Abstract
The present study reports the findings of experiments carried out to optimize super phosphoric acid-catalyzed esterification of palm fatty acid distillate—a low-cost by-product of the palm oil refining process for biodiesel production. The main objectives were to develop an approach that would enable us to better understand the relationships between the governing variables, i.e., oil to methanol ratio, temperature and catalyst concentration, and the response (conversion of the biodiesel), and to obtain the optimum conditions for biodiesel production using central composite design (CCD) and response surface methodology (RSM). All the three variables significantly affected the acid value of the product, oil to methanol ratio being the most effective followed by temperature and catalyst concentration. Using response surface methodology, a quadratic polynomial equation was obtained for acid value by multiple regression analysis. Verification experiments confirmed the validity of the predicted model. Various properties of the biodiesel were evaluated and compared in relation to that of conventional petro-diesel. The prepared biodiesel blended with different proportion of petro-diesel was then subjected to performance and emission tests at varying loads in order to evaluate its actual performance, when used as a diesel engine fuel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biodiesel, a mixture of alkyl esters, is an alternative biomass-based fuel derived from vegetable oils, waste vegetable oils, and animal fats. Technically, biodiesel is fatty acid methyl ester (FAME) formed by replacing the glycerol from each triglyceride molecule of vegetable oil with methyl from methanol (that is by the reaction of free fatty acid and methanol) [1]. The major advantage of biodiesel fuel includes nontoxic preparation from renewable resources, high biodegradability, and low emission profile [2]. Moreover, having excellent lubricity and comparable energy density with petro-diesel, it is compatible with current commercial diesel engines as a feasible energy source [3]. The biodiesel molecules are simple hydrocarbon chains sans sulfur, ring molecules, or aromatics that are otherwise associated with fossil fuels. Biodiesel is made up of almost 10 % oxygen, making it a naturally “oxygenated” fuel [2, 3]. While biodiesel has a myriad of advantages and benefits to its credit, there is a flip side as well. Being renewable and having mass production potential, vegetable oils are widely touted as promising feedstocks for biodiesel production. A plethora of feedstocks including most common vegetable oils (e.g., soybean, cottonseed, palm, peanut, rapeseed/canola, sunflower, safflower, coconut) and animal fats (usually tallow) can be used for the production of biodiesel [4, 5]. The cost of vegetable oil has a crucial role in the economics of the biodiesel. Due to the higher cost of raw vegetable oils, the manufacturing cost of biodiesel is exorbitantly high posing a deterrent towards its commercial viability. Palm oil is one of the most widely used and versatile vegetable oils in the world. During the fatty acid stripping and deodorization stages of the palm oil refining process, a lower-value by-product, known as palm fatty acid distillate (PFAD) is generated. PFAD consists of 85–95 % fatty acids and 5–15 % triglycerides, both of which are available for biodiesel production. A number of works with PFAD as the feedstock for biodiesel are cited in literature using both catalytic [6, 7] and non-catalytic esterification [8, 9]. The price of PFAD is much cheaper than other refined oils which are currently the major feedstocks for most of the biodiesel plants.
On the other hand, a major drawback of homogeneous catalyzed esterification reaction involving strong acid such as sulfuric acid is the high consumption of energy and the separation of the catalysts from the homogeneous reaction mixtures is costly and chemically wasteful [10]. Chances of acid corrosion of the reactors pose additional problems. In comparison, super phosphoric acid is less corrosive since its dissociation constant value is much less. Therefore, the cost of handling, storage, and materials of construction is less as compared to sulfuric acid. In the present work, super phosphoric acid (SPA), which has not been reported earlier in literature, has been used as catalyst. Oil to methanol ratio, temperature, and catalyst concentration in an appropriate range could increase the conversion of biodiesel production during esterification, although much higher values of these parameters could affect adversely as well. Thus, appropriate levels of the above three factors can be chosen to optimize the biodiesel production process. There have been several studies on the optimization of biodiesel production process by one-factor-at-a-time method, but it is laborious and time consuming to perform this operation using this method, which does not depict the interactive effect among the variables. On the contrary, the statistically based experimental method such as response surface methodology (RSM) is a time-saving method and can depict the interactive effect among the variables [11]. Response surface has been applied successfully for optimization of biodiesel production in fat and oil feedstocks having high FFA content, including mahua oil [12], Jatropha oil [13], waste rapeseed oil [14], animal fat [15], and PFAD [16].
