Abstract
Over the past few decades, there is a paradigm shift towards recovering value-added products from contaminated water bodies due to the high cost and energy consumption associated with their treatment and disposal. Microbial electrochemical technologies, including electrofermentation (EF), represent a promising option for the production of a wide range of useful products from waste streams. EF technology holds a great promise to improve the output of traditional fermentation by controlling the microbial metabolism through regulating the intracellular and extracellular redox balance, leading to produce chemicals of interest with improved selectivity, specificity, and product recovery. This chapter provides a state-of-the-art analysis for the recent research advancement and technology development. This chapter also discusses the possible microbial community interactions and how it might affect the overall efficiency of EF systems. An overview is given on the integration possibilities of EF with the existing wastewater treatment process that most likely will lead to successful utilization of waste streams and biomass treatment towards developing value-added biorefinery for sustainable circular economy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Electrofermentation
- Electrochemically-active bacteria
- Microbial electrochemical technology;
- Microbial competition
- Value-added products
6.1 Background
The global energy demand is currently about 13,864 million tons of oil equivalent (Mtoe) annually with over 85% of this demand is being provided from fossil fuels combustion (BP 2019), leading to ~35 gigatons of carbon dioxide (CO2) emission released into the atmosphere (Dowell et al. 2017; IPCC 2014). Replacing the current means for energy production with more sustainable, carbon-neutral energy sources remains a challenge that is facing our society (Brockway et al. 2019; Liu and Rajagopal 2019). Fortunately, this demand can be met from the bioconversion of waste streams to value-added products, such as biochemicals and biofuels. For example, approximately 2.2 million m3 of wastewater and 2 billion tons of municipal solid waste (MSW) are globally released to the environment every year (Kaza et al. 2018; WWAP (United Nations World Water Assessment Programme) 2017). The release of these potentially harmful waste streams into environment without proper treatment has been a serious cause for concern over the past few decades. Therefore, converting waste streams into various forms of renewable energy creates a “win-win” scenario that solves a wicked waste-management challenge, reduces the cost associated with conventional waste streams treatment, produces renewable energy, and recovers value-added products (Rittmann 2008). Theoretically, energy produced from organic-rich waste streams is approximately a few order of magnitude higher than energy required for wastewater treatment processes (Dubrawski et al. 2019; Logan and Rabaey 2012; Heidrich et al. 2011).
One option for bioenergy production from waste streams is the “anaerobic digestion (AD),” in which different microbial groups convert complex organic compounds under strict anaerobic condition to organic acids and hydrogen gas (H2), which are subsequently consumed by methanogens to generate methane gas (CH4) (Fig. 6.1) (Li et al. 2015; Metcalf and Eddy 2003; Rittmann and McCarty 2001). Despite the benefit of producing CH4 from waste streams digestion, the low conversion yield of CH4 to electricity (i.e., 30–40%) limits the application of AD to treat low- and medium-strength wastewater. In addition, AD technology is susceptible to process instability and low biogas production, mainly due to the low organic contents in donor substrates, organic acids accumulation, decrease in the reactor pH, and/or high-level of free ammonia (Mao et al. 2015; Rajagopal et al. 2013; Chen et al. 2008). Thus, AD has often been used to stabilize concentrated waste streams, such as waste activated sludge generated during aerobic domestic wastewater treatment and food wastes (Peccia and Westerhoff 2015; McCarty et al. 2011). Although the coupling of aerobic treatment of wastewater with anaerobic stabilization of waste activated sludge to generate biogas seems to be beneficial, this integration allows only a tiny fraction of this organic matter to be recovered, making the current wastewater treatment practices energy-negative processes (McCarty et al. 2011).
Anaerobic food web, which involves several groups of microorganisms to mediate the biotransformation of complex waste streams into value-added products (Adopted from Mahmoud 2016)
A more recent technology for waste valorization is the “microbial electrochemical technologies (METs),” which are unique platforms that utilize electrochemically-active bacteria (EAB) to catalyze bioelectrochemical reactions. EAB have the capability to exchange electrons beyond their outermost membranes with an electron acceptor (i.e., anode) or an electron donor (i.e., cathode), leading to convert organic compounds into electricity, methane, hydrogen, hydrogen peroxide, or other value-added products (Zou and He 2018; Malvankar and Lovley 2014; Rittmann 2008; Lovley 2008). Despite the growing interest in METs research, only a few studies have addressed the scaling-up of METs with the majority of published research were performed using laboratory-scale MET reactors (i.e., <<1 liter) (Heidrich et al. 2013; Cusick et al. 2011; Logan 2010). Therefore, the main challenge to commercialize the METs is to improve the electron recovery and the productivity of value-added products, while reducing its high capital, and operation and maintenance (O&M) cost, especially when complex waste streams used as donor substrates (Logan 2010; Rittmann 2008).
Similar to the biodegradation of complex organic matter in AD (Fig. 6.1), the biodegradation of organic matter in METs must be occurred through a cascade of anaerobic reactions, including fermentation and anode respiration. Given that EAB have limited ecological capability to consume a limited number of donor substrates (such as acetate), fermentation represents a crucial step to generate simple products that EAB can efficiently consume (Pant et al. 2010). For example, Ge et al. (2014) showed that the energy recovery of MET systems was inversely proportional to the degree of substrate complexity (expressed as chemical oxygen demand (COD)). They observed that the highest energy recovery was achieved when acetate used as the main sole donor substrate (i.e., 0.40 kWh/kgCOD compared to only 0.17 kWh/kgCOD for domestic wastewater and 0.04 kWh/kgCOD for industrial wastewater). The main cause for this low energy recovery, especially for substrate of low solid contents (e.g., landfill leachate), is the inhibition of fermentation not anode respiration (Mahmoud et al. 2016). Despite the fact that fermentation and anode respiration can be occurred in the same reactor (Mahmoud et al. 2014), the integration of METs with anaerobic digestion to perform some or the majority of fermentation in a separate reactor seems to be beneficial for METs (Katuri et al. 2019; Escapa et al. 2016; Mahmoud et al. 2014).
6.2 Fermentation as an Essential Step in Wastewater Biodegradation
Fermentation represents a crucial step in anaerobic food web, in which soluble organic matter (i.e., the products of particulate organic matter hydrolysis) is converted to organic acids, alcohols, and H2 (Temudo et al. 2007; Bolzonella et al. 2005). It is considered a central step whether the final product is CH4 in AD, electric current or H2 in METs (Rittmann 2008). The hallmark of the fermentation process is that fermenting bacteria extract energy from biodegradable donor substrates without the need of external electron acceptors (e.g., nitrate and oxygen), where the electron acceptors are originated from the initial donor substrates. Fermentation often relies on substrate-level phosphorylation to drive adenosine triphosphate (ATP) generation (Rodríguez et al. 2006; Metcalf and Eddy 2003; Rittmann and McCarty 2001). Thus, fermentation involves a rearrangement of donor substrate molecules into simpler products (i.e., organic acids, alcohols, and H2) (Fig. 6.2).
