Abstract
Gibberellic acid (GA) is a well-established group of phytohormones with growth eliciting properties. Considering the substantial damage by salt stress, we investigated whether foliar sprays of 10−6 M GA3 could reverse salinity implicated constraints in fenugreek plants and up to what extent. Our study suggested that exogenous GA3 could significantly (p ≤ 0.05) mitigate the effects of salinity in the fenugreek plants. This treatment maximised the growth and yield variables, as well. The activities of various assimilatory enzymes, such as carbonic anhydrase and nitrogen reductase, observed an increment of about 17% each over salt-stressed plants (50 mg L−1). Further metabolomic analyses revealed an upregulated antioxidant defence system with increased activities of superoxide dismutase (18%), catalase (13%), and ascorbate peroxidase (15%). The enhanced proline content (19%) in tandem with upregulated antioxidant enzymes minimised cellular damage through restricting TBARS and H2O2 contents by about 16% and 14%, respectively. Thus, in the light of sufficient data, we are convinced that foliar sprays of 10−6 M GA3 could be used for minimising the salinity induced growth and yield constraints in the fenugreek crop.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Fenugreek (Trigonella foenum-graceum L.) is an aromatic leguminous plant belonging to the family Fabaceae (Beyzi 2020). The plant is exploited for the broad spectrum of its medicinal properties, which includes antidiabetic, anticancer, analgesic, antimicrobial, antioxidant, hypocholesterolemic, along with lactation and appetite enhancing attributes (Ouzir et al. 2016; Choudhary et al. 2021a). These therapeutic characteristics can be attributed to the high concentration of bioactive phytochemicals present in the fenugreek, such as flavonoids, alkaloids, amino acids, vitamins, saponins, and fibres (Ouzir et al. 2016; Bitarafan et al. 2019). However, the concentration of these secondary metabolites, as well as the overall growth and development of the fenugreek plant, can be influenced by various environmental stimuli (Bitarafan et al. 2019; Mickky et al. 2019).
Soil salinity is one of the leading environmental stresses that hamper crop growth and physiology, causing a reduction in plant productivity (Van Zelm et al. 2020). The salt stress affects plants in two ways: first limiting the plant water uptake and subsequently building an ion excess in the plant system (Munns and Tester 2008). The first phase creates oxidative stress, while the later one contributes to the ionic stress (Shabala and Cuin 2008). Most crops, including fenugreek, are glycophytes and cannot grow well in high salt concentrations, and thus, depending on the severity, salinity can regulate seed germination, cell expansion, stomatal conductance, photosynthesis, and other metabolic and development pathways in such plants (Shabala and Cuin 2008; Mickky et al. 2019; Van Zelm et al. 2020). An essential aspect of salinity induced damage lies in the overproduction of reactive oxygen species (ROS) by salt stress (Zhu 2001). Although ROS acts as a secondary messenger for various vital physiological processes, their accumulation can cause oxidative damage to proteins, lipids, and nucleic acids (Apel and Hirt 2004; Foyer 2018; Mukarram et al. 2021a). As a counter mechanism, plants possess an efficient antioxidant defence system to regulate the ROS build-up (Gill and Tuteja 2010; Mukarram et al. 2021b, c). Principal ROS scavenging antioxidants are superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX), and the harmony among their activities to adjust superoxide radicals (O2−) and hydrogen peroxide (H2O2) can determine the plant tolerance level to the stress (Mittler 2002; Corpas et al. 2017; Zehra et al. 2020; Choudhary et al. 2021b).
Phytohormones are chemical messengers that interact with various physiological processes to ensure normal growth, development, and productivity of the plants (Javid et al. 2011). Gibberellin (GA) is a well-known plant hormone that promotes a wide range of phenomena pertaining to plant growth and development, such as seed germination, cell expansion, stem and leaf elongation, flower induction, and pollen development (Ueguchi-Tanaka et al. 2007; Choudhary et al. 2021c). Although more than 130 types of GAs have been discovered by far in different organisms, only a few of them can impart the aforementioned effects (Davière and Achard 2013). Nevertheless, external stimuli such as salt stress can influence the endogenous level of gibberellin and its biosynthesis through regulating plant water relation and membrane permeability (Tuna et al. 2008). Given salinity causes damage by reducing gibberellin content in the fenugreek plants, the present study hypothesised that these effects could be minimised if additional GA is provided to the plants. To test our hypothesis, we exogenously applied GA3 to salt-stressed fenugreek plants as foliar treatments. Data analyses suggested that GA3 can assist in the reversal of salt-induced constraints and oxidative damage through enzymatic enhancements and upregulating antioxidant metabolism in fenugreek, and thus can ensure enhanced plant growth and productivity.