Although, various optimization approaches have been used to investigate the production of biodiesel using a number of feedstocks and catalyst; to the best of the knowledge of the authors, this is probably the first report of response surface optimization for super phosphoric acid-catalyzed production of biodiesel using PFAD as feedstock. The focal themes of the present work were to investigate the individual and interactive effects of the important governing variables (PFAD to methanol ratio, temperature, and catalyst concentration) and the response (conversion) and to obtain the optimum conditions for biodiesel production using central composite design (CCD) and response surface methodology (RSM). In addition, the fuel properties and emission characteristics of PFAD–biodiesel were also analyzed to assess its suitability as a fuel in diesel engines.
2 Material and methods
2.1 Chemicals used
PFAD was procured from Gujarat Ambuja Exports Limited, Kadi, Gujarat. It was a light yellow solid at room temperature consisting of <90 wt % FFA. Its purity was 92 wt %, moisture content 0.50 %, and saponification value 263. Super phosphoric acid (density 2.05 g/cm3; boiling point 530 °C) and methanol were purchased from S.D. Fine Chemicals Limited, Baroda, Gujarat, India. All other chemicals used in this study were of AR grade, supplied by Merck, India and were used as received without further purification. Deionized water (resistivity 18 MΩ cm−1 at 25 °C) was used for preparing stock solution.
2.2 Fatty acid profile of the PFAD
The fatty acid profile of the PFAD was determined by gas chromatography (model: GC-2010 Shimadzu, Japan) with flame ionization detector (FID) and capillary column (ZB-5HT Inferno, 15 m × 0.32 mm × 0.10 μm). The initial temperature of 50 °C for 1 min was used; the heating rates were 15 °C/min up to 180, 7 °C/min up to 230, and 10 °C/min up to 380 °C. The carrier gas was high purity hydrogen.
2.3 Experimental details
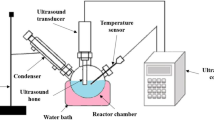
The schematic of the experimental setup is presented in Fig. 1. Esterification of FFA was carried out in a 1-l capacity three-necked round bottom flask equipped with mechanical stirrer, digital temperature controller, dean and stark water condenser, and heating mantle. Different oil to methanol ratios ranging from 1:8 to 1:12 were employed, and the catalyst amount was varied between 5 and 9 % by weight and temperature in the range 50 to 70 °C. The progress of the reaction was monitored by measuring the elimination of the FFAs by way of acid value using American Oil Chemists’ Society Official Method (AOCS Ca 3a–63 for Acid Value, AOCS, 1990 [17]. After completion of reaction, the content was allowed to settle for overnight. The lower layer of methyl ester was separated out for further purification. The separated lower layer was purified by gentle washing with hot distilled water to remove residual catalyst, glycerol, and soaps. The washing process was continued (twice more) until a pH of about 7 was achieved. Finally, the methyl ester phase was distilled to remove the residual water and methanol.
Further, the transesterification of the ester phase was carried out using KOH as catalyst under optimum conditions of catalyst 1 % by weight at 60 °C with oil to methanol ratio of 1:6. The amount of KOH (10 g per liter of pretreated PFAD oil) was calculated at based on the amount needed to neutralize the unreacted acids (i.e., 5 mg KOH/g) in the second stage product plus 0.5 % for catalyst. The reaction was carried out at 60 °C for half an hour. The reaction product was allowed to settle overnight before removing the glycerol layer from the bottom in a separating funnel to get the ester layer on the top, separated as biodiesel.
2.4 Estimation of fuel properties
Laboratory tests were carried out using American Society for Testing and Materials (ASTM) tests standards to determine the properties, namely, density, flash point, pour point, and Conradson carbon residue of biodiesel following ASTM D4052, D56, D97, and D189, respectively [18]. Cetane number of biodiesel was evaluated using a correlation suggested by Mohibbe et al. [19] based on saponification number and iodine value, which again were determined using standard methodologies [18]. The measurement of iodine number was carried out by the iodometric titration method using Wijs solution. Saponification number was also determined titrimetrically following ASTM D5558 method [18]. Kinematic viscosity was measured with a Canon–Fenske capillary viscometer immersed in a constant temperature (40 °C) bath (TAMSON TV 2000) following the European norm EN ISO 3104 [20].
