Abstract
Increased activities of enzymatic and non-enzymatic antioxidants provide a common reaction to environmental stress in most crops. To investigate the effect of foliar application of stress moderators on enzymatic and non-enzymatic antioxidant changes, field experiments were conducted as split-plot design based on a randomized complete block design with three replications in 2017 and 2018. Planting date (early and late) was considered as the main plot, and foliar applications with 50 and 100 mM of Glycine Betaine, 1 and 2 mM of salicylic acid, and 100 and 200 µM of sodium nitroprusside, along with the control treatment, were regarded as the subplot. The results showed that the amounts of chlorophyll pigments and the levels of enzymatic antioxidants (glutathione peroxidase, ascorbate peroxidase, superoxide dismutase, catalase), and proline increased, and H2O2 level decreased with the foliar application of stress moderators. Glycine betaine and sodium nitroprusside had the lowest and highest effects on the enzymatic antioxidant activities, respectively. The analysis of the main components revealed that in the early and delay planting date, the characteristics of enzymatic antioxidants and photosynthetic properties had the greatest effects on the enhancement of tolerance to salinity stress. In general, the results of this research demonstrated that the elevated activities of enzymatic antioxidants compared to non-enzymatic ones were more effective in reducing the impacts of salinity stress. Moreover, the foliar application of sodium nitroprusside at a concentration of 100 µM served as the most suitable moderator for augmenting the activities of enzymatic antioxidants in salinity stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biotic and abiotic stresses are the most important factors hindering the growth of crops and lowering the yield by affecting morphological, physiological, biochemical, and molecular processes. In addition to reducing the yield due to environmental stresses, abiotic stresses lead to oxidative stress in most cases. The production of reactive oxygen species (ROS) is one of the major effects of oxidative stress on plants, causing irreversible damage to plants by destroying cell membranes, oxidizing proteins, lipids, carbohydrates, nucleic acids, and chlorophylls, and inhibiting enzyme activities (Venkateswarlu et al. 2011). Plants use two effective protective mechanisms, enzymatic and non-enzymatic antioxidant defenses, to counteract the damage caused by oxidative stress. In the non-enzymatic antioxidant defense system, low-molecular-weight compounds with high solubility, which do not interfere with cellular functions, accumulate. Non-enzymatic antioxidants include ascorbic acid, glutathione, phenolic compounds, alkaloids, carotenoids, and α-tocopherols accumulating in various organs, such as chloroplasts, mitochondria, peroxisomes, and apoplasts. Among enzymatic antioxidant groups, catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), ascorbate peroxidase (APX),glutathione reductase (GR), dihydro ascorbate reductase (DHAR), guaiacol peroxidase (GP), and glutathione S-transferases (GSTs) are involved in purifying ROS and reducing the effects of environmental stress (Hossain et al. 2019).
Although the syntheses and accumulations of enzymatic and non-enzymatic antioxidants increase in response to environmental stress in most plants, research in recent decades has shown that the exogenous application of signaling molecules such salicylic acid, sodium nitroprusside (SNP), jasmonic acid (JA), or organic osmolytes such as proline, glycine betaine (GB), ascorbic acid, can enhance their syntheses or accumulations as well (Hasanuzzaman et al. 2013). SNP is a plant growth regulator that can act as an intermediary in plant growth regulation. In many studies, it has been reported to be involved in transmitting messages and responding to biological and non-biological stresses. Zheng et al. (2009) reported the effects of increased tolerance to salinity stress with no application due to the enhanced activities of SOD and CAT enzymes, reduced MDA level, and accelerated production rate of single O2− in mitochondria. The external use of NO augments Na+/H+ proton-pump and antiport activity (Wang et al. 2009). Dong et al. (2014) reported that SNP application increased the growth and inhibited accelerated production rate of superoxide anion (O2−). Besides, the activities of antioxidant enzymes, such as CAT, SOD, and APX, were enhanced by SNP foliar application. Increased levels of K+ and antioxidant enzyme activities, along with the reduction of Na+/K+ and MAD ratios, were also reported with 0.1 mM of SNP application. Glycine betaine is a quaternary ammonium compound found amply in chloroplasts, which plays a vital role in regulating and protecting the thylakoid membrane. Hu et al. (2012) reported that the external use of GB increased the tolerance to the oxidative stress triggered by salinity stress in the cells of tobacco via modulation of GB activity in external stress conditions via modulating the activities of the Ascorbate–Glutathione (AsA–GSH) pathway, and subsequently reducing glutathione peroxidase (GPX), enzyme activities of the glycolytic system, and protein oxidation. Also, it has been reported that the external use of GB in salinity stress conditions causes tolerance to salinity stress in mung bean through the activation of antioxidant enzymes and the reduction of H2O2 and lipid peroxidation (Hossain and Fujita 2010). Nawaz and Ashraf (2010) also reported that the external use of GB in corn increased the activities of CAT, SOD, and POD enzymes. Kotb and Elhamahmy (2014) showed that GB application at an appropriate concentration (50 mM) in wheat under salinity stress conditions significantly enhanced antioxidant enzyme activities, total chlorophyll content, osmotic potential of leaves, and the K+ content in leaves and seeds. Salicylic acid is another growth regulator increasing the activity of enzymatic and non-enzymatic antioxidant enzymes in environmental stress conditions. Salicylic acid or ortho-hydroxy benzoic acid is a growth regulator regulating the physiological processes of plants. Flowering, plant growth, ethylene production, respiration, and opening and closing of stomata are among the functions of salicylic acid (Hayat and Ahmad 2007). The enhanced expression of APX and SOD genes were reported by using salicylic acid at the appropriate time in salinity stress conditions. On the other hand, salicylic acid treatments during salinity stress in arabidopsis increased the SOD levels of glutathione peroxidase and guaiacol peroxidase (Jayakannan et al. 2013). The use of SA elevated the total contents of phenols and sugars and POD, CAT, and APX activities (Arif et al. 2020).
