Abstract
This study determined the influence of substrate degradation on power generation in microbial fuel cells (MFCs) and microbial community selection on the anode. Air cathode MFCs were fed synthetic medium containing different substrates (acetate, glucose and starch) using primary clarifier sewage as source of electroactive bacteria. The complexity of the substrate affected the MFC performance both for power generation and COD removal. Power output decreased with an increase in substrate complexity from 99 ± 2 mW m−2 for acetate to 4 ± 2 mW m−2 for starch. The organic matter removal and coulombic efficiency (CE) of MFCs with acetate and glucose (82% of COD removal and 26% CE) were greater than MFCs using starch (60% of COD removal and 19% of CE). The combined hydrolysis–fermentation rate obtained (0.0024 h−1) was considerably lower than the fermentation rate (0.018 h−1), indicating that hydrolysis of complex compounds limits current output over fermentation. Statistical analysis of microbial community fingerprints, developed on the anode, showed that microbial communities were enriched according to the type of substrate used. Microbial communities producing high power outputs (fed acetate) clustered separately from bacterial communities producing low power outputs (fed complex compounds).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cells (MFCs) represent an innovative method for combining waste treatment and power generation. Extensive studies on MFC reactor design, microbial communities, electrode materials and operating parameters have been carried out to improve MFC performance (e.g. Cheng et al. 2006; Min and Logan 2004). Although modifications to reactor configuration and materials have significantly enhanced the power output of MFCs (Du et al. 2007), further understanding of process performance in relation to microbial communities is required for better process optimisation. The degradation of complex organic compounds in MFCs is thought to follow pathways similar to anaerobic digestion, such as the hydrolysis and fermentation (or acidogenesis; Metcalf and Eddy 2003). In order to determine the chemical and biological processes occurring in the MFC anode, it is important to understand the degradation pathway of complex organic compounds. Figure 1 shows the degradation pathway (through glycolysis) of carbohydrates in an MFC anode. Complex organic compounds are first hydrolysed to simple molecules which are assimilated by microorganisms. Hydrolysis occurs if polymeric carbohydrates such as sucrose, trehalose, starch and molasses are used as fuel. In systems requiring hydrolysis, there may be energy losses as a consequence of less effective energy transfer (due to utilization of hydrolysis and fermentation products by non-electrogenic organisms present in the system) from the primary electron donor to electron donors which are used by electrogenic bacteria. After hydrolysis, nutrient carbon is likely to be further degraded by bacterial cells through fermentation as MFC anodes are under anaerobic conditions (Torres et al. 2007). Fermentation involves the biochemical breakdown of organic compounds to compounds that have the same oxidation state but lower energy content (Vogel 1983). In fermentation, unlike in respiration, organic compounds which are reaction products within microbial cells are used as electron acceptors. The general products of fermentation are volatile fatty acids (VFA, e.g. propionate, acetate and butyrate), as well as carbon dioxide, hydrogen and other organic products (e.g. lactate and alcohols; Metcalf and Eddy 2003). VFAs can then be consumed by either anode-respiring bacteria, secondary fermenters or methanogenic archaea. The consumption of VFAs by respiring bacteria can lead to the transfer of electrons to the MFC anode (Fig. 1).
Energy losses during both hydrolysis and fermentation can be reflected in MFCs’ coulombic efficiency (CE). Numerous studies have reported higher CE with simple substrates than with complex substrates. The highest CE (around 80%) is obtained when acetate, that does not require fermentation, is used as substrate (Lee et al. 2007; Thygesen et al. 2009; Torres et al. 2007; Xue et al. 2006). Current outputs are also higher with simple compared to complex compounds. For example, Niessen et al. (2004) obtained higher current densities from glucose (1.3 mA cm−2) than from starch (0.8–1 mA cm−2) when using Clostridium beinjenrinckii as an inoculum. This indicates that the use of complex compounds reduces current output and overall process performance. However, the use of complex compounds is needed since the composition of wastewater can be multifarious. In order to optimise current outputs when using complex substrates, the fundamentals of the substrate degradation stages (hydrolysis and fermentation) occurring in MFC should be determined. In anaerobic digestion, the hydrolysis of complex wastes is considered the major limiting factor with first-order rate constants between 0.025 and 0.47 day−1 (Mata-Alvarez et al. 2000; van Lier et al. 2001).
