Ov21) enrolled 295 patients including 196 in the olaparib arm and showed significantly higher PFS as compared with the placebo arm (19.1 months vs. 5.5 months p < 0.0001 respectively) (Pujade-Lauraine et al. 2017). More recently, results from SOLO-1 (NCT01844986) phase III trial that assessed olaparib (n = 260) versus placebo (n = 131) as maintenance therapy this time for newly diagnosed OC with BRCA mutations and after first-line standard chemotherapy demonstrated a gain of 3 years in PFS (despite not reached) in the group who received olaparib after 41 months of follow-up (HR: 0.30; 95% CI: 0.23–0.41; p < 0.001) (Moore et al. 2018). Remarkably, a recent meta-analysis that enrolled 8 randomized trials (1957 patients) including SOLO-2 found that patients with BRCA carriers exhibited significant survival benefits from olaparib and thus showing decisive additional evidence for this genetic biomarker but with an increased risk of severe anemia which requires regular hematologic surveillance (Guo et al. 2018). Promisingly, further evidence will be released by the ongoing SOLO3 phase III trial that randomizes relapsed OC patients who have received at least 2 prior lines of platinum-based chemotherapy and with BRCA carriers to receive olaparib versus standard of care (NCT02282020). Moving beyond BRCA biomarkers, it seems that a subset of OC patients with mutations in HRR genes other than traditional BRCA may also benefit from olaparib which can expand the use of this drug in the future (Hodgson et al. 2018). Similarly, findings from a comparative molecular analysis of the NCT00753545 trial showed that long-term responders to olaparib maintenance may be multifactorial and related to HRR profile (Lheureux et al. 2017). In the confirmatory SOLO-3 phase III trial, patients with BRCA mutated status were randomly assigned to receive olaparib or a non-platinum drug for the platinum-sensitive setting for which objective response rate was the primary endpoint as mandated by the FDA (Penson et al. 2020). The superiority of olaparib was noticed and reached 72.2 as compared to 51.4% in patients treated with standard of care (Penson et al. 2020). The addition of olaparib to bevacizumab for the first-line maintenance therapy was investigated in the PAOLA-1 phase III trial (Ray-Coquard et al. 2019). This study randomized 806 OC patients with mutated BRCA to receive olaparib and bevacizumab or bevacizumab + placebo in a 2:1 fashion. A significant hazard ratio of 0.59 resulted in the comparison for PFS. In patients with HRR deficiency, the hazard ratio for progression or death reached a value of 0.33 suggesting the clinical benefits of adding olaparib to anti-angiogenesis in this setting (Ray-Coquard et al. 2019).
4.3.2 Rucaparib
Women with OC who have BRCA mutant tumors that were enrolled in the ARIEL-3 randomized and controlled phase III (n = 564) for the recurrent platinum-sensitive disease had superior median PFS (HR: 0.23, 95% CI: 0.16–0.34, p < 0.0001) (Coleman et al. 2017). Similarly, patients with HRR deficiency had also improved PFS (HR: 0.32, 0.24–0.42, p < 0.0001). In the ARIEL-2 phase II trial for the recurrent platinum-sensitive setting that stratified patients into multi-cohorts including those with BRCA status, median PFS was also improved in the group treated with rucaparib and having BRCA mutations (HR: 0.27, 95% CI: 0.16–0.44, p < 0.0001) (Swisher et al. 2017). Notably, RAD51C and RAD51D genetic variants were found associated with acquired resistance to this PARP inhibitor in OC (Kondrashova et al. 2017). Furthermore, reversion mutations in BRCA were also identified in circulating tumor DNA of OC patients with reduced rucaparib PFS as compared to women with no reversion mutations at baseline (median 1.8 vs. 9 months; HR: 0.12; p < 0.0001). Thus, combinatorial approaches may be promising to overcome drug resistance to rucaparib (Lin et al. 2019).
4.3.3 Niraparib
To the best of our knowledge, niraparib has been investigated in two randomized phase III trials for OC, NOVA (n = 553) and PRIMA (n = 733) (see Chap. 3). In the NOVA study that explored the efficacy of niraparib in the recurrent platinum-sensitive setting, 203 women had germline mutated BRCA and had superior PFS as compared to those treated with placebo (HR: 0.27; 95% CI: 0.17–0.41) (Mirza et al. 2016). Remarkably, women with HRR deficiency had also improved PFS (HR: 0.38; 95% CI: 0.24–0.59) (Mirza et al. 2016). When niraparib was investigated as a monotherapy in the maintenance setting after response to front line therapy in NOVA study, enrolled women with HRR deficient tumors had clinically and statistically improved PFS (HR: 0.43; 95% CI: 0.31–0.59; p < 0.001) (González-Martín et al. 2019). In late lines of recurrent OC therapy, the QUADRA phase II trial explored the efficacy of niraparib in heavily pre-treated patients and showed a clinical activity of this PARPi in women with HRR deficiency including those with or without BRCA mutations (Moore et al. 2019).
4.3.4 Veliparib
Veliparib is a new synthetically lethal therapeutic approach for treating OC (Boussios et al. 2020). Previously and based on early signs of efficacy in a phase II trial (Coleman et al. 2015), veliparib as a single agent was studied for platinum-resistant or partially sensitive recurrent OC in a combined phase I/II trial (Steffensen et al. 2017). Veliparib was given to women that have exclusively germline mutated BRCA showed clinical activity in this heavily pretreated population including 65% of overall response rate, PFS of 5.6 months, and OS of 13.7 months (Steffensen et al. 2017). VELIA (n = 1140) was a landmark three arms phase III trial that explored the efficacy of veliparib in the first-line therapy of OC (Coleman et al. 2019). Women with BRCA mutant and HRR deficient tumors treated with veliparib in combination with carboplatin/paclitaxel doublets had favorable outcomes including superior PFS (HR: 0.44 and HR: 0.68 respectively, p < 0.001 for both) (Coleman et al. 2019). In a recent biomarker analysis of a phase II study, homeobox A9 (HOXA9) promoter methylation in circulating tumor DNA was demonstrated to confer resistance to veliparib (Rusan et al. 2020). Longitudinal monitoring of OC patients based on this liquid biopsy approach showed that methylated HOXA9 at baseline was significantly correlated with worse outcomes included reduced PFS and OS (p < 0.0001 and p = 0.002, respectively) (Rusan et al. 2020). Therefore, this may provide perspectives for real-time monitoring using this potential predictive biomarker.
