Abstract
The neuropeptide oxytocin (OT) has a solid reputation as a facilitator of social interactions such as parental and pair bonding, trust, and empathy. The many results supporting a pro-social role of OT have generated the hypothesis that impairments in the endogenous OT system may lead to antisocial behavior, most notably social withdrawal or pathological aggression. If this is indeed the case, administration of exogenous OT could be the “serenic” treatment that psychiatrists have for decades been searching for.
In the present review, we list and discuss the evidence for an endogenous “hypo-oxytocinergic state” underlying aggressive and antisocial behavior, derived from both animal and human studies. We furthermore examine the reported effects of synthetic OT administration on aggression in rodents and humans.
Although the scientific findings listed in this review support, in broad lines, the link between a down-regulated or impaired OT system activity and increased aggression, the anti-aggressive effects of synthetic OT are less straightforward and require further research. The rather complex picture that emerges adds to the ongoing debate questioning the unidirectional pro-social role of OT, as well as the strength of the effects of intranasal OT administration in humans.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Oxytocin (OT) has a solid reputation as a pro-social neuropeptide, promoting affiliation and bonding in various species ranging from fish and birds to rodents and primates (Donaldson and Young 2008). This raises an important question: if OT indeed promotes bonding, care-giving, trust, empathy, sharing, and affiliation, does this mean that impairment of OT neurotransmission leads to antisocial and egocentric behaviors, in particular social rule-breaking and aggression? And, if this is so, is it feasible to treat pathologically aggressive individuals with drugs targeting the OT system?
In the present review, we first describe the various methods used to quantify aggression and antisocial behavior in rodents, primates, and humans. We point out some inconsistencies and translational gaps that still exist. Then, we provide an overview of studies exploring associations between variability in the endogenous OT system (at the genetic, epigenetic, or protein level of OT and/or its receptor) and aggressive behavior in rodents and humans. Next, we discuss if and how various pharmacological manipulations altering OT neurotransmission affect aggression-related behaviors. Finally, we integrate these findings to describe a working model of how the OT system may modulate aggression and we list the most urgent research questions that need to be answered.
2 Categorizing and Quantifying Aggression
Translational animal models have proven to be highly useful in the identification of biomarkers predicting high aggression and the detection of “serenic” effects of drug treatments (Blanchard and Blanchard 2003). However, the translation of data obtained in animal models of aggression to violent human individuals is not without caveats (Blanchard et al. 2003). In this context, major methods of quantifying and categorizing aggression in both animal models and humans need to be discussed in order to define potential translational gaps.
In behavioral neuroscience, psychology, and psychiatry, the term “aggression” is typically used to describe behaviors aimed at another individual with the goal to subordinate or to cause physical or psychological harm. These behaviors generally include threatening postures, actions that limit the movements of the other individual (such as pinning them down or pushing them against a wall), and actions that physically injure the other individual (biting, clawing, punching, kicking, etc.). These behaviors are similar in animals and humans, although humans have added verbal aggression (shouting, cussing, insulting) and indirect aggression (gossiping, social exclusion) to their repertoire (Krahé 2013), as well as the use of highly efficient firearms and weapons of mass destruction. The term “violence” is often used interchangeably with human aggression; however, in the present review, we will use it to signify extreme human aggression resulting in considerable physical injury of the victim.
In laboratory animals, especially rats and mice, the resident-intruder test (RIT) is most often used to quantify inter-male aggressive behavior (Koolhaas et al. 2013; Neumann et al. 2010), and this has recently been extended to inter-female aggression using the female intruder test (FIT) (De Jong et al. 2014). In this standardized behavioral test, the experimental animal (the resident) is first allowed to establish its home cage territory and is then confronted with a smaller same-sex conspecific intruder in that territory for a brief, defined period (typically 10 min). Trained observers score the latency time until the resident first attacks, the frequency or duration in which aggressive behaviors occur, as well as qualitative aspects such as ferociousness of attacks, attacks of vulnerable body parts, or the presence/absence of warning signals prior to attack. In sum, these behavioral data are interpreted as the aggressive state of the resident animal, which can be associated with endogenous markers or manipulated pharmacologically.
Of course, such a paradigm is not feasible in human subjects for obvious ethical reasons. Therefore, the acute quantification of human aggression is realized using competitive computer games. Popular options are (versions of) the point subtraction aggression paradigm (PSAP) and the response choice aggression paradigm (RCAP) (Giancola and Chermack 1998). In the PSAP, subjects can both earn and steal points by rapidly pushing buttons, but so can their “opponent” (who is virtual, unknown to the subject). Aggressive behavior is quantified in terms of proactive or retaliatory point stealing. In the RCAP, subjects are playing a competitive reaction time test and are being punished by the (again, virtual) opponent when losing, for example by a loud aversive sound or a painful electric shock. Test subjects are then allowed to punish the opponent as well and their level of aggression is quantified as the frequency and severity of punishment they deliver. These and other comparative paradigms have been under considerable debate with respect to their validity as a reflection of true aggression (Ferguson and Rueda 2009). Nevertheless, the major factors influencing acute aggression in real life (sex, trait aggression, alcohol, and many others) influence aggression in these laboratory paradigms in the same direction (Anderson and Bushman 1997), supporting the external validity of these measures.
A second approach in both animal and human aggression research is the categorization of individuals in high- or low-aggressive subgroups. In animals, this may be achieved via elaborate behavioral screening of large cohorts in order to select individuals displaying stable high or low aggressive traits. Subsequently, these individuals can be compared with respect to selected neurobiological or neuroendocrine markers of interest, or their response to pharmacological manipulations. This approach has been proven particularly fruitful in Wildtype Groningen (WTG) rats, a feral rat strain of which the males display a marked individual variability in aggression (De Boer et al. 2003). Furthermore, animals with contrasting aggressive traits may be selected for parallel breeding lines resulting in offspring with predictable levels of aggression (Natarajan et al. 2009). Such contrasting traits have also been found in rats selectively bred for high and low anxiety behavior (HAB and LAB rats, respectively), in which especially male LAB rats display excessive aggression (Neumann et al. 2010; Beiderbeck et al. 2012). Additional approaches include the knocking-out of potential aggression-related genes to generate high- or low-aggressive strains (Takahashi and Miczek 2014) or manipulating the early-life social environment resulting in alterations in trait aggression in juvenile and adult male rats (Veenema and Neumann 2009; Veenema et al. 2006).