Regarding microbial selection in the MFC anode, it has been emphasized that more research is needed to determine how process variables, such as the type of substrate used, affect the metabolism of microbial communities and current production (Pham et al. 2009). The study of bacterial metabolism from Pham et al. (2009) showed that the microbial communities can be affected by the type of substrate used in MFCs.
Fermentation and hydrolysis can be undertaken by different or the same types of microbial communities. Specific microorganisms responsible for hydrolysis may include Proteus vulgaris (Matsubara et al. 1981) and Acetovibrio celluliticus (Mackenzie et al. 1985). Microorganisms only catalysing fermentation comprise Lactobacillus sp., Succinomonas amylolytica and Ruminococcus flavefaciens (Abasiekong 1991). Microbial communities performing both hydrolysis and fermentation include Clostridium sp. and Staphylococcus sp. (Andreesen et al. 1973; Arvidson 1973; Kataeva et al. 2002). Several reports have concentrated in identifying the type of communities developed when using different organic fuels in MFCs (Jung and Regan 2007; Logan 2009; Xing et al. 2009). However, few have considered how the community is assembled when the complexity of the substrate is changed, an interesting variable when evaluating the range of microbial communities present when different degradation processes are needed.
This work estimates, for the first time, the hydrolysis and fermentation rate constants in MFCs which are important parameters in the modelling of MFC substrate degradation and for process understanding. This was studied using three substrates differing in complexity (starch, glucose and acetate). Starch is a complex carbohydrate that requires hydrolysis to glucose and fermentation; glucose is a simple carbohydrate that will only need fermentation and finally, acetate is a fermentation product that neither needs hydrolysis nor fermentation; as a result, acetate can be directly consumed by anode-respiring bacteria. The relationship between different simple and complex fuels, current output and the anodic bacterial community was determined. It was hypothesised that the decreased MFC performance observed when using complex compounds may be due to the rate of hydrolysis as it occurs in anaerobic fermentation. In addition, the anodic bacterial communities may present greater diversity when using complex substrates than using simple substrates due to the increased range of degradation processes required.
Materials and methods
Reactor configuration and operation
Experiments were conducted using six single-chambered glass MFCs under anoxic conditions. The anodic chamber had a volume of 200 ml (Fig. 2) and contained an anode electrode. The anode was a carbon cloth (38 cm2), which was boiled twice in 0.1 M H2SO4 for 1 h to activate and sterilise the anode surface, then washed with sterile distilled water until a neutral pH was reached. The cathode (35 cm2) was Toray carbon paper prepared according to the procedures of Cheng et al. (2006) and contained 0.5 mg/cm2 of Pt and four diffusion layers. The top of the anodic chamber was equipped with a sample port to remove samples of the anolyte solution without disturbing the anodic biofilm and a port to insert a reference electrode. Before use, all reactor components were autoclaved. Anode and cathode were positioned at a distance of 4 cm. Anode and cathodes were connected to the electrical circuit using a resistor box.
