Abstract
B cells play a multidimensional role in host immunity. Regulatory B (Breg) cells are a class of B lymphocytes with immunomodulatory properties that play an important role in maintaining immunological tolerance along with dampening harmful immune responses. Bregs suppress various immune pathologies through the production of interleukin (IL)-10, IL-35, and transforming growth factor-β (TGF-β). They act by inhibition of T helper 1 (Th1) and Th17 cells proliferation, suppression of dendritic cell (DC), differentiation and simultaneous enhancement of the expression and differentiation of fork head transcription factor P3-positive regulatory T cells (FoxP3+ Tregs). In this chapter, we discuss the induction, function, and phenotypes of the various Breg cell subsets defined in both mice and humans along with their proposed mechanism of action in various immune responses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Regulatory B cells (Bregs)
- Plasma Bregs
- BR2 Bregs
- B10 Bregs
- T2-MZP Bregs
- TIM1+ Bregs
- B1 B cells
- Br1 Bregs
- Plasmablast
- iBregs
- IgA+ Bregs
- GrB+Bregs
5.1 Discovery of Breg Cells
The concept of B cells regulating immune responses dates back to 1974, when the suppressive nature of B cells in modulating delayed type hypersensitivity in guinea pigs was described [1]. Wolf et al. suggested a regulatory subset of B cells (Bregs) exhibiting immunomodulatory properties in an experimental autoimmune encephalomyelitis (EAE) model of mice in 1996 [2]. From 2002 to 2003, Fillatreau et al., Mizoguchi et al., and Mauri et al. through independent studies demonstrated that B cells produce IL-10 and suppress inflammatory conditions such as EAE, inflammatory bowel disease and collagen-induced arthritis respectively [3,4,5]. Further, Parekh et al. were the first to show a IL-10-independent mechanism of action in 2003, demonstrating TGF-β-dependent B cell–mediated regulation of CD8+ T cell responses, though they did not name these as Bregs at the time [6]. It was only after 3 years that Mizoguchi and Bhan proposed the concept of Bregs while studying their role in colitis, demonstrating that B cell–deficient mice experienced higher severity of colitis than normal [7]. Moreover, Mizoguchi et al. also established that a specific B cell subset induced in gut-associated lymphoid tissue was secreting higher levels of IL-10 and had increased CD1d expression during intestinal inflammatory condition [4]. Till date, numerous studies have been carried out to illustrate the role of various Breg subsets via IL-10-dependent or IL-10-independent manner in modulating host immunity. In 2008, Yanaba et al. also showed the role of CD1dhiCD5+ cells in negatively regulating T-cell responses through IL-10 in contact hypersensitivity model [8]. Dittel et al. observed that mice with B cell deficiency have reduced numbers of both Foxp3+ regulatory T cells (Tregs) and IL-10 levels in EAE and demonstrated a novel IL-10, B7, and MHC class II-independent regulatory role for B cells in suppressing autoimmunity by the maintenance of Tregs via glucocorticoid-induced TNFR family–related gene ligands [9, 10]. In 2010, Amu et al. reported that helminths-induced Bregs were responsible for Treg induction that could suppress allergic airway inflammation (AAI) in the murine model [11]. Carter et al. demonstrated the unique ability of Bregs in inhibiting Th1/Th17 cells during arthritic conditions in mice [12]. Strikingly, the regulatory function of B cells is mediated by the production of various regulatory cytokines such as IL-10, IL-35, and TGF-β1, which are responsible for suppressing autoreactive B cells and pathogenic T cells in a cytokine or cell-cell contact-dependent manner [7, 13]. Another mechanism of immune regulation by B cells involve expression of FAS ligand on CD5+ B cells, known as killer B cells that regulate effector immune responses by inducing cell death [14]. Kaku et al. showed a population of B cells that express both CD73 and CD39, ectoenzymes responsible for the production of adenosine, which inhibited the severity of colitis [15]. Khan et al. described additional phenotype of Bregs, PD-L1hi B cells, which regulate humoral immunity through their interaction with CD4+CXCR5+PD-1+ follicular helper T cells and ameliorate EAE [16]. Recently, Oleinika et al. reported a novel role of CD1d+ T2-MZP Bregs in the induction of immunosuppressive iNKT cells that downregulate excessive Th1/Th17 responses partially via secreting IFN-γ and limit inflammation in experimental arthritis [17]. Together, these studies indicate that Bregs suppress inflammation by inhibiting the differentiation of pro-inflammatory cells and inducing a population of immunosuppressive cells. In addition, studies on exacerbation of colitis and development of psoriasis in patients treated with anti-CD20 mAb (rituximab) suggest the regulatory function of B cells in human subjects [18, 19]. Bregs constitute fewer than 10% of immature B cells in healthy individuals and play an important role in functioning of the immune system by maintaining tolerance and immune homeostasis [20]. Over the last decade, numerous studies in both mice and human have extensively shown the importance of Bregs in regulating various diseases, including inflammatory disorders, autoimmunity, and cancer [21, 22] Bregs with their wide range of immunomodulatory functions can thus be exploited for therapy in various B cell–mediated diseases. Thus, it is important to exhaustively consider the known Breg cell phenotypes, their induction, and function in a chronological manner (Fig. 5.1 and Table 5.1).
