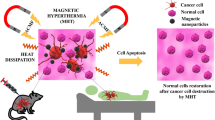
Abstract
Magnetic nanoparticles are an important class of nanomaterials. Several types of magnetic nanoparticles have been discovered in the recent years. The major mechanism of action of magnetic nanoparticles in cancer therapy is the production of hyperthermia. Hyperthermia increases local tissue heat and kills cancer cells. The major drawback which limits clinical utility of magnetic nanoparticles is the inherent toxicity, since normal surrounding tissue is also killed by hyperthermia. This review describes the latest advances in the field of magnetic nanoparticles, describing the important types with examples and major applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
In the 1900s, infectious diseases were responsible for the major share of disease deaths. By the next century, most of these diseases were brought under control. In the current generation, cancer and heart diseases account for the major share of disease burden. Cancer is probably the most dreaded disease of the present century. Several treatment methods have been tried, but we have not been successful in eliminating cancer. Chemotherapy is one of the well-known modes of cancer therapy. Traditional chemotherapeutic agents had limited success, and some of them were also highly toxic. Hence, there is a need for newer modalities of cancer chemotherapy.
Magnetic nanoparticles (NPs) have a magnetic core, biocompatible coating, and surface functionalizations. Such a structure allows integration of targeting agents, chemotherapeutic and biotherapeutic agents. They have theranostic properties [1].
2 Hyperthermia Therapy
It is well-known that cancer cells die when exposed to higher temperatures. This is the basis of hyperthermia therapy, which is receiving increasing attention. The body is exposed to higher temperatures. In the case of cancer, local hyperthermia is used.
Heat can be produced by different means, like microwave, radiofrequency, ultrasound or magnetic hyperthermia. For smaller tumors, radio-ablation is used by a technique known as superficial hyperthermia, where the tissue is exposed to radiation. If needles are inserted into the tissues, it is known as interstitial hyperthermia [2,3,4].
Hyperthermia kills cells directly, but the more significant effects are produced when it is combined with other modalities of cancer therapy [5]. It increases blood perfusion as well as produced hyperbaric conditions, proving to be toxic to cancer cells [6]. There is hypothesis that the size of cancer cells may decrease with heat. However, it should be remembered that hyperthermia is not specifically toxic to cancer cells; it can also damage normal cells. Hence, hyperthermia is not without side effects. Side effects of heat include surface burns, swelling, blood clots, and bleeding complications [6]. Systemic toxicities including cardiovascular toxicity and systemic shock syndrome may occur [7]. The most important aspect is to be able to control the temperature inside the cells/body.
Targeted radiotherapy refers to delivery of higher dose of radiation to cancer cells without causing damage to nearby tissues. It is used in many types of cancers, including head and neck, brain, lung, and prostate cancers. Hyperthermia assists this process significantly [8].
Magnetic hyperthermia may trigger differentiation of cancer cells, preventing progression of cancer [9]. Dendrimers can form effective hybrid platforms with magnetic nanoparticles to form the basis of cancer therapy [10].
3 Magnetic Nanoparticles
Magnetic nanoparticles (MNPs) are a group of nanoparticles whose properties can be modified by application of magnetic fields. MNPs are particular in that they produce controlled increase in temperature. Temperature distribution can be controlled by the velocity, size, and distribution of MNPs within the body [11, 12].
Upon application of external magnetic field, MNPs are able to convert electromagnetic energy to thermal energy and thereby increase the temperature [13]. The increase in temperature enhances tumor oxygenation and chemo- and radio-sensitivity. The net effect is shrinkage in tumor size [14]. Heat may be dissipated as well, especially when alternating magnetic current is applied. Nanoparticles, including magnetic NPs, bring about thermal ablation of tumors. They have advantages over conventional heating methods [15].
Utilizing intra-tumoral Fenton reaction for cancer therapy is a new field known as chemodynamic therapy. The acidic pH of tumor environment is not exactly suited for normal Fenton reaction and hence requires enhancement [16].
Suitable methods need to be developed to produce localized hyperthermia and targeted release of chemotherapy drug. Many modifications of the basic technology have been investigated, and further, methods may need to be evaluated [17, 18].
Magnetic hyperthermia has been useful in the treatment of oral cancer [19, 20]. When exposed to alternating magnetic field, certain NPs can generate heat through hysteresis [21]. This phenomenon forms the basis of cancer therapy using MNPs [22]. Multifunctional magnetic gold nanomaterials have been used for the treatment of cancer [23,24,25].
4 Superparamagnetic Nanoparticles
5 Multifunctional Nanoparticles
Functionalized magnetoparticles sensitize tumors to X-rays and protons and act as radio-enhancers. These are thus used in cancer therapy [28]. Multifunctional nanoparticles (MFNPs) have wide theranostic potential; some examples of studies where they have been used are given below [29].
Mesoporous polydopamine nanosponges on a multifunctional platform have been used for the treatment of cancer [30]. Fe3O4-TMZ-ICG MNPs are produced by incorporating iron oxide NPs with temozolomide (TMZ) and indocyanine green (ICG). These agents enhance apoptosis-mediated death through various genes involved in the pathway. Such selective chemo-phototherapy using these agents has found applications in the treatment of brain cancer [31].
Core-shell-structured iron carbide (Fe5C2@Fe3O4) nanoparticles (NPs) produce reactive oxygen species (ROS), through the catalysis of the Fenton reaction. Normally, ROS-mediated therapy is an inefficient process, but these agents have showed great promise because of high efficiency and high specificity [32]. Biocompatible core-shell magnetic nanocomposite based on cross-linked chitosan hydrogels (using synthetic terephthaloyldiisothiocyanate as a cross-linker) is used for cancer therapy based on in vitro hyperthermia [33].