Experiments were initiated using 180 ml of a mixture of 50% clarifier effluent and 50% synthetic medium (1 g COD/L) until a stable current output was obtained after five batches of 50% clarifier effluent and 50% synthetic medium had been fed to the reactor. The synthetic medium contained either starch, glucose or acetate as substrate, and two reactors were run per substrate. This was done to allow MFC anode enrichment and adaptation of electrochemically active bacteria. After enrichment, experiments were done using 180 ml of synthetic medium made up with the corresponding substrate (acetate, glucose or starch) which had been used in the enrichment stage. Each substrate was tested in duplicate. All MFCs were operated at room temperature (19 ± 2 °C) for 8 months. During that time, different concentrations of the organic substrate were tested, and electrochemical measurements were conducted. Cell voltages were recorded using a data acquisition system (PICO Data logger, UK), and linear sweep voltammograms were used to characterise MFC performance with various substrates. Internal resistance of MFCs was measured by electrochemical impedance spectroscopy. When analysing the anodic anolyte, no more than 10% (18 ml) of the medium volume was removed. Anolyte samples taken for chemical analyses were filtered through a 0.2-μm syringe filter (Whatman, UK) and then stored at −20 °C prior to analysis.
Substrate and inoculum
The synthetic medium was prepared using one substrate (acetate, glucose or starch) and the following compounds (analytical grade, Sigma-Aldrich): 40 mg/L NH4Cl, 10 mg/L MgCl2, 0.1 mg/L CuSO4·5H2O, 5 mg/L CaCl2·2H2O, 0.1 mg/L MnSO4·4H2O and 0.1 mg/L ZnCl2. Sodium phosphate buffer (pH 7.0) was added to a concentration of 25 mM to increase the conductivity of the electrolyte to 3 mS/cm. The amount of substrate in the synthetic medium was changed to give different COD (Chemical Oxygen Demand) concentrations. A calibration curve was constructed to determine the COD per gram of substrate ratio. The source of bacteria was primary clarifier effluent collected from a municipal sewage treatment plant located in Northumberland, UK.
Chemical and microbial measurements
The anodic electrolyte was chemically characterized under different process conditions by analysing the COD and VFA. COD was estimated according to APHA standard method 5220-C (APHA 1995). VFAs were measured by ion chromatography using a Dionex ICS-1000 (ICE-AS1 column; mobile phase, 1 mM heptafluorobutyric acid; flow rate, 0.16 mL/min; backpressure, ∼700 psi and a 25-μL capacity sample loop). Quantification was achieved by integrating the peak areas from a conductivity detector, relative to external standards. The detection limit was 0.2 mg/L for lactic, formic, acetic, propionic and butyric acids.
Anodic microorganisms developed with all substrates were characterized at the end of the experiment by sequentially performing DNA extraction, PCR and denaturing gradient gel electrophoresis (DGGE) analyses as reported previously (Velasquez-Orta et al. 2010). Two additional MFCs fed with wastewater rather than synthetic medium, and operated for the same length of time, were included in the microbial community analysis. Images obtained from DGGE were processed using the Bionumerics software package (version 3.5, Applied Maths, USA). Bionumerics was used to construct a character table of bands in DGGE profiles according to their position and intensity in relation to markers run alongside samples in the gel (van Verseveld and Roling 2008). The character table was exported to Microsoft Excel and Primer 6 for subsequent analysis. Bray–Curtis similarity matrices were obtained using Primer 6 for Windows (Version 6.1.5, Primer-E Ltd, Plymouth, UK) and analysed using non-metric multidimensional scaling (MDS). Similarities were statistically analysed using band designation as variables of presence and absence and analysis of similarities (ANOSIM) (Clarke and Warwick 2001). The MDS analysis and algorithm were followed as mentioned in Gray et al. (2007). SPSS 15.0 for Windows was used for all other statistical analysis using analysis of variance (ANOVA).
Calculations
To calculate the theoretical open circuit voltage (OCV) value for MFCs, it was assumed that MFCs used only acetate as a source of electrons. Therefore, MFCs using glucose and starch had to transform the substrate to acetate prior to being used for current production. As an example, the reaction potential when using acetate is as below for the following conditions: [CH3COO−] = [HCO −3 ] = 4.5 mM = 250 mg COD/L, pH = 7, T = 298.15 K, pO2 = 0.2 bar;
The rates of acetate consumption, fermentation (conversion of glucose to acetate) and hydrolysis–fermentation (conversion of starch to acetate) were calculated assuming a first-order reaction according to previous literature (Lavarack et al. 2000; Mata-Alvarez et al. 2000).The first-order reaction (R → P) is expressed using: r = −k [R], where r is the rate of reaction, R is the reactant concentration and P is the product concentration. The linear plot of t (time) vs In R was constructed to obtain the kinetic rate constant: k, using Eq. 4.