Considering the mixed-culture fermentation of glucose, one mole of glucose could theoretically produce 2 moles of acetate, and 4 moles of H2 (Eq. (6.1)) that its partial pressure should be maintained at a very low level in order to make fermentation thermodynamically favorable (Mahmoud et al. 2017; Angelidaki et al. 2011; Rodríguez et al. 2006; Thauer et al. 1977).
In order to make fermentation thermodynamically feasible, hydrogen partial pressure must be maintained at a low level (Hallenbeck 2009; Stams and Plugge 2009; McInerney et al. 2008). However, increasing the hydrogen partial pressure would induce a metabolic shift in the fermentation pathways and stoichiometry towards producing more reduced organic acids (e.g., butyrate, lactate, and propionate) instead of producing acetate and H2. The main reason for this detouring is that at high hydrogen partial pressure microbes tend to replenish the NAD+ and oxidized ferredoxin pools to continue fermentation, resulting in production of more reduced organic acids (Angenent et al. 2004).
Another major challenge for fermentations is that the product spectrum as well as microbial population structure and diversity can be significantly altered by changing the operating conditions, including pH (Lu et al. 2011; Metcalf and Eddy 2003), organic matter loading (Temudo et al. 2008), the degree of substrate complexity (Saint-amans et al. 2001; Himmi et al. 2000), the presence of inhibitory compounds (Mahmoud et al. 2017), and temperature (Batstone et al. 2002). For instance, Velasquez-Orta et al. (2011) showed that the microbial fuel cell (MFC) performance, in terms of COD removal and power density generation, was significantly affected by the degree of substrate complexity. The highest power density was reported when acetate used as the sole donor substrate (99 ± 2 mW/m2) compared to only 4 ± 2 mW/m2 for starch, mainly due to that different initial donor substrates have distinct degree of substrate degradation and fermentation pathways. In another study, Zhang et al. (2014) revealed that the microbial community structure of AD bioreactors significantly changes as a function of the influent donor substrate composition, probably due to the inability of many microbes to use certain substrates to grow, leading to a dramatic change in mixed-culture community structure towards species that have the ability to consume these substrates.
6.3 Overcoming the Fermentation Bottlenecks Through Electricity-Driven Fermentation
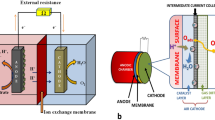
In an early review, Rabaey and Rozendal (2010) discussed the possibility to alter the fermentation pathways towards the production of targeted chemicals and bioproducts by inserting polarized electrodes in the bulk solution of AD reactors, which was later called “electrofermentation (EF)” (Rabaey and Rozendal 2010). The role of polarized electrodes is to provide an external source of either oxidizing or reducing power, leading to stimulate microbial metabolism in traditional fermentation bioreactor towards producing targeted chemicals and enhancing the microbial growth (Agler et al. 2011). In this platform, the supplied electric current allows the organic matter fermentation to proceed under imbalanced redox conditions by altering the extracellular and intracellular NAD+/NADH balance and oxidation-reduction potential (ORP) (Moscoviz et al. 2016).
In anodic EF, organic-rich substrates (e.g., carbohydrate and protein), which are the main source of electrons and energy, are fermented into more oxidized final products. In this case, the anode acts as the terminal electron acceptor. In contrast, the working electrode in cathodic EF (i.e., cathode) supplies electrons to the microbial cells, leading to convert the initial substrates into more reduced final products (Kracke and Krömer 2014).
EF systems have been commonly used to improve the production of a wide spectrum of value-added products from different waste streams (Table 6.1). Similar to AD, the complex organic matter in METs is biodegraded through a cascade of bioelectrochemical reactions under strict anaerobic condition. First, the particulate organic matter is hydrolyzed to soluble monomer, which is then converted into organic acids, alcohols, and H2 by fermenting bacteria. Then, the majority of fermentation by-products are further converted into acetate and H2. Finally, acetate and H2 are consumed by either EAB (the desired pathway) or methanogens to generate CH4 (the undesired pathway) (McCarty et al. 2011; Parameswaran et al. 2010; Thauer et al. 2008; Rittmann and McCarty 2001). Generally, there are two main H2-consumers in the anode of MET systems rather than EAB: hydrogenotrophic methanogens and homoacetogens (Mahmoud et al. 2017). Hydrogenotrophic methanogens consume H2 as the main donor substrate to produce CH4 (Eq. (6.2)) (Stams and Plugge 2009; Thauer et al. 2008), while homoacetogens also consume H2 to yield acetate (Eq. (6.3)) (Schuchmann and Müller 2014). Thus, it is a challenge to minimize the conversion of H2 to CH4, in the presence of hydrogenotrophic methanogens; however, there are several attempts to limit or inhibit the activity of methanogens, such as using chemical inhibitors (Zhu et al. 2015; Parameswaran et al. 2010), employing active harvesting of H2 (Lu et al. 2016), altering operational conditions (Mahmoud et al. 2017), and genetically modifying EAB (Awate et al. 2017).
Recently, Zhao et al. (2015) studied the role of polarized electrodes for enhancing CH4 production in AD bioreactor fed with waste activated sludge. Despite the obvious increase in CH4 production compared to control experiments (without polarized anodes), mass balance revealed that >50% of CH4 production was originated from unknown pathway. Microbial community analysis as well as fluorescence in situ hybridization (FISH) revealed the dominance of Methanosaeta and Geobacter species in electric-anaerobic sludge digester bioreactors. Owing to the increase in biofilm conductivity, they concluded that polarized anode facilitated organic matter degradation and electron exchange between methanogens and Geobacter species. In more recent study, Luo et al. (2016) documented the positive role of polarized electrode for enhancing CH4 production under ammonia stress.
Taken together, these available laboratory-scale studies demonstrate that EF platform opens up new opportunities to integrate METs with the existing AD technology in order to solve the problems associated with conventional AD technology, such as low product yield, slow hydrolysis/fermentation rate (Park et al. 2018), the requirement of long sludge and hydraulic retention times (Song et al. 2016), and the process instability at low temperature (Liu et al. 2016).