5.2 Identification and Phenotypes of Breg Cells
B cell subsets with strong immunomodulatory functions have been reported both in vitro and in vivo (Figs. 5.2 and 5.3) (Table 5.1). Phenotypic identification of Breg cells using the immunomodulatory cytokine IL-10 continues to be a matter of debate due to difficulties in assessing the functionality of Bregs, because IL-10 detection requires intracellular staining. Therefore, other surrogate markers have been employed to identify various Breg subsets. Different overlapping markers are presently being used to describe these cells. Here we discuss both murine and human Breg subsets under separate heads for clarity and distinction among these subsets.
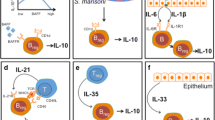
Breg subsets in mice and humans. Mice have a total of five defined Breg subsets: Plasma B cells (CD138+MHC-11lo B220+), B1 Bregs (CD5+), BR2 Bregs (CD40+TGFβ1), B10 Bregs (CD19hiCD1dhiCD5+), and T2-MZP Bregs (CD19+CD21hiCD23hiCD24hi). Humans, on the contrary, have seven defined human Breg subsets: Br1 Bregs (CD19+CD25+CD71+ CD73−), CD19+CD24hiCD38hi Bregs, CD19+CD24hiCD27hi Bregs, Plasmablasts (CD19+ CD27intCD38+), iBregs (IDO, TGFβ), GrB+Bregs (CD19 +CD38+CD1d+IgM+CD147+), and IGA+Bregs (IgA+)
Regulatory mechanisms of Bregs in various immune responses. Bregs lead to the suppression and inhibition of pro-inflammatory lymphocytes such as Th1, Th17, cytotoxic CD8+ T cells, monocytes, and IL-12-producing dendritic cells through the production of various factors like IL-10, IL-35, TGF-β, IDO, GZB, and so on. IL-10 production by Bregs is primarily responsible for restoring the Th1/Th2 balance, where it is shifted toward Th2. One more mechanism of inhibiting inflammatory cascades is via tweaking the Treg/Th17 balance, leading to suppression of Th17 cells. The Breg population is reportedly responsible for enhancing the differentiation of Foxp3+Treg cells and helps in the maintenance of iNKT cells
5.2.1 Mouse Breg Subsets
In mice, Plasma B cells, B-1 cells, CD5+CD1dhi B10 B cells, CD21hiCD23hiCD24hi transitional type 2 marginal zone precursors (T2-MZP) Breg cells, and TIM-1+ B cells have been proposed with regulatory functions in a variety of infections, in autoimmune and transplantation settings [21, 23]. IL-10+ Bregs have also been observed to inhibit IFN-γ production in hepatitis B virus (HBV) infection by modulating CD8+ T cell responses [24, 25]. Furthermore, IL-10+ Bregs inhibit TNF-α production by activated monocytes following stimulation with LPS and bacterial CpG DNA [9, 22]. Bacterial components such as LPS and CpG are known to induce the expansion, differentiation, and activation of murine Bregs through TLR signaling in vitro [26, 27]. Furthermore, mice harboring TLR2- or TLR4-deficient B cells fail to recover from EAE. Alltogether these studies clearly indicate that inflammation acts as stimuli for the activation and differentiation of Bregs.
5.2.1.1 Plasma Bregs
Plasma B cells are representative antibody-secreting cells (ASCs) [28] present in all lymphoid organs. Plasma cells have also been found to occur in significant numbers in the bone marrow compared to their lower numbers in the spleen. Indeed, the bone marrow is primarily responsible for the long-term maintenance of plasma cells arising from immunization [29]. Recently, Lino et al. described a subset of resident Plasma B cells specialized for producing IL-10 upon TLR stimulation and are found to occur naturally, i.e., prior to antigenic challenge [30]. Genome-wide approaches have shown that this Breg lineage is triple-positive for the following markers: IL-10+LAG-3+CD138hi. The lymphocyte activation gene 3 (LAG-3+) helps in regulating humoral immunity and in maintaining immunological tolerance toward endogenous T-independent type 2 antigens, which are normally not detected by CD4+Foxp3+ T regulatory cells. Unlike conventional plasma cell differentiation, which requires several days for proliferation, the detection of IL-10+LAG-3+CD138hi plasma cells at day one post-infection with Salmonella typhimurium in the spleen of mice, confirmed that this subset is derived from already existing cells LAG-3+CD138hi cells. These LAG-3+CD138hi cells are likely induced by self-antigen and remain in a quiescent state. Further, genome-wide methylome, transcriptome, and gene-set enrichment analysis of LAG-3+CD138hi cells in naïve mice and at day one post-Salmonella infection showed that after antigenic challenge, LAG-3+CD138hi cells express IL-10 and become IL-10+LAG-3+CD138hi plasma Bregs [30]. Thus, these results indicate that plasma Bregs provide a first layer of immune regulation in response to stimuli. In contrast, Matsumoto et al. showed that mice lacking genes such as Prdm1 and IRF4, which are required for plasma cell differentiation, develop a severe form of EAE compared to control mice. This study suggested that Bregs are inducible in nature. Thus, these studies clearly establish both the innate and inducible nature of Bregs. During EAE, plasma B cells are known to be the main source of IL-35 and facilitate recovery from EAE. IL-35 secreted by plasma Bregs exhibits anti-inflammatory properties by expanding the immunosuppressive CD4+CD25+ Tregs population which inhibits CD4+CD25− T effector cell proliferation when cultured in vitro [31]. IL-35 also inhibits the differentiation of inflammatory Th17 cells. Recent studies have indicated the role of BATF/IRF-4/IRF-8 axis in regulating IL-35 and IL-10 expression in activated B cells [32]. IL-35 cytokine can act as a potential target in the treatment of both autoimmune and inflammatory conditions. Interestingly, declined populations of LAG-3+CD138hi cells have been reported in mice deficient in CD19 or Bruton’s tyrosine kinase [33], further establishing that differentiation of LAG-3+CD138hi cells to plasma cells is under the control of BCR. Taken together, these studies establish that B cell differentiation into LAG-3+CD138hi cells is a steady-state process driven primarily by BCR signaling rather than TLR-mediated signaling or T cells.