The rate of depletion of glucose and starch was derived by subtracting the VFA produced through time from initial substrate concentrations (1,000 mg/L). For reactors fed with starch, it was assumed that the rate of VFA accumulation in MFC reactors was proportional to the rate of VFA production, while for reactors fed with glucose, the acetate consumption values were added to the VFA accumulation to calculate the rate of fermentation.
Results
Effect of the type of substrate used in MFC performance
MFCs produced a reproducible current after 200 h of enrichment (Fig. 3). The initial increase in current production was faster in MFCs using acetate and glucose than in MFCs using starch. MFC performance was evaluated using different substrates (acetate, glucose and starch) and organic loads (125, 250 and 500 mg COD/L; Table 1). The highest current outputs, power densities and coulombic efficiencies for all substrates were obtained using a concentration of 250 mg COD/L; therefore, subsequent current and power production measurements were performed using this substrate concentration.
Enrichment of MFC reactors using primary clarifier effluent. Current was obtained using a 200-Ω resistor. Experiments were done using primary clarifier effluent (50% of total electrolyte) and synthetic fuel (50% of total electrolyte) to give a concentration of 1 g COD/L and a conductivity of 3 mS/cm. Arrows indicate replacement of the MFC electrolyte with fresh electrolyte
Coulombic efficiencies decreased with all substrates when the concentration of organic matter increased to 500 mg/L.There were no statistical differences in the maximum current produced between MFCs using acetate and glucose (p = 0.78, t test). However, the current produced by MFCs using starch was low and statistically different from MFCs using acetate and glucose (p = 0.01, ANOVA). The highest coulombic efficiencies (CE) were obtained by MFCs using acetate and glucose (around 30%), and the lowest coulombic efficiency was obtained in MFCs using starch (around 1%). There were also no significant differences in the COD removed between MFC reactors using glucose and acetate (p = 0.78, t test), but COD removed in MFCs using starch was lower in all cases (between 30% and 60%).
Current production in MFCs using a substrate concentration of 250 mg COD/L remained constant for 40 h when using acetate, 80 h when using glucose and 60 h when using starch (Fig. 4a). A comparison of four batch runs (Fig. 4b) revealed significant differences in the maximum current obtained when using different synthetic substrates (p < 0.0001, two-way ANOVA). There was greater variance in the mean current produced by MFCs using starch than by MFCs using acetate or glucose. This is due to the slower increase in current in MFCs using starch (Fig. 4a).
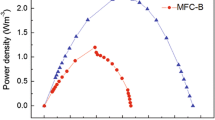
Peak power densities obtained by polarizing the MFCs (Fig. 5) decreased as the complexity of the substrate increased (99 ± 2 mW m−2, 78 ± 5 mW m−2 and 4 ± 2 mW m−2 for acetate, glucose and starch, respectively). It can be observed from the polarisation curves that the open circuit voltage (OCV) in MFCs using acetate and glucose was similar (around 800 mV) while the OCV in MFCs using starch was low (about 600 mV). Measured OCV values in MFCs using acetate were 20% lower than the theoretical OCV of 1,101 mV. This indicates that there were potentially voltage losses in MFCs due to overpotential. MFCs using starch had a high cell voltage drop (from 600 mV to 30 mV for a current density of 30 mA m−2), implying a higher polarisation/resistance and lower activity than in MFCs using acetate and glucose. The internal resistance of MFCs increased as the complexity of the substrate increased. Internal resistance in MFCs using starch was double (329 ± 63 Ω) than in MFCs using acetate (145 ± 16 Ω). MFCs fed with glucose had an internal resistance of 244 ± 41 Ω.