In cathodic EF, the electrode acts as the source of electrons to stimulate EF towards production of more reduced final products, including 1,3-propandiol (1,3-PDO), butanol, and polyhydroxyalkanoates (PHA), with high purity and rate (Xue et al. 2017; Moscoviz et al. 2016; Kracke and Krömer 2014). For instance, Choi et al. (2014) showed that a positive working potential (i.e., +0.045 V vs. standard hydrogen electrode (SHE)) triggered a metabolic shift in Clostridium pasteurianum towards production of NADH-consuming metabolite, such as 1,3-PDO from glycerol fermentation and butanol from glucose. More recently, there are efforts to use mixed-culture microbial community for glycerol electrofermentation to selectively produce 1,3-PDO (Roume et al. 2016; Xafenias et al. 2015; Zhou et al. 2013).
Although the use of electric current has been proved to be effective tool to drive the microbial, allowing the production of targeted chemicals of interest, it seems to be a challenge to build successful mixed-culture microbiomes that are resilient to improve the EF selectivity and specificity (Schievano et al. 2016). Dennis et al. (2013) revealed that change in microbial community structure was significantly associated with change in electrofermentation pathway of glycerol and product spectrum. Zhou et al. (2015) observed that the sharp decrease of 1,3-PDO production from glycerol over long time of operation (>150 days) was associated with loss of the dominant Citrobacter spp.
Polyhydroxyalkanoates (PHA) is another targeted chemical that can be produced during the cathodic EF of glucose. In a proof-of-concept study, Srikanth et al. (2012) observed high accumulation of PHA (19% of dry cell weight) with high hydroxybutyrate concentration(~89%) by providing a microaerophilic environment in the cathode of an MET with glucose as the sole carbon source.
6.4 Towards Building Successful Microbiome: Teamwork or Coexistence?
Despite the fact that the EET mechanism in METs has not yet been fully elucidated, there are 2 main mechanisms through which EAB can exchange electrons with electrodes: direct electron transfer and indirect (or mediated) electron transfer (Fig. 6.3) (Torres et al. 2010). Indirect electron transfer relies on redox active shuttles that transfer electrons between EAB and solid surfaces by altering their oxidation states. The extracellular shuttles can be either a secondary microbial metabolite (e.g., phenazine and flavins) or synthetic molecules (e.g., Anthraquinone-2,6-disulfonic acid, neutral red, and cobalt(III) sepulchrate) (Kracke et al. 2018; Torres et al. 2010; Marsili et al. 2008; Emde and Schink 1990). For direct electron transfer, EAB community has the ability to exchange electrons with solid surfaces via direct contact of redox proteins embedded within the EAB outer membrane (e.g., nanowires) (Malvankar and Lovley 2014; Lovley 2008). These mechanisms have been commonly postulated in anodic and cathodic EF systems (Moscoviz et al. 2016; Torres et al. 2010; Rabaey and Rozendal 2010). Although the study of EET in the anode of METs in early studies has focused only on two Gram-negative, mesophilic EAB: Shewanella oneidensis MR-1 and Geobacter sulfurreducens, there are so far over 100 isolated EAB that have the ability to transfer the electron to/from solid surfaces (Logan et al. 2019; Doyle and Marsili 2018).
EAB community performing indirect electron transfer (e.g., S. oneidensis MR-1) is often characterized with low current density generation (i.e., ≤1 A/m2) mainly due to the slow diffusion of redox shuttles, although they are capable of using fermentable donor substrates, such as glucose, as the main source of energy and electrons. On the other hand, EAB community performing respiration via solid-conductive mechanism (e.g., G. sulfurreducens) are capable of producing much higher current density; however, their metabolic capability is limited to only consume simple substrates, including acetate and H2 (Torres et al. 2010; Marsili et al. 2008). In order to overcome this limited metabolic capability, Speers et al. (2014) proposed a successful strategy to use a co-culture of an EAB (G. sulfurreducens) and a fermenting bacterium (Clostridium cellobioparum) to enhance glycerol fermentation into ethanol. Interestingly, Lusk et al. (2015) used a pure-culture thermophilic bacterium—Thermoanaerobacter pseudethanolicus—that has the ability to ferment complex donor substrates (e.g., xylose, glucose, and cellobiose) and perform anode respiration without the addition of redox mediators.
Despite the benefits of using pure cultures in EF systems for higher selectivity and specificity of fermentation reactions, mixed-culture EF systems may be advantageous to simplify the fermentation process. Owing to the high robustness and functional stability of mixed-culture microbial community compared to pure cultures, mixed-culture EF systems can handle a wide range of complex waste streams, such as real wastewater. It was previously demonstrated in different MET configurations that EAB rely on fermenting bacteria to provide their “fuel” by converting complex organic matter into simple donor substrates (Logan et al. 2019; Mahmoud et al. 2017; Parameswaran et al. 2010). These syntrophic interactions—either by mediated interspecies electron transfer (MIET) via the diffusion of electron carriers (i.e., H2 and formate) (Parameswaran et al. 2010) or direct interspecies electron transfer (DIET) in presence of conductive materials (Lovley 2017)—are required to maintain the concentrations of fermentation by-products below a threshold limit to make the fermentation thermodynamically favorable (Kiely et al. 2011).
Although methanogens represent the main trophic guild in anaerobic digesters to produce CH4from organic wastes, methanogens represent undesired competitors for EAB, since they can compete for space and food (Siegert et al. 2015). They can produce CH4 by two pathways: (1) aceticlastic methanogenesis by oxidizing acetate (Eq. (6.4)) and (2) hydrogenotrophic methanogenesis by oxidizing H2 (Eq. (6.2)). Owing to the thermodynamic and kinetic advantages of EAB over aceticlastic methanogens, EAB usually outcompete aceticlastic methanogens (Parameswaran et al. 2010; Esteve-Nunéz et al. 2005); hence, they are not a competitor for acetate-consuming EAB. However, hydrogenotrophic methanogens have metabolic advantage over EAB for H2 consumption, thereby minimizing the possibility of H2 harvesting or its conversion into another useful product, such as electric current (Mahmoud et al. 2017). Among several possibility to inhibit methanogens, chemical inhibitors (e.g., 2-bromoethanesulfonate) seem to be the most effective option for inhibiting methanogens, although using chemical inhibitors is not practically feasible for industrial applications of EF and other MET as well as they are toxic (Karthikeyan et al. 2017; Mahmoud et al. 2017; Lu et al. 2016; Zhu et al. 2015; Parameswaran et al. 2010; Chae et al. 2010; Freguia et al. 2008).