4.4 Ovarian Cancer Genetics and Surgical Outcomes
Usually, cytoreductive debulking surgery is performed for OC patients after primary diagnosis and staging, followed by adjuvant platinum-based chemotherapy or after receiving neoadjuvant chemotherapy (NACT) for women with poor performance status, large tumors, and important volumes of ascites (Vitale et al. 2013). Furthermore, secondary debulking surgery can be performed during recurrences but its role in improving outcomes is still controversial (Lorusso et al. 2012). Resectability and optimal cytoreduction are influenced by several factors such as disease location, the expertise of surgeons as well as probably genetic status such as BRCA mutations (Narod 2016; Ponzone 2021). Interestingly, to see whether OC patients with BRCA mutations have superior surgical outcomes as compared with those with wild status, some recent reports looked into this matter based on different observational study designs. Earlier in 2012, a retrospective report of 367 stage IIIC-IV high-grade serous OC from the Memorial Sloan Kettering Cancer Center investigated germline BRCA mutation status as a predictor of optimal cytoreduction compared to wild-type tumors (Hyman et al. 2012). OC patients with mutated BRCA and who underwent surgery had relatively superior rates of optimal debulking as compared with wild-type patients (84.1% vs. 70.1% respectively, p = 0.02) (Hyman et al. 2012). However, based on multivariate analysis, this study demonstrated that mutated BRCA status is not associated with residual tumor volume (OR: 0.63; 95% CI: 0.31–1.29; p = 0.21) suggesting that optimal cytoreduction may be due to surgery alone instead of OC genetics (Hyman et al. 2012). In another retrospective study that enrolled 27 cases with recurrent OC treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) and 84 matched controls treated with systemic chemotherapy alone, women with positive BRCA carriers were found to have longer PFS in the HIPEC group as compared with the controls (20.9 vs. 12.6 months, p = 0.048) (Safra et al. 2014). Consequently, this confirms the recently published data supporting the impact of the emerging HIPEC in treating OC (van Driel et al. 2018; Spiliotis et al. 2015; Cascales-Campos et al. 2015) especially in patients with BRCA mutational status. However, an opposing conclusion from a recent study found that patients with BRCA1 mutated OC are less likely to achieve no residual disease after debulking surgery than wild-type patients (19% vs. 39%; p < 0.0001) (Kotsopoulos et al. 2016). Importantly, the same study found that improved survival outcomes observed in OC patients with mutated BRCA status may be due to higher initial sensitivity to platinum-based therapy and, notably, no residual disease at debulking is the strongest predictive factor of long-term survival (Kotsopoulos et al. 2016). Recently, Petrillo et al. evaluated the impact of BRCA mutational status on outcomes including optimal debulking in a large multicenter report of women with newly diagnosed high-grade serous OC with stage IIIc and IV disease (Petrillo et al. 2017). Patients with mutated BRCA had significantly higher peritoneal tumor load but without having different median PFS when treated with NACT or debulking surgery (p = 0.268). Remarkably, patients with wild-type BRCA status and who benefited from primary debulking surgery had superior median PFS as compared to those treated with NACT (26 vs. 18 months; p = 0.003) (Petrillo et al. 2017). Similarly, Marchetti et al. showed in their recent retrospective cohort that women with BRCA wild-type ovarian tumors who underwent complete secondary cytoreductive surgery had superior 5-year post-recurrence survival as compared to those with no surgical intervention (54% vs. 42%; p = 0.048) (Marchetti et al. 2018). However, Naumann et al. showed that optimally resected high-grade OC had frequent BRCA mutations and dramatically improved median OS (110.4 vs. 67.1 months; HR: 0.28, 95% CI: 0.11–0.73, p = 0.009) when treated with HIPEC compared with patients wild type tumors (Naumann et al. 2018). More recently, Gordonova et al. analyzed the medical record of 283 consecutive women who underwent complete or optimal debulking and compared their outcomes based on BRCA status (Gorodnova et al. 2019). Again, this study showed that BRCA status did not predict outcomes in patients subjected to primary surgery (p = 0.56) (Gorodnova et al. 2019). To the best of our knowledge, only one report has prospectively assessed the impact of BRCA status on optimal debulking. This was a cohort report that enrolled 107 OC patients including 51.4% of BRCA mutated cases (Rudaitis et al. 2014). No significant difference between OC patients harboring BRCA mutations and those with wild-type status was seen in terms of optimal debulking surgery (58.2% vs. 53.9%, p = 0.6994). However, BRCA mutated OC patients had improved median PFS (19 months, 95%; CI: 13–25) compared with wild-type subjects (13 months, 95%; CI: 10–16) (p = 0.039) (Rudaitis et al. 2014). In conclusion, it seems that BRCA carriers have no impact on optimal debulking for OC patients. However, most of these studies are retrospective in their design and thus, should be commented with caution because of the high risk of biases. Until to date, no definitive answers were provided and most current studies especially clinical trials are investigating BRCA as biomarkers for chemotherapy and targeted therapies.
4.5 Conclusion
The genetics of OC is becoming actionable with the arrival of precision medicine in gynecologic oncology. This progress is also supported by the recent development of sequencing technology. To date, several therapies require genetic information of OC patients before their use. Remarkably, this approach has deeply improved outcomes in some settings of this aggressive women’s cancer. More research on biomarkers is needed to ensure that patients can achieve maximal clinical benefits from the emerging targeted agents in OC. In this perspective, the currently active clinical trials using BRCA status for patients’ selection and stratification can improve personalized medicine in the near future (Tables 4.5 and 4.6). For additional reading, see Box 4.1.