MFC polarisation curves with different substrates. The organic load used was 250 mg COD/L of synthetic fuel with a conductivity of 3 mS/cm. Filled symbols are for power densities and open symbols are for voltage losses. Polarizations were carried out using linear sweep voltammetry at a scan rate of 1 mV s−1
Generation and consumption of volatile fatty acids in MFC using different substrates
As can be seen in Fig. 6, MFCs using acetate exhibited more rapid current production and a slow rate of acetate consumption (12 ± 3 ppm/h). The VFA analysis showed acetate production with marginal quantities of propionate. In the case of MFC using glucose, VFA accumulated at a rate of 10 ± 2 ppm/h until reaching a plateau at 28 h. For MFCs using starch, VFA did not accumulate during the first 20 h and corresponded with the slow increase in current production. Presumably, bacterial communities for MFCs using starch were consuming the VFA at a rate faster than they were produced during the first 20 h, and therefore, they did not accumulate in the bulk liquid. Once a steady current output was achieved in MFCs using starch (at 20 h), VFA started to slowly accumulate at a rate of 4 ± 1 ppm/h. The accumulation of VFA in MFC reactors using glucose and starch suggests that VFA production occurred at a faster rate than VFA consumption. Fermentation and hydrolysis rates were obtained from the VFA concentrations in Fig. 6. The hydrolysis/fermentation rate constant was some eight times lower (k = 0.0024 h−1) than the rate constant for fermentation (k = 0.018 h−1). The rate constant for acetate consumption (k = 0.017 h−1) was in the same range as the fermentation rate. This can also explain the plateau reached by all MFC reactors after 20 h.
MFC volatile fatty acid production/consumption and current output. Current was obtained using a 200-Ω resistor. MFC experiments were done using 1,000 mg COD/L of synthetic fuel to allow for VFA identification at conductivity of 3 mS/cm. Filled symbols are for VFA concentrations and open symbols are for current outputs
Bacterial community composition of MFC anode biofilms using different substrates
DGGE profiles obtained from anode biofilms (Fig. 7a) showed differences in the number of bands between MFCs using complex compounds (starch, wastewater and glucose) and MFCs using acetate with 25 ± 2 and 15 ± 1 bands, respectively (p < 0.0001, ANOVA). Comparison of the community structure in MFCs using different substrates by MDS analysis (Fig. 7b) showed that bacterial communities from the inoculum were very different from communities developed in anode biofilms (bounded by a 20% similarity contour). This indicates that microorganisms were selected during the 8 months of MFC operation as has been previously reported (Kim et al. 2004; Rabaey et al. 2004). Biofilm communities from MFCs using different types of substrates were also only bounded by a 40% similarity contour. Statistical analysis indicated a significant substrate effect in the bacterial community structure of biofilms (R = 0.96 and p = 0.01, ANOSIM). Figure 7b also shows that microbial biofilms from MFCs using acetate produced the highest current output and were widely separated along the ordinates from MFCs using glucose, starch or wastewater (R = 1 and p = 0.33, ANOSIM). In contrast, biofilm communities in MFCs using glucose, starch or wastewater were similar at the 94% level (R = 1 and p = 0.06, ANOSIM).