In humans, the categorization into high- and low-aggressive subtypes can be done by assessing an individual’s life history of aggression or tendency to be aggressive using convictions for violent crimes or, more commonly, questionnaires. It is of note here that a multitude of questionnaires exist for this purpose and that rarely the same questionnaire was used more than once throughout the publications cited in the present review. A third method of categorization is the diagnosis of an aggression-related psychiatric disorder such as conduct disorder (CD), antisocial personality disorder (ASPD), intermittent explosive disorder (IED), or borderline personality disorder (BPD). The selection of human subjects with high or low levels of trait aggression is then most often used to associate their behavior with certain biomarkers, such as hormone levels or single nucleotide polymorphisms (SNPs). In this context, an additional topic deserving some attention is the increasing scientific interest in “callous and unemotional traits” (CU traits) as a quantifiable correlate of aggression, at least in humans. CU traits are defined by a lack of feelings of guilt, a lack of empathy, and callous use of others for egoistic reasons (Frick and White 2008). These traits are associated with instrumental aggression used for personal gain (as opposed to reactive and defensive aggression used to prevent or retaliate a personal loss) and, together with stealing, lying, and vandalism, form the main antisocial symptoms of CD and ASPD. Importantly, the recent interest in CU traits in humans is not yet complemented with translational animal models. The investigation of endogenous variability in, or pharmacological manipulation of, empathy and altruism in laboratory rodents is therefore a promising future research topic (Bartal et al. 2011; Hernandez-Lallement et al. 2014; Sato et al. 2015).
3 Associations Between the Endogenous OT System and Aggressive Phenotypes
Aggression is a highly variable trait. It can vary across species, across individuals, and even within individuals over time. How much of this behavioral variability can be explained by a relative up- or down-regulation of the endogenous OT system? We will look at the evidence from studies in rodents as well as in humans.
3.1 Markers of the Endogenous OT System in the Periphery and the Brain
The mammalian OT system is mainly constituted of magnocellular neurons in the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei that project to the neurohypophysis and secrete OT into the blood stream in response to various physiological stimuli. Thus, an important marker of the OT system, accessible both in animals and humans, is the concentration of OT in plasma or saliva under basal conditions or in response to a relevant challenge such as emotional stress or physical exercise (Crockford et al. 2014; De Jong et al. 2015; Neumann and Landgraf 2012). Whereas basal concentrations assessed in the laboratory may vary depending on previous (uncontrolled) activities of the individual, controlled stimulation of the OT system (for example by physical exercise, emotional challenge, or sex) allows to directly assess the responsiveness of the system. Especially the quantification of OT in saliva, which can be collected under completely stress-free conditions even at home, could be an excellent option to assess OT levels in children, adolescents, or patients (De Jong et al. 2015). Importantly, we have to keep in mind that plasma or saliva OT does not reflect the activity of the brain OT system, although most physiological stimuli which trigger OT secretion into blood also stimulate OT release in selected brain regions (Neumann and Landgraf 2012). Another possible peripheral marker of the OT system is OTR binding in different tissues (e.g., skin, heart), although this has, to our knowledge, never been performed in the context of behavioral studies in animals or humans. However, genetic (SNPs) or epigenetic (e.g., methylation patterns) variability in the gene coding for OTR can be easily assessed in human blood cells and may serve as biomarkers of aggression (see Sect. 3.3).
Reliable and relevant biomarkers of extreme aggression (or other types of pathological or abnormal social behavior) are far more likely to be found in the central OT system. Due to its limited access in humans, the brain OT system is rather a target for rodent studies. Here, the local expression of the genes for OT (OT) or OTR (OTr) can be studied under basal conditions or, for example, in response to behavioral challenges such as the RIT. Moreover, the amount of OT-immunoreactive (OT-IR) neurons or the strength of local OTR binding may provide associative indicators in the context of aggressive behavior. Also, the expression of immediate early genes at mRNA or protein level in hypothalamic OT neurons provides at least a punctual picture of stimulus-dependent activation of hypothalamic OT neurons, e.g., in response to an intruder. Finally, OT content in the cerebrospinal fluid (CSF) has been used in both humans and animals to provide information about the global activity of the central OT system. In animals, a considerably higher temporal and spatial resolution can be reached using intracerebral microdialysis, i.e., the collection of extracellular fluid samples under basal conditions and during and after a challenge followed by quantification of OT content using highly sensitive radioimmunoassays (Neumann et al. 2013).
3.2 Endogenous OT and Aggression in Animals
Most studies in rodents have shown that high aggression is associated with reduced expression of OT or lower activation of OT neurons, as well as altered OTR binding. Thus, excessively aggressive male WTG rats express less OT mRNA in the PVN compared to low-aggressive individuals (Calcagnoli et al. 2014a). Consistently, adult male mice that underwent daily 3-h bouts of maternal separation in the first 2 postnatal weeks showed a marked reduction in aggression, which coincided with an increased number of OT-IR neurons in the PVN in one study (Tsuda et al. 2011), though not in another (Veenema et al. 2007). Using exposure to a female intruder as a social challenge in the FIT, virgin female Wistar residents that attacked the intruder showed less activation of OT neurons (using pERK as a marker) compared with females that tolerated the intruder (De Jong et al. 2014). Likewise, exposure to an intruder increased the activation of OT neurons in the PVN in non-aggressive worker males, but not in aggressive soldier males, in the eusocially organized naked mole rat (Heterocephalus glaber) (Hathaway et al. 2016). The higher activation of the OT system in friendly compared with aggressive encounters was not or very modestly reflected in increased plasma levels of OT (Ebner et al. 2005; Trainor et al. 2010).