Planting date plays a very important role in the use of maximum environmental factors. Determining the best planting time in cotton depends on several factors such as soil temperature, water solubility, the occurrence of diseases, pests, and weeds, and harvesting time of crops in rotation with cotton (Abbas et al. 2019). Low seedling establishment due to low temperatures and high insect pest incidence in early planting date and reduction in cotton yield due to delayed physiological maturity and carbohydrate deficiency in late planting date are the main challenges in cotton production (Afzal et al. 2020). In Iran, farmers delay the planting date for a month after March 30 (the recommended sowing date to get complete winter crop, especially wheat before cotton). Delay in sowing significantly reduced seed cotton, seed index, and dry matter yield, whereas improved the oil content. There was no significant relationship with planting date and fiber traits like fiber length, uniformity, and spinning consistency index. However, delay in sowing decreased micronaire, fiber strength, and fiber elongation, and increased the short fiber content (Killi and Bolek 2006).
Cotton is one of the most important natural fiber-producing plants in the world, in which more than 100 countries are producing cotton, and the total worldwide yearly planted area is 33 M ha. The total demand for cotton is expected to reach 28.7 Mt in 2027 (Khan et al. 2020). Its relative resistance to salinity stress has made cultivation of this crop to be welcomed by farmers in saline soils. However, in saline water or soil conditions, the growth and yield of this plant decrease (Dong et al. 2014; Zhang et al. 2014). Although in stressful conditions, plant cells reduce the negative effects of ROS by accumulating antioxidant and non-antioxidant enzymes, it is well-known that the external use of some substance under these conditions can increase the production of enzymatic and non-enzymatic antioxidants, as well as stress tolerance, in plants (Hossain et al. 2019). In many studies, only the effect of a single moderator has been considered by researchers, and there is little information to determine the most appropriate stress modulator in cotton under salinity stress conditions. Therefore, in this investigation, moderator-type effects on the physiological and biochemical traits of cotton were determined under saline conditions.
2 Materials and Methods
These experiments were arranged in a split-plot design in a completely randomized design. The planting date (early and late) was considered as the main plot and the foliar applications with 50 and 100 mM of Glycine Betaine (GB) (Barzoi et al. 2020), 1 and 2 mM of Salicylic Acid (SA) (Arif et al. 2020), and 100 and 200 µM of Sodium Nitroprusside (SNP) (Timachi et al. 2020), along with the control treatment, were regarded as the subplot. Triton 100× was utilized as a surfactant at 1% concentration to better absorb the foliar application of modulators. In the early flowering stage (61 BBCH scale), the plants were sprayed with the stress moderator.
The experiments were carried out at a private farm located at 57° 44′ east latitude and 36° 13′ north longitude with a height of 990 m above sea level during 2017–2018, Razavi Khorasan, Iran. According to soil analysis results (Table 1), the studied soil was of a loamy clay type with a pH of 7.2, EC of 10.5 ds m−1, and total N, P, and K contents of 0.02%, 110, and 4 mg/kg, respectively. The rainfall and the mean temperature data during the cotton-growing stages are given in Table 2.
The experimental field went under fallow and wheat cultivation during the first and second years. The seed-bed preparation included moldboard plowing in the autumn and harrowing and leveling in the spring. According to soil analysis results (Table 1), 100 kg P2O5 ha−1 as triple superphosphate and 100 kg K2O ha−1 as potassium sulfate, together with one-third of 150 kg N as urea, were uniformly broadcasted before sowing. Another N fertilizer was top-dressed at the first hand weeding (about 40 days after emergence) and early flowering stages. Cotton seeds (Varamin cultivar) were obtained from Agricultural Jihad in Sabzevar and sown on April 15, 2017, and April 20, 2018, in early planting date, and June 17, 2017, and June 20, 2018 in delay planting date. Each plot contained four 5-m-long rows spaced 50 cm from each other, along which the plants were planted at a distance of 20 cm. After completing the planting stage and at the 5–6 leaf stage, the plants were thinned in the rows at a distance of 20 cm from each other to obtain an optimum density. Irrigations were done every 10 days during the growing season according to the local custom. Weeding (weed removal) was done manually. Before sowing, the seeds were disinfected with Carboxin-Thiram (Vitavax) at a ratio of ‰2. To combat thrips pest, they were impregnated with Larvin (Thiodicarb) at a ratio of ‰7.
2.1 Plant Sampling
All the assays were performed on the 4th extended leaf 30 days after spraying.
The content of photosynthetic pigments was determined by using Lichtenthaler and Wellburn (1983). The content of chlorophyll and carotenoids was measured via spectrophotometry at 646, 663, and 470 nm and expressed in mg/g fresh weight (mg g−1F.W).
-
Chlorophyll a = 12.25 A663 − 2.79 A646
-
Chlorophyll b = 21.21 A646 − 5.1 A663
-
Carotenoides = (1000 A470 − 1.8 chl a − 85.02 chl. b)/198
2.2 Proline Concentration
The method applied by Bates et al. (1973) was employed to measure the leaf proline content. To this aim, 0.2 g of the leaf tissue was weighed and thoroughly gelled in 3 mL of 3% sulfosalicylic acid in Chinese mortar. Upon centrifuging the homogenates at 18,000 rpm for 15 min, 2 mL of the filtered extract was transferred to the gut tubes, and 2 mL of ninhydrin reagent and 2 mL of glacial acetic acid were added to all tubes. Then, they were placed in 100 °C water for 1 h. After cooling the tubes, 4 mL of toluene was added to each tube. The tubes were vortexed for 15–20 s to allow the formation of two separate phases. The top phase was read at 520 nm by the spectrophotometer. Proline concentration in mg/g of fresh leaf tissue was determined using the standard curve. The unit was expressed as mg/g fresh weight.