2 Materials and Methods
2.1 Growth Conditions and Experimental Setup
Fenugreek (Trigonella foenum-graceum L.) plant was used as the plant material for the present study. The authorised seeds from the Indian Agricultural Research Institute (IARI), New Delhi, India, were used for propagating fenugreek plants. Subsequent surface sterilisation of the seeds was performed with 0.2% HgCl2 for 5 min and repeated washing with deionised water. Sterilised seeds were sown in the earthen pots (25 cm × 25 cm) in the semi-automatic net house at the Department of Botany, Aligarh Muslim University, Aligarh (27°52′ N latitude, 78°51′ E longitude, and 187.45 m altitude). Each pot of the 5 kg capacity was filled with a mixture of soil and organic manure in a 5:1 ratio (w/w). A temperature range of 17–25 °C (±4 °C) and the relative humidity [68 ± 5%] were recorded during the experimental period. Soil analysis at the IARI, New Delhi of the random samples collected from different pots revealed the following soil attributes: texture- sandy loam, pH (1:2): 7.6, and electrical conductivity (1:2): 0.52 m mhos cm−1. Available nitrogen, phosphorous, and potassium content in the soil was recorded as 94.7, 8.8, and 136.6 mg kg−1 of the soil, respectively. The pots were arranged according to a simple randomised block design with five replicates, and the plants were irrigated daily to keep the soil hydrated. The effects of the salinity and gibberellic acid were assessed in terms of the modulation observed in the growth, physiology, and productivity of the 90 days old fenugreek plant.
2.2 Induction of Salt Stress
Fenugreek plants were maintained under two different NaCl concentrations (50 and 100 mg L−1). These concentrations were supplied in the form of 300 mL of modified full-strength Hoagland’s nutrient solution on alternate days starting from 10 days after the seed germination, while the control group was supplied with 300 mL nutrient solution only.
2.3 Supplementation of Gibberellic Acid
Exogenous application of gibberellic acid was carried in the form of foliar sprays for the amelioration for salinity induced constraints. Based on the literature available, a concentration of 10−6 M of GA3 was supplemented to the fenugreek plants (Miceli et al. 2019a, b). In total, five foliar sprays (50 mL each) were applied every week starting from 30 days after seed germination using a battery sprayer. The control group was supplied with the sprays of deionised water only.
2.4 Determination of the Growth Attributes
Growth parameters were evaluated in terms of plant length and weight. Five plants from each treatment were uprooted after 90 days of seed sowing and were cleansed carefully with deionised water in order to remove stuck foreign particles. The plant surface was dried with blotting paper. The length of the shoot and root was measured using a metric scale and was expressed in cm. Subsequent shoot and root fresh weights were measured using an electric balance. Thereafter, the plants were dried for 40 h in an oven at 80 °C to attain a dry state. Separate weights of the shoot and root were calculated using the electric balance. All the weight measurements (fresh and dry) were expressed in g. The leaf area index was determined using the graph paper sheet by following the procedure of Watson (1958). The average number of leaves for each treatment was counted accordingly.
2.5 Determination of the Total Chlorophyll Content
The total chlorophyll content in the fresh fenugreek leaves was estimated using the methods developed by Lichtenthaler and Buschmann (2001). Fresh tissue from the interveinal leaf area was ground with acetone solution (80%) with the help of a mortar and pestle. The optical density of the chlorophyll extract was recorded at 662 nm for chlorophyll a content and at 645 nm for chlorophyll b content with a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan). Total chlorophyll content was estimated by adding chlorophyll a and b content and was expressed in mg g−1 FW.
2.6 Determination of the Nitrate Reductase (NR) Activity
The intact tissue assay method of Jaworski (1971) was adapted for nitrate reductase (E.C. 1.7.1.1) activity in the fenugreek leaves. 0.2 g of fresh leaves were chopped and transferred into the test tubes. Each test tube contained a mixture of 2.5 mL of phosphate buffer (pH 7.5) with 0.1 M, 0.5 mL of potassium nitrate (0.2 M) and 2.5 mL of isopropanol (5%). The reaction mixture was incubated at 30 °C for 2 h. Thereafter, 0.4 mL of the aliquot was transferred to the test tube containing 0.3 mL of sulfanilamide (1%) and 0.3 mL of NED-HCl (N-1-naphthyl ethylenediamine dihydrochloride) (0.02%) for nitrite generation after azocoupling with sulfanilamide and NED-HCl. The reaction mixture was incubated again at 30 °C for 20 min for maximised colour development. Distilled water was used for subsequent dilution to reach a final volume of 5 mL. The absorbance of the solution was recorded at 540 nm using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan), and the enzyme activity was expressed as the nmol of nitrite generated per gram of fresh weight of the leaf tissue per hour (nmol NO2−1 g−1 FW h−1).
2.7 Determination of Carbonic Anhydrase (CA) Activity
Considering the crucial role in stomatal conductance, carboxylation, and conversion of CO2 into bicarbonates, the activity of carbonic anhydrase (E.C. 4.2.1.1) was determined. CA activity was measured in the fenugreek leaves by following the procedure of Dwivedi and Randhawa (1974). 0.2 g of fresh leaves were chopped and transferred to the petri dishes, followed by dipping them in the 10 mL of cysteine hydrochloride solution (0.2 M). After leaving the setup was at 4 °C for 20 min, 4 mL of sodium bicarbonate solution (0.2 M) and 0.2 mL of bromothymol blue dye (0.022%) was added to each petri dish. Finally, the reaction mixture was titrated using methyl red as an indicator against 0.05 N HCl. The enzyme activity was expressed in μmol CO2 kg−1 leaf FW s−1.
2.8 Determination of H2O2 Content
Hydrogen peroxide (H2O2) content was determined by a peroxidase dependent assay adopting the method of Okuda et al. (1991). Peroxidase was added to initiate the reaction at 25 °C, and the increase in absorbance was observed at 590 nm spectrophotometrically for 3 min. The H2O2 content was expressed as μmol H2O2 g−1 FW.