MFNPs have also been formed by genetically manipulating NK cells by genetic engineering and conjugating them with magnetic and fluorescent NPs. They have enhanced cancer cytotoxicity [34]. Silica-coated iron oxide NPs (SIO-MNPs) have enhanced radio-sensitivity in breast cancer cells [35]. Graphene oxide NPs kill cancer cells better when they are multifunctional, incorporated with iron oxide NPs and hyaluronic acid. This enables the use of magnetic hyperthermia and kills cancer cells better [36]. An oral drug delivery system [polyacrylic acid (PAA) and chitosan (CS) on Gd3+ -doped mesoporous hydroxyapatite nanoparticles (Gd-MHAp NPs)] has been developed which might be useful for orthotopic colon cancer therapy [37].
MFNPs have been used in the treatment of brain cancer [38]. Multifunctional iron oxide nanocomposites have been used for imaging-guided photothermal therapy of cancers [39]. Certain functionalized NPs are used for fluorescence imaging-guided photothermal therapy [40].
Magnetic NPs have also been used for imaging-guided immunotherapy [41]. MFNPs have also been used for metastatic cancers [42]. Cisplatin-functionalized NPs have been used for the treatment of breast cancer [43]. Some MFNPs [Fe3O4@KCTS, a core-shell type of magnetic nanoparticles, prepared by activating Fe3O4 with carbodiimide and cross-linking it with α-ketoglutarate chitosan (KCTS)] are also used for cancer detection [44]. Advantages include excellent loading efficiency, real-time monitoring, and improved cargo bioavailability and bioselectivity [45].
A triple-modal superparamagnetic iron oxide (Fe3O4), IR780, doxorubicin (DOX), and perfluoropentane (PFP) entrapped poly-lactide-co-glycolide (PLGA) nanoparticles (IR780/Fe3O4@PLGA/PFP/DOX NPs) have been used experimentally for breast cancer treatment [46].
Doxorubicin-loaded magnetic mesoporous silica nanoparticles (ND-MMSNs) have been used for targeting glioma cells. The platform has been used for imaging as well as therapeutic purposes [47].
PEGylated branched gold (Au)-iron oxide (Fe3O4) Janus nanoparticles (JNPs) are used for simultaneous trimodal imaging and photothermal therapy of cancer cells [48].
Exceedingly small magnetic iron oxide nanoparticles (ES-MIONs) (<5 nm) are used for magnetic resonance imaging (MRI) as well as for therapeutic purposes in cancer in experimental animals [49].
Dual surfaced dumbbell-like gold magnetic nanoparticles (Au–Fe3O4) are used for targeted aptamer delivery, and these are used as carriers for cancer hyperthermia therapy [50].
Superparamagnetic iron oxide particles have been successfully implemented for the treatment of gastric cancer [51].
Magnetically responsive microbubbles are used in the treatment of pancreatic cancer. Microbubbles are lipid or polymer stabilized gas filled particles [52].
Magnetically and thermally sensitive poly(N-isopropylacrylamide) (PNIPAAm)/Fe3O4–NH2 microgels encapsulated with curcumin (Cur) are used as controlled release cancer therapeutic drugs [53].
Colorectal cancer with liver metastasis has been treated by hybrid functionalized magnetic-gold NPs [54]. Magnetic MFNPs are used in the treatment of prostate cancer [55, 56] and bladder cancer [57].
Core-shell PB@MIL-100(Fe) dual metal-organic-frameworks (d-MOFs) nanoparticles are used for theranostic cancer therapy [58]. MFNPs enables Fenton reaction assisted photodynamic therapy [59].
Magnetotactic bacteria are aquatic organisms having strong biomedical applications. This is because of their hyperthermia effect, affecting cancer cell proliferation [60].
Stable hybrid nanobiocatalyst is formed when biomimetic silica (Si) nanoparticles are entrapped with Horseradish Peroxidase and magnetic nanoparticles. These NPs have wide applications in cancer therapy [61]. The degradable poly(AA-co-DMA) nanohydrogels with surface-tailorable functionalities are used with nanomaterials and drug molecules for cancer therapy [62].
Because of the superparamagnetic, biocompatible and biodegradable properties, iron oxide NPs are used in the treatment of breast cancer [63]. A multifunctional drug-loaded nanosystem (F/A-PLGA@DOX/SPIO) has been used in the treatment of lung cancer [64].
MFNPs are also used as radiosensitizers in the radiation therapy and imaging [65]. Up-conversion MFNPs are used for photodynamic therapy [66].
Copper sulfide NPs have wide applications in therapeutics [67].
SPIONs with gold NPs have been used for phototherapy [68].
Graphene oxide nanosheets are loaded by magnetic iron oxide nanoparticles (mGO), followed by the technique of layer-by-layer (LbL) self-assembly for the production of chitosan/sodium alginate functionalized mGOnaocomposites. They are used in targeted anticancer drug delivery and photothermal therapy [69].
A synergistic treatment platform was developed with plasmonic-magnetic hybrid nanoparticle (lipids, doxorubicin (DOX), gold nanorods, and iron oxide nanocluster (LDGI))-loaded mesenchymal stem cells (MSCs) for the imaging and treatment of triple negative breast cancer [70].