Box 4.1 Recommended reading of particular interest
DOI | |
|---|---|
Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020;371:m3773. | |
Mirza MR, et al. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;31(9):1148–1159. | |
Chan JK, et al. Selecting new upfront regimens for advanced ovarian cancer with biomarker guidance. Gynecol Oncol. 2020;159(3):604–606. | |
Haunschild CE, Tewari KS. The current landscape of molecular profiling in the treatment of epithelial ovarian cancer. Gynecol Oncol. 2020:S0090-8258(20)33953-6. | |
Byrum AK, et al. Defining and Modulating ‘BRCAness’. Trends Cell Biol. 2019;29(9):740–751. | |
Wakefield MJ, et al. Diverse mechanisms of PARP inhibitor resistance in ovarian cancer. Biochim Biophys Acta Rev Cancer. 2019;1872(2):188307. | |
Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–20. | |
Lheureux S, et al. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280–304. |
Acknowledgment and Conflicts of Interest
KE is an editor in Springer Nature Journals and a previous editor for a Springer Book (https://springerlink.bibliotecabuap.elogim.com/book/10.1007/978-3-030-53821-7).
References
Adams SF, Marsh EB, Elmasri W, Halberstadt S, Vandecker S, Sammel MD, Bradbury AR, Daly M, Karlan B, Rubin SC (2011) A high response rate to liposomal doxorubicin is seen among women with BRCA mutations treated for recurrent epithelial ovarian cancer. Gynecol Oncol 123(3):486–491. https://doi.org/10.1016/j.ygyno.2011.08.032
Alsop K, Fereday S, Meldrum C, de Fazio A, Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, Friedlander M, Fox S, Bowtell D, Mitchell G (2012) BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 30(21):2654–2663. https://doi.org/10.1200/JCO.2011.39.8545. Epub 2012 Jun 18. Erratum in: J Clin Oncol. 2012 Nov 20;30(33):4180
Audeh MW, Carmichael J, Penson RT et al (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376(9737):245–251. https://doi.org/10.1016/S0140-6736(10)60893-8
Boussios S, Karihtala P, Moschetta M, Abson C, Karathanasi A, Zakynthinakis-Kyriakou N, Ryan JE, Sheriff M, Rassy E, Pavlidis N (2020) Veliparib in ovarian cancer: a new synthetically lethal therapeutic approach. Investig New Drugs 38(1):181–193. https://doi.org/10.1007/s10637-019-00867-4
Brachova P, Thiel KW, Leslie KK (2013) The consequence of oncomorphic TP53 mutations in ovarian cancer. Int J Mol Sci 14(9):19257–19275. https://doi.org/10.3390/ijms140919257
Brachova P, Mueting SR, Carlson MJ et al (2014) TP53 oncomorphic mutations predict resistance to platinum- and taxane-based standard chemotherapy in patients diagnosed with advanced serous ovarian carcinoma. Int J Oncol 46(2):607–618
Bryant HE, Schultz N, Thomas HD et al (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434(7035):913–917
Cancer Genome Atlas Research Network (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474(7353):609–615. https://doi.org/10.1038/nature10166. Erratum in: Nature. 2012 Oct 11;490(7419):298
Cascales-Campos P, Gil J, Feliciangeli E et al (2015) HIPEC in ovarian cancer: treatment of a new era or is it the end of the pipeline? Gynecol Oncol 139(2):363–368. https://doi.org/10.1016/j.ygyno.2015.06.012
Christie EL, Fereday S, Doig K, Pattnaik S, Dawson SJ, Bowtell DDL (2017) Reversion of BRCA1/2 germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian cancer. J Clin Oncol 35(12):1274–1280. https://doi.org/10.1200/JCO.2016.70.4627
Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, Rubin SC, Rutherford TJ, Chan JK, Chen A, Swisher EM (2015) A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation – an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 137(3):386–391. https://doi.org/10.1016/j.ygyno.2015.03.042
Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp A, Scambia G, Leary A, Holloway RW, Gancedo MA, Fong PC, Goh JC, O'Malley DM, Armstrong DK, Garcia-Donas J, Swisher EM, Floquet A, Konecny GE, IA MN, Scott CL, Cameron T, Maloney L, Isaacson J, Goble S, Grace C, Harding TC, Raponi M, Sun J, Lin KK, Giordano H, Ledermann JA, ARIEL3 Investigators (2017) Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390(10106):1949–1961. https://doi.org/10.1016/S0140-6736(17)32440-6. Erratum in: Lancet. 2017 Oct 28;390(10106):1948
Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat Ben-Baruch N, Werner TL, Cloven NG, Oaknin A, Di Silvestro PA, Morgan MA, Nam JH, Leath CA 3rd, Nicum S, Hagemann AR, Littell RD, Cella D, Baron-Hay S, Garcia-Donas J, Mizuno M, Bell-McGuinn K, Sullivan DM, Bach BA, Bhattacharya S, Ratajczak CK, Ansell PJ, Dinh MH, Aghajanian C, Bookman MA (2019) Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 381(25):2403–2415. https://doi.org/10.1056/NEJMoa1909707
da Costa AABA, do Canto LM, Larsen SJ, ARG R, Stecca CE, Petersen AH, Aagaard MM, de Brot L, Baumbach J, Baiocchi G, Achatz MI, Rogatto SR (2019) Genomic profiling in ovarian cancer retreated with platinum based chemotherapy presented homologous recombination deficiency and copy number imbalances of CCNE1 and RB1 genes. BMC Cancer 19(1):422. https://doi.org/10.1186/s12885-019-5622-4
Davies AA, Masson JY, McIlwraith MJ et al (2001) Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell 7(2):273–282
