Abstract
Aggression takes several forms and can be offensive or defensive. Aggression between animals of the same species or society aims to inflict harm upon another for the purpose of protecting a resource such as food, reproductive partners, territory, or status. This chapter explores the neurobiology of aggression. We summarize the behavior of aggression, rodent models of aggression, and the correlates of aggressive behavior in the context of neuroendocrinology, neurotransmitter systems, and neurocircuitry. Translational implications of rodent studies are briefly discussed, applying basic research to brain imaging data and therapeutic approaches to conditions where aggression is problematic.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Aggression can be defined as delivering harm to another, with the aim of taking advantage of a limited resource (Haller 2018a). Motivation for aggression in animals can be: (i) offensive, which occurs during competition for, or protection of, resources such as food, reproductive partners, social status, or territory; (ii) defensive, to ward off attacks from another animal of the same or different species; (iii) maternal, to protect offspring; (iv) predatory, or hunting, capturing, and consuming prey; (v) play-fighting, shown by adolescent individuals; and (vi) patrol or marking as a form of agnostic behavior (Adams 2006; Veenema 2009). Differences in animal societal levels of aggression can be attributed to increased mating aggression and decreased parental investment (Barber 2008).
Offensive or predatory aggression is expressed in humans as instrumental or proactive aggression, where some goal is aimed for, whereas defensive aggression corresponds to impulsive or reactive aggression in humans, and occurs in response to perceived attack or threat (Blair 2016). Aggression is also associated with lack of empathy (Hernandez-Lallement et al. 2018).
This chapter reviews the neurobiology of aggression, recent findings in aggression research in rodents, and summarizes its translational implications.
Fighting between conspecifics usually follows rules, such as signaling intent to allow for a weaker opponent to withdraw, lunge-and-bite attacks on relatively robust body parts such as rump, while avoiding non-vital body parts such as face and neck (this is not always the case), and cessation of violence when signals of defeat are expressed by the opponent (Adams 2006; Haller 2017). Abnormal or maladaptive aggression is quantitative or qualitative increase in normal aggressive behavior compared to controls, or departure from these species-specific expressions of violence or its intent (Haller and Kruk 2006; Miczek et al. 2015).
Some animals may seek aggression in operant and place preference paradigms, and this aggression seeking shows features common with compulsive and addictive behavior models, such as resistance to abstinence conditioning (Golden et al. 2017a, b). Previous experience in winning or losing fights partially determines outcome of future escalations to contact violence, in addition to multiple other factors, such as prior residency (Hsu et al. 2006). Male animals with a history of winning fights show increased aggression after a period of deprivation from fighting opportunities (Kudryavtseva et al. 2011).
Aggression in animals may show seasonal variations during reproductive periods and to protect territory, and this is associated with changes in circulating gonadal steroids (Munley et al. 2018). Aggression toward prey, or hunting, is obviously behaviorally distinct from that directed at conspecifics.
2.2 Experimental Paradigms to Explore Aggression in Rodents
Resident-intruder paradigm: a male rodent, the resident, is allowed to familiarize with a home cage, with or without a female. An intruder animal, also male, is then introduced to the resident. A fight ensues, in which, all other conditions being relatively equal, the resident is expected to defeat the intruder.
Maternal aggression: a lactating dam with pups actively defends her nest and pups against an intruder, an unfamiliar male or female animal. Attacks can be fierce, may target face and neck of the intruder, and are quickly initiated.
Predatory aggression: a rodent is allowed to attack and consume prey, usually an insect.
Aggression seeking: This is measured using conditioned place preference after exposure of an animal to a conspecific in one compartment for some days, or in an operant setup where a lever is pressed or a nose poke is required to gain access to a conspecific on which to aggress.
Aggression inheritance in rodents is polygenic with a wide variety of strains in which to model, in addition to considering developmental factors such as maternal care, and the experimental paradigm used to test aggression; animal selection for modeling aggression therefore requires scrutiny (Miczek et al. 2001; Natarajan et al. 2009; Nyberg et al. 2004).
Abnormal or excessive aggression can be modeled in rodents through: (i) stress models, which include repeated prolonged maternal separation and early isolation or social subjugation; (ii) drug models such as administration of anabolic steroids during adolescence or alcohol in adulthood; (iii) genetic, by selective breeding of animals showing high aggression or anxiety; and (iv) decreasing circulating glucocorticoid by adrenalectomy and low-dose corticosterone pellet implantation (Haller 2017; Takahashi et al. 2012). Early social deprivation is associated with increased aggression and attack behavior on vulnerable body parts (Tóth et al. 2008).
Exposure to chronic ultrasonic noise increased aggression in the resident-intruder paradigm only in animals showing initial high levels of aggression in one series of experiments, but in all animals in another (Gorlova et al. 2019; Pavlov et al. 2017). Surgical devocalization of rats increased propensity to aggressive behavior during neutral interactions (Kisko et al. 2017).
2.3 Neuroendocrinology of Aggression
During development: Maternal separation is associated with increased play-fighting in adolescence and aggression in adulthood, and increased basal corticosterone and hypothalamic vasopressin (Veenema and Neumann 2009). Impacting dam–pup interaction, including reduced bedding material, is associated with higher circulating corticosteroids, lower corticotropic hormone, and increased aggression (Rice et al. 2008). Adolescent animals attacked by an adult show changes in the vasopressinergic system, namely vasopressinergic fibers are increased and serotoninergic terminals are decreased (Ferris 2000). Down-regulated or impaired oxytocinergic activity is associated with increased aggression, but exogenous administration does not ameliorate aggressive behavior (de Jong and Neumann 2018).
In Males: Testosterone as a biological root of aggression is contested (Albert et al. 1993). Deletion of androgen receptors in the nervous system is associated with impaired display of masculine behavior (Juntti et al. 2010). Development of play-fighting is dependent on androgen- and estrogen-mediated effects (Field et al. 2006). Higher aggression in male animals was correlated with higher adrenocorticotropin hormone responsiveness, lower trait anxiety, and great Fos immunoreactivity in paraventricular nucleus (Veenema et al. 2007).
In Females: Maternal aggression (actions mediated by a pregnant or lactating dam against others) is modulated by ovarian steroids, stimulated by suckling pups, and increased if the pups are handled (de Almeida et al. 2014; Giovenardi et al. 2005). During estrus in a lactating dam, a male intruder is either attacked or solicited, and this may be associated with changes in perception of male-specific urinary proteins (Agrati et al. 2011; Martín-Sánchez et al. 2015). However, male-specific urinary proteins alone do not instigate aggression in animals (Mucignat-Caretta et al. 2004; however see also Chamero et al. 2007), indicating that other sensory stimuli are also involved. Vasopressin release in central amygdala and oxytocin release in central amygdala and paraventricular nucleus contribute to maternal aggression (Bosch and Neumann 2010; Bosch et al. 2005; Bosch 2013). Female aggression has not received the same attention as male aggression; due to ethological background, neurocircuitry of femal aggression is better investigated in rodent models other than C57BL/6 mice, such as Swiss Webster mice, rats, and Syrian hamsters (Been et al. 2019).