2.5 Exhaust gas analysis of biodiesel blends
Biodiesel blends of different combination were used in a four-stroke water-cooled single cylinder diesel engine (3.67 kW, 1500 rpm, compression ratio: 16.5:1; fuel injection pressure: 185 kg/cm2), and the exhaust gases were analyzed using a Technovation gas analyzer by measuring gas concentrations in the sample cell by using the unique Infra Non-Dispersive-Red (NDIR) absorption spectra of each particular gas of interest. It calculates the amount of energy absorbed as proportional to gas concentration. A beam of infrared energy was passed through the sample cell. The energy at a specific wavelength was determined after the beam exiting from the sample cell, passed through an optical filter, and impinged on a detector.
2.6 Response surface and statistical analysis
A three-factor and three-level central composite design with 20 individual design points was adopted for this study. The method includes a full factorial design with center points that are augmented with a group of star points. As the distance from the center of the design space to a factorial point is defined as ±1 unit for each factor, the distance from the center of the design space to a star point is ±α with |α| = 1.
To avoid bias, 20 runs were performed in a totally random order. The independent variables, or factors studied, were the molar ratio (oil to methanol; A), the temperature (°C; B), and amount of catalyst (% w/w; C). The response or dependent variable (Y) studied was PFAD conversion (%). The effect of these independent variables A, B, and C on the response Y was investigated using the second-order polynomial regression equation with backward elimination (Eq. 1):
where β 0, β i, β ii, and β ij are regression coefficients for intercept, linear, quadratic and interaction terms, respectively. The A, B, and C are uncoded values for independent variables. An analysis of variance (ANOVA) was performed to determine the lack of fit and the effect of linear, quadratic, and interaction terms on conversion. The analysis of data and the optimizing process were generated using Design Expert Version 8 Statease INC, USA. Model verification was carried out using a combination of variables at different levels within the experimental range.
3 Results and discussion
3.1 Fatty acid composition of PFAD
The properties of the triglyceride and the biodiesel fuel are determined by the amounts of each fatty acid that are present in the precursor feedstock. Chain length and number of double bonds determine the physical characteristics of both fatty acids and triglycerides [21]. The chromatogram of the GC analysis of palm fatty acid distillate is given in Fig. 2. The figure indicates the distinct presence of three major and several minor peaks. The major peaks correspond to the presence of palmitic acid (38.79 %), oleic acid (27.83 %), and erucic acid (20.15 %), whereas the minor peaks stand for stearic (2.47 %) and linoleic acid (7.07 %). In general, there are three main types of fatty acids that can be present in a triglyceride: saturated (Cn: 0), monounsaturated (Cn: 1), and polyunsaturated with two or three double bonds (Cn: 2, 3) [22]. The chromatogram in the present study reveals that PFAD predominantly contains saturated palmitic acid (C16:0) and stearic acid (C18:0) and monosaturated oleic acid (C18:1) and erucic acid (C22:1) along with polysaturated linoleic acids (C18:3). The initial acid value, a measure of the FFA content of the oil, was found to be 177.49 mg KOH/g corresponding to FFA level of 89.19 %, which was far above the 1 % limit for satisfactory transesterification reaction using alkaline catalyst. Therefore, FFAs were first converted to esters in a pretreatment process with methanol using super phosphoric acid as an acid catalyst. Transesterification does not alter the fatty acid composition of the feedstock, and this composition plays an important role in some critical parameters of the biodiesel, as cetane number and cold flow properties. This has been discussed little later.
3.2 Response surface analysis
In order to optimize the reaction condition of PFAD biodiesel synthesis, the central composite design was selected with three-level-three-factors: i.e., oil to methanol molar ratio, reaction temperature, and catalyst amount. The coded and uncoded levels of the independent variables are given in Table 1. All 20 of the designed experiments were conducted, and the results were analyzed via multiple regression. The coefficients of a full model were evaluated via regression analysis and tested for significance. Finally, the best fitting model was determined via regression. This showed that three linear coefficients (A, B, C), three quadratic coefficients (A 2, B 2, C 2), and three cross-product coefficients (AB, AC, BC) were significant.