5.2.1.2 B1 Bregs
B-1 cells represent a class of innate immune cells that are responsible for higher antibody production, especially IgMs for mounting rapid immune responses against pathogens [34]. This subset of CD5+ B cells was initially identified in the early 90s in mice, as a set of distinctive fetal B cells to differentiate them from B-2 cells that usually develop in the adult bone marrow [35, 36]. B-1 cells represent a population of B cells found predominantly in the pleural and peritoneal cavities (35–70%). A smaller number of B-1 cells are also found in the spleen [37], bone marrow, mucosal sites, lymph nodes, and blood [38]. Despite their very low frequency in lymphoid tissues, B-1 cells are important regulators of immune defense and tissue homeostasis. B-1 B cells are chiefly produced in the absence of any antigen exposure [39, 40] and are a major source (>80%) of naturally occurring antibodies [41]. Higher levels of natural IgMs are produced by B-1 cells residing in the spleen and bone marrow [38]. These polyreactive [42, 43] antibodies help in recognizing self as well as foreign antigens [44, 45], act as the first line of defense, and are analogously linked to innate immune responses. B-1 cells are categorized into different functioning sub-subsets based on the relative CD5 expression. B-1a represents a class of CD5+(Ly-1) B-1 cells that chiefly express IL-10 upon innate activation [46] whereas B-1b represents a class of CD5− B-1 cells [34, 45]. B-1a cells are major producers of B-cell-derived IL-10 [46], and their activation and expansion are regulated by cross-regulatory cytokines such as IL-12 and IFN-γ [47]. Using Schistosomal infection model, Vellupillai P et al. demonstrated that the outgrowth of IL-10 producing B-1 after infection is genetically restricted and regulated by polylactosamine sugars. Interestingly, it has also been shown that B-cell defect in BALB.Xid mice impart susceptibility to develop filariosis and is associated with lack of antibody production and IL-10 production in response to dominant surface molecule of invading pathogen [48]. B-1a cells were shown to inhibit TLR-mediated excessive inflammation in neonatal mice in an IL-10-dependent manner [49]. Another subset of B-1a, FAS ligand expressing B-1a cells also known as killer B cells, has been shown to mediate T cell apoptosis during schistosomal infection and prevent granulomatous inflammation [14]. Interestingly, the regulatory role of IgM-producing B-1a cells has also been associated with the suppression of colitis in mice that were kept in conventional facility as compared to mice kept under specific pathogen free facility [50]. Thus, B-1a cells play an important role in immune regulation and tissue homeostasis.
5.2.1.3 BR2 (mTGFβ+) Bregs
Here, we propose a novel subset of Bregs called “BR2” Bregs. These Bregs were first reported and studied by Parekh et al. in 2003. They found that B cells activated via T-independent mechanisms such as LPS showed membrane expression of TGFβ1, leading to CD8+ T cell anergy. These Bregs thus have the unique phenotype of mTGFβ+ Bregs. This manner of B cell activation is a major factor influencing CD8+ T cell responses as T-dependent activated B cells provide higher stimulatory properties to CD8+ T cells [6]. Membrane expression of TGFβ1 was found to be solely responsible for conferring these B cells with regulatory properties, thus influencing CD8+ T cell responses. Thus, we now name these Bregs as BR2 (mTGFβ+Bregs), with regulatory properties governed by membrane TGFβ expression. These findings provide insights into the immune evasion strategies adopted by retroviruses and gram-negative bacteria that target toll-like receptor-4 (TLR-4) signaling in B cells. Recent reports have also shown that Bregs producing TGF-β induce Tregs for promoting transplantation tolerance [51]. These results illuminate the importance of novel modes of B-cell activation in the development of therapeutic strategies to modulate the balance between active immunity and tolerance [6].