2.4 Neurotransmitter Systems in Aggression
Neurotransmitters directly implicated in physiology and pathology of aggression include serotonin, dopamine, and GABA (de Almeida et al. 2005). Factors altering serotonin and dopamine neurotransmission during development are associated with life-long behavioral alteration (de Almeida et al. 2005).
Low brain serotonin has been correlated with high aggression but mechanistic explanations or a direct relationship is not agreed upon. Serotonin levels were lower in some brain areas of animals showing high aggression, and these same individual animals show the greatest increase in serotonin after the stress of agonistic behavior (Summers et al. 2005). Treatment with systemic specific serotonin agonists and antagonists suggests that normal and abnormal aggressive behaviors are mediated by different serotonin subtypes and mediate different types, phasic vs. tonic, of serotoninergic activity (de Boer and Koolhaas 2005). Chronically enhanced activity of 5-HT1A serotonin auto-receptors is associated with increased aggression (Caramaschi et al. 2007).
It was suggested that aggression has different phases including appetitive and executive phases, which were influenced by pre- and post-synaptic serotoninergic neurotransmission respectively (Olivier and van Oorschot 2005). Modulation of aggression by serotoninergic neurotransmission interacts with glucocorticoids released during stress in a phase-, context-, and history-dependant manner (Summers and Winberg 2006). Studies have shown that reduced serotoninergic activity in prefrontal cortex is associated with altered serotonin receptor expression in forebrain, poorly regulated dopamine secretion in nucleus accumbens and aggressive impulsivity (Nautiyal et al. 2015; Niederkofler et al. 2016; Seo et al. 2008). Knockout of serotonin autoreceptors 1B decreased serotonin in brain and spinal cord regions probably through increased serotonin turnover, increased dopamine turnover in nucleus accumbens, decreased dopamine, and was associated with increased aggressiveness and heightened cocaine sensitivity (Ase et al. 2008). Increased alcohol-induced aggression is associated with expression of certain GABAA receptor subtypes, whereas activation of serotonin receptor subtypes is associated with decreased alcohol-induced aggression (Miczek et al. 2006).
Dopamine in nucleus accumbens increased in anticipation of aggression and peaked after confrontation, while serotonin in prefrontal cortex decreased in association with termination (Ferrari et al. 2003; van Erp and Miczek 2000). Lack of monoamine oxidase A but not B is associated with increased aggression (Cases et al. 1995; Shih et al. 1999).
Lack of endothelial nitric oxide synthase greatly decreases aggression in male mice but does not influence maternal aggression, whereas deficiency of neuronal nitric oxide synthase decreases maternal aggression in female mice (Demas et al. 1999; Gammie and Nelson 1999; Gammie et al. 2000). Lack of neuronal nitric oxide synthase increased aggression, decreased social investigation, and was associated with decreased serotonin turnover and deficient serotoninergic receptors (Chiavegatto et al. 2001; Trainor et al. 2007a).
2.5 Neurocircuitry of Aggressive Behavior
2.5.1 Amygdala
The amygdala plays a vital role in mediating many aspects of innate and learned emotional behaviors such as fear-conditioning, predation, and aggression. In rat muricide model, c-Fos immunoreactivity increased in medial, central, and basolateral amygdala, as well as lateral hypothalamus; periaqueductal gray activations shifted from dorsal to ventral columns (Tulogdi et al. 2015).
Estrogen receptors α and β in medial preoptic area and medial amygdala differentially modulate aggressive behavior in males (Nakata et al. 2016). Site-specific knockdown of an estrogen receptor β gene in medial preoptic area decreased aggressive but not sexual behavior in adulthood; in amygdala knockdown of estrogen receptor β and α did not impact aggression (ibid). GABAergic neurons in medial amygdala promote aggression and are inhibited by neighboring glutamatergic neurons; the latter promote solitary grooming (Hong et al. 2014).
Aromatase expressing neurons in posterodorsal medial amygdala modulate intermale aggression and maternal aggression (Unger et al. 2015). Estrogen-dependent gene expression increased in bed nucleus of stria terminalis during long days and was associated with decreased aggression (Laredo et al. 2014; Trainor et al. 2007b).
Protein expression in oxytocin- and vasopressin-positive neurons in hypothalamus and bed nucleus of stria terminalis after intermale aggression is associated with medial amygdalar connectivity and activity (Wang et al. 2013).
Increased early growth response factor 1 in medial amygdala was associated with increased maternal aggression (Hasen and Gammie 2006).
2.5.2 Hypothalamus
For decades, it was generally accepted that the aggression center in the brain is the hypothalamic attack area, which is located in mediobasal hypothalamus and receives inputs from medial prefrontal neurons, septal regions, bed nucleus of stria terminalis, medial amygdala, amygdalo-hippocampal subiculum, locally from hypothalamus, and from lateral parabrachial nucleus (Toth et al. 2010). Electrical stimulation of the hypothalamic attack area promptly induced attack in cats and rodents, and increased c-Fos immunoreactivity in the lateral septum, bed nucleus of stria terminalis, medial and central amygdala, mediodorsal thalamic nucleus, and piriform and cingular cortex (Halász et al. 2002), suggesting these brain areas are also involved in aggression. Fos immunoreactivity in medial preoptic area and nucleus accumbens correlates with mating and experience of aggression (McHenry et al. 2016). Bilateral lesions of medial preoptic area are associated with attenuated aggression (Albert et al. 1986). Maternal aggression in lactating mice toward a male intruder is associated with increased Fos immunoreactivity in medial preoptic area, extended amygdala, accessory olfactory bulb, claustrum, and other brain regions (Gammie and Nelson 2001; Hasen and Gammie 2005).
In 2011, the ventrolateral part of ventromedial hypothalamus (VMHvl) was identified as a node structure to initiate attack (Lin et al. 2011). Optogenetic activation of neurons in VMHvl initiated male attack against conspecifics and females, as well as inanimate objects. Single unit activity measured widespread activation during aggressive encounters, but low and diminishing activity during mating (ibid). Consistently, single unit recording showed that the activity of VMHvl neurons is correlated with investigating olfactory cues of male conspecifics and attack; neuronal activity increased as the male–male distance decreased (Falkner et al. 2014). Optogenetic activation of VMHvl potentiated aggression-seeking and attack ferocity; inhibition had an opposite effect (Falkner et al. 2016).