2.9 Quantification of Lipid Peroxidation
The method of Cakmak and Horst (1991) was adapted to quantify lipid peroxidation in fenugreek leaves through estimating total thiobarbituric acid reactive substances (TBARS) content. TBARS content was determined as malondialdehyde (MDA) equivalents and expressed in nmol MDA g−1 FW. Conclusively, 0.5 g of fresh fenugreek leaves were crushed in 5 ml of 0.1% (w/v) trichloroacetic acid (TCA). The mixture was centrifuged at 12,000 × g for 5 min. Thereafter, 0.5% (w/v) tetrabutylammonium (4 mL) in 20% (w/v) TCA was mixed with an aliquot of 1 mL of the supernatant. The setup was left for incubation for 30 min at 90 °C. Subsequent termination of the reaction was carried out in an ice bath. The mixture was centrifuged again at 10,000 × g for 5 min. Spectrophotometric analysis (Shimadzu UV-1700, Tokyo, Japan) of the supernatant was performed at 532 nm, and the values were corrected for non-specific turbidity by subtracting the absorbance at 600 nm.
2.10 Determination of Proline Content
The proline content was estimated according to the methods described by Bates et al. (1973). 0.25 g of the fresh leaves were ground in the 2.5 mL aqueous solution of sulfosalicylic acid (3%). The mixture was centrifuged for 10 min at 10,000 × g. The aliquot (2 mL) of the supernatant collected afterwards was transferred to a test tube containing sulfosalicylic acid (2.5 mL), glacial acetic acid (1 mL), and acid ninhydrin solution (1 mL). The test tube was boiled at 100 °C for 1 h using a hot water bath. Termination of the reaction was performed using an ice bath while extraction was carried by adding toluene (3 mL) and subsequent rousing of the mixture for 20–25 s. The reaction mixture was left for some time to separate the aqueous portion from the toluene layer. The optical density of the toluene aspired layer possessing chromophore was recorded at 520 nm using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan). The proline content was calculated from against a standard curve and was expressed in mg g−1 FW using the following equation:
3 Quantification of the Enzymatic Antioxidant Defence System
3.1 Preparation of Enzyme Extract
For the enzymatic assays, 0.2 g of fresh fenugreek leaves was ground in liquid N2 using a mortar and pestle at 4 °C. The resulting coarse powder (0.5 g) was transferred to 5 ml (w/v) of chilled extraction medium containing potassium phosphate buffer (100 mM and pH 7.8), 1% (w/v) polyvinylpyrrolidone and 0.5% (v/v) Triton-X-100. Homogenates were centrifuged at 15,000 × g for 5 min at 4 °C. The supernatants acquired after centrifugation was used for the determination of enzymatic antioxidants activity (Kuo et al. 1982).
3.2 Superoxide Dismutase (SOD) Activity
The estimation of SOD (E.C. 1.15.1.1) activity was done according to the procedure of Beauchamp and Fridovich (1971). Riboflavin (1 mM), methionine (9.9 mM), nitro blue tetrazolium (55 mM), EDTA (2 mM), and Triton-X-100 (0.02%) was added to the 40 mL of freshly prepared enzyme extract and illuminated and maintained for one hour at 30 °C. The reaction mixture was analysed by a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan), and absorbance was recorded at 560 nm. One SOD unit is the amount of the enzyme needed for half inhibition of nitro blue tetrazolium reaction at the set wavelength.
3.3 Catalase (CAT) Activity
The activity of CAT (E.C. 1.11.1.6) was determined with the methods of Chandlee and Scandalios (1984) with slight modification. In the 0.04 mL of the enzyme extract, 2.6 mL of potassium phosphate buffer (50 mM with pH 7) and 0.4 mL of H2O2 (15 mM) was added. The solution was centrifuged afterwards at 12,500 × g for 20 min at 4 °C. Enzyme activity was measured by determining the disappearance of H2O2 at 240 nm for 2 minutes with 5 seconds interval.
3.4 Ascorbate Peroxidase (APX) Activity
Enzyme activity for APX (E.C. 1.11.1.11) was measured according to Nakano and Asada (1981). A reaction mixture was prepared with enzyme extract containing phosphate buffer (50 mM with pH 7), EDTA (0.1 mM), ascorbate (0.5 mM), and H2O2 (0.1 mM). Enzyme activity was measured by determining the reduction in the substrate absorbance at 290 nm using the extinction coefficient of 2.8 mM−1 cm−1. One APX unit is the amount required per min for the decomposition of 1μmol substrate at 25 °C.
3.5 Determination of Yield and Quality Variables
The yield parameters in the fenugreek plant were quantified in terms of seed attributes. The total number of seeds per pod and pods per plant was calculated accordingly. The weight of 1000 seeds and seed yield were weighed using an electric balance and were expressed in g while the length of the pods was expressed in cm using a metric scale.
The quality of fenugreek seeds was determined in terms of alkaloid content. Seed alkaloid content was estimated by grounding 1 g of seed powder with methanol (80%) and magnesium oxide using a mortar and pestle. The mixture was incubated for 30 min at 60 °C, followed by centrifugation. The supernatant collected was allowed to dry and then transferred to a flask. The Seed alkaloid content was calculated using the following equation:
3.6 Statistical Analyses and Graphics
SPSS-25.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis of the data. The standard errors were calculated, and analysis of variance (ANOVA) was performed on the data with five replicates to determine the least significant difference (LSD) between treatment means with the level of significance at p ≤ 0.05. The graphs presented in the study were generated using SigmaPlot 12 (Systat Software Inc., California, USA).