The effect of the variables as linear, quadratic, or interaction coefficients on the response was tested for significance by ANOVA. As shown in Table 2, it can be found that the variable with the most significant effect on the oil conversion was the linear term of molar ratio (p < 0.01) and temperature (p < 0.01), followed by all interaction effect of independent variables (p < 0.01). The quadratic effect of catalyst concentration (p < 0.05) and molar ratio (p < 0.1) are found to be significant as well. The significance of these effects is also verified by the final estimative response model equation (based on the actual value) as:
in which Y is the response factor, conversion of PFAD into biodiesel (%). A, B, and C are the values of the independent factors, oil to methanol molar ratio, reaction temperature (°C), and the amount of catalyst (% (w/w)), respectively. RSM was used to optimize esterification reaction and the experimental results were presented in Table 3. Experimental conversions were analyzed to get a regression model. The estimated coefficients of the regression model given in Table 2 were obtained by employing a least square technique to predict quadratic polynomial model for the conversion. The large value of the coefficient of multiple determination (R 2 = 0.957) reveals that the model adequately represents the experimental results.
3.3 Interactive effect of various parameters
The estimative response model equation proved suitable for the adequate representation of the real relationship among the selected factors. Biodiesel conversion as a function of temperature and oil to methanol molar ratio (at catalyst concentration of 7 % wt), catalyst concentration and temperature (at oil to methanol ratio of 0.1), and oil to methanol ratio and catalyst concentration (at a temperature of 60 °C) are presented in three-dimensional response surface plots of Figs. 3, 4, and 5, respectively. Figure 3 also denotes the reciprocal interactions of the indicated variables. It shows that temperature and molar ratio of oil to methanol have a direct effect on the conversion of methyl ester. At the minimum reaction temperature 50 °C, an increase in oil to methanol molar ratio resulted in the increase in conversion of methyl ester as well. The highest conversion was found out to be 92.6 % at 7 % catalyst concentration and was achieved at the vicinity of boiling point of methanol. However, at low oil to methanol molar ratio, the effect of temperature and oil to methanol molar ratio on conversion of methyl ester was marginal. Most of the reported literature works have focused on the transesterification/esterification at near boiling point of alcohol. Temperature plays a pivotal role on speed of reaction and often leads to higher conversion of ester. The relatively low conversion of methyl ester at low temperature was probably due to the subcritical state of methanol.
A perusal of Fig. 3 indicates that for a particular catalyst quantity in the range 5 to 7 % by wt, the rise in percentage conversion of methyl ester results in a linear increase in reaction temperature. Nevertheless, further increase in concentration beyond 7 % could neither enhance the reaction rate nor the equilibrium conversion. The maximum conversion was found out at a catalyst quantity of 7 %, 70 °C, and 1: 12 methanol to oil molar ratio.
Similar effects of catalyst concentration on the ultrasound-assisted esterification reactions have been observed elsewhere [23, 24]. Plausibly large quantity of acid catalyst could promote ether formation by alcohol dehydration [25]. The presence of polar compounds during acid-catalyzed alcoholysis reactions significantly reduces reaction rates by competing for hydrogen ions, hindering the availability of these ions for catalysis. [25].
After studying the interaction effect of varying catalyst concentration and oil to methanol molar ratio on methyl ester conversion at a constant reaction temperature, it appears that the effect of catalyst concentration on the methanol to oil ratio is rare and the value of interaction coefficient (p > 0.004) demonstrates this fact (data not shown). At low quantity of catalyst, the conversion was slightly affected by oil to methanol molar ratio, whereas at high catalyst amounts, the oil to methanol molar ratio has shown the increasing effect on conversion of methyl ester in PFAD-esterified biodiesel. The optimal reaction temperature, according to catalyst concentration, was between 60 and 70 °C as shown in Figs. 3 and 4. The reaction temperature has shown the significant effect on methyl ester conversion. Of course, high temperature above 65 °C causes the evaporation of methanol. At any defined reaction temperature, with the increase in catalyst quantity, there is linear enhancement of methyl ester conversion. Comparing the results of Figs. 3, 4, and 5, the optimum condition for the maximum conversion of fatty acid methyl ester was achieved with a high reaction temperature and oil to methanol molar ratio, and catalyst quantity of 7–8 % by wt.