Another potential competitor for EAB is sulfate-reducing bacteria (SRB). Sulfate reduction process in the anodic EF systems is likely to occur, particularly for sulfate-rich organic waste streams, such as food wastewater, petrochemical effluents, and pulp and paper wastewater (Hao et al. 2014). Although there is no comprehensive study showing the impact of sulfate on the performance of EF systems, a recent study revealed the applied current in a microbial electrolysis cell favored sulfate removal from sulfate-rich wastewater (Wang et al. 2017). Their results suggest that EAB can integrate with SRB to remove organic matter and sulfate, although they did not study the effect of sulfate on anode respiration. In another study, Lee et al. (2012) showed that increasing the sulfate concentration had a negative effect on the performance of MFCs as indicated from low power density generation and electron recovery.
Given that nitrate can be reduced in strict anaerobic conditions, it represents a real risk for EAB in the anode of MET reactors, including anodic EF systems. Nitrate reduction by nitrate-reducing bacteria (NRB) (or denitrifiers) is an undesired process, since it would limit the substrate availability for EAB (Sukkasem et al. 2008). For instance, Jin et al. (2019) showed that supplementing the anode of an MFC with nitrate (100 mg-N/L) decreased Coulombic efficiency (CE) by ~2.2-fold (from 63.9% to 29.4%). In another study, Kashima and Regan (2015) studied the impact of nitrate on the efficiency of pure-culture electrochemically-active bacterium (i.e., Geobacter metallireducens). The addition of nitrate (10 mM) resulted in a remarkable reduction of CE (from ~78% to ~4%). A likely reason for low CE and electron losses is the competition between EAB and NRB for substrate and space.
6.5 EAB–Electrode Interaction and EF Systems Architecture
So far, the majority of published research were performed using small-scale EF systems. Thus, the successful scaling-up of EF systems (and other MET as well) will greatly depend on the selection of biocompatible electrodes that favor the microbe-electrode interactions as well as the system design and architecture (Logan et al. 2006). The ideal electrodes for EF systems should have: (1) a relatively high electrical conductivity; (2) high chemical stability; (3) low cost; (4) large accessible specific surface area; and (5) high mechanical strength (Hindatu et al. 2017; Xie et al. 2015).
Owing to their biocompatibility, low cost, and high electrical conductivity, carbon-based electrodes, including graphite brush, carbon cloth, graphite felt, carbon brush, granular activated carbon, and carbon fibers, have been widely used for METs research (ElMekawy et al. 2017; Xie et al. 2015). In addition, altering the surface chemistry of electrodes by either adding conductive catalysts (e.g., carbon nanotube, graphene, and iron oxide) or conducting polymers (e.g., polyaniline and hydrogels) has resulted in enhancing the bacterial colonization and microbe-electrode interactions, thereby reducing the surface electron transfer resistance and improving the extracellular electron (EET) rate (Hindatu et al. 2017). Other surface treatment approaches (e.g., acid treatment (Feng et al. 2010), ammonia treatment (Call et al. 2009), surfactant treatment (Guo et al. 2014a), heat treatment (Wang et al. 2009), and flame oxidation (Guo et al. 2014b) have been applied to alter the surface chemistry of electrodes, facilitating the microbe-electrode interaction and enhancing biocompatibility properties of electrodes. In addition, other non-carbon electrodes (e.g., stainless steel, gold, and titanium) have also been used for METs research; however, their small accessible specific surface area and relatively high cost would limit their application for large-scale MET reactors (Fan and Liu 2014; Richter et al. 2008; Dumas et al. 2008).
Most of recent EF studies have paid more attention to understand the fundamental aspects of EF systems rather than the reactor design and architecture. Single-chamber EF system (i.e., without using ion-exchange membranes) seems to be ideal to upgrade the existing AD technologies for wastewater treatment, such as municipal sludge and food-processing wastewater. This approach would enhance the efficiency of AD, while keeping its O&M relatively low. However, if the purpose of EF system is to recover high-purity chemicals, EF should occur in multi-chambered EF systems equipped with of ion-exchange membranes (bipolar membrane, anion-exchange membrane, and cation-exchange membrane). For example, Roume et al. (2016) used a 3-chambered cathodic EF system to enhance the bioelectrochemical reduction of glycerol to 1,3-PDO. Using arrays of selective membranes, they reported a high production yield of 1,3-PDO (i.e., 0.72 mole of 1,3-PDO per 1 mole of glycerol). Similarly, Andersen et al. (2014) revealed that using an anion-exchange membrane remarkably enhanced the extraction and upgrading of short-chain carboxylates into esters during bioelectrochemical fermentation.
6.6 Conclusion and Future Perspectives
EF is an emerging platform that integrates electrochemistry with traditional fermentation. In EF systems, the polarized electrodes can act as either electron acceptor (i.e., anodic EF) or electron donor (i.e., cathodic EF), leading to stimulate microbial metabolism in traditional fermentation bioreactor to produce targeted chemicals with high purity, to enhance microbial growth, to improve the microbial interspecies interactions, and/or to achieve carbon breakdown or chain elongation. Despite the great promise of this hybrid technology, it is still in its infancy. Multidisciplinary studies are required:
-
1.
to understand the microbial community interaction and how it affects the microbial metabolism as well as cultivating new microbial isolates that are capable of improving the selectivity and specificity of EF. Owing to the recent advances in molecular biology and culture-independent tools and techniques, both options seem to be easily achievable,
-
2.
to improve the EF system architecture and design, including the electrode materials and shapes. This remarkably improvement of EF efficiency has to be accompanied with novel reactors design that should have a relatively low cost and high availability,
-
3.
to investigate the possibility of integration with other existing wastewater treatment processes, such as anaerobic digestion, and
-
4.
to find a practical way to improve the extraction of the produced chemicals.
References
Agler, M. T., Wrenn, B. A., Zinder, S. H., & Angenent, L. T. (2011). Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends in Biotechnology, 29, 70–78.
Andersen, S. J., Hennebel, T., Gildemyn, S., Coma, M., Desloover, J., Berton, J., et al. (2014). Electrolytic membrane extraction enables production of fine chemicals from biorefinery sidestreams. Environmental Science & Technology, 48, 7135–7142.
Angelidaki, I., Karakashev, D., Batstone, D. J., Plugge, C. M., & Stams, A. J. (2011). Biomethanation and its potential. Methods in Enzymology, 494, 327–351.
Angenent, L. T., Karim, K., Al-Dahhan, M. H., Wrenn, B. A., & Domíguez-Espinosa, R. (2004). Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends in Biotechnology, 22, 477–485.