Within the VMHvl, a group of estrogen receptor α-positive neurons has been shown to play an essential role in aggression. Calcium activity and optogenetic activation of estrogen receptor α-positive (Esr1+) neurons in the anterior part of VMHvl were associated with defense behavior against a conspecific, including non-threatening female; optogenetic inhibition impaired defense behavior against an aggressive conspecific (Wang et al. 2019a). Fos immunoreactivity shows overlapping hypothalamic and amygdalar activation after an aggressive or sexual encounter in males, indicating aggression and mating behaviors may be regulated by the same type of neurons (Veening et al. 2005). This concept is supported by a recent study showing that increasing photostimulation power on VMHvl Esr1+ neurons in male mice shifted behavior during a single interaction with a male or female mouse from investigation, to mounting, to attack, whereas non-cell-specific optogenetic activation in the same area was associated with attack but not mounting (Lee et al. 2014). Collectively, these results suggested that the VMHvl Esr1+ neurons coordinate scalable control of two distinct behaviors, namely aggression and mating.
Interestingly, roles of VMHvl Esr1+ neurons seem to be varied in different mouse strains and sexual history. Optogenetic activation of VMHvl Esr1+ neurons in virgin C57 female mice was associated with attack on an intruder female mouse, whereas activation of the same cells in virgin Swiss Webster and lactating C57 female mice was associated with mounting of a female intruder (Hashikawa et al. 2017). Unexpectedly, knockdown of estrogen receptor α in ventromedial hypothalamus increased female aggression against juveniles (Spiteri et al. 2010). In addition to Esr1+ neurons, other cell types in VMH are also involved in aggression. For example, genetic ablation of progesterone expressing neurons in ventromedial hypothalamus inhibited sexual receptivity in females and mating and aggression in males (Yang et al. 2013). In addition, a subset of neurons in VMHvl has been shown to mediate social fear (Sakurai et al. 2016).
Pheromone and olfactory receptors participate in hypothalamic circuits modulating aggressive behavior, but comprehensive descriptions are lacking (Sternson 2013). Deficiency of TRP2-expressing neurons in vomeronasal organ impairs intermale aggression and sex discrimination (Stowers 2002). A component of urine acts as a phermone to instigate intermale aggression in mice via vomeronasal organ neuronal circuits (Chamero et al. 2007).
2.5.3 Prefrontal Cortex
Post-weaning social isolation was associated with increased aggression in later life, reduced prefrontal cortical thickness, and was associated with abnormal aggressive behavior such as reduced signaling and attack of vulnerable body areas (Biro et al. 2017). Optogenetic activation of excitatory neurons decreased the intensity of an aggressive bout and the propensity to initiate it, but was not associated with change in aggression termination; optogenetic suppression was associated with opposite effects (Takahashi et al. 2014).
2.5.4 Lateral Septum
Projections from lateral septum to ventrolateral part of ventromedial hypothalamus are inhibitory, and photoactivation of lateral septum cells terminates attack behavior (Wong et al. 2016). Loss of a calcium-activated chloride channel in a subpopulation of lateral septum neurons increased aggressive display in the resident-intruder paradigm (Wang et al. 2019b).
GABAA receptor agonist injected into lateral septum increased aggression (McDonald et al. 2012).
Animals bred for short attack latency or high anxiety behavior are more aggressive, and show reduced vassopressinergic neurotransmission in lateral septum, in addition to altered hypothalamo-pituitary-adrenal axis response and increased serotoninergic neurotransmission (Veenema and Neumann 2007). Vasopressin released in lateral septum modulates social behavior but not aggression (Beiderbeck et al. 2007).
2.5.5 Other Brain Areas
Winning fights increased neurogenesis in hippocampus and aggression in males, and decreased Fos immunoreactivity in amygdala; these effects discontinue with absence of further opportunities to aggress (Smagin et al. 2015). Dopamine decreased in nucleus accumbens in anticipation of an aggressive episode (Ferrari et al. 2003). Knockdown of progestin receptors in ventral tegmental area in female mice is associated with increased male rejection and aggression (Frye et al. 2014). Neural activity in dorsal midbrain central gray is associated with offensive and defensive aggressive behavior (Adams 2006). pCREB-positive cells increased in caudal periaqueductal gray and lateral septum after maternal aggression (Gammie and Nelson 2001).
2.5.6 Synthesis
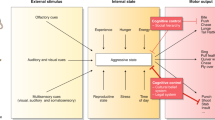
Research summarized above suggests environmental signals to aggress are relayed through one of two possible main systems as shown in Fig. 2.1: (i) medial amygdala to extended amygdala, lateral septum, and hypothalamic areas, and therefrom to periaqueductal gray; (ii) hypothalamic attack area, the ventrolateral part of the ventromedial hypothalamus coordinating afferent and efferent brain signaling in the initiation and processing of aggression with afferents and efferents from and to prefrontal cortex, lateral septum, amygdala, other areas of hypothalamus, and brainstem (Aleyasin et al. 2018; de Boer et al. 2015; Nelson and Trainor 2007). Olfactory cues obviously play an important role in rodent social dynamics, but their contribution to human aggression is unknown. Signals from prefrontal cortex modulate or inhibit aggressive behavior. Data from human studies support a central role for amygdala, hypothalamus, and periaqueductal gray for processing and initiating aggressive impulses, and prefrontal cortex in processing action values and the decision to aggress (Blair 2016).
Models of brain regions and circuitry mediating aggression. Neurocircuitry mediating aggressive behavior is processed either mainly through MeA, to BNST, LS, and hypothalamic areas, or through the hypothalamic attack area and VMHvl which communicates with amygdala, PFC, and LS. The output of these pathways is the PAG. Environmental stimuli of aggression are mostly social in nature. Olfactory cues are received by the olfactory bulb and relayed to MeA, hypothalamic areas, or both. Visual and auditory cues are processed by MeA as well as PFC. BNST bed nucleus of the stria terminalis, LS lateral septum, MeA medial amygdala, MPOA medial preoptic area, PAG periaqueductal gray, PFC prefrontal cortex
2.6 Translational Implications
Aggression in human history is obviously complex, and attempts at interpretation are placed in a socioeconomic context (Fortman and Bas de 2005).
