Abstract
This study was intended to optimize the reaction parameters of formulation of the rapeseed oil-based pentaerythritol ester (PE) biolubricant using response surface methodology (RSM) based on the central composite design (CCD).The parameters chosen for the reaction process to synthesize pentaerythritol ester were catalyst concentration (0.5–1.5 wt%), temperature (140–160 °C), and rate of reaction time (1–5 h). The outcome results of process variables revealed that an optimum condition for the biolubricant synthesis was at the temperature of 145.09 °C, and 1.45 wt% of catalyst capacity and 1.89 h of reaction time were acquired with the 81.6% yield of pentaerythritol ester. The catalyst capacity and reaction process time were found to be the highly considerable interaction variables. The yield predicted after the optimization process has produced adequate results with the experimental value with a coefficient of determination (R2) of 0.9667.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The rapid expansion and utilization of innovative resource of lubricants for various industrial sectors are increasing every year [1]. The petroleum oil-based synthetic lubricants may have a contamination hazard with respect to ecosystems, agricultural land, and groundwater reserves owing to the toxicity and non-biodegradable nature [2, 3]. Vegetable oils are the primary outrider by its uses, and because of their sustainable energy resources, they offer energy independence [4]. Fatty acid esters derived from vegetable oil have gained importance as an alternative lubricant for various applications. Plant oil transesterification with polyol produce biolubricants with high thermo-oxidative stability and pour point for the reason of glycerol replacements by the polyol [5, 6].
Extensive research works on formulation of biolubricants had been conceded out previously on polyols especially pentaerythritol ester [7]. Hamizah Ammarah et al. [8] analyzed the synthesis optimization of trimethylolpropane (TMP) ester and pentaerythritol (PE) ester through the esterification of oleic acid with polyhydric alcohols and stated that optimum condition for the TMP and PE synthesis was the temperature at 150 and 180 °C, catalyst content of 1.5 wt%, molar ratio 3.9:1 and 4.9:1, and reaction time of 5 h and 6 h, respectively, obtained with the yield of 91.2% ester TMP and 92.7% ester PE. Musa et al. [9] investigated the optimization process of transesterification of jatropha methyl ester with TMP using response surface methodology. The model was satisfactorily predicting the yield of jatropha biolubricant with a coefficient of determination (R2) of 0.9068. Venu and Goud [10] analyzed the optimization of RSM-based castor oil synthesis and inferred that the optimal condition was the temperature of 52.81 °C, catalyst capacity of 15.14 wt%, 1.65:1 molar ratio, and reaction time of 2.81 h. The present work aimed to synthesize the rapeseed oil-based pentaerythritol ester by successive transesterification process. The reaction parameters of transesterification process were optimized, and different interaction parameters were also examined by using response surface methodology (RSM) and further CCD was used to determine the effect of process variables using analysis of variance (ANOVA).
2 Materials and Methods
2.1 Transesterification Reaction of Pentaerythritol Ester of Rapeseed Oil
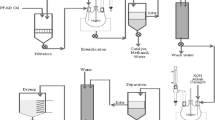
Methanol, sodium hydroxide (NaOH), pentaerythritol (99%), para-toluene sulfonic acid (p-TSA), and xylene were purchased from M/s. Sigma Aldrich, USA, was used in the synthesis of pentaerythritol ester. Rapeseed oil extracted from rapeseed was transesterified to prepare the rapeseed oil methyl ester. A mixture of two-liter of rapeseed oil with 880 ml of methanol and 18.46 g of NaOH catalyst was poured into a round bottom flask equipped with a mechanical stirrer. The reaction mixture was heated up to 55 °C in stirring condition of 300 rpm for 1 h. The mixture was later cooled and drained in a funnel separation of methyl ester. To remove the excess methanol and excess catalyst, the obtained methyl ester was heated to 70 °C and washed with distilled water. The rapeseed oil methyl ester of one liter, 85 g of pentaerythritol, and 55 g of para-toluene sulfonic acid was mixed with xylene. This reaction product was then heated in the nitrogen atmosphere up to 160 °C for 5 h. Finally, the product obtained from rapeseed oil was designated as pentaerythritol ester [11].
2.2 Experimental Design
The CCD design was used in the design expert software to find out the three factors which were the capacity of catalyst (sodium hydroxide, weight %), temperature, and the reaction process duration. Table 1 depicts the coded and actual levels of variables for design determined based on earlier literature findings. The temperature was assorted between 140 and 160 °C, catalyst concentration between 0.5 and 1.5% w/w, and rate of reaction time between 1 and 3 h. Three factors in 23 full factorial CCD with three levels resulted in 20 runs of experiments (2k + 2k + 6), and k stands for number of independent variables/factors chosen. Center point experiments of six runs have analyzed the pure error augmented with 8 factorial and 6 axial experimental runs. The response evaluated from the experiments was the percentage of pentaerythritol ester composition obtained in terms.