It can also be concluded that linear and quadratic effects of A and B are more significant followed by quadratic effect of C as shown in Table 2 and model equation (1). All Eigen values obtained for the analysis were positive (data not shown) indicating that the stationary point for the response was least. The response with respect to the second-order model indicated that, for small catalyst quantity, FFA conversion increases with increasing oil to methanol molar ratio and temperature. Maximum conversion was obtained for large oil to methanol molar ratio, as it was the most significant factor with positive impact. However, at large catalyst quantity, it seems to be less effective if oil to methanol molar ratio increased. This could be due to the positive effects of catalyst to temperature and molar ratio to temperature. Moreover, at low catalyst concentration, there is moderate increase in conversion with temperature and oil to methanol molar ratio.
3.4 Optimization of extraction condition
In order to optimize reaction condition, the first partial derivatives of the regression model were equated to zero according to A, B, and C, respectively. The result was calculated as: A = 0.09, B = 70 °C, and C = 8.99. Under such condition, the conversion of biodiesel was predicted to be 94.3 %. The experimental work at this condition was performed due to maximum experimental conversion predicted. In this work, highest conversion of methyl ester at temperature of 70 °C, catalyst concentration of 8.99 %wt, reaction time of 5 h, and oil to methanol ratio of 1:11 is obtained 96.3 %. By substituting levels of the factors into the regression equation, the maximum predictable response for PFAD biodiesel production was calculated and was experimentally verified. But it merits mentioning that albeit to the less extent, RSM has under predicted the conversion of PFAD biodiesel in the present study. This difference between predicted and experimental conversion can be attributed to the extent of deviation in predictive capacity of the model to reach the global optimum. RSM is most widely used method in optimization of different physicochemical parameters affecting biodiesel production. It is one of the efficient methods for non-linear optimization. But RSM suffers from the limitation that it assumes only quadratic non-linear correlation. Thus, the effective application of RSM should narrow down search window appropriately to enable the linear correlations adequately suitable. This makes the search process highly dependent upon search space. It might require either additional experiments or good priory knowledge of the system to fix search window [26].
3.5 PFAD biodiesel characterization
American Society for Testing and Methods (ASTM) has prescribed certain tests and their limits for diesel fuel to be used in CI engines. For any alternative fuel to be suitable for long-term engine operation without engine modifications, it must be in conformity or within close range to these ASTM permissible limits. Several tests were conducted for various physical, chemical, and thermal properties to characterize PFAD biodiesel obtained in this work and were compared with the American standards and Indian petro-diesel standards. These comparative data are presented in Table 4. The process of esterification brings about a radical change in the density of PFAD as a result; the obtained biodiesel has almost similar density to that of petro-diesel [27]. PFAD biodiesel was miscible in any proportion with that of mineral diesel oil. The optimized blend of biodiesel has density very close of diesel oil. Among the general parameters for biodiesel, viscosity controls the characteristics of the injection from the diesel injector. The viscosity of fatty acid methyl esters is important to control within an acceptable level to avoid negative impacts on fuel injector system performance. The process of esterification and transesterification reduced the viscosity from 10.75 to 5.4 mm2/s. This achievement paved the way to use the produced biodiesel without any engine modifications. Generally, the diesel oil viscosity lies between 3 and 4 mm2/s at 40 °C and it varies depending upon its constituents. Thus, fuel oil, which has viscosity within this range, does not pose any handling problems to the fuel handling systems in the existing diesel engines. The flash point, however, strictly corresponds to the content of methanol, and the viscosity correlates with the content of unreacted triglycerides [28].