2.3 Enzyme Extraction
For enzyme extraction, 0.5 g(fresh weight) of the frozen leaf tissue was grounded with mortar in liquid N2 to obtain a fine powder, which was then homogenized in 5 mL of an extraction buffer, containing 50 mM cold buffered monophosphate (pH 7.8), 0.1 mM EDTA, 0.3% TritonX-100, and 4% polyvinylpolypyrrolidone. The homogeneous material was centrifuged for 15 min at the gravity of 15,000 g at 4 °C. The upper transparent phase was applied to measure enzyme activity.
2.4 Catalase Activity Measurement
The CAT(E.C. 1.11.1.6) activity was determined using the method of Aebi (1984). For a total volume of 3 mL, 30 μL of the extract was applied to 50 mM buffered monophosphate (pH 7.0) and 2% H2O2. The enzyme activity was measured at 240 nm for 2 min using a spectrophotometer (Cary 100) and expressed as changes in the absorbance units (ΔA) min−1 mg protein−1
2.5 Superoxide Dismutase Activity Measurement
The superoxide dismutase (SOD; E.C. 1.15.1.1) activity in the leaf extracts was measured using the inhibition measurement of nitroblue tetrazolium (NBT) photochemical reduction (Giannopolitis and Ries 1977). The reaction mixture had a final volume of 3 mL and contained 2.25 mL of 50 mM potassium phosphate buffer (pH = 7.8), 200 μL of EDTA (0.66 mM), 300 μL of methionine (10 mM), 150 μL of NBT (66 μM), 50 μL of riboflavin (3.35 μM), and 50 μL of the enzyme extract. The reaction was carried out in a transparent glass cuvette at 25 °C under the same white light intensity for all samples. A special cuvette for each sample was placed in the dark as a blank (zero reaction time control). After shining the light for 15 min, the light absorption at 560 nm was read and the absorption difference was set to zero for 15 min. The NIT inhibitory ability in each sample was determined by the enzymatic extract of each sample, as compared to the light absorption difference of 0 and 15 min for the control reaction (without enzyme) according to the following formula: One unit of superoxide dismutase activity equivalent to 50% inhibition of the NBT photoreduction. Finally, the enzyme activity was reported based on the superoxide dismutase unit/min/g fresh weight.
2.6 Ascorbate Peroxidase Activity Measurement
The ascorbate peroxidase (APX; E.C. 1.11.1.1) activity was determined as described by Nakano and Asada (1981). The 3-mL reaction mixture contained 312.5 mM sodium phosphate buffer (pH = 7), 0.5 mM sodium ascorbate, 0.1 mM ethylenediaminetetraacetic acid, 2.2 1 mM hydrogen peroxide, and 50 µL of the enzyme extract. The reaction was started by adding hydrogen peroxide to the reaction mixture, and then, changes in the light absorption relative to the control reaction without hydrogen peroxide were recorded. The conversion rate of ascorbate to dehydroascorbate was measured for 60 s at 290 nm, and finally, the enzyme activity was determined by using an ascorbate extinction coefficient of 18 mM−1 cm−2 expressed as the change in μM ascorbate consumed per min/g fresh weight.
2.7 Guaiacol Peroxidase (GPX) Measurement
The guaiacol peroxidase (EC 1.11.1.7) activity was measured according to the method of Lin and Kao (2001). In this approach, 3 mL of the reaction mixture containing 50 mM potassium phosphate buffer (pH = 7), 9 mM guaiacol, 19 mM H2O2, and 50 mM enzyme extract was allowed to increase absorption via guaiacol oxidation at 470 nm, which was measured for 1 min. One unit of peroxidase was defined as the amount of enzyme, causing the formation of 1 mM of tetraguaiacol per minute by using the extinction coefficient of 26.6 mM−1 cm−2.
The data were subjected to the analysis of variance (ANOVA) using SAS software. The means were compared using the least significant difference test (p < 0.05), and the correlation coefficients between the traits were done by using proc CORR of SAS. The principal component analysis (PCA) was performed based on the correlation matrix to reduce the multiple dimensions of data space, and the biplot was drawn using Stat Graphics software (Stat Graphics centurionXVI version 18, StatPoint Technologies, Inc).
3 Result and Discussion
3.1 Chlorophyll a Content
Chlorophyll a content was affected by moderator type and the interaction effects of planting date and moderator type (Table 3). The mean comparison of treatments indicated that SA at the 1 mM in early planting date and SA at the concentration of 2 mM in late planting date produced the highest amount of chlorophyll a. As shown in Fig. 1, GB at a concentration of 50 mm led to 60.8% less chlorophyll a content in the delayed planting compared to the early planting date, while the difference reached 31% at the concentration of 100 mm. In contrast to GB, the higher concentration of SNP resulted in higher differences in the content of chlorophyll a, while changes in chlorophyll a content were almost identical at varied SA concentrations. Under early planting, the increasing and decreasing amount of chlorophyll a by respectively enhancing SA and GB concentrations and SNP concentration indicated that high SNP concentration could intensify stress effects by producing more oxygen-free radicals. The augmented amount of chlorophyll a, observed in delayed planting with the increase in SNP concentration, might be also caused by the availability of fewer leaves or less development of leaf area and more exposure of leaves to high temperatures, leading to its more absorption than its inhibitory concentration. The reduction in chlorophyll a content exposed to salinity stress due to chloroplast and photosynthetic system degradation, chlorophyll photooxidation, reaction with ROSs, degradation of chlorophyll precursors, inhibition of new chlorophyll biosynthesis, activation of chlorophyll-degrading enzymes, such as chlorophyllase, and hormonal abnormalities are the reasons for lowered chlorophyll content in saline conditions. In this condition, use of stress modulator such as SA, GB and SNP mitigated salinity stress on Chlorophyll a content. The SA increasing role of chlorophyll content is attributed to the stimulation of the enzymes related to chlorophyll biosynthesis or inhibition of photosynthetic system disturbance, thereby alleviating chlorophyll degradation.