Human aggression can be reactive or impulsive associated with anger and autonomic arousal, and instrumental which is thought to be more goal-oriented and involves less autonomic arousal; similarly aggression can be clustered into impulsive-affective and controlled-predatory subtypes (Nelson and Trainor 2007; Vitiello and Stoff 1997). Human conditions in which aggressive behavior is problematic include antisocial personality disorder, borderline personality disorder, intermittent explosive disorder, post-traumatic stress disorder, irritable and depression-linked aggression, schizophrenia, bipolar disorder, attention-deficit hyperactivity disorder, dementia and associated illness, and alcohol-related aggression (Coccaro et al. 2011; Nelson and Trainor 2007; Pompili et al. 2017). It was suggested that aggressive conditions can be classified into those associated with neurocircuitry pathology, hypoarousal and low circulating glucocorticoids, and emotional and physiological hyperarousal (Haller and Kruk 2006). Establishing animal model validity as related to human conditions is suggested (Haller 2018b).
Studies in animals and humans suggest neurocircuitry underlying aggression involves subcortical systems producing aggressive impulses, circuits predicting outcome of aggressing and making a decision to aggress or not, and notably circuits in prefrontal and medial temporal cortex regulating emotion (Anderson et al. 1999; Bufkin and Luttrell 2005; Coccaro et al. 2011; Davidson 2000). Aggression in humans associated with neurological damage or degeneration includes frontotemporal lesions, epilepsy, and Alzheimer’s disease (Haller and Kruk 2006). Violent behavior in humans is associated with functional impairments in prefrontal cortex (Yang and Raine 2009). Bilateral amygdalar destruction in humans to treat intractable aggression has a “taming effect” but does not abolish aggressive outbursts (Lee et al. 1998). Deep brain stimulation of posterior medial hypothalamus and nucleus accumbens reduced aggression (Harat et al. 2015).
Borderline personality disorder is associated with volume loss in amygdala, hippocampus, and left orbitofrontal and right anterior cingulate cortex (van Elst et al. 2003). Psychopathy is associated with hypoactive frontolimbic circuit and hippocampal asymmetry (Raine et al. 2004; Veit et al. 2002).
Early to exposure to violence and cruelty may consolidate an attraction to aggression in an individual, and is associated with higher incidence of post-traumatic stress disorder (Hinsberger et al. 2016; Raine et al. 2004). Aggression during development is highly predictive of maladaptive behavior in adulthood; theories of aggression development combine genetics of neurotransmitter-receptor systems, most notably monoamine oxidase A, brain structure, micro- and macrodynamic psychosocial factors such as parenting and sociocultural background, and hormonal factors (Austerman 2017; Kim-Cohen et al. 2006; Lansford 2018).
Experiments in rodents are needed to improve pharmacotherapy of aggressive conditions, and which presently includes the following: (i) atypical antipsychotics such as clozapine and risperidone; (ii) anticonvulsants such as topiramate; (iii) mood stabilizers such as lithium; (iv) adrenergic receptor agonists such as clonidine; (v) typical antipsychotics such as haloperidol; (vi) benzodiazepines such as midazolam; (vii) combinations of drugs which may include histamine blockers such as promethazine; (viii) drugs acting on brain serotonin notably selective serotonin reuptake inhibitors; (ix) beta blockers such as propranolol; (x) drugs acting on nicotine receptors (Brieden et al. 2002; Buitelaar et al. 2001; Granic 2014; Hoptman 2015; Huf et al. 2016; Knapp et al. 2012; Pompili et al. 2017; Robb et al. 2019; Swann 2003). Studies in animals and humans show that oxidative stress plays an important role in alcohol toxicity and aggressive behavior (Tobore 2019).
In short, we have a wealth of data spanning across genetics, neurophysiology, brain structure, pharmacology, and behavior from animal and human studies on aggression; a comprehensive integration of this data may provide novel insights into how we can better screen for and manage conditions associated with or predisposing to aggression early in life, and shed light on common and disparate mechanisms underlying this complex phenomenon. Further integrating neuroscientific findings into the broader context of society would need a meaningful science of neurosociology, which has not yet developed a common language between the sociological and phenomenological on the one hand, and the biological and deterministic on the other (Meloni et al. 2016). There is reason for optimism toward further integrating the neurology of development within a sociological context (Vasileva and Balyasnikova 2019).
References
Adams DB (2006) Brain mechanisms of aggressive behavior: an updated review. Neurosci Biobehav Rev 30(3):304–318. https://doi.org/10.1016/j.neubiorev.2005.09.004
Agrati D, Fernández-Guasti A, Ferreño M, Ferreira A (2011) Coexpression of sexual behavior and maternal aggression: the ambivalence of sexually active mother rats toward male intruders. Behav Neurosci 125(3):446–451. https://doi.org/10.1037/a0023085
Albert DJ, Walsh ML, Gorzalka BB, Mendelson S, Zalys C (1986) Intermale social aggression: suppression by medial preoptic area lesions. Physiol Behav 38(2):169–173. https://doi.org/10.1016/0031-9384(86)90151-4
Albert DJ, Walsh ML, Jonik RH (1993) Aggression in humans: what is its biological foundation? Neurosci Biobehav Rev 17(4):405–425
Aleyasin H, Flanigan ME, Russo SJ (2018) Neurocircuitry of aggression and aggression seeking behavior: nose poking into brain circuitry controlling aggression. Curr Opin Neurobiol Neurobiol Behav 49:184–191. https://doi.org/10.1016/j.conb.2018.02.013
de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA (2005) Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol 526(1–3):51–64. https://doi.org/10.1016/j.ejphar.2005.10.004
de Almeida RM, Ferreira A, Agrati D (2014) Sensory, hormonal, and neural basis of maternal aggression in rodents. In: Miczek KA, Meyer-Lindenberg A (eds) Neuroscience of aggression, Current Topics in Behavioral Neurosciences. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 111–130. https://doi.org/10.1007/7854_2014_312
Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR (1999) Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci 2(11):1032–1037. https://doi.org/10.1038/14833
Ase AR, Reader TA, Hen R, Riad M, Descarries L (2008) Altered serotonin and dopamine metabolism in the CNS of serotonin 5-HT(1A) or 5-HT(1B) receptor knockout mice. J Neurochem 75(6):2415–2426. https://doi.org/10.1046/j.1471-4159.2000.0752415.x
Austerman J (2017) Violence and aggressive behavior. Pediatr Rev 38(2):69–80. https://doi.org/10.1542/pir.2016-0062
Barber N (2008) Evolutionary social science: a new approach to violent crime. Aggress Violent Behav 13(3):237–250. http://www.sciencedirect.com/science/article/pii/S1359178908000190
Been LE, Gibbons AB, Meisel RL (2019) Towards a neurobiology of female aggression. Neuropharmacology 156:107451. https://doi.org/10.1016/j.neuropharm.2018.11.039
Beiderbeck DI, Neumann ID, Veenema AH (2007) Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci 26(12):3597–3605. https://doi.org/10.1111/j.1460-9568.2007.05974.x
Biro L, Toth M, Sipos E, Bruzsik B, Tulogdi A, Bendahan S, Sandi C, Haller J (2017) Structural and functional alterations in the prefrontal cortex after post-weaning social isolation: relationship with species-typical and deviant aggression. Brain Struct Funct 222(4):1861–1875. https://doi.org/10.1007/s00429-016-1312-z
Blair RJR (2016) The neurobiology of impulsive aggression. J Child Adolesc Psychopharmacol 26(1):4–9. https://doi.org/10.1089/cap.2015.0088
de Boer SF, Koolhaas JM (2005) 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol 526(1–3):125–139. https://doi.org/10.1016/j.ejphar.2005.09.065
de Boer SF, Olivier B, Veening J, Koolhaas JM (2015) The neurobiology of offensive aggression: revealing a modular view. Physiol Behav 146(July):111–127. https://doi.org/10.1016/j.physbeh.2015.04.040
Bosch OJ (2013) Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Philos Trans R Soc Lond B Biol Sci 368(1631):20130085. https://doi.org/10.1098/rstb.2013.0085
Bosch OJ, Neumann ID (2010) Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci 31(5):883–891. https://doi.org/10.1111/j.1460-9568.2010.07115.x
Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID (2005) Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci Off J Soc Neurosci 25(29):6807–6815. https://doi.org/10.1523/JNEUROSCI.1342-05.2005
Brieden T, Ujeyl M, Naber D (2002) Psychopharmacological treatment of aggression in schizophrenic patients. Pharmacopsychiatry 35(3):83–89. https://doi.org/10.1055/s-2002-31523
Bufkin JL, Luttrell VR (2005) Neuroimaging studies of aggressive and violent behavior: current findings and implications for criminology and criminal justice. Trauma Violence Abuse 6(2):176–191. https://doi.org/10.1177/1524838005275089
Buitelaar JK, van der Gaag RJ, Cohen-Kettenis P, Melman CT (2001) A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry 62(4):239–248. https://doi.org/10.4088/jcp.v62n0405
Caramaschi D, de Boer SF, Koolhaas JM (2007) Differential role of the 5-HT1A receptor in aggressive and non-aggressive mice: an across-strain comparison. Physiol Behav 90(4):590–601. https://doi.org/10.1016/j.physbeh.2006.11.010
Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Müller U, Aguet M, Babinet C, Shih JC (1995) Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science (New York, N.Y.) 268(5218):1763–1766. https://doi.org/10.1126/science.7792602
Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L (2007) Identification of protein pheromones that promote aggressive behaviour. Nature 450(7171):899–902. https://doi.org/10.1038/nature05997
Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ (2001) Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci U S A 98(3):1277–1281. https://doi.org/10.1073/pnas.031487198
Coccaro EF, Sripada CS, Yanowitch RN, Luan Phan K (2011) Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry 69(12):1153–1159. https://doi.org/10.1016/j.biopsych.2011.02.032
Davidson RJ (2000) Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science 289(5479):591–594. https://doi.org/10.1126/science.289.5479.591
Demas GE, Kriegsfeld LJ, Blackshaw S, Huang P, Gammie SC, Nelson RJ, Snyder SH (1999) Elimination of aggressive behavior in male mice lacking endothelial nitric oxide synthase. J Neurosci Off J Soc Neurosci 19(19):RC30
van Elst T, Ludger BH, Thiel T, Geiger E, Haegele K, Lemieux L, Lieb K, Bohus M, Hennig J, Ebert D (2003) Frontolimbic brain abnormalities in patients with borderline personality disorder. Biol Psychiatry 54(2):163–171. https://doi.org/10.1016/S0006-3223(02)01743-2
van Erp AM, Miczek KA (2000) Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci Off J Soc Neurosci 20(24):9320–9325
Falkner AL, Dollar P, Perona P, Anderson DJ, Lin D (2014) Decoding ventromedial hypothalamic neural activity during male mouse aggression. J Neurosci Off J Soc Neurosci 34(17):5971–5984. https://doi.org/10.1523/JNEUROSCI.5109-13.2014
Falkner AL, Grosenick L, Davidson TJ, Deisseroth K, Lin D (2016) Hypothalamic control of male aggression-seeking behavior. Nat Neurosci 19(4):596–604. https://doi.org/10.1038/nn.4264
Ferrari PF, Van Erp AMM, Tornatzky W, Miczek KA (2003) Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats: microdialysis and anticipation of aggression. Eur J Neurosci 17(2):371–378. https://doi.org/10.1046/j.1460-9568.2003.02447.x
Ferris CF (2000) Adolescent stress and neural plasticity in hamsters: a vasopressin-serotonin model of inappropriate aggressive behaviour. Exper Physiol 85 Spec No:85S–90S
Field EF, Whishaw IQ, Pellis SM, Watson NV (2006) Play fighting in androgen-insensitive tfm rats: evidence that androgen receptors are necessary for the development of adult playful attack and defense. Dev Psychobiol 48(2):111–120. https://doi.org/10.1002/dev.20121
Fortman G, Bas de. (2005) Violence among peoples in the light of human frustration and aggression. Eur J Pharmacol 526(1–3):2–8. https://doi.org/10.1016/j.ejphar.2005.09.035
Frye CA, Walf AA, Kohtz AS, Zhu Y (2014) Progesterone-facilitated lordosis of estradiol-primed mice is attenuated by knocking down expression of membrane progestin receptors in the midbrain. Steroids 81:17–25. https://doi.org/10.1016/j.steroids.2013.11.009