4 Results
4.1 Exogenous GA3 Supplementation Protected Growth and Development in Fenugreek Under Salinity
We observed that salinity reduced growth and development modules to a minimum in a dose-dependent manner. Nevertheless, plants supplied with 10−6 M GA3 exhibited better vegetative growth over the control group, even under salt stress. Although gibberellin mitigated growth and development in both NaCl 50 mg L−1 and NaCl 100 mg L−1 stressed fenugreek, the optimum amelioration was observed with NaCl 50 mg L−1. Plants under this concentration exhibited mitigation in shoot length (24.89%), root length (25.38%), shoot fresh weight (28.21%), shoot dry weight (15.70%), fresh root weight (20.18%), and root dry weight (16.22%) with the GA3 application. Similarly, the average number of leaves plant−1 and leaf area was increased with 10−6 M GA3 sprays by 23.36% and 8.64%, respectively, over its salt-stressed counterpart (NaCl 50 mg L−1) (Table 6.1).
4.2 GA3 Treated Plants Exhibited Upregulated Photosynthetic Pigment and Assimilatory Enzymes Activities
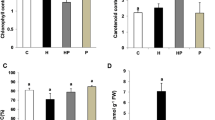
Foliar sprays of 10−6 M GA3 boosted photosynthetic pigment and the activities of enzymes pertaining to carbon, nitrogen, and sulphur assimilation. This concentration also assuaged salt-induced constraints through upregulating chlorophyll content by 19.81% in plants exposed to NaCl 50 mg L−1. Similarly, the best amelioration of enzymatic activities through gibberellin supplementation was observed in NaCl 50 mg L−1. The obtained data suggest an upregulation of 16.94% and 17.22%, in the activities of CA and NR, respectively, with exogenous gibberellin application in salt-stressed (NaCl 50 mg L−1) fenugreek. Additionally, this degree of palliation was followed by gibberellin application in plants treated with NaCl 100 mg L−1 concentration (Fig. 6.1).
Foliar sprays of 10−6 M GA3 enhanced photosynthetic pigment and enzymatic activities in fenugreek plants under salinity stress. Although both the salt concentrations restricted photosynthetic pigment content and activities of CA and NR, the maximum reduction was observed with 100 mg L−1 NaCl treatment. 10−6 M GA3 upregulated chlorophyll content as well as the enzymatic activities in fenugreek. Foliar sprays of GA3 also mitigated salinity induced restrictions in chlorophyll content (a) and the activities of CA (b) and NR (c). CA carbonic anhydrase, NR nitrate reductase. Each bar represents the mean ± SE (n = 3). Means followed by the same letter(s) do not differ by LSD test at 5% probability level (p ≤ 0.05)
4.3 GA3 Application Reduced Lipid Peroxidation and Electrolyte Leakage Through Upregulating Antioxidant Defence
Salinity-imposed substantial membrane damage through lipid peroxidation in a dose-dependent manner. The maximum membrane damage was observed with NaCl 100 mg L−1, followed by NaCl 50 mg L−1 concentration. Salt stress-induced electrolyte leakage also followed a similar pattern through H2O2 overproduction. Exogenous application of 10−6 M GA3 minimised the lipid peroxidation and electrolyte leakage in both NaCl concentrations. The optimum mitigation in TBARS content (16.36%) and H2O2 content (14.11%) was observed foliar application of GA3 in salt-stressed (50 mg L−1) fenugreek plants.
The antioxidant defence system in fenugreek plants was found hyperactivated in both stressful (with NaCl treatments) and assuaging environment (with GA3 treatments). Plants under the highest salt concentration (100 mg L−1) possessed the maximum activity for antioxidant enzymes to counter the induced damage. Nevertheless, the best amelioration in the activities of key antioxidant enzymes such as SOD (17.96%), CAT (13.02%), and APX (15.38%) was observed after the GA3 application in NaCl 50 mg L−1 stressed plants. The content of proline, a key osmolyte, followed the analogous trend with the antioxidant enzymes. The proline content was increased by 18.88% with the 10−6 M GA3 application compared to its salt-stressed (NaCl 50 mg L−1) counterpart (Fig. 6.2).
Effect of 10−6 M GA3 application on the oxidative damage marker and antioxidant metabolism in the fenugreek plants under salinity stress. The marker for oxidative damage, i.e., H2O2 (a) and TBARS (b) contents were significantly (p ≤ 0.05) reduced with 10−6 M GA3 application. Moreover, this treatment also enhanced osmoprotectant content, i.e., proline (c) and the activities of antioxidant enzymes, e.g., CAT (d), SOD (e), and APX (f) when applied alone. Additionally, the same treatment substantially ameliorated salinity induced oxidative stress and helped maintain cellular homeostasis. H2O2 hydrogen peroxide, TBARS thiobarbituric acid reactive substances, SOD superoxide dismutase, CAT catalase, APX ascorbate peroxidase. Each bar represents the mean ± SE (n = 3). Means followed by the same letter(s) do not differ by LSD test at 5% probability level (p ≤ 0.05)
4.4 Foliar Sprays of GA3 Mitigated Salinity Induced Yield Constraints in Fenugreek
The fenugreek plants supplied with 10−6 M GA3 treatments experienced maximum production. Although both concentrations of NaCl (50 and 100 mg L−1) restricted crop yield significantly (p ≤ 0.05), the best placation of this reduction was observed in 50 mg L−1 NaCl-stressed plants with foliar GA3 supplementation. This treatment exhibited an increment of 62.90%, 38.27%, and 30.92% in seed yield, alkaloid content, and pod length, respectively. Moreover, a similar enhancement was observed in seeds pod−1 (11.75%) and pods plant−1 (25.25%) (Table 6.2).