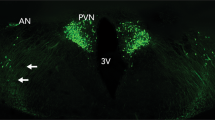
Thus far, no reports have been published about the central release of OT during aggressive behavior, except in the context of maternal aggression (see below). Preliminary data from our lab indicate that OT is robustly released in the PVN of male Wistar residents during aggressive encounters with an intruder as revealed by intracerebral microdialysis. In contrast, in female rats, both the release of OT within the PVN and the duration of aggression displayed during the FIT were lower in virgin female residents (Fig. 1a). Although this may at first sight suggest that high OT release is linked to high aggression, when looking more closely, it appears that among virgin females (but not males), the relative increase in OT in the PVN correlated negatively with the duration of aggression (Fig. 1b). These preliminary results form a starting point for further microdialysis studies encompassing additional target areas, which may each display a unique pattern of aggression-related OT release (as shown for AVP release; Veenema et al. 2010). These data indicate a clear sex difference in the role of OT in the regulation of aggressive behavior that requires follow-up research.
Relative OXT content in microdialysates sampled from the PVN of adult male (n = 8) and virgin female (n = 15) Wistar rats during (a) four consecutive 30-min microdialysis samples collected before (BASAL-1/2), during (FIT/RIT), and after (POST) a 10-min interaction with an intruder, with insert depicting the average duration of aggression toward the intruder, and (b) the FIT/RIT sample correlated with the duration of aggression toward the intruder in females, with insert depicting the same correlation in males. Microdialysis procedures were followed as described earlier (Waldherr and Neumann 2007). Until surgery, male residents were co-housed with one female for at least 12 days and female residents were group-housed with 2–3 females since weaning (females). All residents underwent one RIT/FIT (“pre-test”), 4–7 days prior to surgery. A two-way mixed model ANOVA revealed a main effect of time (within-subjects factor, F[1.72] = 7.43, P = 0.003), a main effect of sex (F[1] = 11.97, P = 0.002), and a significant interaction of time × sex (F[1.72] = 9.16, P = 0.001). a/bSample differs from corresponding aBASAL-1 or bBASAL-2 sample (P < 0.05). **Sample differs from corresponding female sample (P < 0.01). #Significant Pearson’s correlation coefficient (P < 0.05)
A few studies have quantified OTR binding associated with aggression. Specific emphasis has been placed on a number of OTR-positive brain areas that are known to be involved in aggressive behavior in rodents, such as the central and medial amygdala (CEA/MEA), bed nucleus of the stria terminalis (BNST), lateral septum (LS), medial preoptic area (MPOA), lateral hypothalamic area, ventromedial hypothalamus, and the PVN (Nelson and Trainor 2007). Thus, high-aggressive WTG rats show elevated OTR binding in the CEA and BNST, but not the LS, compared with low-aggressive males (Calcagnoli et al. 2014a). Reduced OTR binding in the caudate putamen and LS and elevated OTR binding in the MPOA were found in male Wistar rats that had become highly aggressive in response to daily 3-h bouts of maternal separation in the first 2 postnatal weeks (Lukas et al. 2010). Although it is not quite clear whether locally increased OTR binding indeed reflects increased or even reduced (due to reduced availability of the ligand) OT neurotransmission in that brain area, these associative binding studies are particularly useful for the selection of target areas for future local manipulations or measurements.
Although the results described above have linked variability in endogenous OT expression and activation, as well as OTR binding, with aggressive traits and/or acute aggression, they have not revealed whether variability in the OT system is the cause or the effect. To tackle the problem of causality, knockout mouse strains missing the gene for either OT (OT−/−) or its receptor (OTr−/−) were generated and their aggressive tendencies compared. The first wave of studies was performed in OT−/− mice, but results were inconsistent and both increases and decreases in aggression were reported (DeVries et al. 1997; Lazzari et al. 2013; Ragnauth et al. 2005; Winslow et al. 2000). The contrasting results were later explained by the fact that, in some studies, OT−/− mice were born to OT−/− mothers, whereas in others they were born to OT+/− mothers, the latter condition leading to transient exposure to maternal OT during gestation and lactation and, as a result, reduced aggression in adulthood (Dhakar et al. 2012; Takayanagi et al. 2005). A second problem in OT−/− mouse strains is the ability of AVP to bind to OTR, which may result in considerable compensatory mechanisms that are difficult to unravel in terms of behavioral outcome (Ragnauth et al. 2004). The generation of OTr−/− mice resulted in a second wave of studies that equivocally reported increased levels of aggression in the absence of OTR (Dhakar et al. 2012; Takayanagi et al. 2005; Hattori et al. 2015; Sala et al. 2011, 2013). If, however, the OTr knockout was induced after weaning, rather than at fertilization, and was limited to forebrain areas rather than the entire body, the effect on aggression disappeared (Dhakar et al. 2012). Limiting the ablation of OTr to serotonergic neurons in the dorsal and median raphe nucleus, on the other hand, reduced aggression in males (Pagani et al. 2015).
Clearly, the majority of studies rather point towards an anti-aggressive effect of an up-regulated endogenous OT system, at least in male and non-lactating virgin female rodents. Maternal aggression, which is the violent response of dams in late pregnancy, and early lactation, towards intruders threatening their offspring, is part of the complex patterns of maternal behavior only seen in the peripartum period and appears to be regulated in a different manner (Bosch 2013; Lonstein and Gammie 2002). Thus, as part of their specific behavioral profile initiated by a variety of neurooendocrine changes, including an up-regulated activity of the brain OT system (Russell et al. 2003; Slattery and Neumann 2008), lactating mammals generally display heightened levels of aggression in order to protect their offspring. Interestingly, a peak in OTR binding in the LS occurs simultaneously with the peak in maternal aggression (Caughey et al. 2011). Furthermore, OT is released within the PVN and CEA during the display of maternal aggression (Bosch et al. 2004, 2005). This local OT release is particularly pronounced in high-aggressive and highly maternal HAB dams as compared with low-aggressive LAB dams (Bosch et al. 2005). Consistently, lesion of the PVN (including the majority of magnocellular and parvocellular OT neurons in the brain) inhibits maternal aggression (Consiglio and Lucion 1996). Although these results point to a positive link between the endogenous OT and maternal aggression, some studies have found the opposite. Thus, selective lesion of parvocellular brainstem-projecting OT neurons facilitated maternal aggression and this effect could be mimicked with the selective down-regulation of OT synthesis in the PVN via antisense administration (Giovenardi et al. 1998). Furthermore, high-aggressive rat dams (as a result of social stress during pregnancy) had lower levels of OT mRNA in the MEA (Murgatroyd et al. 2015) and high-aggressive mouse dams (as a result of early life stress) had lower levels of OT-IR in the PVN (Veenema et al. 2007).