Gammie SC, Nelson RJ (1999) Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci Off J Soc Neurosci 19(18):8027–8035
Gammie SC, Nelson RJ (2001) CFOS and PCREB activation and maternal aggression in mice. Brain Res 898(2):232–241. https://doi.org/10.1016/s0006-8993(01)02189-8
Gammie SC, Huang PL, Nelson RJ (2000) Maternal aggression in endothelial nitric oxide synthase-deficient mice. Horm Behav 38(1):13–20. https://doi.org/10.1006/hbeh.2000.1595
Giovenardi M, de Azevedo MS, da Silva SP, do E S Hermel E, Gomes CM, Lucion AB (2005) Neonatal handling increases fear and aggression in lactating rats. Physiol Behav 86(1–2):209–217. https://doi.org/10.1016/j.physbeh.2005.07.011
Golden SA, Aleyasin H, Heins R, Flanigan M, Heshmati M, Takahashi A, Russo SJ, Shaham Y (2017a) Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes Brain Behav 16(1):44–55. https://doi.org/10.1111/gbb.12310
Golden SA, Heins C, Venniro M, Caprioli D, Zhang M, Epstein D, Shaham Y (2017b) Compulsive addiction-like aggressive behavior in mice. Biol Psychiatry 82(4):239–248. https://doi.org/10.1016/j.biopsych.2017.03.004
Gorlova AV, Pavlov DA, Ushakova VM, Zubkov EA, Morozova AY, Zorkina YA, Inozemtsev AN, Chekhonin VP (2019) Chronic exposure to ultrasonic frequencies selectively increases aggression in rats. Dokl Biol Sci 486(1):69–71. https://doi.org/10.1134/S0012496619030074
Granic I (2014) The role of anxiety in the development, maintenance, and treatment of childhood aggression. Dev Psychopathol 26(4 Pt 2):1515–1530. https://doi.org/10.1017/S0954579414001175
Halász J, Liposits Z, Meelis W, Kruk MR, Haller J (2002) Hypothalamic attack area-mediated activation of the forebrain in aggression. Neuroreport 13(10):1267–1270. https://doi.org/10.1097/00001756-200207190-00010
Haller J (2017) Studies into abnormal aggression in humans and rodents: methodological and translational aspects. Neurosci Biobehav Rev 76(Pt A):77–86. https://doi.org/10.1016/j.neubiorev.2017.02.022
Haller J (2018a) The role of central and medial amygdala in normal and abnormal aggression: a review of classical approaches. Neurosci Biobehav Rev 85:34–43. https://doi.org/10.1016/j.neubiorev.2017.09.017
Haller J (2018b) Preclinical models of conduct disorder - principles and pharmacologic perspectives. Neurosci Biobehav Rev 91:112–120. https://doi.org/10.1016/j.neubiorev.2016.05.032
Haller J, Kruk MR (2006) Normal and abnormal aggression: human disorders and novel laboratory models. Neurosci Biobehav Rev 30(3):292–303. https://doi.org/10.1016/j.neubiorev.2005.01.005
Harat M, Rudaś M, Zieliński P, Birska J, Sokal P (2015) Deep brain stimulation in pathological aggression. Stereotact Funct Neurosurg 93(5):310–315. https://doi.org/10.1159/000431373
Hasen NS, Gammie SC (2005) Differential Fos activation in virgin and lactating mice in response to an intruder. Physiol Behav 84(5):681–695. https://doi.org/10.1016/j.physbeh.2005.02.010
Hasen NS, Gammie SC (2006) Maternal aggression: new insights from Egr-1. Brain Res 1108(1):147–156. https://doi.org/10.1016/j.brainres.2006.06.007
Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, Piper WT, Lee H, Rudy B, Lin D (2017) Esr1+ cells in the ventromedial hypothalamus control female aggression. Nat Neurosci 20(11):1580–1590. https://doi.org/10.1038/nn.4644
Hernandez-Lallement J, van Wingerden M, Kalenscher T (2018) Towards an animal model of callousness. Neurosci Biobehav Rev 91(August):121–129. https://doi.org/10.1016/j.neubiorev.2016.12.029
Hinsberger M, Sommer J, Kaminer D, Holtzhausen L, Weierstall R, Seedat S, Madikane S, Elbert T (2016) Perpetuating the cycle of violence in south African low-income communities: attraction to violence in young men exposed to continuous threat. Eur J Psychotraumatol 7(January):29099. https://doi.org/10.3402/ejpt.v7.29099
Hong W, Kim D-W, Anderson DJ (2014) Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell 158(6):1348–1361. https://doi.org/10.1016/j.cell.2014.07.049
Hoptman MJ (2015) Impulsivity and aggression in schizophrenia: a neural circuitry perspective with implications for treatment. CNS Spectr 20(3):280–286. https://doi.org/10.1017/S1092852915000206
Hsu Y, Earley RL, Wolf LL (2006) Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc 81(1):33–74. https://doi.org/10.1017/S146479310500686X
Huf G, Alexander J, Gandhi P, Allen MH (2016) Haloperidol plus promethazine for psychosis-induced aggression. Cochrane Database Syst Rev 11:CD005146. https://doi.org/10.1002/14651858.CD005146.pub3
de Jong TR, Neumann ID (2018) Oxytocin and aggression. Curr Top Behav Neurosci 35:175–192. https://doi.org/10.1007/7854_2017_13
Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S-I, Harada N, Shah NM (2010) The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66(2):260–272. https://doi.org/10.1016/j.neuron.2010.03.024
Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE (2006) MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry 11(10):903–913. https://doi.org/10.1038/sj.mp.4001851
Kisko TM, Wöhr M, Pellis VC, Pellis SM (2017) From play to aggression: high-frequency 50-khz ultrasonic vocalizations as play and appeasement signals in rats. Curr Top Behav Neurosci 30:91–108. https://doi.org/10.1007/7854_2015_432
Knapp P, Chait A, Pappadopulos E, Crystal S, Jensen PS, T-MAY Steering Group (2012) Treatment of maladaptive aggression in youth: CERT guidelines I. engagement, assessment, and management. Pediatrics 129(6):e1562–e1576. https://doi.org/10.1542/peds.2010-1360
Kudryavtseva NN, Smagin DA, Bondar NP (2011) Modeling fighting deprivation effect in mouse repeated aggression paradigm. Prog Neuro-Psychopharmacol Biol Psychiatry 35(6):1472–1478. https://doi.org/10.1016/j.pnpbp.2010.10.013
Lansford JE (2018) Development of aggression. Curr Opin Psychol Aggres Viol 19(February):17–21. https://doi.org/10.1016/j.copsyc.2017.03.015
Laredo SA, Orr VN, McMackin MZ, Trainor BC (2014) The effects of exogenous melatonin and melatonin receptor blockade on aggression and estrogen-dependent gene expression in male California mice (Peromyscus Californicus). Physiol Behav 128(April):86–91. https://doi.org/10.1016/j.physbeh.2014.01.039
Lee GP, Bechara A, Adolphs R, Arena J, Meador KJ, Loring DW, Smith JR (1998) Clinical and physiological effects of stereotaxic bilateral amygdalotomy for intractable aggression. J Neuropsychiatry Clin Neurosci 10(4):413–420. https://doi.org/10.1176/jnp.10.4.413