El Bairi K, Amrani M, Kandhro AH, Afqir S (2017a) Prediction of therapy response in ovarian cancer: where are we now? Crit Rev Clin Lab Sci 54(4):233–266. https://doi.org/10.1080/10408363.2017.1313190
El Bairi K, Kandhro AH, Gouri A, Mahfoud W, Louanjli N, Saadani B, Afqir S, Amrani M (2017b) Emerging diagnostic, prognostic and therapeutic biomarkers for ovarian cancer. Cell Oncol (Dordr) 40(2):105–118. https://doi.org/10.1007/s13402-016-0309-1
El Bairi K, Amrani M, Afqir S (2018) Starvation tactics using natural compounds for advanced cancers: pharmacodynamics, clinical efficacy, and predictive biomarkers. Cancer Med 7(6):2221–2246. https://doi.org/10.1002/cam4.1467
El Bairi K, Atanasov AG, Amrani M, Afqir S (2019) The arrival of predictive biomarkers for monitoring therapy response to natural compounds in cancer drug discovery. Biomed Pharmacother 109:2492–2498. https://doi.org/10.1016/j.biopha.2018.11.097
Farmer H, McCabe N, Lord CJ et al (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434(7035):917–921
Fong PC, Boss DS, Yap TA et al (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361(2):123–134. https://doi.org/10.1056/NEJMoa0900212
Fong PC, Yap TA, Boss DS et al (2010) Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 28(15):2512–2519. https://doi.org/10.1200/JCO.2009.26.9589
Franzese E, Centonze S, Diana A, Carlino F, Guerrera LP, Di Napoli M, De Vita F, Pignata S, Ciardiello F, Orditura M (2019) PARP inhibitors in ovarian cancer. Cancer Treat Rev 73:1–9. https://doi.org/10.1016/j.ctrv.2018.12.002
Fuh K, Mullen M, Blachut B, Stover E, Konstantinopoulos P, Liu J, Matulonis U, Khabele D, Mosammaparast N, Vindigni A (2020) Homologous recombination deficiency real-time clinical assays, ready or not? Gynecol Oncol 159(3):877–886. https://doi.org/10.1016/j.ygyno.2020.08.035
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G (2012) Molecular mechanisms of cisplatin resistance. Oncogene 31(15):1869–1883. https://doi.org/10.1038/onc.2011.384
Garsed DW, Alsop K, Fereday S et al (2018) Homologous recombination DNA repair pathway disruption and retinoblastoma protein loss are associated with exceptional survival in high-grade serous ovarian cancer. Clin Cancer Res 24(3):569–580. https://doi.org/10.1158/1078-0432.CCR-17-1621
González-Martín A, Pothuri B, Vergote I, De Pont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D, Hoskins P, Freyer G, Baumann K, Jardon K, Redondo A, Moore RG, Vulsteke C, O’Cearbhaill RE, Lund B, Backes F, Barretina-Ginesta P, Haggerty AF, Rubio-Pérez MJ, Shahin MS, Mangili G, Bradley WH, Bruchim I, Sun K, Malinowska IA, Li Y, Gupta D, Monk BJ, PRIMA/ENGOT-OV26/GOG-3012 Investigators (2019) Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381(25):2391–2402. https://doi.org/10.1056/NEJMoa1910962
Gorodnova TV, Sokolenko AP, Ivantsov AO, Iyevleva AG, Suspitsin EN, Aleksakhina SN, Yanus GA, Togo AV, Maximov SY, Imyanitov EN (2015) High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation. Cancer Lett 369(2):363–367. https://doi.org/10.1016/j.canlet.2015.08.028
Gorodnova T, Sokolenko A, Ni V, Ivantsov A, Kotiv K, Petrik S, Amelina I, Berlev I, Imyanitov E (2019) BRCA1-associated and sporadic ovarian carcinomas: outcomes of primary cytoreductive surgery or neoadjuvant chemotherapy. Int J Gynecol Cancer 29(4):779–786. https://doi.org/10.1136/ijgc-2018-000175
Grisham RN, Iyer G, Garg K, Delair D, Hyman DM, Zhou Q, Iasonos A, Berger MF, Dao F, Spriggs DR, Levine DA, Aghajanian C, Solit DB (2013) BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer 119(3):548–554. https://doi.org/10.1002/cncr.27782
Guo XX, Wu HL, Shi HY, Su L, Zhang X (2018) The efficacy and safety of olaparib in the treatment of cancers: a meta-analysis of randomized controlled trials. Cancer Manag Res 10:2553–2562. https://doi.org/10.2147/CMAR.S169558
Harter P, Johnson T, Berton-Rigaud D, Park SY, Friedlander M, Del Campo JM, Shimada M, Forget F, Mirza MR, Colombo N, Zamagni C, Chan JK, Imhof M, Herzog TJ, O’Donnell D, Heitz F, King K, Stinnett S, Barrett C, Jobanputra M, Xu CF, du Bois A (2016) BRCA1/2 mutations associated with progression-free survival in ovarian cancer patients in the AGO-OVAR 16 study. Gynecol Oncol 140(3):443–449. https://doi.org/10.1016/j.ygyno.2015.12.027
Hodgson DR, Dougherty BA, Lai Z et al (2018) Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer 119(11):1401–1409. https://doi.org/10.1038/s41416-018-0274-8
Hoppenot C, Eckert MA, Tienda SM, Lengyel E (2018) Who are the long-term survivors of high grade serous ovarian cancer? Gynecol Oncol 148(1):204–212. https://doi.org/10.1016/j.ygyno.2017.10.032
Hsu LC, White RL (1998) BRCA1 is associated with the centrosome during mitosis. Proc Natl Acad Sci U S A 95(22):12983–12988. https://doi.org/10.1073/pnas.95.22.12983
Huang YW (2018) Association of BRCA1/2 mutations with ovarian cancer prognosis: an updated meta-analysis. Medicine (Baltimore) 97(2):e9380. https://doi.org/10.1097/MD.0000000000009380
Hyman DM, Zhou Q, Arnold AG et al (2011) Topotecan in patients with BRCA-associated and sporadic platinum-resistant ovarian, fallopian tube, and primary peritoneal cancers. Gynecol Oncol 123(2):196–199. https://doi.org/10.1016/j.ygyno.2011.07.019