5.2.1.4 B10 Bregs
B10 cells are defined by their ability to express IL-10 following ex vivo stimulation with PMA and ionomycin and are enriched within CD1dhiCD5+ B cell subset [8]. Mouse B10 cells represent around 1–3% of cells in the spleen. Other tissues like the lymph nodes, central nervous system, Peyer’s patches, and intestinal tissues comprise a very small number of B10 cells. Their presence in peritoneal cavity is also prominent [29, 52, 53]. Mouse B10 cells have a typical phenotype as IgDloIgMhi cells, although a very small number of B10 cells are also reported to co-express IgA or IgG [54]. B10 cells secrete polyreactive or Ag-specific IgMs and IgGs upon differentiation [53, 54]. T-cell Ig mucin domain-1 (TIM-1) is a type of transmembrane glycoprotein responsible for immunomodulatory responses [55], and its expression was found to be important for the induction and maintenance of IL-10-producing B cells, whereas a defect in TIM-1 expression leads to increased production of proinflammatory cytokines such as IL-1 and IL-6 [56]. During allotransplantation, TIM-1 is particularly responsible for Breg stimulation to prolong allograft survival. TIM-1+ B cells usually express IL-4 and IL-10 and promote Th2 responses with subsequent allograft tolerance [57]. Numerous studies have shown the potential of B10 cells in inhibiting disease initiation and subsequent pathology after their adoptive transfer in models of contact hypersensitivity [8], EAE [3, 52, 58], lupus [59], IBD [53, 60], and graft-versus-host disease [61]. Mauri et al. were the first to elucidate the therapeutic potential of B cells using agonistic CD40 mAbs for treating mice with collagen-induced arthritis [5, 62]. Depletion of B10 cells can have either therapeutic or detrimental effects in the course of various human pathological mouse models. Depletion of IL-10-producing B cells is known to enhance the innate, humoral, and cellular immune responses in mice [62, 63]. This intensifies the severity of disease-related symptoms in various autoimmune diseases in mice such as EAE, skin transplant rejection, and contact hypersensitivity [27, 58, 64].
5.2.1.5 T2-MZP Bregs
The T2-MZP Breg cell subset was discovered by Evans et al. in 2007 [65]. T2-MZP Bregs are immature transitional B cells found in the spleen with a CD19+CD21hiCD23hiCD24hiIgMhiIgDhiCD1dhi phenotype. Among the different B-cell subsets residing in the spleen of mice with arthritis, this specific Breg cell type is responsible for IL-10 production after collagen stimulation. T2-MZP Bregs were discovered to have decisive suppressing properties both in vitro and in vivo, and the mechanism of suppression includes inhibition of pathogenic Th1 responses via producing IL-10 [65]. IL-10-producing T2-MZP B cells are shown to exert immunomodulatory properties in various immune-mediated pathologies, including autoimmune diseases, cancer, and allergy [21, 65, 66]. Recently, Oleinika et al. reported a novel role of CD1d+ T2-MZP Bregs in the induction of immunosuppressive in-variant Natural Killer T (iNKT)-cells that downregulate excessive Th1/Th17 responses partially via secreting IFN-γ and limit inflammation in experimental arthritis [17]. Recently, T2-MZP Breg cells have been linked as the precursors of B10 Bregs, but the interrelation between these two Breg subsets needs to be further established [21].
5.2.2 Human Breg Subsets
Similar to mouse Bregs, human Breg cells also play an important role in the maintenance of tissue homeostasis. Mauri et al. in an extensive study demonstrated that CD19+CD24hiCD38hi B cells with a phenotype very similar to immature B cells produce the highest fraction of IL-10 in healthy human peripheral blood upon CD40 stimulation [20]. Separately, Tedder et al. also categorized human Breg cells as CD24hiCD27+, a phenotype related to memory B cells [22]. Furthermore, Bosma et al. reported that due to altered CD1d recycling in B cells, defect in B-cell-mediated iNKT expansion was observed in SLE patients [67]. Human Bregs exert immunomodulatory properties through their actions on various immune cell types such as inhibiting cytokine production in monocytes [22]; inducing immunosuppressive NKT cells [67], restraining IFN-α production from pDCs [68]; and regulating CD4+ T cell proliferation [69], inhibition of Th1 and Th17 differentiation, and conversion of CD4+ T-cells into CD4+CD25+ cells along with enhancing FOXP3 and PD-1 expression on Tregs [20, 70, 71]. In humans, research on Bregs is mainly restricted due to lack of access to the human spleen, the primary site of the Bregs population. Thus, the majority of identified human Bregs are from peripheral blood where Bregs ranging from immature B cells to differentiated plasmablasts are found. Other phenotypes of human Bregs comprise CD19+CD25+CD71+CD73− B regulatory 1 (Br1) cells [72], CD19+CD27intCD38+ plasmablasts [73]. Furthermore, human Bregs (i.e., equivalent to B10 of mice) with the CD19+CD24hiCD27+ phenotype along with Tim1+ Bregs are preferentially found in the transitional B cells [22, 74]. Thus, it is important to describe different defined subsets of human Bregs.