Analysis of similarity between DGGE profiles of 16S rRNA gene fragments from bacterial communities developed in MFC anode biofilms and the initial inoculum. a Bands obtained from DGGE analysis. b Statistical analysis of band patterns using non-metric multidimensional scaling (MDS). Maximum current outputs were obtained in experiments using 250 mg COD/L
Discussion
Results from this study showed differences in MFC process performance in relation to substrate complexity. Process performance was better in MFCs using simple substrates (acetate) than in MFCs using complex substrates (starch), which is in agreement with previous studies (Catal et al. 2008; Kim et al. 2000; Lee et al. 2007; Niessen et al. 2004). As current output increased, COD removal and CE also increased, indicating a relationship between current and COD removal by bacteria on the anode. For this experiment, the electron production from the different substrates limited the overall process performance. Maximum CE obtained in this experiment for MFCs using acetate (32%) or starch (19%) was lower than CE reported in literature using acetate (65%) or starch (21%) (Min and Logan 2004).CEs for MFCs using glucose were similar to the 29% maximum CE reported for a similar MFC configuration (Catal et al. 2008; Kim et al. 2000). It was found that increasing the concentration of substrates to 500 mg COD/L decreased the MFC process performance (as CE) compared to that with 250 mg/L (Table 1). This may be due to organic matter saturation in the anode due to a low ratio of the surface area/organic matter availability which can limit MFCs’ performance and enhance secondary reactions such as bacterial growth, storage of energy in cells and/or methanogenesis (Velasquez-Orta et al. 2010). Recently, Sharma and Li (2010) reported a substrate inhibition effect when using high glucose concentrations. This was attributed to the random binding of substrate molecules in the active sites of an enzyme at the same time. For their experiments, this substrate inhibition effect was produced at concentrations higher than 900 mg/L.
Several reports have shown that electrogenic microbial communities utilise VFAs to produce electrons (Thygesen et al. 2009; Kim et al. 2004), making hydrolysis and fermentation essential processes when using complex substrates as electron donors. In this experiment, the first-order rate constant obtained for fermentation (0.018 h−1) was ten times higher than that for the combined fermentation–hydrolysis processes (0.0024 h−1). This means that the rate of hydrolysis limited MFC performance. This is comparable with anaerobic digestion where fermentation occurs rapidly because fermentative bacteria can replicate rapidly (Gujer and Zehnder 1983; Metcalf and Eddy 2003), while hydrolysis is considered the rate-limiting step (Mata-Alvarez et al. 2000; Vavilin et al. 2008). It was also observed that the rate constant for fermentation (k = 0.018 h−1) was in the same order with that of the rate of acetate consumption (k = 0.017 h−1). However, MFCs still accumulated VFA (mostly acetate) in the bulk liquid. This suggests that the rate of fermentation was initially occurring faster than the rate of acetate consumption, but these processes reached equilibrium when the VFA concentration curve showed a plateau. CEs obtained in MFCs using acetate were less than 33%. This shows that approximately one third of the acetate was oxidised, and electrons were produced by anode-respiring bacteria, while the two thirds of acetate were consumed by secondary reactions such as methanogenesis. These secondary reactions may have faster kinetics than the electrogenesis process.
In order to optimize MFC fed with complex substrates (such as wastewater), the rate of hydrolysis, fermentation and acetate consumption for electron transport should ideally be standardised. For example, the rate of hydrolysis could be increased by promoting the growth of hydrolytic bacteria or by adding hydrolytic enzymes. Rezaei et al. (2008) obtained a tenfold improvement in current output when cellulases were added to enhance hydrolysis in MFCs using cellulose. Another option is the use of a two-stage process to conduct hydrolysis and fermentation in a pre-treatment process such as anaerobic digestion (Lalaurette et al. 2009). The rate of acetate consumption for electron transport to the anode could also be raised by increasing the concentrations of redox mediator compounds (Velasquez-Orta et al. 2010).
Analysis of the structure of microbial communities using different substrates suggested that the diversity in communities was wider in MFCs using complex wastes (glucose, starch and wastewater) than in MFCs using acetate. This is likely to be due to the increased number of degradation processes performed by different types of communities. MFCs using starch had the lowest MFC performance (Figs. 3 and 4). Presumably, bacteria present in MFCs using starch transferred fewer electrons to the anode due to the increased number of degradation processes performed by a wide bacterial community (Fig. 6).