Lee H, Kim D-W, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, Anderson DJ (2014) Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509(7502):627–632. https://doi.org/10.1038/nature13169
Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ (2011) Functional identification of an aggression locus in the mouse hypothalamus. Nature 470(7333):221–226. https://doi.org/10.1038/nature09736
Martín-Sánchez A, McLean L, Beynon RJ, Hurst JL, Ayala G, Lanuza E, Martínez-Garcia F (2015) From sexual attraction to maternal aggression: when pheromones change their behavioural significance. Horm Behav 68(February):65–76. https://doi.org/10.1016/j.yhbeh.2014.08.007
McDonald MM, Markham CM, Alisa N, Elliott Albers H, Huhman KL (2012) GABAA receptor activation in the lateral septum reduces the expression of conditioned defeat and increases aggression in Syrian hamsters. Brain Res 1439(February):27–33. https://doi.org/10.1016/j.brainres.2011.12.042
McHenry JA, Robison CL, Bell GA, Bolaños-Guzmán CA, Vialou VV, Nestler EJ, Hull EM (2016) The role of ΔFosB in the medial preoptic area: differential effects of mating and cocaine history. Behav Neurosci 130(5):469–478. https://doi.org/10.1037/bne0000160
Meloni M, Williams S, Martin P (2016) The biosocial: sociological themes and issues. Sociol Rev Monogr 64(1):7–25. https://doi.org/10.1002/2059-7932.12010
Miczek KA, Maxson SC, Fish EW, Faccidomo S (2001) Aggressive behavioral phenotypes in mice. Behav Brain Res 125(1–2):167–181. https://doi.org/10.1016/s0166-4328(01)00298-4
Miczek KA, Fish EW, Almeida RMM, Faccidomo S, Debold JF (2006) Role of alcohol consumption in escalation to violence. Ann N Y Acad Sci 1036(1):278–289. https://doi.org/10.1196/annals.1330.018
Miczek KA, Takahashi A, Gobrogge KL, Hwa LS, de Almeida RMM (2015) Escalated aggression in animal models: shedding new light on mesocorticolimbic circuits. Social Behavior 3(June):90–95. https://doi.org/10.1016/j.cobeha.2015.02.007
Mucignat-Caretta C, Cavaggioni A, Caretta A (2004) Male urinary chemosignals differentially affect aggressive behavior in male mice. J Chem Ecol 30(4):777–791
Munley KM, Rendon NM, Demas GE (2018) Neural androgen synthesis and aggression: insights from a seasonally breeding rodent. Front Endocrinol 9:136. https://doi.org/10.3389/fendo.2018.00136
Nakata M, Sano K, Musatov S, Yamaguchi N, Sakamoto T, Ogawa S (2016) Effects of prepubertal or adult site-specific knockdown of estrogen receptor β in the medial preoptic area and medial amygdala on social behaviors in male mice. Eneuro 3(2):ENEURO.0155-15.2016. https://doi.org/10.1523/ENEURO.0155-15.2016
Natarajan D, de Vries H, Saaltink D-J, de Boer SF, Koolhaas JM (2009) Delineation of violence from functional aggression in mice: an ethological approach. Behav Genet 39(1):73–90. https://doi.org/10.1007/s10519-008-9230-3
Nautiyal KM, Tanaka KF, Barr MM, Tritschler L, Le Dantec Y, David DJ, Gardier AM, Blanco C, Hen R, Ahmari SE (2015) Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron 86(3):813–826. https://doi.org/10.1016/j.neuron.2015.03.041
Nelson RJ, Trainor BC (2007) Neural mechanisms of aggression. Nat Rev Neurosci 8(7):536–546. https://doi.org/10.1038/nrn2174
Niederkofler V, Asher TE, Okaty BW, Rood BD, Narayan A, Hwa LS, Beck SG, Miczek KA, Dymecki SM (2016) Identification of serotonergic neuronal modules that affect aggressive behavior. Cell Rep 17(8):1934–1949. https://doi.org/10.1016/j.celrep.2016.10.063
Nyberg J, Sandnabba K, Schalkwyk L, Sluyter F (2004) Genetic and environmental (inter)actions in male mouse lines selected for aggressive and nonaggressive behavior. Genes Brain Behav 3(2):101–109
Olivier B, van Oorschot R (2005) 5-HT1B receptors and aggression: a review. Eur J Pharmacol 526(1–3):207–217. https://doi.org/10.1016/j.ejphar.2005.09.066
Pavlov DA, Gorlova AV, Ushakova VM, Zubkov EA, Morozova AY, Inozemtsev AN, Chekhonin VP (2017) Effects of chronic exposure to ultrasound of alternating frequencies on the levels of aggression and anxiety in CBA and BALB/c mice. Bull Exp Biol Med 163(4):409–411. https://doi.org/10.1007/s10517-017-3815-x
Pompili E, Carlone C, Silvestrini C, Nicolò G (2017) Focus on aggressive behaviour in mental illness. Riv Psichiatr 52(5):175–179. https://doi.org/10.1708/2801.28344
Raine A, Ishikawa SS, Arce E, Lencz T, Knuth KH, Bihrle S, LaCasse L, Colletti P (2004) Hippocampal structural asymmetry in unsuccessful psychopaths. Biol Psychiatry 55(2):185–191. https://doi.org/10.1016/S0006-3223(03)00727-3
Rice CJ, Sandman CA, Lenjavi MR, Baram TZ (2008) A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149(10):4892–4900. https://doi.org/10.1210/en.2008-0633
Robb AS, Schwabe S, Ceresoli-Borroni G, Nasser A, Yu C, Marcus R, Candler SA, Findling RL (2019) A proposed anti-maladaptive aggression agent classification: improving our approach to treating impulsive aggression. Postgrad Med 131(2):129–137. https://doi.org/10.1080/00325481.2019.1574401
Sakurai K, Zhao S, Takatoh J, Rodriguez E, Lu J, Leavitt AD, Min F, Han B-X, Wang F (2016) Capturing and manipulating activated neuronal ensembles with cane delineates a hypothalamic social-fear circuit. Neuron 92(4):739–753. https://doi.org/10.1016/j.neuron.2016.10.015
Seo D, Patrick CJ, Kennealy PJ (2008) Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress Violent Behav 13(5):383–395. https://doi.org/10.1016/j.avb.2008.06.003
Shih JC, Chen K, Ridd MJ (1999) Monoamine oxidase: from genes to behavior. Annu Rev Neurosci 22:197–217. https://doi.org/10.1146/annurev.neuro.22.1.197
Smagin DA, Park J-H, Michurina TV, Peunova N, Glass Z, Sayed K, Bondar NP, Kovalenko IN, Kudryavtseva NN, Enikolopov G (2015) Altered hippocampal neurogenesis and amygdalar neuronal activity in adult mice with repeated experience of aggression. Front Neurosci 9:443. https://doi.org/10.3389/fnins.2015.00443
Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Agmo A (2010) The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav Brain Res 210(2):211–220. https://doi.org/10.1016/j.bbr.2010.02.033
Sternson SM (2013) Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77(5):810–824. https://doi.org/10.1016/j.neuron.2013.02.018
Stowers L (2002) Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295(5559):1493–1500. https://doi.org/10.1126/science.1069259