5 Discussion
Soil salinity is one of the leading abiotic stresses that can restrict plant growth and yield to a minimum (Zelm et al. 2020). Salt stress can cause damage depending on certain variables, including salt concentration and plant adaptability (Munns and Tester 2008). Fenugreek being a salt-sensitive crop afflicts severe oxidative damage on the advent of salinity (Mickky et al. 2019). In the present study, different salt concentrations (50 and 100 mg L−1) reduced growth variables related to plant length, weight, and leaves. A similar impact was observed in yield variables of fenugreek plants. Seed production, pod length, and alkaloid content were all restricted with increasing salinity concentration. These alterations are understandable as salinity limits water uptake, stomatal conductance, and mineral uptake that negatively influence plant water relation and source-sink potential (Vetrano et al. 2020). As a result, plants experience retarded growth and yield, as were observed in our study. Notably, salinity could reduce GA3 biosynthesis, and improving plant GA3 status through exogenous gibberellin supplementation could have upregulated cell expansion, leaf area, and stem length, resulting in improved growth and productivity (Wang et al. 2019). Additionally, GA3 has also been attributed as a growth elicitor for various crops (Khan et al. 2006; Vetrano et al. 2020). We perceived similar promoting activity of GA3 in the fenugreek as well. Exogenous application of GA3 could reverse salinity drawn growth and production constraints through improving water and ion uptake besides stomatal adjustments (Javid et al. 2011). Additionally, GA3 might promote leaf expansion and shoot elongation, contributing to the growth and yield enhancements in fenugreek (Ueguchi-Tanaka et al. 2007).
In the present study, we observed limited chlorophyll content in salt-stressed plants. The higher salt concentration had a more severe effect on chlorophyll synthesis. Salinity can achieve such an effect by destabilising the chlorophyll-protein complex through chlorophyll oxidation or damaging the enzymes that synthesise chlorophyll (Wang et al. 2019). Additionally, salinity could induce chlorophyllase synthesis in the mesophyll cells. Chlorophyllase is a proteolytic enzyme with chlorophyll digesting potential and could also disrupt the photosynthetic machinery and, thus, regulates the chlorophyll content (Tuna et al. 2008). This view is also shared by other plant physiologists who made similar observations in different crops during salt stress. Salinity induced Na+ accumulation increases the osmotic potential that could disrupt the photosynthetic electron transport system and damage the chloroplast. Salt stress could restrict photosynthetic enzymes, gaseous exchange, and poses structural and functional threats to the thylakoid membrane (Hendawey 2015). The low chlorophyll content is generally considered as a disadvantage, but interestingly, plants could utilise the same phenomenon to protect the photosynthetic electron transport system from over reduction, and thus, reducing the ROS production (Belmecheri-Cherifi et al. 2019). Gibberellin could enhance the ultra-structural morphogenesis of plastids and chlorophyll retention (Ahmad 2010).
Salt stress influenced the activities of CA and NR in a dose-dependent manner causing significant reduction at the highest salt concentration. Carbonic anhydrase (CA) is a metal-containing ubiquitous enzyme that catalyses the reversible conversion reaction of CO2 and H2O to bicarbonate ions and plays an essential role in stomatal conductance and carboxylation (Naeem et al. 2020). The salinity reduces CA activity by restricting stomatal conductance and CO2 fixation and subsequently reducing CO2 availability for CA (Singh et al. 2016). Additionally, salinity induced ion excess could make it harder for the plant to uptake other ions and minerals. In this context, salinity could downregulate CA activity by reducing Zn uptake, which is a crucial component of CA (Chakraborty et al. 2016; Singh et al. 2016). Exogenous application of GA3 overcame the constraints in CA activity under salt stress. This upregulation could be because of the positive influence of GA3 on stomatal conductance and CO2 metabolism (Ribeiro et al. 2012). Moreover, GA3 is also attributed to improve plant water relations and minerals uptake (Ueguchi-Tanaka et al. 2007; Wang et al. 2019). As a result, we observed enhancement in CA activity with GA3 supplementation. Several studies are in harmony with our observation, where GA3 application enhanced CA activity in different plants under salt stress (Afroz et al. 2006; Siddiqui et al. 2008; Tuna et al. 2008).