Cold atmospheric plasma (CAP) combined with magnetic NPs has been used in the treatment of lung cancer [71].
Magnetic nanogels made of thermosensitive and biocompatible polymers and core-shell nanoparticles with a magnetic core and molecularly imprinted polymer shell are both used for cancer therapy [72].
Dual-responsive multifunctional magnetic complex micelle (sPEG/HA/CSO-SS-Hex/Fe3O4/GA) consisting of reducible hexadecanol-modified chitosan oligosaccharide polymer micelle (CSO-SS-Hex) coated with hyaluronic acid (HA) and DCA grafted sheddable PEG-PLL (sPEG) copolymers and loaded with gambogic acid (GA) and Fe3O4 nanoparticles is used for the treatment of triple negative breast cancer [73].
Polymer-coated gold-ferric oxide superparamagnetic nanoparticles have theranostic applications [74].
Hydrophilic graphene-based yolk-shell magnetic nanoparticles functionalized with copolymer pluronic F-127 (GYSMNP@PF127) produces hyperthermia and is used for cancer therapy [75].
Multifunctional iron–gold alloy nanoparticles are used for combined hyperthermia and dual stimuli-responsive drug delivery [76]. Poly lactic-co-glycolic acid (PLGA)-modified magnetic nanoplatform was synthesized with iron oxide NPs for enhanced apoptosis and therapy in human brain cancer [77].
Functionalized boron nitride nanotubes (BNNTs) are efficient tools for magnetohyperthermia treatment [78].
Gold MFNPs have significant theranostic properties. They have autophagy-based chemotherapeutic applications [79].
Nearly monodispersed magnetic Fe3O4@MTX-LDH/Au nanoparticles (NPs) containing methotrexate (MTX) produce hyperthermia and are used in cancer therapy [80].
The chemotherapeutic drug, sorafenib with PVA/SPIONs showed better anticancer efficiency than free sorafenib in the treatment of hepatocellular carcinoma [81].
Amphipathic chitosan-based nanomicelle with doxorubicin and SPIONs are used in the treatment of metastatic breast cancer [82].
Hydroxyapatite-coated iron oxide NPs are used for producing magnetic hyperthermia for the treatment of cancer cells [83].
Curcumin-loaded magnetic alginate/chitosan nanoparticles were used for therapy in MDA-MB-231 breast cancer cells [84].
6 Hybrid Nanoparticles
Hybrid NPs are formed by integrating Gd doped silicon nanoparticles (Si–Gd NPs), chlorine e6 (Ce6), doxorubicin (DOX), zeoliticimidazolate framework-8 (ZIF-8), poly(2-(diethylamino)ethyl methacrylate) polymers (HOOC-PDMAEMA-SH), and folic acid-polyethylene glycol-maleimide (MaL-PEG-FA) into one single nanoplatform. Such hybrid NPs are good theranostic agents [85].
Her2 functionalized gold-nanoshelled magnetic hybrid NPs are used as theranostic agents for dual-modal imaging and photothermal therapy of breast cancer cells. The advantages are non-invasive diagnosis and used as adjuvant therapy in SKBR3 cells [86].
7 Synthesis of Magnetic NPs
Production of sub-10 nm SPIONs is a challenging task. Several methods are available for the same. One such involves poly(ethylene glycol) (PEG) reactor adsorbed onto reduced graphene oxide nanosheets (rGO) via the microwave hydrothermal route [87].
Microrobots are small, non-invasive and can be subjected to robotic control. They are important vehicles for targeted therapy. Degradable hyperthermia microrobot (DHM) containing poly(ethylene glycol) diacrylate (PEGDA) and pentaerythritoltriacrylate (PETA) and magnetic Fe3O4 nanoparticles (MNPs) and 5-fluorouracil (5-FU) are useful agents for targeted therapy and hyperthermia [88].
8 Other NPs Producing Hyperthermia
Magnetic NPs are the main agents producing hyperthermia. However, there are some other NPs which also produce hyperthermia and hence may be used for the treatment of cancer.
Hyaluronic acid-based NPs include micelles, polymersomes, hydrogels, and nanoparticles. They bind to receptors over-expressed in certain cancers. They are important platforms for hyperthermic cancer therapy [89].
Near-infrared (NIR)-based iron oxide nanomaterials (NIR-IO) are excellent vehicles for tumor ablation and were found to have good biocompatibility and low cytotoxicity. They have great potential as theranostic agents in cancer [90].
Reactive oxygen species (ROS)-producing NPs have the natural ability to produce hyperthermia. Indeed magnetic NPs can also produce ROS by Fenton reaction and otherwise. This has been discussed earlier in this chapter [91].
Others include glutathione producing particles including ultra-small gadolinium oxide NPs. These are used for CT/MR-guided photothermal and radio-combination cancer therapy [92]. Iron-doped copper sulfide NPs also can produce hyperthermia and are used for MRI [93]. Iron-containing multifunctional nanozymes kills tumor cells efficiently [94]. Carboxymethyl chitosan (CMCS) is used as a nanodelivery system carrier for sustained intracellular release of rose bengal (RB) and doxorubicin (DOX) to achieve combinational drug treatment [95].
Magnetoliposomes containing MgFe2O4 nanoparticles are used in cancer therapy, allowing combined magnetic hyperthermia and chemotherapy [96]. Co-delivery system of (DOX/MEL)-loaded citric acid-functionalized Fe3O4 magnetic nanoparticles (CA-MNPs) is highly capable to be used in magnetically targeted cancer therapy [97].