Awate, B., Steidl, R. J., Hamlischer, T., & Reguera, G. (2017). Stimulation of electro-fermentation in single-chamber microbial electrolysis cells driven by genetically engineered anode biofilms. Journal of Power Sources, 356, 510–518.
Batstone, D. J., Keller, J., Angelidaki, I., Kalyuzhnyi, S. V., Pavlostathis, S. G., Rozzi, A., et al. (2002). Anaerobic digestion model no. 1: IWA Task Group for mathematical modelling of anaerobic digestion processes. London: IWA Publishing.
Bolzonella, D., Fatone, F., Pavan, P., & Cecchi, F. (2005). Anaerobic fermentation of organic municipal solid wastes for the production of soluble organic compounds. Industrial and Engineering Chemistry Research, 44, 3412–3418.
British Petroleum. (2019). Statistical review of world energy (68th edition). Retrieved from https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf
Brockway, P. E., Owen, A., Brand-Correa, L. I., & Hardt, L. (2019). Estimation of global final-stage energy-return on-investment for fossil fuels with comparison to renewable energy sources. Nature Energy, 4, 612–621.
Call, D. F., Merrill, M. D., & Logan, B. E. (2009). High surface area stainless steel brushes as cathodes in microbial electrolysis cells. Environmental Science & Technology, 43, 2179–2183.
Chae, K. J., Choi, M. J., Kim, K. Y., Ajayi, F. F., Park, W., Kim, C. W., et al. (2010). Methanogenesis control by employing various environmental stress conditions in two-chambered microbial fuel cells. Bioresource Technology, 101, 5350–5357.
Chen, Y., Cheng, J. J., & Creamer, K. S. (2008). Inhibition of anaerobic digestion process: A review. Bioresource Technology, 99, 4044–4064.
Choi, O., Kim, T., Woo, H. M., & Um, Y. (2014). Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridium pasteurianum. Scientific Reports, 4, 6961.
Choi, O., Um, Y., & Sang, B. I. (2012). Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnology and Bioengineering, 109, 2494–2502.
Cusick, R. D., Bryan, B., Parker, D. S., Merrill, M. D., Mehanna, M., Kiely, P. D., et al. (2011). Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Applied Microbiology and Biotechnology, 89, 2053–2063.
Dennis, P. G., Harnisch, F., Yeoh, Y. K., Tyson, G. W., & Rabaey, K. (2013). Dynamics of cathode-associated microbial communities and metabolite profiles in a glycerol-fed bioelectrochemical system. Applied and Environmental Microbiology, 79, 4008–4014.
Dowell, N. M., Fennell, P. S., Shah, N., & Maitland, G. C. (2017). The role of CO2 capture and utilization in mitigating climate change. Nature Climate Change, 7, 243.
Doyle, L. E., & Marsili, E. (2018). Weak electricigens: a new avenue for bioelectrochemical research. Bioresource Technology, 258, 354–364.
Dubrawski, K. L., Shao, X., Milton, R. D., Deutzmann, J. S., Spormann, A. M., & Criddle, C. S. (2019). Microbial battery powered enzymatic electrosynthesis for carbon capture and generation of hydrogen and formate from dilute organics. ACS Energy Letters, 4, 2929–2936.
Dumas, C., Basseguy, R., & Bergel, A. (2008). Electrochemical activity of Geobacter sulfurreducens biofilms on stainless steel anodes. Electrochimica Acta, 53, 5235–5241.
ElMekawy, A., Hegab, H. M., Losic, D., Saint, C. P., & Pant, D. (2017). Applications of graphene in microbial fuel cells: The gap between promise and reality. Renewable and Sustainable Energy Reviews, 72, 1389–1403.
Emde, R., & Schink, B. (1990). Enhanced propionate formation by Propionibacterium freudenreichii subsp. freudenreichii in a three-electrode amperometric culture system. Applied and Environmental Microbiology, 56, 2771–2776.
Escapa, A., Mateos, R., Martínez, E. J., & Blanes, J. (2016). Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renewable and Sustainable Energy Reviews, 55, 942–956.
Esteve-Nunéz, A., Rothermich, M., Sharma, M., & Lovley, D. R. (2005). Growth of Geobacter sulfurreducens under nutrient limiting conditions in continuous culture. Environmental Microbiology, 7, 641–648.
Fan, Y., & Liu, H. (2014). Materials for microbial fuel cells. In B. Ladewig, S. P. Jiang, & Y. Yan (Eds.), Materials for low-temperature fuel cells (pp. 145–164). New York: John Wiley & Sons.
Feng, Y., Yang, Q., Wang, X., & Logan, B. E. (2010). Treatment of carbon fiber brush anodes for improving power generation in air-cathode microbial fuel cells. Journal of Power Sources, 195, 1841–1844.
Freguia, S., Rabaey, K., Yuan, Z., & Keller, J. (2008). Syntrophic processes drive the conversion of glucose in microbial fuel cell anodes. Environmental Science & Technology, 42, 7937–7943.
Ge, Z., Li, J., Xiao, L., Tong, Y., & He, Z. (2014). Recovery of electrical energy in microbial fuel cells. Environmental Science & Technology Letters, 1, 137–141.
Guo, K., Donose, B. C., Soeriyadi, A. H., Prévoteau, A., Patil, S. A., Freguia, S., et al. (2014b). Flame oxidation of stainless steel felt enhances anodic biofilm formation and current output in bioelectrochemical systems. Environmental Science & Technology, 48, 7151–7156.
Guo, K., Soeriyadi, A. H., Patil, S. A., Prévoteau, A., Freguia, S., Gooding, J. J., et al. (2014a). Surfactant treatment of carbon felt enhances anodic microbial electrocatalysis in bioelectrochemical systems. Electrochemistry Communications, 39, 1–4.
Hallenbeck, P. C. (2009). Fermentative hydrogen production: Principles, progress, and prognosis. International Journal of Hydrogen Energy, 34, 7379–7389.
Hao, T., Xiang, P., Mackey, H. R., Chi, K., Lu, H., Chui, H., et al. (2014). A review of biological sulfate conversions in wastewater treatment. Water Research, 65, 1–21.
Harrington, T. D., Mohamed, A., Tran, V. N., Biria, S., Gargouri, M., Park, J. J., et al. (2015). Neutral red-mediated microbial electrosynthesis by Escherichia coli, Klebsiella pneumoniae, and Zymomonas mobilis. Bioresource Technology, 195, 57–65.
Heidrich, E. S., Curtis, T. P., & Dolfing, J. (2011). Determination of the internal chemical energy of wastewater. Environmental Science & Technology, 45, 827–832.