Salt stress also inhibited the NR activity, a crucial enzyme associated with nitrate metabolism, facilitating protein synthesis at various stages of plant growth (Afroz et al. 2006). Given the salinity interfere with the mineral uptake and assimilation, decreased NR activity could be an outcome of salt stress-induced restriction in the nitrogen and sulphur uptake (Nazar et al. 2011; Chakraborty et al. 2016). Moreover, salt stress might also enhance the activity of DNase, RNase, and protease enzymes that could affect NR activity negatively (Siddiqui et al. 2008). However, the GA3 application curbed the salt-induced effects on NR in the present study and enhanced its activity. Previous reports favour these correlations, where the similar eliciting pattern in NR activity was observed under salt stress with phytohormones action (Eleiwa et al. 2011; Iqbal et al. 2014).
The ROS overproduction and ion accumulation are the chariots that enable salinity to interfere with plant metabolism (Munns and Tester 2008). While ROS poses an osmo-oxidative threat in the fenugreek system, ion accumulation could regulate membrane permeability, stomatal conductance, and the uptake of other ions (Belmecheri-Cherifi et al. 2019). Fenugreek has antioxidant and osmolyte defence to counter such physiological complications to a certain extent (Mickky et al. 2019). Foliar sprays of GA3 were noticed to enhance antioxidant generation in fenugreek plants compared to the control treatment. In this context, Maggio et al. (2010) suggested that GA3-induced protein synthesis could have increased antioxidant enzymes production and upregulated their activities. However, salt severity might exceed the threshold defence capacity and cause severe damage at higher concentrations, as was observed in our study with 100 mg L−1 of NaCl. It could be noticed that although at this dose, the activities of SOD, CAT, and APX were increased, the natural biochemical defence of the plant was failing to tolerate salinity effectively. Nevertheless, the exogenous application of GA3 might have ameliorated cellular stress by relieving antioxidant hyperproduction, as suggested by comparing salt-stressed fenugreek with GA3 supplied plants under salt stress. This could probably be the reason for GA3 induced reduction in antioxidant content in salt-stressed fenugreek.
High TBARS and H2O2 contents directly intoxicate the plasma membrane and negatively influence cytosolic metabolism through their cell structure breaking tendency (Wang et al. 2019). Thus, from the data, we can conceive an increased lipid peroxidation and electrolyte leakage in salt-stressed fenugreek plants. Nevertheless, the foliar supplementation of GA3 ameliorated salinity-imposed lipid peroxidation and electrolyte leakage, as indicated by lower TBARS and H2O2 contents. The antioxidant defence could likely have scavenged H2O2, while the osmolytes such as proline might have helped maintain membrane permeability through osmotic adjustments. In this context, it is noteworthy that plants can accumulate a very high concentration of organic and inorganic osmolytes and use them as a defencive measure against various stress conditions. The present study offered the same analogy with proline, where the highest proline content was found with the most severe salt stress. Apart from being an osmolyte, proline could also act as a storage house offering carbon and nitrogen for plant defence and development in dire situations (Matysik et al. 2002). Different studies have made similar observations where fenugreek accumulated proline besides other osmolytes in response to salinity exposure (Nair et al. 2017; Mickky et al. 2019). Additionally, proline can also assist the plant in cell expansion, pH homeostasis, and membrane stabilisation (Mickky et al. 2019). Interestingly, gibberellin seems to increase as well as decrease the proline content depending on the stress presence (Tuna et al. 2008). In the absence of stress, gibberellin enhances proline content while in stressed plants, it reduces proline content, probably by inhibiting lipid peroxidation (Tuna et al. 2008; Javid et al. 2011).
6 Conclusion and Future Perspectives
Fenugreek is an aromatic plant with substantial pharmacological potential. However, the growth, development, and yield of fenugreek crop are greatly restricted under salt stress. Increasing soil salinity is posing an imminent threat to fenugreek along with other agronomic crops. In the present study, we suggested an alternative for the salinity reversal through the exogenous supplementation of 10−6 GA3 in the fenugreek crop. This treatment was proved beneficial to a broad spectrum of morpho-physiological parameters in the fenugreek plants. Plant length and weight were elicited with this treatment as well as the seed number and yield were also enhanced. Plant sprayed with GA3 experienced better tolerance against salinity, and thus, salt concentration was unable to pose more significant damage in such plants than those without GA3. Moreover, such plants exhibited an upregulated antioxidant defense and osmoprotection. Overall, the present investigation suggested that crop enhancement in fenugreek could be conferred through exogenous application of GA3 in the plants under salinity stress. Considering the eliciting effects of GA3 in this study, similar treatments could also be instigated for conferring stress tolerance in other related crops with agronomical potential.
References
Afroz, S., Mohammad, F., Hayat, S., & Siddiqui, M. H. (2006). Exogenous application of gibberellic acid counteracts the ill effect of sodium chloride in mustard. Turkish Journal of Biology, 29(4), 233-236.
Ahmad, P. (2010). Growth and antioxidant responses in mustard (Brassica juncea L.) plants subjected to combined effect of gibberellic acid and salinity. Archives of Agronomy and Soil Science, 56(5), 575-588.
Apel, K., & Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol., 55, 373-399.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205-207.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44(1), 276-287.
Belmecheri-Cherifi, H., Albacete, A., Martínez-Andújar, C., Pérez-Alfocea, F., & Abrous-Belbachir, O. (2019). The growth impairment of salinized fenugreek (Trigonella foenum-graecum L.) plants is associated to changes in the hormonal balance. Journal of Plant Physiology, 232, 311-319.