Table 4 reveals that PFAD–biodiesel obtained in present study has a higher cetane number than petroleum diesel oil. Even 20 % blend of biodiesel showed improvement in cetane number. It is well known that biodiesel cetane number depends on the feedstock used for its production [29, 30]. According to Knothe et al. [31], high cetane numbers were observed for esters of saturated fatty acids such as palmitic (C16:0) and stearic (C18:0) acids. PFAD sample used in the present study containing about 38.79 % of palmitic acid (although less amount of stearic acid) thus presumably gave higher value of cetane number. Similar results were reported by Van Gerpen [32] observing an increase of the cetane number with increasing the percentage of methyl palmitate in a blend. Flash point of a fuel is the temperature at which it will ignite when exposed to a flame or spark. Fuels with flash point above 66 °C are regarded as safe. The flash point of biodiesel is higher than that of the petro-diesel, which is safe for transport purpose. Thus, biodiesel is an extremely safe fuel to handle compared to diesel oil. Even 20 % biodiesel blend has a flash point much above that of diesel oil, making it a preferable choice as far as safety is concerned. A perusal of the properties indicates that the process could yield biodiesel having comparable properties with the standards. But the pour point of the PFAD biodiesel was a bit higher than that of the standard as the principal components of the PFAD were saturated FFA. However, the pour point could be depressed by blending PFAD biodiesel with diesel in suitable ratios. Therefore, the PFAD biodiesel obtained in the present study has the potential to be an alternative to the petro-diesel for using in unmodified diesel engines.
3.6 Exhaust gas analysis
Exhaust gas analysis was carried out on an engine using diesel and biodiesel blends separately as fuels at 1500 rpm. Emission test was conducted on various biodiesel blends to assess the emission level of CO2, CO, and hydrocarbons (HC). Five different combinations of diesel–biodiesel blends, namely, B-0, B-10, B-20, B-30, and B-50, were used in the present study. The baseline data were generated using unblended petro-diesel. Figure 6 represents percentage emission of CO2 with different blends of PFAD–biodiesel and petro-diesel as a function of engine load. The general trend of Fig. 6 indicates that percentage emission of CO2 increased with the increase in engine load for all the combinations of biodiesel blends as well as unblended petro-diesel. However, the magnitude of CO2 emission was appreciably less in all blends tested compared to the unblended petro-diesel regardless of engine load. Among all the blends, B-30 had the least percent emission of CO2 in the exhaust gas. CO2 emissions of B-10 were close to petro-diesel. On the other hand, no significant trend could be observed in the emission profile of CO, which largely remained same for most of the blends, although there was marginal increase in CO content of B-10 blend (data not shown). Factors causing combustion deterioration (such as high latent heat of evaporation) could be responsible for the increased CO emission. CO emission increases gradually with blending of higher concentration of biodiesel to diesel. This may be due to increase in viscosity with blending leading to less homogenous mixtures [33]. However, this observation was not entirely consistent with the present experiments. CO concentration in the exhaust is a measure of the combustion efficiency of the system. Normally, better combustion can be achieved at a medium speed and with a medium-sized load and the presence of oxygen in the biodiesel might have resulted in complete combustion [34]. It is worth mentioning that the impact of diesel–biodiesel blends on CO emissions varies with engine operating conditions and was not conclusive.
Percentage emission of CO2 with different blends of PFAD–biodiesel and petro-diesel as a function of engine load (B-10: a mixture of 10 % biodiesel and 90 % petro-diesel; B-20: a mixture of 20 % biodiesel and 80 % petro-diesel; B-30: a mixture of 30 % biodiesel and 70 % petro-diesel; B-50: a mixture of 50 % biodiesel and 50 % petro-diesel)
Figure 7 presents emission profile of hydrocarbons with different blends of PFAD–biodiesel and petro-diesel as a function of engine load. It reveals that there was considerable reduction of hydrocarbon emission for all the blends compared to unblended petro-diesel. At the highest engine load (3 kw) under the present experimental conditions, the maximum reduction of hydrocarbon was roughly 66 % for B-50, 50 % for B-30, and 44 % for B-20. Biodiesel contains oxygen in its structure. When biodiesel is added to diesel fuel, the oxygen content of fuel blend is increased; thus, less oxygen is needed for combustion. The reduction in HC is mainly due to the result of improved combustion of biodiesel blends within the combustion period due to the presence of excess oxygen atom in biodiesel [33]. Hydrocarbon (HC) emissions generally result from unburned fuel. HC formation is attributed to fuel/air mixtures that are too lean to auto-ignite or to support a propagating flame or attributed to fuel/air mixtures that are too rich to auto-ignite. The long carbon chains and the absence of aromatic content make cetane number of biodiesel higher than that of petro-diesel. Thus, the blends improve the overall cetane value, promoting complete combustion and reducing the level of unburned fuel [35]. Although there are considerable emissions, benefits for certain gases can be realized but a small NOx increased was measured in some studies. Although engine modifications were not necessary, some researchers have reported that engine optimum calibrations lowered biodiesel emissions, especially NOx emissions. However, the NOx emissions could not be carried out with the present experimental setup.