Summers CH, Winberg S (2006) Interactions between the neural regulation of stress and aggression. J Exp Biol 209(Pt 23):4581–4589. https://doi.org/10.1242/jeb.02565
Summers CH, Korzan WJ, Lukkes JL, Watt MJ, Forster GL, Øverli Ø, Höglund E et al (2005) Does serotonin influence aggression? Comparing regional activity before and during social interaction. Physiol Biochem Zool 78(5):679–694. https://doi.org/10.1086/432139
Swann AC (2003) Neuroreceptor mechanisms of aggression and its treatment. J Clin Psychiatry 64(Suppl 4):26–35
Takahashi A, Quadros IM, de Almeida RMM, Miczek KA (2012) Behavioral and pharmacogenetics of aggressive behavior. Curr Top Behav Neurosci 12:73–138. https://doi.org/10.1007/7854_2011_191
Takahashi A, Nagayasu K, Nishitani N, Kaneko S, Koide T (2014) Control of intermale aggression by medial prefrontal cortex activation in the mouse. PLoS One 9(4):e94657. https://doi.org/10.1371/journal.pone.0094657
Tobore TO (2019) On the neurobiological role of oxidative stress in alcohol-induced impulsive, aggressive and suicidal behavior. Substance Use Misuse 54(14):2290–2303. https://doi.org/10.1080/10826084.2019.1645179
Tóth M, Halász J, Mikics É, Barsy B, Haller J (2008) Early social deprivation induces disturbed social communication and violent aggression in adulthood. Behav Neurosci 122(4):849–854. https://doi.org/10.1037/0735-7044.122.4.849
Toth M, Fuzesi T, Halasz J, Tulogdi A, Haller J (2010) Neural inputs of the hypothalamic ‘aggression area’ in the rat. Behav Brain Res 215(1):7–20. https://doi.org/10.1016/j.bbr.2010.05.050
Trainor BC, Workman JL, Jessen R, Nelson RJ (2007a) Impaired nitric oxide synthase signaling dissociates social investigation and aggression. Behav Neurosci 121(2):362–369. https://doi.org/10.1037/0735-7044.121.2.362
Trainor BC, Lin S, Sima Finy M, Rowland MR, Nelson RJ (2007b) Photoperiod reverses the effects of estrogens on male aggression via genomic and nongenomic pathways. Proc Natl Acad Sci U S A 104(23):9840–9845. https://doi.org/10.1073/pnas.0701819104
Tulogdi A, Biro L, Barsvari B, Stankovic M, Haller J, Toth M (2015) Neural mechanisms of predatory aggression in rats—implications for abnormal intraspecific aggression. Behav Brain Res 283(April):108–115. https://doi.org/10.1016/j.bbr.2015.01.030
Unger EK, Burke KJ, Yang CF, Bender KJ, Fuller PM, Shah NM (2015) Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep 10(4):453–462. https://doi.org/10.1016/j.celrep.2014.12.040
Vasileva O, Balyasnikova N (2019) (Re)Introducing Vygotsky’s thought: from historical overview to contemporary psychology. Front Psychol 10:1515. https://doi.org/10.3389/fpsyg.2019.01515
Veenema AH (2009) Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front Neuroendocrinol 30(4):497–518. https://doi.org/10.1016/j.yfrne.2009.03.003
Veenema AH, Neumann ID (2007) Neurobiological mechanisms of aggression and stress coping: a comparative study in mouse and rat selection lines. Brain Behav Evol 70(4):274–285. https://doi.org/10.1159/000105491
Veenema AH, Neumann ID (2009) Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin MRNA expression in juvenile male rats. Psychoneuroendocrinology 34(3):463–467. https://doi.org/10.1016/j.psyneuen.2008.10.017
Veenema AH, Torner L, Blume A, Beiderbeck DI, Neumann ID (2007) Low inborn anxiety correlates with high intermale aggression: link to acth response and neuronal activation of the hypothalamic paraventricular nucleus. Horm Behav 51(1):11–19. https://doi.org/10.1016/j.yhbeh.2006.07.004
Veening JG, Coolen LM, de Jong TR, Joosten HW, de Boer SF, Koolhaas JM, Olivier B (2005) Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur J Pharmacol 526(1–3):226–239. https://doi.org/10.1016/j.ejphar.2005.09.041
Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N (2002) Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Lett 328(3):233–236. https://doi.org/10.1016/S0304-3940(02)00519-0
Vitiello B, Stoff DM (1997) Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psychiatry 36(3):307–315. https://doi.org/10.1097/00004583-199703000-00008
Wang Y, He Z, Zhao C, Li L (2013) Medial amygdala lesions modify aggressive behavior and immediate early gene expression in oxytocin and vasopressin neurons during intermale exposure. Behav Brain Res 245(May):42–49. https://doi.org/10.1016/j.bbr.2013.02.002
Wang L, Talwar V, Osakada T, Kuang A, Guo Z, Yamaguchi T, Lin D (2019a) Hypothalamic control of conspecific self-defense. Cell Rep 26(7):1747–1758.e5. https://doi.org/10.1016/j.celrep.2019.01.078
Wang L, Simms J, Peters CJ, Fontaine MT-L, Kexin L, Michael Gill T, Jan YN, Lily Y (2019b) TMEM16B calcium-activated chloride channels regulate action potential firing in lateral septum and aggression in male mice. J Neurosci 39:3137–3118. https://doi.org/10.1523/JNEUROSCI.3137-18.2019
Wong LC, Wang L, D’Amour JA, Yumita T, Chen G, Yamaguchi T, Chang BC et al (2016) Effective modulation of male aggression through lateral septum to medial hypothalamus projection. Curr Biol 26(5):593–604. https://doi.org/10.1016/j.cub.2015.12.065
Yang Y, Raine A (2009) Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res Neuroimaging 174(2):81–88. https://doi.org/10.1016/j.pscychresns.2009.03.012
Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM (2013) Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell 153(4):896–909. https://doi.org/10.1016/j.cell.2013.04.017
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31970940, 31671100, and 31622027), the Zhejiang Provincial Natural Science Foundation of China (LR18H090001), the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2018PT31041), the Program for Introducing Talents in Discipline to Universities, and the Fundamental Research Funds for the Central Universities (2019QNA5001).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Helmy, M., Zhang, J., Wang, H. (2020). Neurobiology and Neural Circuits of Aggression. In: Wang, H. (eds) Neural Circuits of Innate Behaviors. Advances in Experimental Medicine and Biology, vol 1284. Springer, Singapore. https://doi.org/10.1007/978-981-15-7086-5_2
Download citation
DOI: https://doi.org/10.1007/978-981-15-7086-5_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7085-8
Online ISBN: 978-981-15-7086-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)