Silk-PEI nanoparticles (SPPs) and magnetic-silk/PEI core-shell nanoparticles (MSPPs) were used for targeted delivery of c-myc antisense oligodeoxynucleotides (ODNs) into MDA-MB-231 breast cancer cells [98]. Gold nanocages (AuNCs) modified with hyaluronic acid (HA) and conjugated with anti-Glypican-1 (anti-GPC1) antibody, oridonin (ORI), gadolinium (Gd), and Cy7 dye have been used for the treatment of pancreatic cancer [99].
Folic acid (FA)-conjugated poly (lactic-co-glycolicacid) (PLGA)-polyethylene glycol (PEG) nano-noisome has been used for the treatment of cervical cancer [100]. Indocyanine green (ICG)-conjugated NPs are used for photothermaltumor therapy [101].
9 Magnetic Liposomes
Magnetic liposomes are used in cancer therapy [102]. Bacterial magnetosomes have been developed recently co-loaded with siRNA and doxorubicin, using polyethylineamine as a cross-linking agent. These nanocarriers are used in cancer therapeutics [103]. Magnetic nanoclusters have been also used for delivery of cisplatin for chemotherapy [104].
Neoadjuvantnano-photothermal therapy (NNPT) has been used in breast cancer treatment. When it is done before surgery, NNPT is found to improve the benefits of surgery [105].
Multifunctional nanohybrids have various applications in improving drug delivery of magnetic NPs [106]. Gadolinium-based nanoplatforms have been used for cancer therapy [107].
Superparamagnetic nanoparticles (SPIONs) have been coated with the amphiphilic copolymer INU-LA-PEG-FA and loaded with doxorubicin (DOXO-SPIONs) to function as smart agents for colon cancer therapy [108].
10 Ferroptosis
Iron-dependent cell death mediated by lipid peroxidation is known as ferroptosis. Fenton reaction produces reactive oxygen species, which also contributes to lipid peroxidation. Iron-based NPs can produce ferroptosis [109]. Ferroptosis is also used in the treatment of brain tumors [110]. Ferroptosis contributes to the hyperthermia in producing the effects of magnetic NPs.
11 Challenges
12 Conclusion
Various magnetic NPs have been described in the recent years. A complete description of the complete armamentarium of these species is beyond the scope of any publication. However, we have tried to focus on the latest and most important of this important group of NPs. The most important mechanism by which magnetic NPs work is by producing hyperthermia. In addition, ROS production and Fenton reaction, as well as ferroptosis, contribute to the same. There are other NPs which produce hyperthermia, but magnetic NPs are the most important among them. Future years shall reveal newer magnetic NPs, including MFNPs, with better functionalities. Indeed, as is the case with any NP, the main issue shall remain as toxicity. Once, we solve the toxicity paradox, magnetic NPs shall become one of the most important tools available to the physician for the treatment of a variety of cancers.
References
Nandwana V, De M, Chu S et al (2015) Theranostic magnetic nanostructures (MNS) for cancer. In: Nanotechnology-based precision tools for the detection and treatment of cancer. Springer, pp 51–83
Javidi M, Heydari M, Attar MM et al (2015) Cylindrical agar gel with fluid flow subjected to an alternating magnetic field during hyperthermia. Int J Hyperth 31(1):33–39
Javidi M, Heydari M, Karimi A, Haghpanahi M, Navidbakhsh M, Razmkon A (2014) Evaluation of the effects of injection velocity and different gel concentrations on nanoparticles in hyperthermia therapy. J Biomed Phys Eng 4(4):151
Heydari M, Javidi M, Attar MM et al (2015) Magnetic fluid hyperthermia in a cylindrical gel contains water flow. J Mech Med Biol 15(05):1550088
Dai C, Wang C, Hu R et al (2019) Photonic/magnetic hyperthermia-synergistic nanocatalytic cancer therapy enabled by zero-valence iron nanocatalysts. Biomaterials 219:
Mejías R, Hernández Flores P, Talelli M et al (2018) Cell-promoted nanoparticle aggregation decreases nanoparticle-induced hyperthermia under an alternating magnetic field independently of nanoparticle coating, core size, and subcellular localization. ACS Appl Mater Interfaces 11(1):340–355
Vangijzegem T, Stanicki D, Laurent S (2019) Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin Drug Deliv 16(1):69–78
Akbari-Karadeh S, Aghamiri SMR, Tajer-Mohammad-Ghazvini P, Ghorbanzadeh-Mashkani S (2019) Radiolabeling of biogenic magnetic nanoparticles with Rhenium-188 as a novel agent for targeted radiotherapy. Appl Biochem Biotechnol 1–11
Moise S, Byrne JM, El Haj AJ, Telling ND (2018) The potential of magnetic hyperthermia for triggering the differentiation of cancer cells. Nanoscale 10(44):20519–20525
Jędrzak A, Grześkowiak BF, Coy E et al (2019) Dendrimer based theranostic nanostructures for combined chemo-and photothermal therapy of liver cancer cells in vitro. Colloids Surf B Biointerfaces 173:698–708