3.3 Endogenous OT and Aggression in Humans
Based on the results obtained in rodent models, it can be hypothesized that high-aggressive humans differ from the normal population with respect to their OT system. As mentioned above, the available methods to assess the activity of the endogenous OT system in humans in order to verify this hypothesis are relatively limited and largely comprise of (1) the measurement of OT levels in blood, saliva, or cerebrospinal fluid and (2) the assessment of SNPs in, or methylation patterns of, OTr.
The first study, using plasma OT levels as a readout parameter for the OT system in humans, surprisingly showed a positive correlation with indirect aggression and irritability, as quantified with the Karolinska Scales of Personality questionnaire (Uvnäs-Moberg et al. 1991). Since then, all other studies have reported a negative relationship. Thus, basal plasma OT levels correlated negatively with aggression (measured using the Buss-Perry Aggression questionnaire) and positively with empathy (measured using the Bryant’s Empathy Index) in boys with attention deficit and hyperactivity disorder (Demirci et al. 2016). In young adult women with BPD, basal plasma OT levels were lower compared with healthy controls and correlated negatively with trait aggression, as assessed by the Buss-Durkee Hostility Inventory (Bertsch et al. 2013a). Basal salivary OT levels correlated negatively with CU traits within a cohort of boys with conduct problems (Levy et al. 2015). Similarly, OT-reactive immunoglobulin G and M autoantibodies were increased in the plasma of adult men diagnosed with conduct disorder or convicted for a violent crime, compared to healthy, normal males (Fetissov et al. 2006); however, it is not known whether this reflects a general up- or down-regulation of OT system activity. Similar to the negative correlations between aggression and peripheral OT levels, increased aggression as measured with Life History of Aggression interviews correlated negatively with basal levels of OT in the CSF of men and women (including both healthy subjects and patients with various diagnosed psychiatric disorders) (Lee et al. 2009). A somewhat weaker trend in the same direction was found among women, but not men, in a cohort of suicide attempters and healthy controls, using the Karolinska Interpersonal Violence Scale to assess aggression (Jokinen et al. 2012).
Together, these results from both animal and human studies support the hypothesis that reduced peripheral and central basal OT levels (potentially reflecting a “hypo-oxytocinergic state” (Malik et al. 2012)) seem to predict aggression in some contexts, although the data is in dire need of both replication and extension. Aside from a decreased availability of OT itself, a hypo-oxytocinergic state could also be the result of alterations in the expression pattern, sensitivity, or intracellular signaling pathways of OTR, possibly caused by genetic or epigenetic modifications of OTr. Indeed, using a broad approach with rigorous statistical procedures, it could be demonstrated that variability in OTr in general (without defining specific SNPs) strongly predicts aggressive behavior in the RCAP (LoParo et al. 2016). In addition, another study showed that the level of methylation of OTr was positively correlated with CU traits in boys with conduct problems and negatively correlated with circulating plasma OT levels, supporting the “hypo-oxytocinergic state” hypothesis (Dadds et al. 2014a). Various other investigations have searched for specific SNPs underlying aggression or antisocial behavior, but the emerging picture is inconsistent. Importantly, the functional consequences of the reported SNPs, with respect to OTr expression and OTR binding as determinants of OT neurotransmission, need to be demonstrated. Nevertheless, such results generally support the search for OT-related treatment options. Thus, one research group genotyped multiple cohorts of high-aggressive children and healthy controls and reported an association between SNP rs237885AA and high CU-traits within high-aggressive subjects (Beitchman et al. 2012), as well as a sex-specific link between high aggression and SNP rs677032T (in girls) and SNP rs1042778C (in boys) (Malik et al. 2012). However, in a later study they could only confirm their finding in girls, as well as a direct link between high aggression and SNPs rs237898A and rs237902C in boys (Malik et al. 2014). Interestingly, SNP rs1042778TT (not C!) was found to be associated with high CU traits in boys and girls with conduct problems (Dadds et al. 2014b), as well as with increased amygdala activation upon exposure to angry faces in adult men, which is considered a consistent neuropsychological marker of antisocial behavior (Waller et al. 2017). SNP rs7632287AA was strongly associated with Life History of Aggression interview scores and Self-Reported Delinquency scores in two cohorts of children representing the normal Swedish population (Hovey et al. 2016).
Two additional lines of research deserve to be mentioned here. In the first line, two independent studies could demonstrate that SNP rs53576GG predicts aggression or antisocial behavior under circumstances of high social stress in females (Buffone and Poulin 2014; Smearman et al. 2015). In the second line, it could be shown that SNPs rs4564790C and rs1488467C were predictive of higher levels of aggression, as measured in the RCAP and various questionnaires, but only in adult males that were under the influence of alcohol (Johansson et al. 2012a, b). These studies clearly emphasize the importance of the context in which aggression is measured and confirm that further research is needed to establish how the genetic or epigenetic variability in OTr can be utilized as a marker of abnormal aggressive or antisocial behavior.
4 Effects of Exogenous OT on Aggressive Behavior
The correlational studies described above clearly point towards a modulatory role for the endogenous OT system in aggressive and antisocial behavior. If this is indeed the case, then the administration of synthetic OT could be an effective treatment for highly aggressive individuals, in particular for those characterized by a hypo-OT state. Numerous animal and a few human studies have explored this promising possibility. In this context, a clear methodological limitation, especially in human studies, is the inability of OT to cross the blood–brain barrier under physiological circumstances. In rodents and primates, this problem can be solved by the administration of OT directly into the ventricles (intracerebroventricular [ICV] infusion) or into target brain areas. Treatment of human subjects with synthetic OT is mainly performed using intranasal administration and this has been reported to affect various (social) behaviors. Although the uptake of intranasally applied OT into the brain compartment is likely (Neumann and Landgraf 2012; Neumann et al. 2013; Modi et al. 2014; Striepens et al. 2013), the scientific strength of many of the reported findings and the underlying mechanisms is critically debated (Leng and Ludwig 2016; Walum et al. 2016).