Heidrich, E. S., Dolfing, J., Scott, K., Edwards, S. R., Jones, C., & Curtis, T. P. (2013). Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Applied Microbiology and Biotechnology, 97, 6979–6989.
Himmi, E. H., Bories, A., Boussaid, A., & Hassani, L. (2000). Propionic acid fermentation of glycerol and glucose by Propionibacterium acidi-propionici and Propionibacterium freudenreichii ssp. shermanii. Applied Microbiology and Biotechnology, 53, 435–440.
Hindatu, Y., Annuar, M. S. M., & Gumelc, A. M. (2017). Mini-review: Anode modification for improved performance of microbial fuel cell. Renewable and Sustainable Energy Reviews, 73, 236–248.
Hou, Y., Zhang, R., Yu, Z., Huang, L., Liu, Y., & Zhou, Z. (2017). Accelerated azo dye degradation and concurrent hydrogen production in the single-chamber photocatalytic microbial electrolysis cell. Bioresource Technology, 224, 63–68.
IPCC. (2014). Climate Change 2014: Impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. In Contribution of working group II to the Fifth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press.
Jia, X., Li, M., Wang, Y., Wu, Y., Zhu, L., Wang, X., et al. (2020). Enhancement of hydrogen production and energy recovery through electro-fermentation from the dark fermentation effluent of food waste. Environmental Science & Ecotechnology, 1, 100006.
Jin, X., Guo, F., Ma, W., Liu, Y., & Liu, H. (2019). Heterotrophic anodic denitrification improves carbon removal and electricity recovery efficiency in microbial fuel cells. Chemical Engineering Journal, 370, 527–535.
Karthikeyan, R., Cheng, K. Y., Selvam, A., Bose, A., & Wong, J. W. C. (2017). Bioelectrohydrogenesis and inhibition of methanogenic activity in microbial electrolysis cells - A review. Biotechnology Advances, 35, 758–771.
Kashima, H., & Regan, J. M. (2015). Facultative nitrate reduction by electrode-respiring Geobacter metallireducens biofilms as a competitive reaction to electrode reduction in a bioelectrochemical system. Environmental Science & Technology, 49, 3195–3202.
Katuri, K. P., Ali, M., & Saikaly, P. E. (2019). The role of microbial electrolysis cell in urban wastewater treatment: Integration options, challenges, and prospects. Current Opinion in Biotechnology, 57, 101–110.
Kaza, S., Yao, L. C., Bhada-Tata, P., & van Woerden, F. (2018). What a waste 2.0: A global snapshot of solid waste management to 2050 (urban development series knowledge papers). Washington, DC: World Bank.
Kiely, P. D., Regan, J. M., & Logan, B. E. (2011). The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Current Opinion in Biotechnology, 22, 378–385.
Kim, C., Kim, M. Y., Michie, I., Jeon, B. H., Premier, G. C., Park, S., et al. (2017). Anodic electro-fermentation of 3-hydroxypropionic acid from glycerol by recombinant Klebsiella pneumoniae L17 in a bioelectrochemical system. Biotechnology for Biofuels, 10, 199.
Kracke, F., & Krömer, J. O. (2014). Identifying target processes for microbial electrosynthesis by elementary mode analysis. BMC Bioinformatics, 15, 410–423.
Kracke, F., Lai, B., Yu, S., & Krömer, J. O. (2018). Balancing cellular redox metabolism in microbial electrosynthesis and electro fermentation – A chance for metabolic engineering. Metabolic Engineering, 45, 109–120.
Lee, D. J., Lee, C. Y., & Chang, J. S. (2012). Treatment and electricity harvesting from sulfate/sulfide-containing wastewaters using microbial fuel cell with enriched sulfate-reducing mixed culture. Journal of Hazardous Materials, 243, 67–72.
Lewis, A. J., & Borole, A. P. (2016). Understanding the impact of flow rate and recycle on the conversion of a complex biorefinery stream using a flow-through microbial electrolysis cell. Biochemical Engineering Journal, 116, 95–104.
Li, W. W., Yu, H. Q., & Rittmann, B. E. (2015). Chemistry: Reuse water pollutants. Nature, 528(7580), 29–31.
Liu, B., & Rajagopal, D. (2019). Life-cycle energy and climate benefits of energy recovery from wastes and biomass residues in the United States. Nature Energy, 4, 700–708.
Liu, D., Zhang, L., Chen, S., Buisman, C., & TerHeijne, A. (2016). Bioelectrochemical enhancement of methane production in low temperature anaerobic digestion at 10°C. Water Research, 99, 281–287.
Logan, B. E. (2010). Scaling up microbial fuel cells and other bioelectrochemical systems. Applied Microbiology and Biotechnology, 85, 1665–1671.
Logan, B. E., Hamelers, H. V. M., Rozendal, R. A., Schroder, U., Keller, J., Freguia, S., et al. (2006). Microbial fuel cells: Methodology and technology. Environmental Science & Technology, 40, 5181–5192.
Logan, B. E., & Rabaey, K. (2012). Conversion of wastes into bioelectricity and chemicals using microbial electrochemical technologies. Science, 337, 686–690.
Logan, B. E., Rossi, R., Ragab, A., & Saikaly, P. E. (2019). Electroactive microorganisms in bioelectrochemical systems. Nature Reviews. Microbiology, 17, 307–319.
Lovley, D. R. (2008). The microbe electric: Conversion of organic matter to electricity. Current Opinion in Biotechnology, 19, 564–571.
Lovley, D. R. (2017). Syntrophy goes electric: Direct interspecies electron transfer. Annual Review of Microbiology, 71, 643–664.
Lu, L., Hou, D., Wang, X., Jassby, D., & Ren, Z. J. (2016). Active H2 harvesting prevents methanogenesis in microbial electrolysis cells. Environmental Science & Technology, 3, 286–290.
Lu, Y., Slater, F. R., Mohd-Zaki, Z., Pratt, S., & Batstone, D. J. (2011). Impact of operating history on mixed culture fermentation microbial ecology and product mixture. Water Science and Technology, 64, 760.
Luo, L., Xu, S., Selvam, A., & Wong, J. W. C. (2016). Assistant role of bioelectrode on methanogenic reactor under ammonia stress. Bioresource Technology, 217, 72–81.
Lusk, B. G., Khan, Q. F., Parameswaran, P., Hameed, A., Ali, N., Rittmann, B. E., et al. (2015). Characterization of electrical current generation capabilities from thermophilic bacterium Thermoanaerobacter pseud ethanolicus using xylose, glucose, cellobiose, or acetate with fixed anode potentials. Environmental Science & Technology, 49, 14725–14731.