Brady LW, Wazer DE, Perez CA (2013) Perez & Brady’s principles and practice of radiation oncology. Lippincott Williams & Wilkins
Dollinger M, Rosenbaum EH (2002) Everyone’s guide to cancer therapy;: how cancer is diagnosed, treated, and managed day to day. Andrews McMeel Publishing
John Ł, Janeta M, Szafert S (2017) Designing of macroporous magnetic bioscaffold based on functionalized methacrylate network covered by hydroxyapatites and doped with nano-MgFe2O4 for potential cancer hyperthermia therapy. Mater Sci Eng, C 78:901–911
Kumar CSSR, Mohammad F (2011) Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev 63(9):789–808
Ashikbayeva Z, Tosi D, Balmassov D, Schena E, Saccomandi P, Inglezakis V (2019) Application of nanoparticles and nanomaterials in thermal ablation therapy of cancer. Nanomaterials 9(9):1195
Chen Q, Luo Y, Du W et al (2019) Clearable theranostic platform with pH-independent chemodynamic therapy enhancement strategy for synergetic photothermal tumor therapy. ACS Appl Mater Interfaces
Minaei SE, Khoei S, Khoee S, Vafashoar F, Mahabadi VP (2019) In vitro anti-cancer efficacy of multi-functionalized magnetite nanoparticles combining alternating magnetic hyperthermia in glioblastoma cancer cells. Mater Sci Eng, C 101:575–587
Izadi A, Meshkini A, Entezari MH (2019) Mesoporous superparamagnetic hydroxyapatite nanocomposite: a multifunctional platform for synergistic targeted chemo-magnetotherapy. Mater Sci Eng, C 101:27–41
Legge CJ, Colley HE, Lawson MA, Rawlings AE (2019) Targeted magnetic nanoparticle hyperthermia for the treatment of oral cancer. J Oral Pathol Med
Wang Z, Xue X, He Y et al (2018) Novel redox-responsive polymeric magnetosomes with tunable magnetic resonance property for in vivo drug release visualization and dual-modal cancer therapy. Adv Funct Mater 28(33):1802159
Carrey J, Mehdaoui B, Respaud M (2011) Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: application to magnetic hyperthermia optimization. J Appl Phys 109(8):83921
Roet M, Hescham S-A, Jahanshahi A, Rutten BPF, Anikeeva PO, Temel Y (2019) Progress in neuromodulation of the brain; a role for magnetic nanoparticles? Prog Neurobiol
Das P, Fatehbasharzad P, Colombo M, Fiandra L, Prosperi D (2019) Multifunctional magnetic gold nanomaterials for cancer. Trends Biotechnol
Abed Z, Beik J, Laurent S et al (2019) Iron oxide–gold core–shell nano-theranostic for magnetically targeted photothermal therapy under magnetic resonance imaging guidance. J Cancer Res Clin Oncol 145(5):1213–1219
Manshadi MKD, Saadat M, Mohammadi M et al (2018) Delivery of magnetic micro/nanoparticles and magnetic-based drug/cargo into arterial flow for targeted therapy. Drug Deliv 25(1):1963–1973
Zuvin M, Kuruoglu E, Kaya VO et al (2019) Magnetofection of green fluorescent protein encoding DNA-bearing polyethyleneimine-coated superparamagnetic iron oxide nanoparticles to human breast cancer cells. ACS Omega 4(7):12366–12374
Fang Z, Li X, Xu Z et al (2019) Hyaluronic acid-modified mesoporous silica-coated superparamagnetic Fe3O4 nanoparticles for targeted drug delivery. Int J Nanomed 14:5785
Hafsi M, Preveral S, Hoog C et al (2019) RGD-functionalized magnetosomes are efficient tumor radioenhancers for X-rays and protons. Nanomed Nanotechnol Biol Med 102084
Zheng T, Wang W, Wu F, Zhang M, Shen J, Sun Y (2019) Zwitterionic polymer-gated Au@ TiO2 core-shell nanoparticles for imaging-guided combined cancer therapy. Theranostics 9(17):5035
Wang Y, Song S, Lu T et al (2019) Oxygen-supplementing mesoporous polydopamine nanosponges with WS2 QDs-embedded for CT/MSOT/MR imaging and thermoradiotherapy of hypoxic cancer. Biomaterials 220:
Kwon YM, Je J, Cha SH, Oh Y, Cho WH (2019) Synergistic combination of chemo-phototherapy based on temozolomide/ICG-loaded iron oxide nanoparticles for brain cancer treatment. Oncol Rep
Yu J, Zhao F, Gao W et al (2019) Magnetic reactive oxygen species nanoreactor for switchable magnetic resonance imaging guided cancer therapy based on pH-sensitive Fe5C2@Fe3O4 nanoparticles. ACS Nano
Eivazzadeh-Keihan R, Radinekiyan F, Maleki A, Bani MS, Hajizadeh Z, Asgharnasl S (2019) A novel biocompatible core-shell magnetic nanocomposite based on cross-linked chitosan hydrogels for in vitro hyperthermia of cancer therapy. Int J Biol Macromol
Kim K-S, Han J-H, Park J-H et al (2019) Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials 221:
Fathy MM, Fahmy HM, Saad OA, Elshemey WM (2019) Silica-coated iron oxide nanoparticles as a novel nano-radiosensitizer for electron therapy. Life Sci 234:
Pramanik N, Ranganathan S, Rao S et al (2019) A composite of hyaluronic acid-modified graphene oxide and iron oxide nanoparticles for targeted drug delivery and magnetothermal therapy. ACS Omega 4(5):9284–9293