Hyman DM, Long KC, Tanner EJ et al (2012) Outcomes of primary surgical cytoreduction in patients with BRCA-associated high-grade serous ovarian carcinoma. Gynecol Oncol 126(2):224–228. https://doi.org/10.1016/j.ygyno.2012.05.001
Ignatov T, Eggemann H, Costa SD, Roessner A, Kalinski T, Ignatov A (2014) BRCA1 promoter methylation is a marker of better response to platinum-taxane-based therapy in sporadic epithelial ovarian cancer. J Cancer Res Clin Oncol 140(9):1457–1463. https://doi.org/10.1007/s00432-014-1704-5
Joukov V, Groen AC, Prokhorova T, Gerson R, White E, Rodriguez A, Walter JC, Livingston DM (2006) The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell 127(3):539–552. https://doi.org/10.1016/j.cell.2006.08.053
Kais Z, Chiba N, Ishioka C, Parvin JD (2012) Functional differences among BRCA1 missense mutations in the control of centrosome duplication. Oncogene 31(6):799–804. https://doi.org/10.1038/onc.2011.271
Kalachand RD, Stordal B, Madden S, Chandler B, Cunningham J, Goode EL, Ruscito I, Braicu EI, Sehouli J, Ignatov A, Yu H, Katsaros D, Mills GB, Lu KH, Carey MS, Timms KM, Kupryjanczyk J, Rzepecka IK, Podgorska A, McAlpine JN, Swisher EM, Bernards SS, O'Riain C, O'Toole S, O'Leary JJ, Bowtell DD, Thomas DM, Prieske K, Joosse SA, Woelber L, Chaudhry P, Häfner N, Runnebaum IB, Hennessy BT (2020) BRCA1 promoter methylation and clinical outcomes in ovarian cancer: an individual patient data meta-analysis. J Natl Cancer Inst 112(12):1190–1203. https://doi.org/10.1093/jnci/djaa070
Kondrashova O, Nguyen M, Shield-Artin K, Tinker AV, Teng NNH, Harrell MI, Kuiper MJ, Ho GY, Barker H, Jasin M, Prakash R, Kass EM, Sullivan MR, Brunette GJ, Bernstein KA, Coleman RL, Floquet A, Friedlander M, Kichenadasse G, O'Malley DM, Oza A, Sun J, Robillard L, Maloney L, Bowtell D, Giordano H, Wakefield MJ, Kaufmann SH, Simmons AD, Harding TC, Raponi M, McNeish IA, Swisher EM, Lin KK, Scott CL, AOCS Study Group (2017) Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor Rucaparib in high-grade ovarian carcinoma. Cancer Discov 7(9):984–998. https://doi.org/10.1158/2159-8290.CD-17-0419
Konstantinopoulos PA, Matulonis UA (2018) Targeting DNA damage response and repair as a therapeutic strategy for ovarian cancer. Hematol Oncol Clin North Am 32(6):997–1010. https://doi.org/10.1016/j.hoc.2018.07.006
Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D'Andrea AD (2015) Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov 5(11):1137–1154. https://doi.org/10.1158/2159-8290.CD-15-0714
Kotsopoulos J, Rosen B, Fan I et al (2016) Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol Oncol 140(1):42–47. https://doi.org/10.1016/j.ygyno.2015.11.009
Kriege M, Jager A, Hooning MJ, Huijskens E, Blom J, van Deurzen CH, Bontenbal M, Collee JM, Menke-Pluijmers MB, Martens JW, Seynaeve C (2012) The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer 118(4):899–907. https://doi.org/10.1002/cncr.26351
Le Page C, Amuzu S, Rahimi K, Gotlieb W, Ragoussis J, Tonin PN (2020a) Lessons learned from understanding chemotherapy resistance in epithelial tubo-ovarian carcinoma from BRCA1and BRCA2mutation carriers. Semin Cancer Biol:S1044-579X(20)30177-2. https://doi.org/10.1016/j.semcancer.2020.08.005
Le Page C, Chung J, Rahimi K, Köbel M, Provencher D, Mes-Masson AM (2020b) Exploring the clinical impact of predictive biomarkers in serous ovarian carcinomas. Curr Drug Targets 21(10):974–995. https://doi.org/10.2174/1389450120666191016143836
Ledermann JA (2019) Do increased tumor infiltrating lymphocytes co-existing with homologous recombination deficiency provide clues to enhance immunotherapy of ovarian cancer? Gynecol Oncol 153(2):213–214. https://doi.org/10.1016/j.ygyno.2019.04.014
Ledermann J, Harter P, Gourley C et al (2014) Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 15(8):852–861. https://doi.org/10.1016/S1470-2045(14)70228-1
Ledermann JA, Harter P, Gourley C et al (2016) Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol 17(11):1579–1589. https://doi.org/10.1016/S1470-2045(16)30376-X
Leunen K, Cadron I, Van Gorp T, Amant F, Berteloot P, Neven P, Legius E, Vergote I (2009) Does paclitaxel-carboplatin chemotherapy in a dose-dense regimen enhance survival of BRCA-related ovarian cancer patients? Int J Gynecol Cancer 19(9):1501–1504. https://doi.org/10.1111/IGC.0b013e3181bb703f
Lheureux S, Lai Z, Dougherty BA et al (2017) Long-term responders on Olaparib maintenance in high-grade serous ovarian cancer: clinical and molecular characterization. Clin Cancer Res 23(15):4086–4094. https://doi.org/10.1158/1078-0432.CCR-16-2615
Lin KK, Harrell MI, Oza AM, Oaknin A, Ray-Coquard I, Tinker AV, Helman E, Radke MR, Say C, Vo LT, Mann E, Isaacson JD, Maloney L, O'Malley DM, Chambers SK, Kaufmann SH, Scott CL, Konecny GE, Coleman RL, Sun JX, Giordano H, Brenton JD, Harding TC, McNeish IA, Swisher EM (2019) BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor Rucaparib in high-grade ovarian carcinoma. Cancer Discov 9(2):210–219. https://doi.org/10.1158/2159-8290.CD-18-0715
Liu Y, Yasukawa M, Chen K et al (2015) Association of somatic mutations of ADAMTS genes with chemotherapy sensitivity and survival in high-grade serous ovarian carcinoma. JAMA Oncol 1(4):486–494
Lord CJ, Ashworth A (2017) PARP inhibitors: synthetic lethality in the clinic. Science 355(6330):1152–1158. https://doi.org/10.1126/science.aam7344