Mahmoud, M. (2016). Towards improving electron recovery and Coulombic efficiency of microbial electrochemical cells fed with fermentable electron donors. PhD dissertation, Arizona State University.
Mahmoud, M., Parameswaran, P., Torres, C. I., & Rittmann, B. E. (2014). Fermentation pre-treatment of landfill leachate for enhanced electron recovery in a microbial electrolysis cell. Bioresource Technology, 151, 151–158.
Mahmoud, M., Parameswaran, P., Torres, C. I., & Rittmann, B. E. (2016). Relieving the fermentation inhibition enables high electron recovery from landfill leachate in a microbial electrolysis cell. RSC Advances, 6, 6658–6664.
Mahmoud, M., Torres, C. I., & Rittmann, B. (2017). Changes in glucose fermentation pathways as a response to the free ammonia concentration in microbial electrolysis cells. Environmental Science & Technology, 51, 13461–13470.
Malvankar, N. S., & Lovley, D. R. (2014). Microbial nanowires for bioenergy applications. Current Opinion in Biotechnology, 27, 88–95.
Mao, C., Feng, Y., Wang, X., & Ren, G. (2015). Review on research achievements of biogas from anaerobic digestion. Renewable and Sustainable Energy Reviews, 45, 540–555.
Marsili, E., Rollefson, J. B., Baron, D. B., Hozalski, R. M., & Bond, D. R. (2008). Microbial biofilm voltammetry: Direct electrochemical characterization of catalytic electrode-attached biofilms. Applied and Environmental Microbiology, 74, 7329.
McCarty, P. L., Bae, J., & Kim, J. (2011). Domestic wastewater treatment as a net energy producer–can this be achieved? Environmental Science & Technology, 45, 7100–7106.
McInerney, M. J., Struchtemeyer, C. G., Sieber, J., Mouttaki, H., Stams, A. J., Schink, B., et al. (2008). Physiology, ecology, phylogeny, and genomics of microorganisms capable of syntrophic metabolism. Annals of the New York Academy of Sciences, 1125, 58–72.
Metcalf and Eddy. (2003). Wastewater engineering–treatment and reuse (4th ed.). New York: McGraw-Hill Inc..
Moscoviz, R., Toledo-Alarcón, J., Trably, E., & Bernet, N. (2016). Electro-fermentation: How to drive fermentation using electrochemical systems. Trends in Biotechnology, 34, 856–865.
Moscoviz, R., Trably, E., & Bernet, N. (2018). Electro-fermentation triggering population selection in mixed-culture glycerol fermentation. Microbial Biotechnology, 11, 74–83.
Pant, D., Van Bogaert, G., Diels, L., & Vanbroekhoven, K. (2010). A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresource Technology, 101, 1533–1543.
Parameswaran, P., Zhang, H., Torres, C. I., Rittmann, B. E., & Krajmalnik-Brown, R. (2010). Microbial community structure in a biofilm anode fed with a fermentable substrate: The significance of hydrogen scavengers. Biotechnology and Bioengineering, 105, 69–78.
Park, J., Lee, B., Tian, D., & Jun, H. (2018). Bioelectrochemical enhancement of methane production from highly concentrated food waste in a combined anaerobic digester and microbial electrolysis cell. Bioresource Technology, 247, 226–233.
Peccia, J., & Westerhoff, A. (2015). We should expect more out of our sewage sludge. Environmental Science & Technology, 49, 8271–8276.
Rabaey, K., & Rozendal, R. A. (2010). Microbial electrosynthesis - Revisiting the electrical route for microbial production. Nature Reviews. Microbiology, 8, 706–716.
Rajagopal, R., Massé, D. I., & Singh, G. (2013). A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresource Technology, 143, 632–641.
Richter, H., McCarthy, K., Nevin, K. P., Johnson, J. P., Rotello, V. M., & Lovley, D. R. (2008). Electricity generation by Geobacter sulfurreducens attached to gold electrodes. Langmuir, 24, 4376–4379.
Rittmann, B. E. (2008). Opportunities for renewable bioenergy using microorganisms. Biotechnology and Bioengineering, 100, 203–212.
Rittmann, B. E., & McCarty, P. L. (2001). Environmental biotechnology: Principles and applications. New York: McGraw-Hill.
Rodríguez, J., Kleerebezem, R., Lema, J. M., & van Loosdrecht, M. C. (2006). Modeling product formation in anaerobic mixed culture fermentations. Biotechnology and Bioengineering, 93, 592–606.
Roume, H., Arends, J. B. A., Ameril, C. P., Patil, S. A., & Rabaey, K. (2016). Enhanced product recovery from glycerol fermentation into 3-carbon compounds in a bioelectrochemical system combined with in situ extraction. Frontiers in Bioengineering and Biotechnology, 4, 73.
Saint-amans, S., Girbal, L., Andrade, J., Ahrens, K., & Soucaille, P. (2001). Regulation of carbon and electron flow in Clostridium butyricum VPI 3266 grown on glucose-glycerol mixtures. Journal of Bacteriology, 183, 1748–1754.
Sasaki, K., Tsuge, Y., Sasaki, D., & Kondo, A. (2014). Increase in lactate yield by growing Corynebacterium glutamicum in a bioelectrochemical reactor. Journal of Bioscience and Bioengineering, 117, 598–601.
Schievano, A., Sciarria, T. P., Vanbroekhoven, K., De Wever, H., Puig, S., Andersen, S. J., et al. (2016). Electro-fermentation – Merging electrochemistry with fermentation in industrial applications. Trends in Biotechnology, 34, 866–878.
Schuchmann, K., & Müller, V. (2014). Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nature Reviews. Microbiology, 12, 809–821.
Siegert, M., Li, X. F., Yates, M. D., & Logan, B. E. (2015). The presence of hydrogenotrophic methanogens in the inoculum improves methane gas production in microbial electrolysis cells. Frontiers in Microbiology, 5, 778.
Song, Y. C., Feng, Q., & Ahn, Y. (2016). Performance of the bioelectrochemical anaerobic digestion of sewage sludge at different hydraulic retention times. Energy & Fuels, 30, 352–359.
Speers, A. M., Young, J. M., & Reguera, G. (2014). Fermentation of glycerol into ethanol in a microbial electrolysis cell driven by a customized consortium. Environmental Science & Technology, 48, 6350–6358.
Sravan, J. S., Butti, S. K., Sarkar, O., Krishna, K. V., & Mohan, S. V. (2018). Electrofermentation of food waste – Regulating acidogenesis towards enhanced volatile fatty acids production. Chemical Engineering Journal, 334, 1709–1718.