Chavoushi et al. (2020) reported that the SA application decreased the content of chlorophyll a in non-stress conditions, but its foliar application increased the chlorophyll a content by 75% in stressful situations. Liu et al. (2014) evidenced that chlorophyll a content in cotton decreased by 25.27% in salinity conditions; however, 0.1 mMSA foliar application increased chlorophyll a content by 2.10% compared to no salinity stress, and 36.46% compared to salinity stress conditions. Foliar spraying with SNP had greater positive effects on the content of chlorophyll a, so that it increased by 27.68% and 70.64% compared to those of non-stress and stress conditions, respectively.
3.2 Chlorophyll b Content
The chlorophyll b content was affected by the planting date and moderator type, but the interaction of planting date and moderator type on its amount was not significant (Table 3). The late planting date led to a 40.1% reduction in its content (Table 4). The highest chlorophyll b content (3.12 mg/g frwt) was obtained by the spraying of 1 mM SA, which was statistically significantly different from those of all the other experimental treatments. The application of 100 µM of SNP and 50 mM of GB did not lead to a statistically significant difference from the control. The lowest content of chlorophyll b was observed with 100 µM of SNP application (Table 4). The role of chlorophyll b in the plant photosynthetic system is receiving light in the light-harvesting complex and transferring it to chlorophyll a. When there is no stress and more leaf area is available, the plant hardly needs to strengthen its light absorption complex, and this can be a reason for the less content of chlorophyll b in non-stress conditions. Nevertheless, due to the negative effects of stress on osmotic potential and cell division and ultimately leaf production, there would be an increased need for chlorophyll b under environmental stress. Under such conditions, the external use of stress modulators enhances the chlorophyll content by inducing gene expression for chlorophyll synthesis. In this study, it seemed that the increasing effects observed with the SA application were due to the greater role of this substance in stimulating chlorophyll b synthesis. However, high SNP concentrations were shown to improve the chlorophyll b synthesis. Researchers have reported that the use of SNP augments the chlorophyll b content in the cotton plant. Liu et al. (2014) reported a 15.24% increase in the content of chlorophyll b in cotton compared to non-foliar application in no-salinity stress conditions, while foliar spraying with 10 µM of SNP under salinity stress caused an enhancement of 63.58% in chlorophyll b content. Foliar spraying with SA in the absence of stress and salinity stress conditions also resulted in a decrease of 8.14% and an increase of 30.37% in the chlorophyll b content, respectively. Moreover, the concomitant use of SNP + SA enhanced the content of chlorophyll b by 27 and 80.36% in non-stress and salinity stress conditions, respectively.
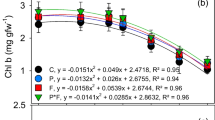
3.3 Total Chlorophyll Content
The total chlorophyll content was affected by the type of stress moderator and the interaction of the planting date and moderator type (Table 3). As with the changes in the content of chlorophyll a, the highest amount of total chlorophyll was produced by using 2 mM of SA, which was statistically significantly different from those of the other experimental treatments. In the early planting date, increasing both SA and GB concentrations led to the enhanced content of total chlorophyll, while augmenting the SNP concentration reduced its amount. However, in the delayed planting date, the increased concentration of SA and SNP was associated with the decreased content of total chlorophyll, and the enhanced concentration of GB was accompanied by the increased content of total chlorophyll (Fig. 2). Chlorophyll and proline are both derived from a precursor called glutamate. It seems that the cells in the precursor become deficient, and chlorophyll synthesis decreases with nitrogen metabolism alteration and proline production enhancement under salinity stress conditions. The increased activity of proteolytic enzymes like chlorophyllase also plays an important role in chlorophyll degradation. On the other hand, the elevated amount of ROS and sodium ions in these conditions would be toxic to many biomolecules, including proteins involved in chlorophyll synthesis, thus alleviating the syntheses of these pigments. The positive effects of nitric oxide in such conditions are due to ROS removal and preservation of photosynthetic pigment structures. The increasing role of SA in chlorophyll content can be attributed to the stimulation of enzymes associated with chlorophyll biosynthesis or photosynthetic system inhibition, which in turn declines chlorophyll degradation. Besides, the effects of SA enhancer on photosynthetic capacity can be ascribed to its stimulatory impacts on Rubisco activity and pigment contents. It was reported that the foliar application with SNP (100 µM) under salinity stress conditions elevated the content of total chlorophyll from 834.24 to 1453.58 mg.kg, while the foliar application with SA enhanced its content to 1121.16 mg/kg. Yet, their combined foliar spraying had a greater positive effect on its content, increasing it to 1716.04 mg/kg (Liu et al. 2014). Also, in wheat, it was shown that the content of total chlorophyll of flag leaf was reduced by 36.81 and 28.83% at the salinity of 10.2% in the first and second years without GB application, while the foliar application at the concentration of 50 mm led to the enhancement of the total chlorophyll content by 25.5 and 44.9% in the mentioned years of study, respectively (Kotb and Elhamahmy 2014).