This study highlighted the importance of promoting efficient degradation of compounds to achieve an increased electron transfer process to the MFC anode. Two proposed mechanisms can be applied to improve MFCs’ performance during wastewater treatment: (1) using anaerobic digestion as pre-treatment of wastewater effluents to improve substrate degradation, and (2) promoting enrichment of bacterial communities which are balanced with respect to hydrolysis, fermentation and electrogenesis in the MFC anode. For the first option, combining MFCs with anaerobic digestion can offer benefits in the treatment of complex wastes such as wastewater. Anaerobic digestion is normally used to treat wastewater with high organic loads, achieving around 80% COD removal and leaving 20% of COD (in the form of VFA and refractory compounds). The COD load reduction after anaerobic digestion could also increase MFC performance as shown in this study. For the second option, a balanced community enrichment might be possible by changing process conditions such as light (Xing et al. 2009), external resistance (Katuri et al. 2011) or momentarily switching the type of substrate used (Zhang et al. 2011).
References
Abasiekong SF (1991) Effects of fermentation on crude protein content of brewers dried grains and spent sorghum grains. Bioresour Technol 35(1):99–102
Andreesen JR, Schaupp A, Neurauter C, Brown A, Ljungdahl LG (1973) Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO2. J Bacteriol 114(2):743–751
APHA (1995) Standard methods for examination of water and wastewater Clescerl LS, Greenberg AE, Eaton AD (eds) American Public Health Association, Washington, DC
Arvidson S (1973) Hydrolysis of casein by three extracellular proteolytic enzymes from Staphylococcus aureus, strain v8. Acta Pathologica Microbiologica Scandinavica 81B(5):538–544
Catal T, Li K, Bermek H, Liu H (2008) Electricity production from twelve monosaccharides using microbial fuel cells. J Power Sources 175(1):196–200
Cheng S, Liu H, Logan BE (2006) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40(7):2426–2432
Clarke K, Warwick R (2001) Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25(5):464–482
Gray ND, Brown A, Nelson DR, Pickup RW, Rowan AK, Head IM (2007) The biogeographical distribution of closely related freshwater sediment bacteria is determined by environmental selection. ISME J 1(7):596–605
Gujer W, Zehnder AJB (1983) Conversion processes in anaerobic digestion. Water Sci Technol 15:127–167
Jung S, Regan J (2007) Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl Microbiol Biotechnol 77(2):393–402
Kataeva IA, Seidel RD III, Shah A, West LT, Li X-L, Ljungdahl LG (2002) The fibronectin type 3-like repeat from the Clostridium thermocellum cellobiohydrolase CbhA promotes hydrolysis of cellulose by modifying its surface. Appl Environ Microbiol 68(9):4292–4300
Katuri KP, Scott K, Head IM, Picireanu C, Curtis TP (2011) Microbial fuel cells meet with external resistance. Bioresour Technol 102(3):2758–2766
Kim BH, Park HS, Kim HJ, Kim GT, Chang IS, Lee J, Phung NT (2004) Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl Microbiol Biotechnol 63(6):672–681
Kim N, Choi Y, Jung S, Kim S (2000) Effect of initial carbon sources on the performance of microbial fuel cells containing Proteus vulgaris. Biotechnol Bioeng 70(1):109–114
Lalaurette E, Thammannagowda S, Mohagheghi A, Maness P-C, Logan BE (2009) Hydrogen production from cellulose in a two-stage process combining fermentation and electrohydrogenesis. Int J Hydrogen Energy 34(15):6201–6210
Lavarack BP, Griffin GJ, Rodman D (2000) Measured kinetics of the acid-catalysed hydrolysis of sugar cane bagasse to produce xylose. Catal Today 63(2–4):257–265