Beyzi, E. (2020). Chemometric methods for fatty acid compositions of fenugreek (Trigonella foenum-graecum L.) and black cumin (Nigella sativa L.) seeds at different maturity stages. Industrial Crops and Products, 151, 112488.
Bitarafan, Z., Asghari, H. R., Hasanloo, T., Gholami, A., Moradi, F., Khakimov, B., Liu, F., & Andreasen, C. (2019). The effect of charcoal on medicinal compounds of seeds of fenugreek (Trigonella foenum-graecum L.) exposed to drought stress. Industrial Crops and Products, 131, 323-329.
Cakmak, I., & Horst, W. J. (1991). Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiologia Plantarum, 83(3), 463-468.
Chakraborty, K., Sairam, R. K., & Bhaduri, D. (2016). Effects of different levels of soil salinity on yield attributes, accumulation of nitrogen, and micronutrients in Brassica spp. Journal of Plant Nutrition, 39(7), 1026-1037.
Chandlee, J. M., & Scandalios, J. G. (1984). Analysis of variants affecting the catalase developmental program in maize scutellum. Theoretical and Applied Genetics, 69(1), 71-77.
Choudhary, S., Zehra, A., Mukarram, M., Wani, K. I., Naeem, M., Hakeem, K. R., & Aftab, T. (2021a). Potential uses of bioactive compounds of medicinal plants and their mode of action in several human diseases. In: Aftab T., Hakeem K.R. (eds) Medicinal and Aromatic Plants. Springer, Cham. https://doi.org/10.1007/978-3-030-58975-2_5
Choudhary, S., Zehra, A., Mukarram, M., Wani, K. I., Naeem, M., Khan, M. M. A., & Aftab, T. (2021b). Salicylic acid-mediated alleviation of soil boron toxicity in Mentha arvensis and Cymbopogon flexuosus: Growth, antioxidant responses, essential oil contents and components. Chemosphere, 130153.
Choudhary, S., Zehra, A., Mukarram, M., Naeem, M., Khan, M. M. A., Hakeem, K. R., & Aftab, T. (2021c). An insight into the role of plant growth regulators in stimulating abiotic stress tolerance in some medicinally important plants. In: Aftab T., Hakeem K.R. (eds) Plant Growth Regulators. Springer, Cham. https://doi.org/10.1007/978-3-030-61153-8_3
Corpas, F. J., Barroso, J. B., Palma, J. M., & Rodriguez-Ruiz, M. (2017). Plant peroxisomes: a nitro-oxidative cocktail. Redox Biology, 11, 535-542.
Davière, J. M., & Achard, P. (2013). Gibberellin signaling in plants. Development, 140(6), 1147-1151.
Dwivedi, R. S., & Randhawa, N. S. (1974). Evaluation of a rapid test for the hidden hunger of zinc in plants. Plant and Soil, 40(2), 445-451.
Eleiwa, M. E., Bafeel, S. O., & Ibrahim, S. A. (2011). Influence of brassinosteroids on wheat plant (Triticum aestivum L.) production under salinity stress conditions. I-Growth parameters and photosynthetic pigments. Australian Journal of Basic and Applied Sciences, 5(5), 58-65.
Foyer, C. H. (2018). Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environmental and Experimental Botany, 154, 134-142.
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909-930.
Hendawey, M. H. (2015). Biochemical changes associated with induction of salt tolerance in wheat. Global Science and Research Journal, 10, 84-99.
Iqbal, N., Umar, S., Khan, N. A., & Khan, M. I. R. (2014). A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environmental and Experimental Botany, 100, 34-42.
Javid, M. G., Sorooshzadeh, A., Moradi, F., Modarres Sanavy, S. A. M., & Allahdadi, I. (2011). The role of phytohormones in alleviating salt stress in crop plants. Australian Journal of Crop Science, 5(6), 726.
Jaworski, E. G. (1971). Nitrate reductase assay in intact plant tissues. Biochemical and Biophysical Research Communications, 43(6), 1274-1279.
Khan, M. M. A., Gautam, C., Mohammad, F., Siddiqui, M. H., Naeem, M., & Khan, M. N. (2006). Effect of gibberellic acid spray on performance of tomato. Turkish Journal of Biology, 30(1), 11-16.
Kuo, T. M., Warner, R. L., & Kleinhofs, A. (1982). In vitro stability of nitrate reductase from barley leaves. Phytochemistry, 21(3), 531-533.
Lichtenthaler, H. K., & Buschmann, C. (2001). Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Current Protocols in Food Analytical Chemistry, 1(1), F4-3.
Maggio, A., Barbieri, G., Raimondi, G., De Pascale, S. (2010). Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J Plant Growth Regul 29(1):63–72.
Matysik, J., Alia, Bhalu, B., & Mohanty, P. (2002). Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Current Science, 525–532.
Miceli, A., Moncada, A., Sabatino, L., & Vetrano, F. (2019b). Effect of gibberellic acid on growth, yield, and quality of leaf lettuce and rocket grown in a floating system. Agronomy, 9(7), 382.
Miceli, A., Vetrano, F., Sabatino, L., D’Anna, F., & Moncada, A. (2019a). Influence of preharvest gibberellic acid treatments on postharvest quality of minimally processed leaf lettuce and rocket. Horticulturae, 5(3), 63.