Lord CJ, Tutt AN, Ashworth A (2015) Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu Rev Med 66:455–470. https://doi.org/10.1146/annurev-med-050913-022545
Lorusso D, Mancini M, Di Rocco R et al (2012) The role of secondary surgery in recurrent ovarian cancer. Int J Surg Oncol 2012:613980
Lorusso D, Scambia G, Pignata S, Sorio R, Amadio G, Lepori S, Mosconi A, Pisano C, Mangili G, Maltese G, Sabbatini R, Artioli G, Gamucci T, Di Napoli M, Capoluongo E, Ludovini V, Raspagliesi F, Ferrandina G (2016) Prospective phase II trial of trabectedin in BRCA-mutated and/or BRCAness phenotype recurrent ovarian cancer patients: the MITO 15 trial. Ann Oncol 27(3):487–493. https://doi.org/10.1093/annonc/mdv608
Lorusso D, Ceni V, Daniele G, Salutari V, Pietragalla A, Muratore M, Nero C, Ciccarone F, Scambia G (2020) Newly diagnosed ovarian cancer: which first-line treatment? Cancer Treat Rev 91:102111. https://doi.org/10.1016/j.ctrv.2020.102111
Madariaga A, Lheureux S, Oza AM (2019) Tailoring ovarian cancer treatment: implications of BRCA1/2 mutations. Cancers (Basel) 11(3):416. https://doi.org/10.3390/cancers11030416
Madariaga A, Bowering V, Ahrari S, Oza AM, Lheureux S (2020) Manage wisely: poly (ADP-ribose) polymerase inhibitor (PARPi) treatment and adverse events. Int J Gynecol Cancer 30(7):903–915. https://doi.org/10.1136/ijgc-2020-001288
Marchetti C, De Leo R, Musella A, D'Indinosante M, Capoluongo E, Minucci A, Benedetti Panici P, Scambia G, Fagotti A (2018) BRCA mutation status to personalize management of recurrent ovarian cancer: a multicenter study. Ann Surg Oncol 25(12):3701–3708. https://doi.org/10.1245/s10434-018-6700-6
Milanesio MC, Giordano S, Valabrega G (2020) Clinical implications of DNA repair defects in high-grade serous ovarian carcinomas. Cancers (Basel) 12(5):1315. https://doi.org/10.3390/cancers12051315
Miller RE, Leary A, Scott CL, Serra V, Lord CJ, Bowtell D, Chang DK, Garsed DW, Jonkers J, Ledermann JA, Nik-Zainal S, Ray-Coquard I, Shah SP, Matias-Guiu X, Swisher EM, Yates LR (2020) ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol 31(12):1606–1622. https://doi.org/10.1016/j.annonc.2020.08.2102
Millstein J, Budden T, Goode EL et al (2020) Prognostic gene expression signature for high-grade serous ovarian cancer. Ann Oncol 31(9):1240–1250. https://doi.org/10.1016/j.annonc.2020.05.019
Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Mądry R, Christensen RD, Berek JS, Dørum A, Tinker AV, du Bois A, González-Martín A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA (2016) ENGOT-OV16/NOVA investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375(22):2154–2164. https://doi.org/10.1056/NEJMoa1611310
Monk BJ, Ghatage P, Parekh T, Henitz E, Knoblauch R, Matos-Pita AS, Nieto A, Park YC, Cheng PS, Li W, Favis R, Ricci D, Poveda A (2015) Effect of BRCA1 and XPG mutations on treatment response to trabectedin and pegylated liposomal doxorubicin in patients with advanced ovarian cancer: exploratory analysis of the phase 3 OVA-301 study. Ann Oncol 26(5):914–920. https://doi.org/10.1093/annonc/mdv071
Monk BJ, Brady MF, Aghajanian C et al (2017) A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: a Gynecologic Oncology Group partners study. Ann Oncol 28(5):996–1004. https://doi.org/10.1093/annonc/mdx049
Monk BJ, Herzog TJ, Wang G, Triantos S, Maul S, Knoblauch R, McGowan T, Shalaby WSW, Coleman RL (2020) A phase 3 randomized, open-label, multicenter trial for safety and efficacy of combined trabectedin and pegylated liposomal doxorubicin therapy for recurrent ovarian cancer. Gynecol Oncol 156(3):535–544. https://doi.org/10.1016/j.ygyno.2019.12.043
Moore K, Colombo N, Scambia G et al (2018) Maintenance Olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379(26):2495–2505. https://doi.org/10.1056/NEJMoa1810858
Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, Wahner Hendrickson AE, Azodi M, DiSilvestro P, Oza AM, Cristea M, Berek JS, Chan JK, Rimel BJ, Matei DE, Li Y, Sun K, Luptakova K, Matulonis UA, Monk BJ (2019) Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 20(5):636–648. https://doi.org/10.1016/S1470-2045(19)30029-4. Erratum in: Lancet Oncol. 2019 May;20(5):e242
Morse CB, Toukatly MN, Kilgore MR, Agnew KJ, Bernards SS, Norquist BM, Pennington KP, Garcia RL, Liao JB, Swisher EM (2019) Tumor infiltrating lymphocytes and homologous recombination deficiency are independently associated with improved survival in ovarian carcinoma. Gynecol Oncol 153(2):217–222. https://doi.org/10.1016/j.ygyno.2019.02.011
Mullee LI, Morrison CG (2016) Centrosomes in the DNA damage response--the hub outside the Centre. Chromosom Res 24(1):35–51. https://doi.org/10.1007/s10577-015-9503-7
Nakayama N, Nakayama K, Shamima Y et al (2010) Gene amplification CCNE1 is related to poor survival and potential therapeutic target in ovarian cancer. Cancer 116(11):2621–2634. https://doi.org/10.1002/cncr.24987
Narod S (2016) Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol 13(4):255–261. https://doi.org/10.1038/nrclinonc.2015.224
Naumann RW, Morris JC, Tait DL, Higgins RV, Crane EK, Drury LK, Amacker-North L, Templin M, Brown J (2018) Patients with BRCA mutations have superior outcomes after intraperitoneal chemotherapy in optimally resected high grade ovarian cancer. Gynecol Oncol 151(3):477–480. https://doi.org/10.1016/j.ygyno.2018.10.003