4.1 Effects of Exogenous OT on Animal Aggression
In general, acute central infusion of OT inhibits aggression, independent of the route of administration and sex of the individual. So, ICV infusion of OT inhibits inter-male aggression in male WTG rats both after acute (1,000 ng) and chronic (10 ng/h for 7 days) infusion (Calcagnoli et al. 2013, 2014b). Acute ICV infusion of OT also reduced inter-female aggression in virgin Wistar rats (at 100 ng) (De Jong et al. 2014). In steroid-primed female prairie voles, ICV OT also lowered female-to-male aggression (at 1 and 1,000 ng) (Witt et al. 1990), whereas it did not affect male-to-female aggression in male prairie voles (Witt et al. 1990; Mahalati et al. 1991). Also, in male C57bl/6 mice, acute ICV infusion of OT (100 ng) reduced aggression after co-housing with unfamiliar male conspecifics, whereas an OTR antagonist (OTRA, 500 ng) had the opposite effect (Arakawa et al. 2015). Acute and repeated (once daily over 7 days) intranasal application of 20 μg OT reduced aggression in high-aggressive WTG rats (Calcagnoli et al. 2015a) and a similar acute effect was found in socially isolated and high aggressive C57Bl/6 mice, in response to 200 ng intranasal OT (Karpova et al. 2016). Some neutral or pro-aggressive effects of OT have also been reported: 24 h of chronic ICV infusion with OT (0.5 ng/h) did not affect mating-induced aggression of male prairie voles against male intruders (Winslow et al. 1993), whereas acute ICV OT (100–1,000 ng) increased aggressive behavior in dominant male squirrel monkeys without affecting aggression in subordinate individuals (Winslow and Insel 1991).
Only a handful of studies have attempted to localize the predominantly anti-aggressive effects of OT via infusion of OT or an OTR antagonist (OTRA) into target brain areas. In male WTG rats displaying normal to high aggression, bilateral infusion of 30 ng OT into the CEA inhibited aggression, whereas bilateral infusion of a selective OTRA into the CEA had only a modest pro-aggressive effect (Calcagnoli et al. 2015b). In virgin female Syrian hamsters, bilateral infusion of OT (at 9 ng/200 nL) into the MPOA and anterior hypothalamus reduced aggression (Harmon et al. 2002), whereas bilateral infusion of an OTRA had a robust effect in the opposite direction.
Keeping in mind that maternal defense is part of the suite of maternal behaviors emerging strictly in the context of complex peripartum adaptations, including those of the OT system, reports of pharmacological OT manipulations affecting maternal aggression should be mentioned in this review. These reports describe somewhat inconsistent results that appear to depend on the experimental conditions. On the one hand, modest anti-aggressive effects of OT were found in Wistar and Sprague-Dawley rat dams. Local infusion of 10–20 ng OT into the CEA (and BNST) reduced maternal aggression, whereas a higher dose of 200 ng OT was not effective (Consiglio et al. 2005). ICV infusion of a selective OTRA did not affect maternal aggression in Wistar rats (Neumann et al. 2001), but infusion of OTRA into the CEA or infralimbic prefrontal cortex enhanced maternal aggression (Lubin et al. 2003; Sabihi et al. 2014). On the other hand, a pro-aggressive effect of OT has been found in HAB and LAB dams: chronic 5-day ICV infusion of OT enhanced the low levels of maternal aggression in LAB dams, whereas acute ICV infusion of a selective OTRA reduced the high levels of maternal aggression in HAB dams (Bosch and Neumann 2012). In support, bilateral application of a selective OTRA into the CEA via retrodialysis during ongoing behavioral testing decreased maternal aggression only in highly aggressive HAB dams (Bosch et al. 2005). Effects in the same direction were found in lactating golden hamsters, where 2 ng of OT infused into the CEA increased maternal aggression (Ferris et al. 1992).
4.2 Effects of Exogenous OT on Human Aggression
Following initial reports of shifts in social decision-making following intranasal OT treatment in humans (Kosfeld et al. 2005), an avalanche of papers claiming pro-social effects of intranasal OT has followed. Relatively few of these papers have measured the hypothetical anti-aggressive effects of OT and the handful of reported results has been far from encouraging.
The effects of intranasal OT treatment on aggressive behavior were mainly assessed in versions of the PSAP. No acute behavioral effects were detected in either aggressive adult males with ASPD or healthy adult males (Alcorn et al. 2015a, b), whereas a modest increase in both reactive and proactive aggression was found in healthy adult men and women using a modified PSAP, the Social Orientation Paradigm (Ne’eman et al. 2016). Acute intranasal OT modestly decreased aggression in the PSAP in (otherwise healthy) women with higher state anxiety while not affecting non-anxious women (Campbell and Hausmann 2013). De Dreu et al. found that intranasal OT shifted behavior of healthy males towards sharing with in-group members combined with punishment of out-group members (De Dreu et al. 2010). In follow-up studies, intranasal OT facilitated in-group conformity and cooperation while increasing aggressive and anti-social tendencies towards out-group individuals (De Dreu and Kret 2016). Finally, Bertsch et al. found that, in young adult female BPD patients with high trait aggression, intranasal OT could reverse their disorder-induced increased threat sensitivity, as reflected by the higher level of eye-fixation changes and increased amygdala reactivity (measured with fMRI) in response to angry faces (Bertsch et al. 2013b).