5.2.2.1 CD19+CD24hiCD38hi Bregs
Human B cells with regulatory function have been described in CD19+CD24hiCD38hi immature subset of peripheral blood B cells. After CD40 stimulation, this subpopulation isolated from peripheral blood of healthy individuals is known to inhibit the differentiation of Th1 cells via IL-10 production and CD80 and CD86 engagement [20]. However, CD24hiCD38hi cells isolated from SLE patients lacked regulatory capacity [20]. Recently, in patients with SLE, an expanded population of CD19+CD24hiCD38hi Bregs was observed with deficient IL-10R expression, which is correlated with compromised Breg function despite showing enhanced IL-10 expression [75]. Thus, targeting the ‘Bregs/IL-10/IL-10R’ axis may prove to be a novel therapeutic approach in the treatment of SLE. In addition to inhibiting Th1 and Th17 differentiation, these cells also convert CD4+CD25− into Tregs [70]. Both numerical and functional impairment has been observed in a number of autoimmune diseases such as SLE [20, 75] and RA [70]. Recent studies showing reduced capacity of CD19+CD24hiCD38hi Bregs to secrete IL-10 in GVHD patients as compared to transplant tolerant and healthy controls indicated their important role in preventing graft rejection by promoting tolerance. Moreover, Cherukuri et al. in 2014 found low IL-10/TNF-α ratio by CD19+CD24hiCD38hi transitional B cells in renal patients with graft rejection when compared with healthy controls, further highlighting their role in establishing transplant tolerance [76] TIM-1 is also a marker for IL-10+ Bregs and around 50% of IL-10+ B cells were TIM-1+. On evaluating TIM-1 expression on human B cell subsets, this transitional subset was enriched in TIM-1+ subset [74]. In the same study, authors found a decreased number as well as impaired function of TIM-1+ in patients with systemic sclerosis [74]. In 2015, Kristensen et al. stated that in humans, 40% of IL-10+ B10 cells expressed TIM-1 [77]. Supporting this study, Liu et al. found that compared to HIV-infected patients, healthy controls have more than 75% of peripheral B10 cells expressing TIM-1. These studies highlight the role of TIM-1 as a marker of Bregs and will open new avenues for the isolation of Bregs that could be utilized for achieving immune homeostasis.
5.2.2.2 CD19+CD24hiCD27hi Bregs
The IL-10-producing B cells, named B10 in humans, are predominantly CD19+CD24hiCD27+ memory subset of B cells, known to be a major source of IL-10 after stimulation with LPS or CpG along with CD40 ligation B cells. B10 cells also express CD48, and CD148 [22]. IL-21 has the potential to further induce IL-10 production from CpG- or LPS-treated CD19+CD27+ memory B10 cells [78]. Among other subsets, B10 cells are also present in the tonsils, spleen, and newborn cord blood [76]. Interestingly, an increase in the number of B10 cells was observed in a number of autoimmune diseases [22, 79, 80]. In patients with RA, B10 cells are highly capable of expressing receptor activator of nuclear factor-κB ligand (RANKL) compared to those in the healthy controls, suggesting a possible mechanism by which B10 cells are involved in RA pathogenesis [81]. At the molecular level, Zheng et al. in 2017 reported that microRNA-155 (miR-155) positively regulates IL-10 expression in B10 cells, which is impaired in patients with Crohn’s disease (CD), leading to miR-155-induced expression of TNF-α by monocytes. These findings further suggest a novel miRNA-mediated approach in developing Breg-based strategies to control the progression of autoimmune diseases.
5.2.2.3 Br1 Bregs
This subset of human Bregs with the CD19+CD25+CD71+CD73− phenotype was identified by Van de Veen et al. in 2013. These IL-10-producing Br1 Bregs share homology with the Tr1 subtype of T cells. Due to the low CD73 expression on their surface, the immunosuppressive function of Br1 cells was considered to be independent of adenosine and could thus be IL-10 dependent. In support of this, further studies substantiated the role of IL-10 in imparting immunosuppressive functions to Br1 cells. This IL-10+ subset of Bregs is reported to induce tolerance toward allergens by repressing the proliferation of allergen-specific CD4+ T cells as well as by producing allergen-specific anti-inflammatory IgG4 antibodies [72], thus contributing to peripheral tolerance. This subset of Bregs can induce tolerance against bee venom allergen and PLA2 (phospholipase A2) in an IL-10-dependent manner and also showed tolerance toward various food allergens like casein (cow milk protein). Van de Veen et al. used flow cytometry and whole-genome sequencing to further show that human Br1 cells express the inhibitory ligand PD-L1 (programmed death ligand-1), which binds PD-1 on T cells to inhibit T cell activation and promote the maintenance of Tregs cells.
5.2.2.4 Plasmablasts
This subset of Bregs is known to be derived from both naïve and immature B cells in humans with the CD19+CD27intCD38+phenotype, which secretes IL-10 [73]. In the presence of IL-2, IL-6, CpG, and IFN-α, immature B cells undergo differentiation, leading to expansion of plasmablasts with increased expression of IRF4, Blimp1, and XBP1 [73]. In normal tissues, CD30 expression is limited to a few T and B cells, whereas in B cell lymphoma, CD30 expression is upregulated on B cells. Recently, in a mouse model of B cell lymphoma, higher CD30 expression on B cells was found to promote the differentiation of plasma B cells to plasmablasts via NF-κB activation and enhanced phosphorylation of STAT3, STAT6, and nuclear factor IRF4 [82]. Interestingly, exacerbation of inflammatory symptoms in MS patients upon treatment with Atacicept, which deplete antibody-secreting cells, further suggests the regulatory function of plasmablasts [83]. Patients with immunoglobulin G4 (IgG4)–related disorder (IgG4-RD), primary Sjögren’s syndrome [84, 85], and SLE [86] have increased plasmablast number, indicating their expansion could be the result of inflammatory conditions. In 2019, Arbore et al. further reported that microRNA-155 (miR-155) plays an important role in the survival and proliferation of plasmablast B cells [87].