Lee H-S, Parameswaran P, Kato-Marcus A, Torres CI, Rittmann BE (2007) Evaluation of energy conversion efficiencies in microbial fuel cells utilizing fermentable and non-fermentable substrates. Water Res 42(6–7):1501–1510
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Micro 7(5):375–381
Mackenzie CR, Bilous D, Patel GB (1985) Studies on cellulose hydrolysis by Acetivibrio cellulolyticus. Appl Environ Microbiol 50(2):243–248
Mata-Alvarez J, Macé S, Llabrés P (2000) Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour Technol 74(1):3–16
Matsubara N, Yotsuji A, Kumano K, Inoue M, Mitsuhashi S (1981) Purification and some properties of a cephalosporinase from Proteus vulgaris. Antimicrob Agents Chemother 19(1):185–187
Metcalf and Eddy (2003) Wastewater engineering: treatment and reuse. Mc Graw Hill, New York, pp 630–631
Min B, Logan BE (2004) Continuous electricity generation from domestic wastewater in a flat plate microbial fuel cell. Environ Sci Technol 38(21):5809–5814
Niessen J, Schroder U, Scholz F (2004) Exploiting complex carbohydrates for microbial electricity generation—a bacterial fuel cell operating on starch. Electrochem Commun 6(9):955–958
Pham TH, Aelterman P, Verstraete W (2009) Bioanode performance in bioelectrochemical systems: recent improvements and prospects. Trends Biotechnol 27(3):168–178
Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70(9):5373–5382
Rezaei F, Richard TL, Logan BE (2008) Enzymatic hydrolysis of cellulose coupled with electricity generation in a microbial fuel cell. Biotechnol Bioeng 101(6):1163–1169
Sharma Y, Li B (2010) The variation of power generation with organic substrates in single-chamber microbial fuel cells (SCMFCs). Bioresour Technol 101:1844–1850
Thygesen A, Poulsen FW, Min B, Angelidaki I, Thomsen AB (2009) The effect of different substrates and humic acid on power generation in microbial fuel cell operation. Bioresour Technol 100(3):1186–1191
Torres CI, Kato Marcus A, Rittmann BE (2007) Kinetics of consumption of fermentation products by anode-respiring bacteria. Appl Microbiol Biotechnol 77(3):689–697
van Lier JB, Tilche A, Ahring BK, Macarie H, Moletta R, Dohanyos M, Pol LWH, Lens P, Verstraete W (2001) New perspectives in anaerobic digestion. Water Sci Technol 41(1):1–18
van Verseveld HW, Roling WFM (2008) Cluster analysis and statistical comparison of molecular community profile data. In: Kowalchuk GA, FJd B, Head IM, Akkermans AD, JDv E (eds) Molecular microbial ecology manual. Springer, London, pp 1373–1397
Vavilin VA, Fernandez B, Palatsi J, Flotats X (2008) Hydrolysis kinetics in anaerobic degradation of particulate organic material: an overview. Waste Manage 28(6):939–951
Velasquez-Orta S, Head IM, Curtis TP, Scott K, Lloyd J, Canstein H (2010) The effect of flavin electron shuttles in microbial fuel cells current production. Appl Microbiol Biotechnol 85(5):1373–138
Vogel HC (1983) Fermentation and biochemical engineering handbook. Noyes, New Jersey, pp 1–46
Xing D, Cheng S, Regan JM, Logan BE (2009) Change in microbial communities in acetate- and glucose-fed microbial fuel cells in the presence of light. Biosens Bioelectron 25(1):105–111
Xue F, Zhang X, Luo H, Tan T (2006) A new method for preparing raw material for biodiesel production. Process Biochem 41(7):1699–1702
Zhang Y, Min B, Huang L, Angelidaki I (2011) Electricity generation and microbial community response to substrate changes in microbial fuel cell. Bioresour Technol 102(2):1166–1173
Acknowledgements
This research was supported by the Mexican Research Council for Science and Technology (CONACyT), PhD contract 196298.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Velasquez-Orta, S.B., Yu, E., Katuri, K.P. et al. Evaluation of hydrolysis and fermentation rates in microbial fuel cells. Appl Microbiol Biotechnol 90, 789–798 (2011). https://doi.org/10.1007/s00253-011-3126-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3126-5