Taken together, these results do not equivocally support the theory that intranasal OT treatment is capable of inhibiting aggression, at least in healthy subjects. In patients with a confirmed impaired endogenous OT system (such as BPD patients with lowered basal plasma OT levels (Bertsch et al. 2013a)), intranasal OT administration may be beneficial. In future research, the possible anti-aggressive effects of intranasal OT will need to be tested in selected cohorts of high-aggressive patient groups (for example, suffering from CD with high CU traits) that are positive for one or more biomarkers indicating altered/reduced OT neurotransmission. Currently, the EU-funded research consortium FemNAT-CD is collecting data towards this goal in girls and boys with CD (Freitag 2014).
5 Integration and Future Research
There is no doubt that both endogenous and exogenous OT are capable of modulating antisocial and aggressive behavior, but the strength of the effects and, in some cases, even the direction, depends on the context. Variability within the endogenous OT system as a result of genetic variations, early life stress, reproductive state, seasonal cues, or unknown other parameters is likely to shift an individual’s tendency to respond aggressively towards an encountered conspecific. According to the hypo-OT state hypothesis, a down-regulated OT system will rather be associated with the facilitation of aggression, whereas an up-regulated OT system will inhibit aggression. This hypothesis clearly fits with the known pro-social effects of OT, which include an increase in social preference, an improvement in social memory, and a reduction in social fear (Neumann and Landgraf 2012; Maroun and Wagner 2016; Neumann and Slattery 2016). A more complex association can be expected with the anxiolytic properties of OT, since the links between aggression and anxiety are not straightforward (Neumann et al. 2010).
In general, we have to critically scrutinize the current knowledge and presume that our work in this field is far from complete. Especially, investigations with a higher spatial resolution (i.e., focusing on variability in OT neurotransmission in specific brain areas associated with aggression and social behavior mentioned above) and in contexts beyond inter-male territorial defense (i.e., females, adolescents, non-territorial instrumental aggression, and CU traits) are needed. Such (animal) studies will help to interpret the low-resolution findings in humans and to define novel hypotheses to be tested in humans. Furthermore, although treatment with synthetic OT is still a promising possibility to inhibit aggression and violent behavior in subgroups of patients with a verified impairment in their endogenous OT system, these effects are not expected to be consistent across subjects in different contexts. In fact, when designing future pharmacological experiments, it needs to be taken into account that selection of subjects (in terms of animal species, age, sex, reproductive state, or patient subgroup) and context may yield vastly different outcomes and results need to be interpreted with this information in mind.
References
Alcorn JL, Green CE, Schmitz J, Lane SD (2015a) Effects of oxytocin on aggressive responding in healthy adult men. Behav Pharmacol 26:798–804
Alcorn JL, Rathnayaka N, Swann AC et al (2015b) Effects of intranasal oxytocin on aggressive responding in antisocial personality disorder. Psychol Rec 65:691–703
Anderson CA, Bushman BJ (1997) External validity of “trivial” experiments: the case of laboratory aggression. Rev Gen Psychol 1:19–41
Arakawa H, Blanchard DC, Blanchard RJ (2015) Central oxytocin regulates social familiarity and scent marking behavior that involves amicable odor signals between male mice. Physiol Behav 146:36–46
Bartal IB-A, Decety J, Mason P (2011) Empathy and pro-social behavior in rats. Science 334:1427–1430
Beiderbeck DI, Reber SO, Havasi A et al (2012) High and abnormal forms of aggression in rats with extremes in trait anxiety – involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology 37:1969–1980
Beitchman JH, Zai CC, Muir K et al (2012) Childhood aggression, callous-unemotional traits and oxytocin genes. Eur Child Adolesc Psychiatry 21:125–132
Bertsch K, Schmidinger I, Neumann ID, Herpertz SC (2013a) Reduced plasma oxytocin levels in female patients with borderline personality disorder. Horm Behav 63:424–429
Bertsch K, Gamer M, Schmidt B et al (2013b) Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am J Psychiatr 170:1169–1177
Blanchard DC, Blanchard RJ (2003) What can animal aggression research tell us about human aggression? Horm Behav 44:171–177
Blanchard RJ, Wall PM, Blanchard DC (2003) Problems in the study of rodent aggression. Horm Behav 44:161–170
Bosch OJ (2013) Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Philos Trans R Soc Lond 368:85
Bosch OJ, Neumann ID (2012) Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav 61:293–303
Bosch OJ, Krömer SA, Brunton PJ, Neumann ID (2004) Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience 124:439–448
Bosch OJ, Meddle SL, Beiderbeck DI et al (2005) Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci 25:6807–6815
Buffone AEK, Poulin MJ (2014) Empathy, target distress, and neurohormone genes interact to predict aggression for others-even without provocation. Personal Soc Psychol Bull 40:1406–1422
Calcagnoli F, de Boer SF, Althaus M et al (2013) Antiaggressive activity of central oxytocin in male rats. Psychopharmacology (Berlin) 229:639–651
Calcagnoli F, de Boer SF, Beiderbeck DI et al (2014a) Local oxytocin expression and oxytocin receptor binding in the male rat brain is associated with aggressiveness. Behav Brain Res 261:315–322
Calcagnoli F, Meyer N, de Boer SF et al (2014b) Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Horm Behav 65:427–433
Calcagnoli F, Kreutzmann JC, de Boer SF et al (2015a) Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology 51:112–121
Calcagnoli F, Stubbendorff C, Meyer N et al (2015b) Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology 90:74–81
Campbell A, Hausmann M (2013) Effects of oxytocin on women’s aggression depend on state anxiety. Aggress Behav 39:316–322
Caughey SD, Klampfl SM, Bishop VR et al (2011) Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J Neuroendocrinol 23:1113–1124