Nientiedt C, Heller M, Endris V et al (2017) Mutations in BRCA2 and taxane resistance in prostate cancer. Sci Rep 7(1):4574. https://doi.org/10.1038/s41598-017-04897-x
Nodin B, Zendehrokh N, Sundström M, Jirström K (2013) Clinicopathological correlates and prognostic significance of KRAS mutation status in a pooled prospective cohort of epithelial ovarian cancer. Diagn Pathol 8:106. https://doi.org/10.1186/1746-1596-8-106
Ow GS, Ivshina AV, Fuentes G, Kuznetsov VA (2014) Identification of two poorly prognosed ovarian carcinoma subtypes associated with CHEK2 germ-line mutation and non-CHEK2 somatic mutation gene signatures. Cell Cycle 13(14):2262–2280
Oza AM, Cibula D, Benzaquen AO et al (2015) Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 16(1):87–97. https://doi.org/10.1016/S1470-2045(14)71135-0
Patch AM, Christie EL, Etemadmoghadam D et al (2015) Whole-genome characterization of chemoresistant ovarian cancer. Nature 521(7553):489–494. https://doi.org/10.1038/nature14410
Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, Agnew KJ, Pritchard CC, Scroggins S, Garcia RL, King MC, Swisher EM (2014) Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 20(3):764–775. https://doi.org/10.1158/1078-0432.CCR-13-2287
Penson RT, Valencia RV, Cibula D, Colombo N, Leath CA 3rd, Bidziński M, Kim JW, Nam JH, Madry R, Hernández C, Mora PAR, Ryu SY, Milenkova T, Lowe ES, Barker L, Scambia G (2020) Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol 38(11):1164–1174. https://doi.org/10.1200/JCO.19.02745
Petrillo M, Marchetti C, De Leo R, Musella A, Capoluongo E, Paris I, Benedetti Panici P, Scambia G, Fagotti A (2017) BRCA mutational status, initial disease presentation, and clinical outcome in high-grade serous advanced ovarian cancer: a multicenter study. Am J Obstet Gynecol 217(3):334.e1–334.e9. https://doi.org/10.1016/j.ajog.2017.05.036
Ponzone R (2021) BRCA1/2 status and chemotherapy response score to tailor ovarian cancer surgery. Crit Rev Oncol Hematol 157:103128. https://doi.org/10.1016/j.critrevonc.2020.103128
Pujade-Lauraine E, Ledermann JA, Selle F et al (2017) Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomized, placebo-controlled, phase 3 trial. Lancet Oncol 18(9):1274–1284. https://doi.org/10.1016/S1470-2045(17)30469-2
Quinn JE, James CR, Stewart GE, Mulligan JM, White P, Chang GK, Mullan PB, Johnston PG, Wilson RH, Harkin DP (2007) BRCA1 mRNA expression levels predict for overall survival in ovarian cancer after chemotherapy. Clin Cancer Res 13(24):7413–7420. https://doi.org/10.1158/1078-0432.CCR-07-1083
Quinn JE, Carser JE, James CR et al (2009) BRCA1 and implications for response to chemotherapy in ovarian cancer. Gynecol Oncol 113:134–142. https://doi.org/10.1016/j.ygyno.2008.12.015
Ratner ES, Keane FK, Lindner R, Tassi RA, Paranjape T, Glasgow M, Nallur S, Deng Y, Lu L, Steele L, Sand S, Muller RU, Bignotti E, Bellone S, Boeke M, Yao X, Pecorelli S, Ravaggi A, Katsaros D, Zelterman D, Cristea MC, Yu H, Rutherford TJ, Weitzel JN, Neuhausen SL, Schwartz PE, Slack FJ, Santin AD, Weidhaas JB (2012) A KRAS variant is a biomarker of poor outcome, platinum chemotherapy resistance and a potential target for therapy in ovarian cancer. Oncogene 31(42):4559–4566. https://doi.org/10.1038/onc.2011.539
Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N, Mäenpää J, Selle F, Sehouli J, Lorusso D, Guerra Alía EM, Reinthaller A, Nagao S, Lefeuvre-Plesse C, Canzler U, Scambia G, Lortholary A, Marmé F, Combe P, de Gregorio N, Rodrigues M, Buderath P, Dubot C, Burges A, You B, Pujade-Lauraine E, Harter P, PAOLA-1 Investigators (2019) Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381(25):2416–2428. https://doi.org/10.1056/NEJMoa1911361
Rudaitis V, Zvirblis T, Kanopiene D et al (2014) BRCA1/2 mutation status is an independent factor of improved survival for advanced (stage III-IV) ovarian cancer. Int J Gynecol Cancer 24(8):1395–1400. https://doi.org/10.1097/IGC.0000000000000247
Rusan M, Andersen RF, Jakobsen A, Steffensen KD (2020) Circulating HOXA9-methylated tumour DNA: a novel biomarker of response to poly (ADP-ribose) polymerase inhibition in BRCA-mutated epithelial ovarian cancer. Eur J Cancer 125:121–129. https://doi.org/10.1016/j.ejca.2019.11.012
Safra T, Grisaru D, Inbar M, Abu-Abeid S, Dayan D, Matceyevsky D, Weizman A, Klausner JM (2014) Cytoreduction surgery with hyperthermic intraperitoneal chemotherapy in recurrent ovarian cancer improves progression-free survival, especially in BRCA-positive patients-a case-control study. J Surg Oncol 110(6):661–665. https://doi.org/10.1002/jso.23688
Sankaran S, Crone DE, Palazzo RE, Parvin JD (2007) BRCA1 regulates gamma-tubulin binding to centrosomes. Cancer Biol Ther 6(12):1853–1857. https://doi.org/10.4161/cbt.6.12.5164
Sharma B, Preet Kaur R, Raut S, Munshi A (2018) BRCA1 mutation spectrum, functions, and therapeutic strategies: the story so far. Curr Probl Cancer 42(2):189–207. https://doi.org/10.1016/j.currproblcancer.2018.01.001
Spiliotis J, Halkia E, Lianos E et al (2015) Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 22(5):1570–1575. https://doi.org/10.1245/s10434-014-4157-9