3.4 Carotenoid
The content of carotenoids was not affected by planting date; however, the moderator type and the interaction effect of planting date and moderator type had significant effects on the content of carotenoids similar to the conditions of salinity stress (Table 3). Changes in the content of carotenoids in the early planting date were less than in those that occurred in the delayed planting. Nevertheless, there were no statistically significant differences between the different types of the consumed moderators in the early planting date; however, significant differences were observed between the treatments in addition to more differences in the content of carotenoids in the delayed cultivation. Increasing the application dose of SA decreased the content of carotenoids in the early planting date, whereas increasing its concentration did not have much effect on their content in the delayed cultivation. These changes for GB were the opposite of SA. The increasing dose of GB application increased and decreased the content of carotenoids in the early and delayed planting date, respectively.
The content of carotenoids was reduced by enhancing SNP concentration both in the early (38.3%) and delayed (32.6%) planting date (Fig. 3). Plants have an antioxidant defense system to deal with oxidative stress caused by enzymes and antioxidant compounds, including carotenoids. Carotenoids convert single oxygen to triplets and play their antioxidant roles in capturing ROS. In the present research, the use of stress modulators had positive effects on carotenoids. It seemed that the application of stress modulators led to their stronger reactions with ROS. Since ROS are the main factors causing damage to and breakdown of photosynthetic pigments, as well as degradation of the structural proteins of the photosynthetic apparatus under stress, the use of stress modulators was able to prevent the reduction of carotenoids triggered by ROS. It was reported that salinity stress enhanced the content of carotenoids by 12.8% compared with those obtained in non-stress conditions. The foliar application of SNP increased the content of carotenoids by 8.99 and 17.81% compared to those achieved in the salinity and non-salinity stress conditions, respectively. The amount of enhancement for the foliar application with SA was 176.2 and 155.1%, respectively (Yadu et al. 2017; Zheng et al. 2009).
3.5 H2O2 Content
The H2O2 content was affected by the date of planting and type of stress moderator; however, the interaction of the planting date and moderator type had no significant effect on its content (Table 3). The delayed cultivation compared to the early cultivation date led to 18.91% higher H2O2 content. The non-foliar application resulted in the highest H2O2 content, and the foliar application with stress moderators reduced its content. The highest reduction (34.19%) was obtained with the foliar application of SA at the concentration of 2 mM. Although foliar application with 50 mM of GB and 200 µM of SNP produced lower H2O2 content in comparison with the control group, they did not prevent H2O2 accumulation as much as the other moderators did (Table 4). Under normal conditions, many metabolic processes in plants produce ROS. Fortunately, plants have effective antioxidant mechanisms to eliminate them. Nonetheless, this balance is disturbed by stress, leading to an increased amount of ROS. The presence of these active species would be harmful to plants, causing damage to cellular structures, such as membranes, proteins, and nucleic acids. In this article, the increase in the content of H2O2 under stress conditions might be also the result of this imbalance. Yet, it is believed that the use of stress modulators mitigates the amount of H2O2 by lowering ROS production. In this research, the internal antioxidant system of cotton could not eliminate ROS, and thus, the highest amount of H2O2 was observed in the control treatment. Moreover, the enhancement of H2O2 content by increasing SNP concentration might be because this substance acts as an oxidative agent at high concentrations and induces ROS production while representing a protective role at low concentrations. Additionally, the lower ability of GB to scavenge ROS might be due to this fact that it has less effect on the production of antioxidant enzymes compared to SA. The enhancement of H2O2 levels in response to environmental stresses like salinity stress has been reported in many studies. The external use of GB or Pro causes ROS scavenging by augmenting the activities of enzymatic and/or non-enzymatic antioxidants in saline conditions, thereby reducing H2O2 levels and alleviating cell membrane damage (Hossain and Fujita 2010). It was reported that 24 and 48 h after applying the salinity stress of 300 mmol in mung bean, H2O2 levels increased by 21 and 48% compared to the control treatment, respectively. Forty-eight hours after treatments with BG and Pro, its levels decreased by 18 and 12%, respectively (Hossain and Fujita 2010). Previous studies have shown that the external application of stress modulator decreases the oxidative damage caused by drought stress. The inhibitory effects of some stress modulators like SNP at high concentrations could be the reason for the increase in the H2O2 content by increasing foliar application in dry conditions. When the plant is under drought stress, synergistic effects are observed to have a counteraction between stress and moderators (Timachi et al. 2020). Kumar and Yadav (2009) reported that salinity stress significantly elevated the H2O2 level in soybean so that it was 245.45% higher in the cells compared to that of the control treatment. The external use of GB had moderating effects on H2O2 levels, and the application of 5, 25, and 50 mM GB in saline conditions lowered its level by 53, 60, and 66% compared to no-GB use in saline conditions, respectively (Malekzadeh 2015).