Emission of hydrocarbons with different blends of PFAD–biodiesel and petro-diesel as a function of engine load (B-10: a mixture of 10 % biodiesel and 90 % petro-diesel; B-20: a mixture of 20 % biodiesel and 80 % petro-diesel; B-30: a mixture of 30 % biodiesel and 70 % petro-diesel; B-50: a mixture of 50 % biodiesel and 50 % petro-diesel)
4 Conclusion
The high FFA level of PFAD could be reduced to the acceptable value by its pretreatment with methanol (0.08 molar ratio) using super phosphoric acid as catalyst (7 % w/w) at 70 °C temperature. Response surface methodology was successfully applied for esterification of PFAD. A second-order model was obtained to predict acid value as a function of methanol to oil ratio, catalyst concentration, and temperature. The high regression coefficients of the second-order polynomial showed that the model was well fitted to the experimental data. The ANOVA test implied that molar ratio of oil to alcohol, reaction temperature, and concentration of catalyst had the great significant factor affecting the conversion of biodiesel. The biodiesel production had a negative quadratic behavior by temperature, molar ratio of oil to alcohol, and concentration of catalyst. The methyl ester which produced at optimum conditions had acceptable fuel properties and compared well with petro-diesel. It had lower carbon residue and acid number than petro-diesel, but kinematic viscosity, cetane number, and flash point of biodiesel were higher as compared to petro-diesel. Analysis of exhaust gas from engine with different blends of PFAD–biodiesel and petro-diesel showed appreciable reduction of CO2 and hydrocarbons as against the unblended petro-diesel; however, reduction of CO was marginal. Production of biofuels from edible oils could result in inevitable displacement of food crops, leading to possible food scarcity and inflation of food prices. In line with the argument, palm fatty acid distillates, from palm kernel oil refinery, and methanol used in this work are not edible, enabling them better raw materials to be used for biodiesel production to avoid soaring of food prices.
References
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresource Technol 70:1–15
Bozbas K (2008) Biodiesel as an alternative motor fuel: production and policies in the European Union. Renew Sustain Ener Rev 12:542–552
Zhang X, Yan S, Tyagi RD, Rao YS (2013) Energy balance and greenhouse gas emissions of biodiesel production from oil derived from wastewater and wastewater sludge. Renew Energy 55:392–403
Dennis YC, Leung XW, Leung MKH (2010) A review on biodiesel production using catalyzed transesterification. Appl Energy 87:1083–1095
Canakci M, Sanli M (2008) Biodiesel production from various feedstocks and their effects on the fuel properties. J Industrial Microbiol Biotechnol 35:431–441
Chongkhong S, Tongurai C, Chetpattananondh P, Bunyakan C (2007) Biodiesel production by esterification of palm fatty acid distillate. Biomass Bioenergy 31:563–568
Mongkolbovornkij P, Champreda V, Sutthisripok W, Laosiripojana N (2010) Esterification of industrial-grade palm fatty acid distillate over modified ZrO2 (with WO3−, SO4 2− and TiO2 −): effects of co-solvent adding and water removal. Fuel Process Technol 91:1510–1516
Cho HJ, Kim SH, Hong SW, Yeo YK (2012) A single step non-catalytic esterification of palm fatty acid distillate (PFAD) for biodiesel production. Fuel 93:373–380
Yujaroen D, Goto M, Sasaki M, Shotipruk A (2009) Esterification of palm fatty acid distillate (PFAD) in supercritical methanol: effect of hydrolysis on reaction activity. Fuel 88:2011–2016
Toda M, Takagaki A, Okamura M, Kondo JN (2005) Green chemistry: biodiesel made with sugar catalyst. Nature 438:178
Kalil SJ, Maugeri F, Rodrigues MI (2000) Response surface analysis and simulation as a tool for bioprocess design and optimization. Process Biochem 35:539–550
Ghadge SV, Raheman H (2006) Process optimization for biodiesel production from mahua (Madhuca indica) oil using response surface methodology. Bioresource Technol 97:379–384