Steffensen KD, Adimi P, Jakobsen A (2017) Veliparib monotherapy to patients with BRCA germ line mutation and platinum-resistant or partially platinum-sensitive relapse of epithelial ovarian cancer: a phase I/II study. Int J Gynecol Cancer 27(9):1842–1849. https://doi.org/10.1097/IGC.0000000000001089
Stewart ML, Tamayo P, Wilson AJ, Wang S, Chang YM, Kim JW, Khabele D, Shamji AF, Schreiber SL (2015) KRAS genomic status predicts the sensitivity of ovarian cancer cells to decitabine. Cancer Res 75(14):2897–2906. https://doi.org/10.1158/0008-5472.CAN-14-2860
Sun C, Li N, Ding D, Weng D, Meng L, Chen G, Ma D (2014) The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS One 9(5):e95285. https://doi.org/10.1371/journal.pone.0095285
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O’Malley DM, Kristeleit RS, Ma L, Bell-McGuinn KM, Brenton JD, Cragun JM, Oaknin A, Ray-Coquard I, Harrell MI, Mann E, Kaufmann SH, Floquet A, Leary A, Harding TC, Goble S, Maloney L, Isaacson J, Allen AR, Rolfe L, Yelensky R, Raponi M, McNeish IA (2017) Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 18(1):75–87. https://doi.org/10.1016/S1470-2045(16)30559-9
Takaya H, Nakai H, Takamatsu S, Mandai M, Matsumura N (2020) Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci Rep 10(1):2757. https://doi.org/10.1038/s41598-020-59671-3
Tobalina L, Armenia J, Irving E, O'Connor MJ, Forment JV (2021) A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann Oncol 32(1):103–112. https://doi.org/10.1016/j.annonc.2020.10.470
Tsimberidou AM, Müller P, Ji Y (2020) Innovative trial design in precision oncology. Semin Cancer Biol:S1044-579X(20)30195-4. https://doi.org/10.1016/j.semcancer.2020.09.006
van Driel WJ, Koole SN, Sikorska K et al (2018) Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 378(3):230–240. https://doi.org/10.1056/NEJMoa1708618
Vencken PMLH, Kriege M, Hoogwerf D, Beugelink S, van der Burg MEL, Hooning MJ, Berns EM, Jager A, Collée M, Burger CW, Seynaeve C (2011) Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol 22(6):1346–1352. https://doi.org/10.1093/annonc/mdq628
Ventriglia J, Paciolla I, Cecere SC, Pisano C, Di Napoli M, Arenare L, Setola SV, Losito NS, Califano D, Orditura M, Pignata S (2018) Trabectedin in ovarian cancer: is it now a standard of care? Clin Oncol (R Coll Radiol) 30(8):498–503. https://doi.org/10.1016/j.clon.2018.01.008
Vitale SG, Marilli I, Lodato M et al (2013) The role of cytoreductive surgery in advanced-stage ovarian cancer: a systematic review. Updat Surg 65(4):265–270. https://doi.org/10.1007/s13304-013-0213-4
Weigelt B, Comino-Méndez I, de Bruijn I, Tian L, Meisel JL, García-Murillas I, Fribbens C, Cutts R, Martelotto LG, Ng CKY, Lim RS, Selenica P, Piscuoglio S, Aghajanian C, Norton L, Murali R, Hyman DM, Borsu L, Arcila ME, Konner J, Reis-Filho JS, Greenberg RA, Robson ME, Turner NC (2017) Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin Cancer Res 23(21):6708–6720. https://doi.org/10.1158/1078-0432.CCR-17-0544
Wong KK, Izaguirre DI, Kwan SY et al (2013) Poor survival with wild-type TP53 ovarian cancer? Gynecol Oncol 130(3):565–569
Xiong B, Li S, Ai JS, Yin S, Ouyang YC, Sun SC, Chen DY, Sun QY (2008) BRCA1 is required for meiotic spindle assembly and spindle assembly checkpoint activation in mouse oocytes. Biol Reprod 79(4):718–726. https://doi.org/10.1095/biolreprod.108.069641
Xu K, Yang S, Zhao Y (2017) Prognostic significance of BRCA mutations in ovarian cancer: an updated systematic review with meta-analysis. Oncotarget 8(1):285–302. https://doi.org/10.18632/oncotarget.12306
Yang D, Khan S, Sun Y et al (2011) Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer [published correction appears in JAMA. 2012 Jan 25;307(4):363]. JAMA 306(14):1557–1565. https://doi.org/10.1001/jama.2011.1456
Yang SYC, Lheureux S, Karakasis K, Burnier JV, Bruce JP, Clouthier DL, Danesh A, Quevedo R, Dowar M, Hanna Y, Li T, Lu L, Xu W, Clarke BA, Ohashi PS, Shaw PA, Pugh TJ, Oza AM (2018) Landscape of genomic alterations in high-grade serous ovarian cancer from exceptional long- and short-term survivors. Genome Med 10(1):81. https://doi.org/10.1186/s13073-018-0590-x
Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC (2002) BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet 30(3):285–289. https://doi.org/10.1038/ng837
Zhong Q, Peng HL, Zhao X, Zhang L, Hwang WT (2014) Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res 21(1):211–220
Authors’ Contribution
KE wrote the chapter. OA and SA revised and supervised the chapter writing. The final draft was reviewed and approved by all the authors. The contents of the chapter reflect the authors’ perspectives and not of their institutions of affiliation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
El Bairi, K., Al Jarroudi, O., Afqir, S. (2021). Genetic Alterations in Ovarian Cancer as Prognostic and Predictive Biomarkers of Therapy Response and Surgical Outcomes. In: El Bairi, K. (eds) Ovarian Cancer Biomarkers. Springer, Singapore. https://doi.org/10.1007/978-981-16-1873-4_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-1873-4_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1872-7
Online ISBN: 978-981-16-1873-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)