3.6 Proline (Pro) Content
The amount of proline was affected by the planting date, the type of stress modulator, and their interaction (Table 3). In the early planting date, enhancing the application dose of the studied moderators increased the Pro contents of cotton leaves. Under delayed cultivation, the foliar application with 100 mM of GB produced the highest Pro content. In the early planting date, only increasing the dose of GB led to enhanced Pro content, while augmenting the consumed doses of SA and SNP slightly reduced its contents, the higher reductions of which were observed with higher doses of SNP (Fig. 4). Accumulation of metabolites that act as adaptive receptors is one of the most common plant reactions in environmental stress conditions. Pro as an organic molecule can alleviate the effects of salinity in salinity stress conditions via osmotic regulation, stabilizations of subcellular structures, and energy sources, and even signalization (Hossain et al. 2019). High proline production can decrease the negative effects of salinity stress by increasing intracellular osmotic pressure. This increase in proline levels persists for about 1 month, even after the stress conditions have been resolved. However, its enhancement, caused by salinity stress, is caused by the activation of enzymes in the glutamate pathway since sodium chloride stimulates the genes that synthesize them. Its elevated amount caused by the application of SNP treatment can be explained by the fact that this substance as a nitric oxide donor can augment the activity of Pyrroline-5-carboxylate synthase (P5CS) enzyme. Nitric oxide also indirectly enhances P5CS activity by inducing ABA synthesis and proline production through the ABA-dependent pathway. Also, the use of SNP in salinity stress conditions can increase proline synthesis by activating enzymes in the ornithine pathway and lowering proline dehydrogenase activity. By using GB after the reduction of stress effects, proline production is expected to decrease; however, its increase in this experiment might be due to GB participation in changing the metabolism of amino acids into the production of proline or other amino acids associated with proline biosynthesis. It was reported that the external use of GB increased Pro accumulation both in saline and non-saline conditions. Under non-saline conditions, the enhanced concentration of GB from 0 to 50 mm led to slight changes in Pro content (2.71%), whereas its content was augmented by 10.40% in saline conditions (Malekzadeh 2015).
3.7 Superoxide Dismutase (SOD) Activity
Superoxide dismutase (SOD) activity was affected by the type of stress moderator; however, the date of planting and the interaction of the planting date and moderator type had no significant effects on its content (Table 3). The highest SOD activity was obtained by the spraying of 2 mM SA, which was statistically significantly different from those of all the other experimental treatments except SNP 100 μM. The application of 50 mM of GB did not lead to a statistically significant difference from the control (Table 4). The increase in superoxide dismutase activity under stress conditions may be due to the increase in H2O2 content. It is believed that the enhanced activity of antioxidant enzymes like superoxide dismutase in stress conditions plays an important role in reducing or eliminating H2O2. In situations where the antioxidant activity of plants is not able to alleviate the negative effects of ROS, the external application of stress modulators can augment antioxidant production in the plant and enhance stress resistance. The similar superoxide dismutase reaction to the SA and SNP application indicated that the growth hormone or pseudo-hormone was more rapidly absorbed and transported compared to non-hormonal compounds like GB, inducing more and faster production of antioxidant enzymes, such as superoxide dismutase. Many researchers believe that SOD is the first defense enzyme in plants to counteract the oxidative stress induced by other environmental factors like salinity by rapidly converting O2 to H2O2 (Alhoshan et al. 2019). The SOD enzyme activity has shown a positive reaction to the external use of GB in both saline and non-saline conditions; however, its reaction to elevated GB concentration is higher in non-saline conditions (Malekzadeh 2015). In wheat, it was reported that the foliar application of GB led to a significant increase in SOD activity in saline conditions. The spraying at a concentration of 50 mM enhanced the SOD activity by 24%; however, it was not statistically significantly different from the case of spraying with 75 mM (Kotb and Elhamahmy 2014).
3.8 Catalase (CAT) Activity
Catalase (CAT) activity was affected by the type of moderator and the interaction of planting date and moderator type, while the planting date did not influence its activity. SNP application at a concentration of 200 µM produced the highest rate of CAT activity in the delayed cultivation. At this condition, GB at the concentration of 100 mM led to higher CAT activity after SNP. Moreover, higher concentrations of moderators resulted in more CAT activity. This mechanism was true with the high doses of SA and SNP under the early planting date (Fig. 5). In the early planting date, the use of SA and SNP decreased CAT activity, caused by SA inhibitors, while the reason for its inhibitory role is not yet clearly known. It is believed that the chelation of heme protein might be the reason for the inhibitory effects of SA and other phenolic compounds. It was reported that SA + SNP foliar application displayed the same effects as SNP in terms of CAT enzyme activity (Liu et al. 2014). In soybean, salinity stress significantly augmented the activity of CAT enzyme, and pre-treatment with GB significantly enhanced its activity in salinity stress conditions; however, increasing GB concentration led to little change in its activity in non-saline conditions. In saline conditions, by augmenting GB concentration, CAT enzyme activity was linearly elevated (Malekzadeh 2015).
3.9 Ascorbate Peroxidase (APX) Activity
The activity of ascorbate peroxidase (APX) enzyme was affected by the planting date and the type of moderator; however, the date of planting and the interaction of the planting date and moderator type had no significant effects on APX activity (Table 3). The delayed planting increased the APX content. The activity of the APX enzyme in the delayed planting was 7.85% higher than that obtained from the early planting date. Foliar spraying with SA at the concentration of 2 mM led to the highest APX activity, which did not significantly differ from the case of an SNP concentration of 200 µM. Among the moderators, foliar spraying with GB at the concentration of 50 mM resulted in the lowest APX activity (Table 4). Ascorbate peroxidase converts hydrogen peroxide into the water by converting ascorbate molecule into monohydrohydrocarbonate thereby regulating ROS levels since ascorbate peroxidase has a higher affinity for reacting with H2O2 compared to catalase enzyme. When widely distributed in the cell, it would be a more efficient enzyme for controlling oxidative damage caused by salinity stress. The application of stress modulators, such as SA or SNP as signaling molecules under salinity stress, would change the expression of transcription factors and thus enhance the activity of antioxidant enzymes like ascorbate peroxidase. Malekzadeh (2015) reported that the external use of GB in salinity stress conditions enhanced the APX activity, which is consistent with the results of this study. In wheat, foliar spraying with GB at a concentration of 50 mM increased the APX enzyme activity by 11% in saline conditions (Kotb and Elhamahmy 2014). The researchers attributed the augmented APX enzyme activity associated with SOD to the enhanced ROS levels triggered by salinity stress (Hossain and Fujita 2010; Hossain et al. 2019; Kotb and Elhamahmy 2014; Liu et al. 2014; Timachi et al. 2020; Zheng et al. 2009).