Srikanth, S., Reddy, M. V., & Mohan, S. V. (2012). Microaerophilic microenvironment at biocathode enhances electrogenesis with simultaneous synthesis of polyhydroxyalkanoates (PHA) in bioelectrochemical system (BES). Bioresource Technology, 125, 291–299.
Stams, A. J., & Plugge, C. M. (2009). Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nature Reviews. Microbiology, 7, 568–577.
Steinbusch, K. J. J., Hamelers, H. V. M., Schaap, J. D., Kampman, C., & Buisman, C. J. N. (2010). Bioelectrochemical ethanol production through mediated acetate reduction by mixed cultures. Environmental Science & Technology, 44, 513–517.
Sturm-Richter, K., Golitsch, F., Sturm, G., Kipf, E., Dittrich, A., Beblawy, S., et al. (2015). Unbalanced fermentation of glycerol in Escherichia coli via heterologous production of an electron transport chain and electrode interaction in microbial electrochemical cells. Bioresource Technology, 186, 89–96.
Sukkasem, C., Xu, S., Park, S., Boonsawang, P., & Liu, H. (2008). Effect of nitrate on the performance of single chamber air cathode microbial fuel cells. Water Research, 42, 4743–4750.
Temudo, M. F., Kleerebezem, R., & van Loosdrecht, M. (2007). Influence of the pH on (open) mixed culture fermentation of glucose: A chemostat study. Biotechnology and Bioengineering, 98, 69–79.
Temudo, M. F., Poldermans, R., Kleerebezem, R., & van Loosdrecht, M. C. (2008). Glycerol fermentation by (open) mixed cultures: A chemostat study. Biotechnology and Bioengineering, 100, 1088–1098.
Thauer, R. K., Jungermann, K., & Decker, K. (1977). Energy conservation in chemotrophic anaerobic bacteria. Bacteriological Reviews, 41, 100–180.
Thauer, R. K., Kaster, A. K., Seedorf, H., Buckel, W., & Hedderich, R. (2008). Methanogenic archaea: Ecologically relevant differences in energy conservation. Nature Reviews. Microbiology, 6, 579–591.
Torres, C. I., Marcus, A. K., Lee, H., Parameswaran, P., Krajmalnik-Brown, R., & Rittmann, B. E. (2010). A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiology Reviews, 34, 3–17.
Velasquez-Orta, S. B., Yu, E., Katuri, K. P., Head, I. M., Curtis, T. P., & Scott, K. (2011). Evaluation of hydrolysis and fermentation rates in microbial fuel cells. Applied Microbiology and Biotechnology, 90, 789–798.
Villano, M., Monaco, G., Aulenta, F., & Majone, M. (2011). Electrochemically assisted methane production in a biofilm reactor. Journal of Power Sources, 196, 9467–9472.
Wang, K., Sheng, Y., Cao, H., Yan, K., & Zhang, Y. (2017). Impact of applied current on sulfate-rich wastewater treatment and microbial biodiversity in the cathode chamber of microbial electrolysis cell (MEC) reactor. Chemical Engineering Journal, 307, 150–158.
Wang, X., Cheng, S., Feng, Y., Merrill, M. D., Saito, T., & Logan, B. E. (2009). Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environmental Science & Technology, 43, 6870–6874.
WWAP (United Nations World Water Assessment Programme). (2017). The United Nations world water development report 2017. Wastewater: The untapped resource. Paris: UNESCO.
Xafenias, N., Anunobi, M. O., & Mapelli, V. (2015). Electrochemical startup increases 1,3-propanediol titers in mixed-culture glycerol fermentations. Process Biochemistry, 50, 1499–1508.
Xie, X., Criddle, C., & Cui, Y. (2015). Design and fabrication of bioelectrodes for microbial bioelectrochemical systems. Energy & Environmental Science, 8, 3418–3441.
Xue, G., Lai, S., Li, X., Zhang, W., You, J., Chen, H., et al. (2017). Efficient bioconversion of organic wastes to high optical activity of l-lactic acid stimulated by cathode in mixed microbial consortium. Water Research, 131, 1–10.
Zeng, X., Borole, A. P., & Pavlostathis, S. G. (2015). Biotransformation of furanic and phenolic compounds with hydrogen gas production in a microbial electrolysis cell. Environmental Science & Technology, 49, 13667–13675.
Zhang, W., Werner, J. J., Agler, M. T., & Angenent, L. T. (2014). Substrate type drives variation in reactor microbiomes of anaerobic digesters. Bioresource Technology, 151, 397–401.
Zhao, Y., Cao, W., Wang, Z., Zhang, B., Chen, K., & Ouyang, P. (2016). Enhanced succinic acid production from corncob hydrolysate by microbial electrolysis cells. Bioresource Technology, 202, 152–157.
Zhao, Z., Zhang, Y., Wang, L., & Quan, X. (2015). Potential for direct interspecies electron transfer in an electric-anaerobic system to increase methane production from sludge digestion. Scientific Reports, 5, 11094.
Zhou, M., Chen, J., Freguia, S., Rabaey, K., & Keller, J. (2013). Carbon and electron fluxes during the electricity driven 1,3-Propanediol biosynthesis from glycerol. Environmental Science & Technology, 47, 11199–11205.
Zhou, M., Freguia, S., Dennis, P. G., Keller, J., & Rabaey, K. (2015). Development of bioelectrocatalytic activity stimulates mixed-culture reduction of glycerol in a bioelectrochemical system. Microbial Biotechnology, 8, 483–489.
Zhu, X., Siegert, M., Yates, M. D., & Logan, B. E. (2015). Alamethicin suppresses methanogenesis and promotes acetogenesis in bioelectrochemical systems. Applied and Environmental Microbiology, 81, 3863–3868.
Zou, S., & He, Z. (2018). Efficiently “pumping out” value-added resources from wastewater by bioelectrochemical systems: A review from energy perspectives. Water Research, 131, 62–73.
Acknowledgements
MM is supported by the Science and Technology Development Fund (STDF) reintegration grant (No. 33475), Egypt.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mahmoud, M. (2020). Electricity-Driven Microbial Factory for Value-Added Resources Recovery from Waste Streams. In: Kumar, P., Kuppam, C. (eds) Bioelectrochemical Systems. Springer, Singapore. https://doi.org/10.1007/978-981-15-6872-5_6
Download citation
DOI: https://doi.org/10.1007/978-981-15-6872-5_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6871-8
Online ISBN: 978-981-15-6872-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)