Tiwari AK, Kumar A, Raheman H (2007) Biodiesel production from Jatropha oil with high free fatty acids: an optimized process. Biomass Bioenergy 31:569–575
Yuan X, Lui J, Zeng G, Shi J, Tong J (2008) Optimization of conversion of waste rapeseed oil with high FFA to biodiesel using response surface methodology. Renew Energy 33:1678–1684
Jeong GT, Yan HS, Park DH (2009) Optimization of transesterification of animal fat ester using response surface methodology. Bioresource Technol 100:25–30
Boey PL, Ganesan S, Maniam GP, Khairuddean M, Efendi J (2013) A new heterogeneous acid catalyst for esterification: optimization using response surface methodology. Energy Conver Manag 65:392–396
American Oil Chemists’ Society (AOCS) (1990) Official methods and recommended practices of the American Oil Chemists’ Society, 1990th edn. AOCS Press, Champaign
ASTM (2003) Standard specification for biodiesel fuel (B100) blend stock for distillate fuels. In annual book of ASTM standards, 1131–1136. American Society for Testing and Materials, West Conshohocken
Mohibbe Azam M, Waris A, Nahar NM (2005) Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy 29:293–302
UNE-EN ISO 3104, (1994) Petroleum products. Transparent and opaque liquids. Determination of kinematic viscosity and calculation of dynamic viscosity
Mittelbach M, Remschmidt C (2004) Biodiesel: the comprehensive handbook. Boersedruck Ges, M.B.H. Vienna
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresource Technol 100:261–268
Kelkar MA, Gogate PR, Pandit AB (2008) Intensification of esterification of acids for synthesis of biodiesel using acoustic and hydrodynamic cavitation. Ultrason Sonochem 15:188–194
Wen B, Eli W, Xue Q, Dong X, Liu W (2007) Ultrasound accelerated esterification of palmitic acid with vitamin C. Ultrasonic Sonochem 14:213–218
Lotero E, Liu Y, Lpez DE, Suwannakarn K, Bruce DA, Goodwin JG Jr (2005) Synthesis of biodiesel via acid catalysis. Ind Eng Chem Res 44:5353–5363
Desai KM, Survase SA, Saudagar PS, Lele SS, Singhal RS (2008) Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: case study of fermentative production of scleroglucan. Biochem Eng J 41:266–273
Agarwal AK, Das LM (2001) Biodiesel development and characterization for use as a fuel in compression ignition engines. J Eng Gas Tur Power 123:441–447
Ramadhas AS, Jayaraj S, Muraleedharan C (2005) Biodiesel production from high FFA rubber seed oil. Fuel 84:335–340
Meher LC, Vidya Sagar D, Naik SN (2006) Technical aspects of biodiesel production by transesterification—a review. Renew Sust Energ Rev 10:248–268
Bajpai D, Tyagi VK (2006) Biodiesel: source, production, composition, properties and its benefits. J Oleo Sci 55:487–502
Knothe G, Matheaus AC, Ryan TW III (2003) Cetane numbers of branched and straight chain fatty esters determined in an ignition quality tester. Fuel 82:971–975
Van Gerpen JH (1996) Cetane number testing of biodiesel. Liquid fuels and industrial products from renewable resources. In: Proceedings of the Third Liquid Fuel Conference, 1996, 15–17 September, Nashville, Tennessee
Sahoo B, Sahoo N, Saha UK (2009) Effect of engine parameters and type of gaseous fuel on the performance of dual fuel gas engines—a critical review. Renew Sustain Energy Rev 13:1151–1184
Nwafor OMI (2004) Emission characteristics of diesel engine running on vegetable oil with elevated fuel inlet temperature. Biomass Bioenergy 27:507–511
Wang WG, Lyons DW, Clark NN, Gautam M, Norton PM (2000) Emissions from nine heavy trucks fueled by diesel and biodiesel blend without engine modification. Environ Sci Technol 34:933–939
Acknowledgments
The authors are thankful to the Sophisticated Instrumentation Centre for Applied Research and Testing (SICART), Vallabh Vidyanagar, Gujarat, for their valuable help to conduct certain analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Metre, A., Nath, K. Process optimization of super phosphoric acid-catalyzed esterification of palm fatty acid distillate using response surface methodology. Biomass Conv. Bioref. 5, 397–407 (2015). https://doi.org/10.1007/s13399-014-0157-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-014-0157-z