3.10 Glutathione Peroxidase (GPX) Activity
The activity of glutathione peroxidase (GPX) was affected by planting date and type of consumed moderator; however, the interaction between the planting date and moderator type did not affect its activity (Table 3). Like other antioxidant enzymes, the GPX activity was further enhanced by delayed planting. Spraying with higher doses of GB, SA, and SNP elevated the GPX activity. Among the moderators employed, spraying with 200 μM of SNP led to the highest GPX activity. Spraying with GB, especially at lower doses (50 mm), could not stimulate GPX enzyme activity, although it resulted in a statistically significant difference from that obtained from the control treatment (Table 4). Peroxidase is a heme protein that utilizes oxidized phenolic materials as electron donors to sweep H2O2. Applying SNP as a signaling molecule of nitric oxide donor can elevate intracellular calcium concentration. The increased content of calcium ions has a significant effect on cellular mechanisms, including activation of such enzymes as peroxidase. For this reason, the enhanced concentration of salinity and SNP in canola is reported to be associated with augmented peroxidase enzyme content. In mung bean, it was reported that salinity stress significantly enhanced the GPX activity. Under this condition, the external use of Pro or GB augmented the activity of GPX enzyme, the highest activity of which was observed 48 h after GB application (Hossain and Fujita 2010). This report suggests that GB plays an important role in GSH biosynthesis and metabolism (Fig. 6).
3.11 Relationship Between the Traits
The correlation coefficients between enzymatic and non-enzymatic antioxidants for the early planting and delayed planting are shown in Table 5. In the early planting date, the CAT enzyme activity had a high correlation with the content of chlorophyll a and total chlorophyll, while there was a very low correlation between Pro content and the CAT enzyme activity. There was a strong correlation between H2O2 levels and the activity of enzymatic antioxidants, such as CAT (25.6%), APX (0.62), SOD (3%), and GPX (54%), while there was a weak correlation between H2O2 levels and the activities of non-enzymatic antioxidants, such as Pro (0.69) and carotenoids (0.46). In the delayed planting date, the correlation between non-enzymatic antioxidants was as follows: Pro and CAT (0.46), APX (0.14), SOD (0.13), and GPX (0.38), and between carotenoid and CAT (0.23), APX (0.29), SOD (35), and GPX (0.19). This indicated that the lower synthesis or accumulation of enzymatic and non-enzymatic antioxidants occurred in the delayed planting; thus, more salinity stress effects on similar physiological traits were documented. Also, in the conditions of drought stress in potatoes, the SOD activity was reported to be positively correlated with the content of chlorophyll a, chlorophyll b, and total chlorophyll and negatively correlated with the ion leakage (Table 6).
3.12 Biplot Analysis
The analysis of the main components demonstrated that the first and second components described 63.71 and 65.77% of the diversity in the data in the early planting date and delayed planting, respectively. In the early planting date, a positive and strong relationship was observed between Pro levels and the activities of APX and GPX enzymes in the first component. The high levels of these enzymes were indicative of their key roles, and thus, the first component could be considered as having antioxidant properties. In the second component, a positive relationship was evidenced between the content of chlorophyll a, chlorophyll b, and total chlorophyll and CAT enzyme activities, according to which this component could be regarded as having photosynthetic properties. Contrary to the early planting date, the delayed planting led to the highest positive relationship of photosynthetic properties, such as chlorophyll a, chlorophyll b, and total chlorophyll contents, in the first component, whereas in the second component, antioxidant enzymes had a stronger relationship with each other. Based on this, it can be said that in conditions of salinity stress with delay in planting, maintaining the photosynthesis of the plant is of higher importance compared to the synthesis or accumulation of enzymatic and non-enzymatic antioxidants, while in early planting condition, plants can find the necessary opportunity and energy to produce enzymatic and non-enzymatic antioxidants. Therefore, enzymatic and non-enzymatic antioxidants and photosynthetic properties play important roles in tolerating salinity stress in the conditions of early and delayed planting dates, respectively. Similar to the above results, positive and strong relationships of photosynthetic pigments and the properties of enzymatic and non-enzymatic antioxidants with SA and GB foliar application in drought stress conditions were reported, although responses of the studied cultivars to the consumed SA and GB concentrations were different. Therefore, the use of GB and SA would increase the tolerance to drought stress in drought stress conditions.
4 Conclusion
Our results revealed that the foliar application of SNP in saline condition was the best treatment for increasing antioxidant enzyme activities and decreasing the H2O2 content, while GB spraying had the lowest effect on antioxidant enzyme activities. In early and late planting dates, enzymatic and non-enzymatic antioxidants and photosynthetic properties play important roles in tolerating salinity stress, respectively. Therefore, the foliar application of SNP at a concentration of 100 µM served as the most suitable moderator for augmenting the activities of enzymatic antioxidants in salinity stress conditions.