Song Q, Jia J, Niu X et al (2019) An oral drug delivery system with programmed drug release and imaging properties for orthotopic colon cancer therapy. Nanoscale 11(34):15958–15970
Zanganeh S, Georgala P, Corbo C et al (2019) Immunoengineering in glioblastoma imaging and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol e1575
Fu S, Ding Y, Cong T et al (2019) Multifunctional NaYF4: Yb, Er@PE3@Fe3O4 nanocomposites for magnetic-field-assisted upconversion imaging guided photothermal therapy of cancer cells. Dalt Trans 48(34):12850–12857
Hu X, Tang Y, Hu Y et al (2019) Gadolinium-chelated conjugated polymer-based nanotheranostics for photoacoustic/magnetic resonance/NIR-II fluorescence imaging-guided cancer photothermal therapy. Theranostics 9(14):4168
Alomari M, Jermy BR, Ravinayagam V et al (2019) Cisplatin-functionalized three-dimensional magnetic SBA-16 for treating breast cancer cells (MCF-7). Artif Cells, Nanomed, Biotechnol 47(1):3079–3086
Guo Y, Ran Y, Wang Z et al (2019) Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials 219:
Zhong D, Zhao J, Li Y et al (2019) Laser-triggered aggregated cubic α-Fe2O3@Au nanocomposites for magnetic resonance imaging and photothermal/enhanced radiation synergistic therapy. Biomaterials 219:
Wu S, Liu X, He J et al (2019) a dual targeting magnetic nanoparticle for human cancer detection. Nanoscale Res Lett 14(1):228
Wei R, Gong X, Lin H et al (2019) Versatile octapod-shaped hollow porous manganese(II) oxide nanoplatform for real-time visualization of cargo delivery. Nano Lett 19(8):5394–5402
Wang L, Chen S, Zhu Y et al (2018) Triple-modal imaging-guided chemo-photothermal synergistic therapy for breast cancer with magnetically targeted phase-shifted nanoparticles. ACS Appl Mater Interfaces 10(49):42102–42114
Wu M, Zhang H, Tie C et al (2018) MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat Commun 9(1):4777
Chen X, Li G, Han Q et al (2017) Rational design of branched Au–Fe3O4 janus nanoparticles for simultaneous trimodal imaging and photothermal therapy of cancer cells. Chem Eur J 23(68):17204–17208
Shen Z, Chen T, Ma X et al (2017) Multifunctional theranostic nanoparticles based on exceedingly small magnetic iron oxide nanoparticles for T1-weighted magnetic resonance imaging and chemotherapy. ACS Nano 11(11):10992–11004
Zhao J, Tu K, Liu Y et al (2017) Photo-controlled aptamers delivery by dual surface gold-magnetic nanoparticles for targeted cancer therapy. Mater Sci Eng, C 80:88–92
Luo X, Peng X, Hou J, Wu S, Shen J, Wang L (2017) Folic acid-functionalized polyethylenimine superparamagnetic iron oxide nanoparticles as theranostic agents for magnetic resonance imaging and PD-L1 siRNA delivery for gastric cancer. Int J Nanomed 12:5331
Sheng Y, Beguin E, Nesbitt H et al (2017) Magnetically responsive microbubbles as delivery vehicles for targeted sonodynamic and antimetabolite therapy of pancreatic cancer. J Control Release 262:192–200
Kuo C-Y, Liu T-Y, Wang K-S et al (2017) Magnetic and thermal-sensitive poly(N-isopropylacrylamide)-based microgels for magnetically triggered controlled release. JoVE (Journal Vis Exp) (125):e55648
White SB, Kim D-H, Guo Y et al (2017) Biofunctionalized hybrid magnetic gold nanoparticles as catalysts for photothermal ablation of colorectal liver metastases. Radiology 285(3):809–819
Chowdhury P, Roberts AM, Khan S et al (2017) Magnetic nanoformulations for prostate cancer. Drug Discov Today 22(8):1233–1241
Nan X, Zhang X, Liu Y, Zhou M, Chen X, Zhang X (2017) Dual-targeted multifunctional nanoparticles for magnetic resonance imaging guided cancer diagnosis and therapy. ACS Appl Mater Interfaces 9(11):9986–9995
Rezaei G, Habibi-Anbouhi M, Mahmoudi M et al (2017) Development of anti-CD47 single-chain variable fragment targeted magnetic nanoparticles for treatment of human bladder cancer. Nanomedicine 12(6):597–613
Wang D, Zhou J, Chen R et al (2016) Controllable synthesis of dual-MOFs nanostructures for pH-responsive artemisinin delivery, magnetic resonance and optical dual-model imaging-guided chemo/photothermal combinational cancer therapy. Biomaterials 100:27–40
Hou H, Huang X, Wei G, Xu F, Wang Y, Zhou S (2019) Fenton reaction assisted photodynamic therapy for cancer with multifunctional magnetic nanoparticles. ACS Appl Mater Interfaces
Gandia D, Gandarias L, Rodrigo I et al (2019) Unlocking the potential of magnetotactic bacteria as magnetic hyperthermia agents. Small 1902626
Correa S, Puertas S, Gutiérrez L et al (2019) Design of stable magnetic hybrid nanoparticles of Si-entrapped HRP. PLoS One 14(4):
Yang WJ, Liang L, Wang X et al (2019) Versatile functionalization of surface-tailorable polymer nanohydrogels for drug delivery systems. Biomater Sci 7(1):247–261
Shakil M, Hasan M, Sarker SR (2019) Iron oxide nanoparticles for breast cancer theranostics. Curr Drug Metab