Consiglio AR, Lucion AB (1996) Lesion of hypothalamic paraventricular nucleus and maternal aggressive behavior in female rats. Physiol Behav 59:591–596
Consiglio AR, Borsoi A, Pereira GAM, Lucion AB (2005) Effects of oxytocin microinjected into the central amygdaloid nucleus and bed nucleus of stria terminalis on maternal aggressive behavior in rats. Physiol Behav 85:354–362
Crockford C, Deschner T, Ziegler TE, Wittig RM (2014) Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Front Behav Neurosci 8:68
Dadds MR, Moul C, Cauchi A et al (2014a) Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Dev Psychopathol 26:33–40
Dadds MR, Moul C, Cauchi A et al (2014b) Polymorphisms in the oxytocin receptor gene are associated with the development of psychopathy. Dev Psychopathol 26:21–31
De Boer SF, van der Vegt BJ, Koolhaas JM (2003) Individual variation in aggression of feral rodent strains: a standard for the genetics of aggression and violence? Behav Genet 33:485–501
De Dreu CKW, Kret ME (2016) Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol Psychiatry 79:165–173
De Dreu CKW, Greer LL, Handgraaf MJJ et al (2010) The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328:1408–1411
De Jong TR, Beiderbeck DI, Neumann ID (2014) Measuring virgin female aggression in the female intruder test (FIT): effects of oxytocin, estrous cycle, and anxiety. PLoS One 9:e91701
De Jong TR, Menon R, Bludau A et al (2015) Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: the Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology 62:381–388
Demirci E, Ozmen S, Kilic E, Oztop DB (2016) The relationship between aggression, empathy skills and serum oxytocin levels in male children and adolescents with attention deficit and hyperactivity disorder. Behav Pharmacol 27:681–688
DeVries AC, Young WS, Nelson RJ (1997) Reduced aggressive behaviour in mice with targeted disruption of the oxytocin gene. J Neuroendocrinol 9:363–368
Dhakar MB, Rich ME, Reno EL et al (2012) Heightened aggressive behavior in mice with lifelong versus postweaning knockout of the oxytocin receptor. Horm Behav 62:86–92
Donaldson ZR, Young LJ (2008) Oxytocin, vasopressin and the neurogenetics of sociality. Science 322:900–904
Ebner K, Wotjak CT, Landgraf R, Engelmann M (2005) Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Horm Behav 47:14–21
Ferguson CJ, Rueda SM (2009) Examining the validity of the modified Taylor competitive reaction time test of aggression. J Exp Criminol 5:121–137
Ferris CF, Foote KB, Meltser HM et al (1992) Oxytocin in the amygdala facilitates maternal aggression. Ann N Y Acad Sci 652:456–457
Fetissov SO, Hallman J, Nilsson I et al (2006) Aggressive behavior linked to corticotropin-reactive autoantibodies. Biol Psychiatry 60:799–802
Freitag C (2014) Neurobiology and treatment of adolescent female conduct disorder: FemNAT-CD consortium: a new European cooperation. Eur Child Adolesc Psychiatry 23:723–724
Frick P, White SF (2008) Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. J Child Psychol Psychiatry 49:359–375
Giancola PR, Chermack ST (1998) Construct validity of laboratory aggression paradigms: a response to Tedeschi and Quigley (1996). Aggress Violent Behav 3:237–253
Giovenardi M, Padoin MJ, Cadore LP, Lucion AB (1998) Hypothalamic paraventricular nucleus modulates maternal aggression in rats: effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav 63:351–359
Harmon AC, Huhman KL, Moore TO, Albers HE (2002) Oxytocin inhibits aggression in female Syrian hamsters. J Neuroendocrinol 14:963–969
Hathaway GA, Faykoo-Martinez M, Peragine DE et al (2016) Subcaste differences in neural activation suggest a prosocial role for oxytocin in eusocial naked mole-rats. Horm Behav 79:1–7
Hattori T, Kanno K, Nagasawa M et al (2015) Impairment of interstrain social recognition during territorial aggressive behavior in oxytocin receptor-null mice. Neurosci Res 90:90–94
Hernandez-Lallement J, van Wingerden M, Marx C et al (2014) Rats prefer mutual rewards in a prosocial choice task. Front Neurosci 8:443
Hovey D, Lindstedt M, Zettergren A et al (2016) Antisocial behavior and polymorphisms in the oxytocin receptor gene: findings in two independent samples. Mol Psychiatry 21:983–988
Johansson A, Bergman H, Corander J et al (2012a) Alcohol and aggressive behavior in men-moderating effects of oxytocin receptor gene (OXTR) polymorphisms. Genes Brain Behav 11:214–221
Johansson A, Westberg L, Sandnabba K et al (2012b) Associations between oxytocin receptor gene (OXTR) polymorphisms and self-reported aggressive behavior and anger: interactions with alcohol consumption. Psychoneuroendocrinology 37:1546–1556
Jokinen J, Chatzittofis A, Hellström C et al (2012) Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology 37:482–490
Karpova IV, Mikheev VV, Marysheva VV et al (2016) Oxytocin-induced changes in monoamine level in symmetric brain structures of isolated aggressive C57Bl/6 mice. Bull Exp Biol Med 160:605–609
Koolhaas JM, Coppens CM, de Boer SF et al (2013) The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp 77:e4367
Kosfeld M, Heinrichs M, Zak PJ et al (2005) Oxytocin increases trust in humans. Nature 435:673–676
Krahé B (2013) The social psychology of aggression, 2nd edn. Psychology Press, Hove
Lazzari VM, Becker RO, de Azevedo MS et al (2013) Oxytocin modulates social interaction but is not essential for sexual behavior in male mice. Behav Brain Res 244:130–136
Lee R, Ferris C, Van de Kar LD, Coccaro EF (2009) Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology 34:1567–1573
Leng G, Ludwig M (2016) Intranasal oxytocin: myths and delusions. Biol Psychiatry 79:243–250
Levy T, Bloch Y, Bar-Maisels M et al (2015) Salivary oxytocin in adolescents with conduct problems and callous-unemotional traits. Eur Child Adolesc Psychiatry 24:1543–1551
Lonstein JS, Gammie SC (2002) Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev 26:869–888
LoParo D, Johansson A, Walum H et al (2016) Rigorous tests of gene-environment interactions in a lab study of the oxytocin receptor gene (OXTR), alcohol exposure, and aggression. Am J Med Genet B Neuropsychiatr Genet 171:589–602
Lubin DA, Elliott JC, Black MC, Johns JM (2003) An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci 117:195–201
Lukas M, Bredewold R, Neumann ID, Veenema AH (2010) Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology 58:78–87