5.2.2.5 Granzyme B (GrB+) Bregs
Granzyme B–expressing Bregs are known to display the characteristic phenotype of CD19+CD38+CD1d+IgM+CD147+ [88]. Expression of Granzyme B on Bregs (GrB+ Bregs) mediates their inhibitory effect on T cells by suppressing their proliferation and inducing apoptosis. In various inflammatory conditions such as SLE [89] and in acute viral infections [90], the percentage of GrB+ Bregs is relatively high. Peripheral B cells stimulated in the presence of IL-21 are reported to produce and secrete GrB. These cells mediate their suppressive function by repressing T cell proliferation, partly via downregulation of the TCR zeta chain, thereby promoting T cell apoptosis [88]. In the case of RA, the proportion of GrB+ Bregs is significantly reduced due to the lowered expression of IL-21R, which in turn impairs the negative regulation of Th1/Th17 by GrB+ Bregs [91], suggesting that impaired GrB+ Bregs are associated with RA pathogenesis.
5.2.2.6 iBregs (Induced Bregs)
B cells like other immunosuppressive cells differentiate into induced Breg (iBreg) cells when subjected to certain stimuli and express indoleamine 2,3-dioxygenase (IDO) and TGFβ. T cells expressing cytotoxic T lymphocyte–associated protein 4 (CTLA-4) enhance the induction of iBregs, which then convert T cells into TGF-β- and IL-10-producing Tregs, thereby modulating various immune responses [92].
5.2.2.7 IgA+ Bregs
This subset of Bregs has been identified recently by Fehres et al. in 2019. They described that overexpression of APRIL (A Proliferation-Inducing Ligand) instead of BAFF induces activation of IL-10+ human Bregs that further repress inflammatory immune reactions. These APRIL-induced IgA+ Bregs suppress the effector function of T cells and macrophages and induce Tregs via IL-10 and PD-L1 expression [93]. These findings collectively suggest the importance of the novel APRIL-induced Breg subset with IgA+ phenotype, both in the immunopathology and homeostasis of immunological reactions. In colorectal cancer patients, a higher proportion of IgA+ Bregs was observed at the tumor site due to lowered expression of microRNA15A (miRNA15A) and microRNA16–1 (miRNA16–1). These microRNAs exhibit the ability to regulate proliferation, drug resistance, and apoptosis. These studies thus concluded that microRNAs and IgA+ Bregs are negatively correlated and that a lower level of microRNAs along with higher proportion of IgA+ Bregs reduces the survival rates in cancer patients [94].
5.3 Bregs in Health and Diseases
The discovery of various defined subsets of Bregs has now compelled researchers to revisit the understanding of B cell biology in the context of various immune-mediated diseases. Vaccines have been ideally responsible for eradicating several diseases via the specific activation of B cells. Similarly, cancer immunotherapies demonstrate their course of action via production of different B cells. Moreover, B cell deficiencies lead to various devastating impacts on health and immunity. It is now well established that B lymphocytes produce antibodies and are associated with various immunomodulatory properties. Bregs are now extensively studied for their novel immune-regulatory roles, as mice deprived of B cells are reported to demonstrate higher incidences of immune-related disorders. Bregs are known to produce various cytokines and immunomodulatory factors responsible for proper functioning of the host immune system [95]. A cohort study indicated that targeted depletion of B cell populations serves as a treatment in autoantibody-mediated autoimmune disorders such as SLE [96]. Thus, Bregs undoubtedly play an important role in host pathology, thereby opening Pandora’s Box in harnessing the potential of Bregs in mediating health. In the following sections, we focus on the role of Bregs in selected diseases/pathologies.