Gao P, Mei C, He L et al (2018) Designing multifunctional cancer-targeted nanosystem for magnetic resonance molecular imaging-guided theranostics of lung cancer. Drug Deliv 25(1):1811–1825
Rajaee A, Zhao L, Wang S et al (2019) Multifunction bismuth gadolinium oxide nanoparticles as radiosensitizer in radiation therapy and imaging. Phys Med Biol
Dong S, Xu J, Jia T et al (2019) Upconversion-mediated ZnFe2O4 nanoplatform for NIR-enhanced chemodynamic and photodynamic therapy. Chem Sci 10(15):4259–4271
Poudel K, Gautam M, Jin SG, Choi H-G, Yong CS, Kim JO (2019) Copper sulfide: an emerging adaptable nanoplatform in cancer theranostics. Int J Pharm
Multari C, Miola M, Laviano F et al (2019) Magnetoplasmonic nanoparticles for photothermal therapy. Nanotechnology 30(25):
Xie M, Zhang F, Peng H et al (2019) Layer-by-layer modification of magnetic graphene oxide by chitosan and sodium alginate with enhanced dispersibility for targeted drug delivery and photothermal therapy. Colloids Surf B Biointerfaces 176:462–470
Xu C, Feng Q, Yang H et al (2018) A light-triggered mesenchymal stem cell delivery system for photoacoustic imaging and chemo-photothermal therapy of triple negative breast cancer. Adv Sci 5(10):1800382
Li W, Yu H, Ding D et al (2019) Cold atmospheric plasma and iron oxide-based magnetic nanoparticles for synergetic lung cancer therapy. Free Radic Biol Med 130:71–81
Cazares-Cortes E, Nerantzaki M, Fresnais J, Wilhelm C, Griffete N, Ménager C (2018) Magnetic nanoparticles create hot spots in polymer matrix for controlled drug release. Nanomaterials 8(10):850
Sang MM, Liu FL, Wang Y et al (2018) A novel redox/pH dual-responsive and hyaluronic acid-decorated multifunctional magnetic complex micelle for targeted gambogic acid delivery for the treatment of triple negative breast cancer. Drug Deliv 25(1):1846–1857
Abedin MR, Umapathi S, Mahendrakar H et al (2018) Polymer coated gold-ferric oxide superparamagnetic nanoparticles for theranostic applications. J Nanobiotechnol 16(1):80
Rodrigues RO, Baldi G, Doumett S et al (2018) Multifunctional graphene-based magnetic nanocarriers for combined hyperthermia and dual stimuli-responsive drug delivery. Mater Sci Eng, C 93:206–217
Li Y-Q, Xu M, Dhawan U et al (2018) Iron–gold alloy nanoparticles serve as a cornerstone in hyperthermia-mediated controlled drug release for cancer therapy. Int J Nanomed 13:5499
Wang X, Yang L, Zhang H et al (2018) Fluorescent magnetic PEI-PLGA nanoparticles loaded with paclitaxel for concurrent cell imaging, enhanced apoptosis and autophagy in human brain cancer. Colloids Surf B Biointerfaces 172:708–717
Ferreira TH, Faria J, Gonzalez IJ et al (2018) BNNT/Fe3O4 system as an efficient tool for magnetohyperthermia therapy. J Nanosci Nanotechnol 18(10):6746–6755
Wang G, Qian K, Mei X (2018) A theranostic nanoplatform: magneto-gold@ fluorescence polymer nanoparticles for tumor targeting T1 & T2-MRI/CT/NIR fluorescence imaging and induction of genuine autophagy mediated chemotherapy. Nanoscale. 10(22):10467–10478
Zhao X-F, Wang W-Y, Li X-D, Li S-P, Song F-G (2018) Core-shell structure of Fe3O4@MTX-LDH/Au NPs for cancer therapy. Mater Sci Eng, C 89:422–428
Tom G, Philip S, Isaac R, Praseetha PK, Jiji SG, Asha VV (2018) Preparation of an efficient and safe polymeric-magnetic nanoparticle delivery system for sorafenib in hepatocellular carcinoma. Life Sci 206:10–21
Manigandan A, Handi V, Sundaramoorthy NS et al (2018) Responsive nanomicellar theranostic cages for metastatic breast cancer. Bioconjug Chem 29(2):275–286
Mondal S, Manivasagan P, Bharathiraja S et al (2017) Hydroxyapatite coated iron oxide nanoparticles: a promising nanomaterial for magnetic hyperthermia cancer treatment. Nanomaterials 7(12):426
Song W, Su X, Gregory D, Li W, Cai Z, Zhao X (2018) Magnetic alginate/chitosan nanoparticles for targeted delivery of curcumin into human breast cancer cells. Nanomaterials 8(11):907
Qin Y-T, Peng H, He X-W, Li W-Y, Zhang Y (2019) pH-responsive polymer-stabilized ZIF-8 nanocomposite for fluorescence and magnetic resonance dual-modal imaging-guided chemo/photodynamic combinational cancer therapy. ACS Appl Mater Interfaces
Dong Q, Yang H, Wan C et al (2019) Her2-Functionalized gold-nanoshelled magnetic hybrid nanoparticles: a theranostic agent for dual-modal imaging and photothermal therapy of breast cancer. Nanoscale Res Lett 14(1):1–16
Alhasan AH, Fardous R, Alsudir SA et al (2019) Polymeric reactor for the synthesis of superparamagnetic-thermal treatment of breast cancer. Mol Pharm 16(8):3577–3587
Park J, Jin C, Lee S, Kim J, Choi H (2019) Magnetically actuated degradable microrobots for actively controlled drug release and hyperthermia therapy. Adv Healthc Mater 8(16):1900213