2.3 Statistical Analysis
The regression coefficients of the second-order polynomial models were utilized to effectuate the pentaerythritol ester biolubricant yield from the CCD optimization data.
where Yyield was the response variable (composition of pentaerythritol ester), i and j indicate linear and quadratic coefficients, regression coefficients were expressed by b0, bi, bij, and bij, k was the number of factors studied and optimized in the experiments, while e denoted the random error [12]. The Design–Expert software was used to produce the regression analysis of the obtained data, and analysis of variance (ANOVA) was utilized to find out the model adequacy.
3 Results and Discussion
3.1 Optimization of Process Parameters using RSM
The CCD based on RSM was used to optimize the reaction parameters for the formulation of rapeseed oil-based pentaerythritol ester. The temperature, time, and catalyst concentration were selected as independent variables. Their interaction between the parameters was examined for finding out the optimum yield of biolubricant. The design layout and experimental results are shown in Table 2. The composition of pentaerythritol ester was determined using an estimated response model, and final equation with respect to actual factors was given in Eq. (2).
where Y was pentaerythritol ester yield composition, X1, X2 and X3 were the temperature, catalyst capacity, and reaction duration, respectively. The highest percentage of pentaerythritol ester (PE) yield of 81.51% was obtained at 150 °C, catalyst content of 1.89%, and reaction time of 1.89 h [13].
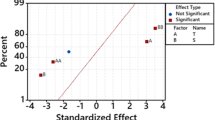
Figure 1 shows the actual versus predicted values plot. The uniformly scattered points in the plot showed that the points were very closer to the straight lines which represent the better agreement of the experimental values with the predicted values of the response. Figure 2 depicts the normal probability of residual plot. The residuals distribution was established by the normal probability of residual plot. The data normality is confirmed by the straight lines [14].
3.2 Fitting of Model and Variance Analysis (ANOVA)
The Model F-value of 62.21 has validated the importance of the model. In ANOVA analysis-catalyst, duration, AB, BC, A2, C2 have considerable model variables shown in Table 3. The P-values higher than 0.1000 disclose the model terms are not noteworthy. The lack of fit F-value of 0.34 complimented to the pure error. It may be 84.28% possibility with this large lack of fit F-value could arise as a result of noise. The fitted model is guaranteed by the good non-significant lack of fit. There is a reasonable agreement with the predicted R2 of 0.9480 and adjusted R2 of 0.9667 [13, 15]. The desirability is assured by the adequate precision (signal-to-noise) ratio more than 4. The acquired signal-to-noise ratio of 31.072 point outs an adequate signal.
3.3 Effect of Process Parameter on Transesterification Reaction
The 3D surface plot of the second-order model was used for the reaction parameters to find out the variables' interaction response on the biolubricant yield.
Figure 3a depicts the catalyst capacity and temperature interactive effect on the biolubricant yield. The catalyst quantity considerably increases the pentaerythritol ester biolubricant yield. This is due to the reason for the inclusion of more catalyst that accelerated the higher reaction process in the lesser duration of time. The increasing catalysts' effect was observed at the reaction condition; the temperature of 140 °C, catalyst intensity of 1.45% w/w, and reaction time of 3 h produce the 81.51% yield, and the pentaerythritol ester yield of 75.19% was recorded at a temperature of 150 °C, catalyst capacity of 0.5% w/w, and reaction time of 3 h. The biolubricant yield decreases with temperatures increase owing to the speeding up of saponification reaction rate which leads to the decrease of transesterification reaction yield [9, 12].
Figure 3b depicts the interactive effect on biolubricant yield of temperature and reaction time. The pentaerythritol ester yield decreases as temperature increases and is attributable to reduced rate of reaction speed resulting in lower pentaerythritol ester conversion [12]. The reduced effect of temperature demonstrates that 77.95% yield was perceived at a temperature of 160 °C, the reaction time of 5 h, and 1.0% catalyst quantity, whereas 72.67% yield was recorded at a temperature of 140 °C, the reaction time of 3 h, and catalyst intensity of 1.0% w/w. The results showed that the pentaerythritol ester yield increases with the increase in duration of the reaction process. This raising effect of reaction time produces the yield of 74.12% for the reaction condition of temperature at 160 °C, catalyst quantity of 1.0%, and 5 h reaction time. However, 77.95% yield was recorded at a temperature of 140 °C, catalyst quantity of 1.0%, and reaction time.