5.3.1 Multiple Sclerosis
Multiple sclerosis (MS) is an autoimmune disorder occurring due to T and B cell hyperactivation, leading to demyelination and axonal damage in the central nervous system (CNS). Apart from the role of B cells as pathogenic cells, they also modulate immune responses in MS. IL-10-producing Bregs were first observed in MS patients infected with helminthes; these Bregs were found to suppress the proliferation and IFN-γ production in T cells in vitro [97]. The role of Bregs in MS was further substantiated by diminished levels of IL-10 production in MS patients. In relapsing-remitting MS patients, a significantly reduced number of IL-10-producing naïve Bregs were observed compared to that in the controls [98]. Further, treatment of MS patients with IFN-β, fingolimod, or alemtuzumab is reported to increase the number and function of Bregs [99, 100]. In EAE, one of the most widely studied animal model of MS, the importance of Bregs in alleviating EAE progression was recently illustrated [52, 58, 101, 102]. The suppressive functioning of Bregs involves binding to the BCR co-receptor CD19, which plays an inhibitory role in the development of EAE by modulating the Th1/Th2 cytokine balance [103]. Fillatreau et al. found that B-cell-depleted mice have a persistent type I immune response in EAE and that their recovery was dependent on myelin oligodendrocyte glycoprotein (MOG)-specific IL-10-producing B cells [3]. Further studies indicate that Bregs with the CD1dhiCD5+ phenotype are effective in inhibiting EAE progression. CD1dhiCD5+ Bregs possess highly decisive immunomodulatory properties in controlling the pathogenesis of the initial and late phase of EAE [52, 58]. Further, depletion of CD20+ B cell enhances the pathogenesis of EAE. This was evident from a simultaneous increase in the expression of various inflammatory cytokines in the CNS and an increased number of autoreactive CD4+ T cells due to absence of the IL-10-producing CD1hiCD5+ Bregs subset [52, 58].
5.3.2 Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a highly deteriorating inflammatory condition of the intestine, usually represented by Crohn’s disease (CD) and ulcerative colitis (UC) [104, 105]. Recently, an alarming rise in the prevalence and incidence of IBD has been observed globally [105]. Numerous studies have reported the functions of Bregs in regulating intestinal inflammation. Mizoguchi et al. [106] credited B cells and autoantibody production as important factors in protecting T cell receptor (TCR) α chain-deficient (TCRα−/−) mice, which are highly susceptible to develop chronic colitis. They showed that CD1+ B cells producing higher levels of IL-10 upon induction in the gut-associated lymphoid tissues in TCRα−/− mice reduced the intestinal inflammation and disease incidence [4]. IL-10-producing Bregs have now been linked with downregulating the inflammatory cascade associated with IL-1 and signal transducer and activator of transcription 3 (STAT3) without tweaking T cell responses. Wei et al. demonstrated that adoptive transfer of B cells from mesenteric lymph nodes could repress IBD by enhancing the Tregs population [107, 108]. A numerical (number/percentage of Bregs) defect in IL-10-producing Bregs has also been described in patients with both CD and UC [109].
5.3.3 Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is designated as a systemic multigene autoimmune disorder characterized by higher production of autoantibodies with simultaneous deposition of immune complexes, resulting in tissue inflammation and damage to the skin, kidneys, and joints. This phenomenon results in proteinuria and large-scale renal tubule inflammation (glomerulonephritis), which eventually affects the immune system [110, 111]. Both B- and T-cell abnormalities have been found to be responsible for the occurrence of SLE in mammals [112]. SLE-affected individuals usually show a reduced number as well as decreased functional activity of circulating Bregs. This defect usually arises as immature B cells (CD19+CD24hiCD38hi) fail to differentiate into Bregs [20, 68, 113]. Various mouse models have been identified to study the role of regulatory B cells in spontaneous lupus. Recently two well-defined models, New Zealand Black (NZB) × New Zealand White (NZW) F1 hybrid (NZB/W) mice and MRL/lpr mice, have been used to investigate the inhibitory role of Bregs in regulating the severity of SLE [59, 112]. Depletion of Bregs in infant mice resulted in higher severity of SLE, whereas deletion of Bregs from adult mice did not affect SLE progression. Thus, Bregs have been found as predominantly effective during the initiation phase of SLE rather than during disease progression [59, 112]. Additionally, the higher therapeutic interventions of Bregs have come into play due to their role in enhancing the number of Tregs after the transfer of splenic CD1dhi CD5+ B cells from wild-type NZB/W F1 mice to CD19 −/− NZB/W F1 [95]. Blair et al. further observed that anti-CD40-induced T2 Breg cells significantly improved the survival rate in MPL/lpr mice via higher expression of IL-10. Collectively, these findings indicate that T2-MZP B cells as well as B10 cells effectively help in protecting mice from severe SLE [21].
5.3.4 Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a systemic autoimmune disease with a worldwide prevalence of 0.3–1%. It is responsible for increased societal dependency with simultaneous reduction of mobility and working ability [114]. RA is characterized by autoimmune inflammatory responses at synovial membranes and joint capsules, resulting in significant morbidity and mortality due to synovial proliferation, cartilaginous injury, and bone erosion [115]. B cells produce various factors including autoantibodies like anti-citrullinated protein antibodies (ACPAs) and rheumatoid factor (RF) that are responsible for severe disease activity in RA [116]. Moreover, reduced numbers of Bregs such as IL-10-producing Bregs, CD19+TIM-1+IL-10+ Bregs, CD19+CD5+CD1dhi B cells, and CD19+CD5+CD1d+IL-10+ Bregs were observed in RA patients compared to those in healthy controls upon stimulation with CpG or LPS along with phorbol myristate acetate and ionomycin [117, 118]. Further, the function of Bregs was found to be impaired in RA. One study demonstrated that CD24hiCD38hi Breg cells from healthy individuals inhibited Th1 and Th17 differentiation and favored the conversion of CD4+CD25− T cells to Tregs via IL-10 expression. In contrast, CD19+CD24hiCD38hi cells from RA patients were unable to reduce Th17 development and induce Tregs differentiation [70]. In 2017, Banko et al. showed that CD19+CD27+IL-10+ Bregs are significantly reduced in RA patients compared to those in the controls and that the existing Bregs showed a reduced ability to suppress IFN-γ production by T helper cells. Breg-deficient mice demonstrate higher incidences of autoimmune arthritic conditions due to enhanced induction of Th1 and Th17 cells along with simultaneous suppression of Treg cells [113]. Bregs have thus been found instrumental in suppressing inflammation via restoring or modulating the Th1/Th2 balance in various T-cell-mediated autoimmune diseases such as EAE and RA [113].