Kim S, Poilil Surendran S, Jeong YY (2019) Biomedical applications of hyaluronic acid-based nanomaterials in hyperthermic cancer therapy. Pharmaceutics 11(7):306
Syu W-J, Huang C-C, Hsiao J-K et al (2019) Co-precipitation synthesis of near-infrared iron oxide nanocrystals on magnetically targeted imaging and photothermal cancer therapy via photoablative protein denature. Nanotheranostics 3(3):236
Cho H-Y, Mavi A, Chueng S-TD et al (2019) Tumor homing reactive oxygen species nanoparticle for enhanced cancer therapy. ACS Appl Mater Interfaces
Cheng Y, Lu T, Wang Y et al (2019) Glutathione-mediated clearable nanoparticles based on ultrasmall Gd2O3 for MSOT/CT/MR imaging guided photothermal/radio combination cancer therapy. Mol Pharm 16(8):3489–3501
Wang L, Xu X, Mu X et al (2019) Fe doped copper sulfide nanoparticles for in vivo magnetic resonance imaging and simultaneous photothermal therapy. Nanotechnology
Ma X, Ren X, Guo X et al (2019) Multifunctional iron-based Metal–organic framework as biodegradable nanozyme for microwave enhancing dynamic therapy. Biomaterials 214:
Zhang X, Li L, Liu Q et al (2019) Co-delivery of rose bengal and doxorubicin nanoparticles for combination photodynamic and chemo-therapy. J Biomed Nanotechnol 15(1):184–195
Cardoso BD, Rio ISR, Rodrigues ARO et al (2018) Magnetoliposomes containing magnesium ferrite nanoparticles as nanocarriers for the model drug curcumin. R Soc Open Sci 5(10):
Hematyar M, Soleimani M, Es-haghi A, Rezaei Mokarram A (2018) Synergistic co-delivery of doxorubicin and melittin using functionalized magnetic nanoparticles for cancer treatment: loading and in vitro release study by LC–MS/MS. Artif Cells, Nanomed Biotechnol 46(sup3):S1226–S1235
Song W, Gregory DA, Al-janabi H, Muthana M, Cai Z, Zhao X (2019) Magnetic-silk/polyethyleneimine core-shell nanoparticles for targeted gene delivery into human breast cancer cells. Int J Pharm 555:322–336
Qiu W, Chen R, Chen X et al (2018) Oridonin-loaded and GPC1-targeted gold nanoparticles for multimodal imaging and therapy in pancreatic cancer. Int J Nanomed 13:6809
You L, Liu X, Fang Z, Xu Q, Zhang Q (2019) Synthesis of multifunctional Fe3O4@PLGA-PEG nano-niosomes as a targeting carrier for treatment of cervical cancer. Mater Sci Eng, C 94:291–302
Xue P, Yang R, Sun L et al (2018) Indocyanine green-conjugated magnetic prussian blue nanoparticles for synchronous photothermal/photodynamic tumor therapy. Nano-micro Lett 10(4):74
Anilkumar TS, Shalumon KT, Chen J-P (2019) Applications of magnetic liposomes in cancer therapies. Curr Pharm Des
Long R-M, Dai Q-L, Zhou X et al (2018) Bacterial magnetosomes-based nanocarriers for co-delivery of cancer therapeutics in vitro. Int J Nanomed 13:8269
Mandriota G, Di Corato R, Benedetti M, De Castro F, Fanizzi FP, Rinaldi R (2018) Design and application of cisplatin-loaded magnetic nanoparticle clusters for smart chemotherapy. ACS Appl Mater Interfaces 11(2):1864–1875
Yan H, Shang W, Sun X et al (2019) Neoadjuvant nano-photothermal therapy used before operation effectively assists in surgery for breast cancer. Nanoscale 11(2):706–716
Wen Y, Xu M, Liu X et al (2019) Magnetofluorescent nanohybrid comprising polyglycerol grafted carbon dots and iron oxides: colloidal synthesis and applications in cellular imaging and magnetically enhanced drug delivery. Colloids Surf B Biointerfaces 173:842–850
Guo H, Zhao X, Sun H, Zhu H, Sun H (2018) Synthesis of gadolinium-based Bi2S3 nanoparticles as cancer theranostics for dual-modality computed tomography/magnetic resonance imaging-guided photothermal therapy. Nanotechnology 30(7):75101
Licciardi M, Scialabba C, Puleio R et al (2019) Smart copolymer coated SPIONs for colon cancer chemotherapy. Int J Pharm 556:57–67
Wang S, Luo J, Zhang Z et al (2018) Iron and magnetic: new research direction of the ferroptosis-based cancer therapy. Am J Cancer Res 8(10):1933
Shen Z, Liu T, Li Y et al (2018) Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano 12(11):11355–11365
Kaur P, Aliru ML, Chadha AS, Asea A, Krishnan S (2016) Hyperthermia using nanoparticles–promises and pitfalls. Int J Hyperth 32(1):76–88
Chang D, Lim M, Goos JACM et al. (2018) Biologically targeted magnetic hyperthermia: potential and limitations. Front Pharmacol 9
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gopalakrishnan, S., Vaidyanathan, K. (2021). Magnetic Nanoparticles for Hyperthermia a New Revolution in Cancer Treatment. In: Joshy, K.S., Sabu, T., Thakur, V.K. (eds) Magnetic Nanoparticles. Gels Horizons: From Science to Smart Materials. Springer, Singapore. https://doi.org/10.1007/978-981-16-1260-2_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-1260-2_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1259-6
Online ISBN: 978-981-16-1260-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)