Figure 3c shows the interaction response of catalyst concentration and reaction process duration on biolubricant yield. The results show that the higher significant effect of the increase in catalyst concentration on the biolubricant yield. This is due to the more catalyst capacity that promotes the higher conversion of pentaerythritol ester [16]. The yield of 81.51% was substantiated at a temperature of 145 °C, catalyst intensity of 1.45%, and reaction process period of 1.89 h, whereas 61.19% yield was observed at a temperature of 135 °C, the reaction time of 1 h, and catalyst strength of 0.5% [17]. The effect of the catalyst with respect to time had a higher considerable impact contributing to the fitted model.
4 Conclusion
The outcome of this investigation is detailed below.
The formulation of the rapeseed oil-based pentaerythritol ester (PE) biolubricant was successfully optimized. The obtained optimum reaction's conditions are temperature at 145.09 °C, the catalyst capacity of 1.45 wt% and process reaction time of1.89 h with the pentaerythritol ester yield of 81.6%. The catalyst effect with time had a higher considerable impact contributing to the fitted model. The yield foreseen after the optimization process produces good agreement with the experimental values with a coefficient of determination (R2) of 0.9667.
References
Kodali DR (2002) High performance ester lubricants from natural oils. Ind Lubr Tribol 54(4):165–170
Erhan SZ, Asadauskas S (2000) Lubricant basestocks from vegetable oils. Ind Crop Prod 11(2–3):277–282
Wagner H, Luther R, Mang T (2001) Lubricant base fluids based on renewable raw materials: their catalytic manufacture and modification. Appl Catal A-Gen 221(1–2):429–442
Arbain NH, Salimon J (2011) Synthesis and characterization of ester trimethylolpropane based Jatropha Curcas oil as biolubricant base stocks. J Sci Tech 2(2):47–58
Hwang HS, Erhan SZ (2006) Synthetic lubricant basestocks from epoxidized soybean oil and Guerbet alcohols. Ind Crop Prod 23(3):311–317
Kamil RNM, Yusup S (2010) Modeling of reaction kinetics for transesterification of palm-based methyl esters with trimethylolpropane. Bioresour Technol 101(15):5877–5884
Kyzimova NS (2008) Thermostable lubricating composition based on pentaerythritol esters. Chem Technol Fuels Oils 44:169–171
Mahmud HA, Salimon J (2014) Optimization of Esterification of Oleic acid and Trimethylolpropane (TMP) and Pentaerythritol (PE). AIP Conf Proc 1614:230–236
Umaru M, Aris MI, Munnir SM, Aliyu AM, Aberuagba F, Isaac AJ (2016) Statistical optimization of biolubricant production from jatropha curcas oil using trimethylolpropane as a polyol. ProcWorld Congr Eng Comp Sci 2:1–6
Borugadda VB, Goud VV (2015) Response surface methodology for optimization of bio-lubricant basestock synthesis from high free fatty acids castor oil. Energy Sci Eng 3(4):371–383
Vithya P, Sriram G, Arumugam S (2018) Tribological analysis of biodegradable refrigeration oil for vapour compression refrigeration system using four ball tribometer. IOP Conf Ser Mater Sci Eng 390:012070. https://doi.org/10.1088/1757-899X/390/1/012070
Aziza NAM, Yunusa R, Rashida U, Syama AM (2014) Application of response surface methodology (RSM) for optimizing the palm-based pentaerythritol ester synthesis. Ind Crop Prod 62:305–312
Shrivastsvs A, Sandagar P, Baja I, Singhal R (2008) Media optimization for the production of U-linolenic acid by cunninghamella Echinulata varielegans MTCC 522 using response surface methodology. Int J Food Eng 4(2):1–32
Ocholi O, Menkiti M, Auta M, Ezemagu I (2018) Optimization of the operating parameters for the extractive synthesis of biolubricant from sesame seed oil via response surface methodology. Egyp J Petro 27:265–275
Russell VL (2009) Response surface methods in R, using RSM. J Stat Softw
Yunus R, Fakhrul-Razi A, Ooi TL, Iyuke SE, Idris A (2003a) Development of optimum synthesis method for transesterification of palm oil methyl esters and trimethylolpropane to environmentally acceptable palm oil-based lubricant. J Oil Palm Res 15:35–41
Yunus R, Fakhrul-Razi A, Ooi TL, Iyuke SE, Idris A (2003b) Preparation and characterization of trimethylolpropane esters from palm kernel oil methyl esters. J Oil Palm Res 15:42–49
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Vithya, P., Sriram, G., Arumugam, S. (2021). RSM-Based Optimization of Process Parameters in Synthesis of Pentaerythritol Ester of Rapeseed Oil. In: Rajmohan, T., Palanikumar, K., Davim, J.P. (eds) Advances in Materials and Manufacturing Engineering. Springer Proceedings in Materials, vol 7. Springer, Singapore. https://doi.org/10.1007/978-981-15-6267-9_58
Download citation
DOI: https://doi.org/10.1007/978-981-15-6267-9_58
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6266-2
Online ISBN: 978-981-15-6267-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)