Mahalati K, Okanoya K, Witt DM, Carter CS (1991) Oxytocin inhibits male sexual behavior in prairie voles. Pharmacol Biochem Behav 39:219–222
Malik AI, Zai CC, Abu Z et al (2012) The role of oxytocin and oxytocin receptor gene variants in childhood-onset aggression. Genes Brain Behav 11:545–551
Malik AI, Zai CC, Berall L et al (2014) The role of genetic variants in genes regulating the oxytocin-vasopressin neurohumoral system in childhood-onset aggression. Psychiatr Genet 24:201–210
Maroun M, Wagner S (2016) Oxytocin and memory of emotional stimuli: some dance to remember, some dance to forget. Biol Psychiatry 79:203–212
Modi ME, Connor-Stroud F, Landgraf R et al (2014) Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology 45:49–57
Murgatroyd CA, Taliefar M, Bradburn S et al (2015) Social stress during lactation, depressed maternal care, and neuropeptidergic gene expression. Behavioral Pharmacology 26:642–653
Natarajan D, de Vries H, Saaltink D-J et al (2009) Delineation of violence from functional aggression in mice: an ethological approach. Behav Genet 39:73–90
Ne’eman R, Perach-Barzilay N, Fischer-Shofty M et al (2016) Intranasal administration of oxytocin increases human aggressive behavior. Horm Behav 80:125–131
Nelson RJ, Trainor BC (2007) Neural mechanisms of aggression. Nat Rev Neurosci 8:536–546
Neumann ID, Landgraf R (2012) Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35:649–659
Neumann ID, Slattery DA (2016) Oxytocin in general anxiety and social fear: a translational approach. Biol Psychiatry 79:213–221
Neumann ID, Toschi N, Ohl F et al (2001) Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur J Neurosci 13:1016–1024
Neumann ID, Veenema AH, Beiderbeck DI (2010) Aggression and anxiety: social context and neurobiological links. Front Behav Neurosci 4(12):1–16
Neumann ID, Maloumby R, Beiderbeck DI et al (2013) Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38:1985–1993
Pagani JH, Williams Avram SK, Cui Z et al (2015) Raphe serotonin neuron-specific oxytocin receptor knockout reduces aggression without affecting anxiety-like behavior in male mice only. Genes Brain Behav 14:167–176
Ragnauth AK, Goodwillie A, Brewer C et al (2004) Vasopressin stimulates ventromedial hypothalamic neurons via oxytocin receptors in oxytocin gene knockout male and female mice. Neuroendocrinology 80:92–99
Ragnauth AK, Devidze N, Moy V et al (2005) Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav 4:229–239
Russell JA, Leng G, Douglas AJ (2003) The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front Neuroendocrinol 24:27–61
Sabihi S, Dong SM, Durosko NE, Leuner B (2014) Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front Behav Neurosci 8:258
Sala M, Braida D, Lentini D et al (2011) Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry 69:875–882
Sala M, Braida D, Donzelli A et al (2013) Mice heterozygous for the oxytocin receptor gene (Oxtr(+/−)) show impaired social behaviour but not increased aggression or cognitive inflexibility: evidence of a selective haploinsufficiency gene effect. J Neuroendocrinol 25:107–118
Sato N, Tan L, Tate K, Okada M (2015) Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn 18:1039–1047
Slattery DA, Neumann ID (2008) No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol 586:377–385
Smearman EL, Winiarski DA, Brennan PA et al (2015) Social stress and the oxytocin receptor gene interact to predict antisocial behavior in an at-risk cohort. Dev Psychopathol 27:309–318
Striepens N, Kendrick KM, Hanking V et al (2013) Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep 3:3440
Takahashi A, Miczek KA (2014) Neurogenetics of aggressive behavior: studies in rodents. Curr Top Behav Neurosci 17:3–44
Takayanagi Y, Yoshida M, Bielsky IF et al (2005) Pervasive social deficits, but normal parturition in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A 102:16096–16101
Trainor BC, Takahashi EY, Silva AL et al (2010) Sex differences in hormonal responses to social conflict in the monogamous California mouse. Horm Behav 58:506–512
Tsuda MC, Yamaguchi N, Ogawa S (2011) Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport 22:259–263
Uvnäs-Moberg K, Arn I, Theorell T, Jonsson CO (1991) Personality traits in a group of individuals with functional disorders of the gastrointestinal tract and their correlation with gastrin, somatostatin and oxytocin levels. J Psychosom Res 35:515–523
Veenema AH, Neumann ID (2009) Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology 34:463–467
Veenema AH, Blume A, Niederle D et al (2006) Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci 24:1711–1720
Veenema AH, Bredewold R, Neumann ID (2007) Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology 32:437–450
Veenema AH, Beiderbeck DI, Lukas M, Neumann ID (2010) Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav 58:273–281
Waldherr M, Neumann ID (2007) Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci U S A 104:16681–16684
Waller R, Corral-Frías NS, Vannucci B et al (2017) An oxytocin receptor polymorphism predicts amygdala reactivity and antisocial behavior in men. Soc Cogn Affect Neurosci 11:1218–1226
Walum H, Waldman ID, Young LJ (2016) Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol Psychiatry 79:251–257
Winslow JT, Insel TR (1991) Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J Neurosci 11:2032–2038
Winslow JT, Hastings N, Carter CS et al (1993) A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365:545–548
Winslow JT, Hearn EF, Ferguson J et al (2000) Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav 37:145–155
Witt DM, Carter CS, Walton DM (1990) Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster). Pharmacol Biochem Behav 37:63–69
Acknowledgements
The authors were financially supported by the European Community’s Seventh Framework Programme (FP7/2007–2013) under Grant Agreement no. 602407 (FemNAT-CD, www.femnat-cd.eu).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
de Jong, T.R., Neumann, I.D. (2017). Oxytocin and Aggression. In: Hurlemann, R., Grinevich, V. (eds) Behavioral Pharmacology of Neuropeptides: Oxytocin. Current Topics in Behavioral Neurosciences, vol 35. Springer, Cham. https://doi.org/10.1007/7854_2017_13
Download citation
DOI: https://doi.org/10.1007/7854_2017_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-63738-9
Online ISBN: 978-3-319-63739-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)