Mickky, B. M., Abbas, M. A., & Sameh, N. M. (2019). Morpho-physiological status of fenugreek seedlings under NaCl stress. Journal of King Saud University-Science, 31(4), 1276-1282.
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9), 405-410.
Mukarram, M., Khan, M. M. A., & Corpas, F. J. (2021a). Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield. Journal of Hazardous Materials, 412, 125254.
Mukarram, M., Khan, M. M. A., Uddin, M., & Corpas, F. J. (2021b). Irradiated chitosan (ICH): An alternative tool to increase essential oil content in lemongrass (Cymbopogon flexuosus). Acta Physiologiae Plantarum (in press).
Mukarram, M., Choudhary, S., Kurjak, D., Petek, A., & Khan, M. M. A. (2021c). Drought: Sensing, signalling, effects and tolerance in higher plants. Physiologia Plantarum, 172, 1291–1300.
Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol., 59, 651-681.
Naeem, M., Sadiq, Y., Jahan, A., Nabi, A., Aftab, T., & Khan, M. M. A. (2020). Salicylic acid restrains arsenic induced oxidative burst in two varieties of Artemisia annua L. by modulating antioxidant defence system and artemisinin production. Ecotoxicology and Environmental Safety, 202, 110851.
Nair, S., Gopalakrishnan, P., Umesh, S., Akshaya, S., Abhilasha, V. (2017). Evaluation of abiotic stress induced physiological and biochemical changes in Trigonella foenum-graecum. J. Biotechnol. Biochem. 3, 89–97.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22(5), 867-880.
Nazar, R., Iqbal, N., Syeed, S., & Khan, N. A. (2011). Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. Journal of Plant Physiology, 168(8), 807-815.
Okuda, T., Matsuda, Y., Yamanaka, A., & Sagisaka, S. (1991). Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiology, 97(3), 1265-1267.
Ouzir, M., El Bairi, K., & Amzazi, S. (2016). Toxicological properties of fenugreek (Trigonella foenum graecum). Food and Chemical Toxicology, 96, 145-154.
Ribeiro, D. M., Araújo, W. L., Fernie, A. R., Schippers, J. H., & Mueller-Roeber, B. (2012). Action of gibberellins on growth and metabolism of Arabidopsis plants associated with high concentration of carbon dioxide. Plant Physiology, 160(4), 1781-1794.
Shabala, S., & Cuin, T. A. (2008). Potassium transport and plant salt tolerance. Physiologia Plantarum, 133(4), 651-669.
Siddiqui, M. H., Khan, M. N., Mohammad, F., & Khan, M. M. A. (2008). Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. Journal of Agronomy and Crop Science, 194(3), 214-224.
Singh, M., Singh, V. P., & Prasad, S. M. (2016). Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiology and Biochemistry, 109, 72-83.
Tuna, A. L., Kaya, C., Dikilitas, M., & Higgs, D. (2008). The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environmental and Experimental Botany, 62(1), 1-9.
Ueguchi-Tanaka, M., Nakajima, M., Motoyuki, A., & Matsuoka, M. (2007). Gibberellin receptor and its role in gibberellin signaling in plants. Annu. Rev. Plant Biol., 58, 183-198.
Van Zelm, E., Zhang, Y., & Testerink, C. (2020). Salt tolerance mechanisms of plants. Annual Review of Plant Biology, 71.
Vetrano, F., Moncada, A., & Miceli, A. (2020). Use of Gibberellic Acid to Increase the Salt Tolerance of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy, 10(4), 505.
Wang, Y. H., Zhang, G., Chen, Y., Gao, J., Sun, Y. R., Sun, M. F., & Chen, J. P. (2019). Exogenous application of gibberellic acid and ascorbic acid improved tolerance of okra seedlings to NaCl stress. Acta Physiologiae Plantarum, 41(6), 93.
Watson, D. J. (1958). The dependence of net assimilation rate on leaf-area index. Annals of Botany, 22(1), 37-54.
Zehra, A., Choudhary, S., Mukarram, M., Naeem, M., Khan, M. M. A., & Aftab, T. (2020). Impact of long-term copper exposure on growth, photosynthesis, antioxidant defence system and artemisinin biosynthesis in soil-grown Artemisia annua genotypes. Bulletin of Environmental Contamination and Toxicology, 104(5), 609–618.
Zhu, J. K. (2001). Plant salt tolerance. Trends in Plant Science, 6(2), 66-71.
Acknowledgements
MM acknowledges Mr. Abbu Zaid (AMU, Aligarh, India) for his sincere help with experimentations.
Author Contributions
FM: Conceptualization, Methodology, Validation, Resources, Supervision. MN: Methodology, Validation, Visualization, Supervision, Writing - Review & Editing. MMAK: Visualization, Writing - Review & Editing. MM: Investigation, Software, Formal analysis, Data curation, Writing -Original Draft.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mukarram, M., Mohammad, F., Naeem, M., Khan, M.M.A. (2021). Exogenous Gibberellic Acid Supplementation Renders Growth and Yield Protection Against Salinity Induced Oxidative Damage Through Upregulating Antioxidant Metabolism in Fenugreek (Trigonella foenum-graceum L.). In: Naeem, M., Aftab, T., Khan, M.M.A. (eds) Fenugreek. Springer, Singapore. https://doi.org/10.1007/978-981-16-1197-1_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-1197-1_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1196-4
Online ISBN: 978-981-16-1197-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)