References
Abbas G. et al. (2019) Effect of Planting Dates on Agronomic Crop Production. In: Hasanuzzaman M. (eds) Agronomic Crops. Springer, Singapore. https://doi.org/10.1007/978-981-32-9151-5_8
Aebi H (1984) Catalase in vitro. In: Wilchek M, Bayer EA (eds) Methods in enzymology, vol 105. Elsevier, Amsterdam, pp 121–126
Afzal MN, Tariq M, Ahmed M, Abbas G, Mehmood Z (2020) Managing planting time for cotton production. In: Ahmad S, Hasanuzzaman M (eds) Cotton production and uses. Springer, Berlin, pp 31–44
Alhoshan M, Zahedi M, Ramin AA, Sabzalian MR (2019) Exogenous application of salicylic acid and glycine betaine as tools to enhance biomass and tolerance of potato cultivars. Gesunde Pflanz 71:25–35. https://doi.org/10.1007/s10343-018-00438-2
Arif Y, Sami F, Siddiqui H, Bajguz A, Hayat S (2020) Salicylic acid in relation to other phytohormones in plant: a study towards physiology and signal transduction under challenging environment. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2020.104040
Barzoi Z, Armin M, Marvi H (2020) Physiological and biochemical responses of cotton to times and type of stress modulator in saline conditions Communications Faculty of Sciences University of Ankara Series C Biology (In press)
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/bf00018060
Chavoushi M, Najafi F, Salimi A, Angaji SA (2020) Effect of salicylic acid and sodium nitroprusside on growth parameters, photosynthetic pigments and secondary metabolites of safflower under drought stress. Sci Hortic-Amst 259:108823. https://doi.org/10.1016/j.scienta.2019.108823
Dong Y, Jinc S, Liu S, Xu L, Kong J (2014) Effects of exogenous nitric oxide on growth of cotton seedlings under NaCl stress. J Soil Sci Plant Nutr 14:1–13. https://doi.org/10.4067/s0718-95162014005000001
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Hasanuzzaman M, Nahar K, Fujita M (2013) Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad P, Azooz MM, Prasad MN (eds) Ecophysiology and responses of plants under salt stress. Springer, Berlin, pp 25–87
Hayat S, Ahmad A (2007) Salicylic acid-a plant hormone. Springer, Berlin
Hossain MA, Fujita M (2010) Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol Mol Biol Pla 16:19–29
Hossain MA, Kumar V, Burritt DJ, Fujita M, Mäkelä PS (2019) Osmoprotectant-mediated abiotic stress tolerance in plants: recent advances and future perspectives. Springer, Berlin
Hu L, Hu T, Zhang X, Pang H, Fu J (2012) Exogenous glycine betaine ameliorates the adverse effect of salt stress on perennial ryegrass. J Am Soc Hortic Sci 137:38–46
Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S (2013) Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J Exp Bot 64:2255–2268. https://doi.org/10.1093/jxb/ert085
Khan MA, Wahid A, Ahmad M, Tahir MT, Ahmed M, Ahmad S, Hasanuzzaman M (2020) World cotton production and consumption: an overview. In: Ahmad S, Hasanuzzaman M (eds) Cotton production and uses. Springer, Berlin, pp 1–7
Killi F, Bolek Y (2006) Timing of planting is crucial for cotton yield. Acta Agric Scand B-Soil Plant Sci 56:155–160. https://doi.org/10.1080/09064710510029178
Kotb M, Elhamahmy M (2014) Improvement of wheat productivity and their salt tolerance by exogenous glycine betaine application under saline soil condition for long-term Zagazig. J Agric Res 41:1127–1143
Kumar V, Yadav SK (2009) Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol Plant 31:261–269
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Ltd., South Portland
Lin CC, Kao CH (2001) Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited root growth of rice seedlings. Plant Soil 230:135–143
Liu S, Dong Y, Xu L, Kong J (2014) Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul 73:67–78
Malekzadeh P (2015) Influence of exogenous application of glycinebetaine on antioxidative system and growth of salt-stressed soybean seedlings (Glycine max L.). Physiol Mol Biol Plant 21:225–232
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nawaz K, Ashraf M (2010) Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J Agron Crop Sci 196:28–37. https://doi.org/10.1111/j.1439-037x.2009.00385.x
Timachi F, Armin M, Jamimoeini M, Abhari A (2020) Physiological response of cumin to times and type of stress modulator in rain-fed and irrigated conditions. Russ J Plant Physl 67:1163–1172. https://doi.org/10.1134/S1021443720060175
Venkateswarlu B, Shanker AK, Shanker C, Maheswari M (2011) Crop stress and its management: perspectives and strategies. Springer, Berlin
Wang H, Liang X, Wan Q, Wang X, Bi Y (2009) Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 230:293–307
Yadu S, Dewangan TL, Chandrakar V, Keshavkant S (2017) Imperative roles of salicylic acid and nitric oxide in improving salinity tolerance in Pisum sativum L. Physiol Mol Biol Plant 23:43–58. https://doi.org/10.1007/s12298-016-0394-7
Zhang L, Ma H, Chen T, Pen J, Yu S, Zhao X (2014) Morphological and physiological responses of cotton (Gossypium hirsutum L.) plants to salinity. PLoS One 9(11):e112807. https://doi.org/10.1371/journal.pone.0112807
Zheng C, Jiang D, Liu F, Dai T, Liu W, Jing Q, Cao W (2009) Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ Exp Bot 67:222–227. https://doi.org/10.1016/j.envexpbot.2009.05.002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Participants
This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shenavaei Zare, M., Armin, M. & Marvi, H. Physiological Responses of Cotton to Stress Moderator Application on Different Planting Date Under Saline Conditions. Iran J Sci Technol Trans Sci 45, 11–25 (2021). https://doi.org/10.1007/s40995-020-01009-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-020-01009-9