5.3.5 Type 1 Diabetes
Type 1 diabetes (T1D) is an autoimmune disease caused by the obliteration of insulin-producing pancreatic β cells mediated by CD4+ and CD8+ T cells [119]. Onset of T1D usually occurs around 13–15 weeks of age in non-obese diabetic (NOD) mice, a model of human T1D. The prevalence of T1D in NOD mice is higher in females with about 80% females and 20% males affected by this disease by 30 weeks [120]. B cells are particularly found to be responsible for the development of pathogenesis of T1D. B cell penetration into the pancreatic islets of NOD mice results in selective propagation of T cells within lymphoid structures, leading to an increased number of autoreactive B cells [121]. Treatment of 5-week-old NOD female mice with anti-CD20 mAbs was found to deplete 95% of B cells, thereby arresting insulitis; however, at 15 weeks, the same treatment was inefficient to hinder the progression of T1D [8, 122]. Grey et al. found that the increased population of CD4+CD25+Foxp3+ Treg cells due to B cell depletion reduced the occurrence of diabetes [123]. Smith and Tedder further postulated that B-cell-depleted NOD mice remained free from diabetes even after reconstitution with B cells [124]. Among various types of B cells, IL-10-expressing B cells have been primarily found to be responsible for decreasing the pathogenicity of insulitis and reducing T1D incidence. Simultaneously, various Th1 immune-related responses were curbed, leading to the diversion of CD4+ T cells toward the Th2 phenotype upon introduction of activated B cells in pre-diabetic NOD mice [125]. Tian and colleagues further established that LPS-activated B cells mediate apoptosis of diabetogenic Th1 cells in NOD mice via expression of FasL and secretion of TGF-β [24]. These findings provide new insights into treating human T1DM via targeting the T cell-B cell interaction. Reduced numbers of IL-10-producing Bregs have been reported in patients with T1D [126]. There is substantial evidence that Bregs are either insufficient in number and/or functionally compromised in autoimmune diseases. Thus, further studies are needed to understand their mechanisms of action in these diseases.
5.3.6 Infectious Diseases
The role of B cells in infectious diseases has been studied extensively. In contrast, the role of Bregs in intracellular infections is unclear. Studies on Bregs in infections will uncover the valuable targets/potent markers in developing therapeutic interventions to treat various infectious diseases. Recent studies have shown that successful treatment of Mycobacterium tuberculosis infection induces Bregs with the ability to express FasL and IL-5RA in TB patients. Thus, these molecules could be potentially utilized as indicators of monitoring treatment responses during infections [127, 128]. Various studies have demonstrated the suppressive role of Bregs in chronic hepatitis B virus infection. Das et al. [129] first demonstrated that Bregs are responsible for regulating antigen-specific CD8+ T cells in hepatitis B virus infection. They also found that inhibition of IL-10 may reestablish HBV-specific CD8+ T cells in vitro. Various studies have reported that in HIV infection, Bregs impaired T cells via expression of IL-10 and programmed death (PD)-L1, contributing to immune dysfunction [130]. In 2014, Jiao et al. found that the frequency of Bregs in HIV patients was negatively correlated with the CD4+ T cell count but was positively correlated with the viral load. Supporting this, it is also observed that following anti-retroviral treatment, the frequency of Bregs was decreased along with a concomitant step-wise increase in the CD4+ T cell count.
5.3.7 Allergy and Asthma
Bregs also exert protection against allergic airway inflammation [131]. Through antigen- specific/non-specific immunomodulatory mechanisms, it is apparent that Bregs demonstrate allergen tolerance and contribute to suppress allergic diseases. Allergic inflammation is reported to be suppressed by IL-10-producing Bregs and involves a delicate balance between IL-10 induced parasite responses and detrimental IL-4-mediated allergic responses [132]. Br1 and Br3 cells increase in response to casein in milk-tolerant individuals [133] but not in milk-allergic individuals. Thus, both Br1 and Br3 cell types are critical for immune tolerance in non-IgE-mediated food allergies related to atopic dermatitis. Patients with allergic asthma and allergic rhinitis have a decreased number of IL-10-producing CD24hiCD27+ Bregs [134]. In a similar manner, beekeepers also develop tolerance against bee venom allergen, i.e., Phospholipase Az (PLAz)–specific to BR1 cells producing IgG4 antibodies by suppressing T cell responses in an IL-10-dependent manner [71, 135]. In allergic asthma, treatment with oral corticosteroids (OCS) significantly affects the frequency of Bregs as well as their ability to express IL-10 in a Breg subset–specific manner [136].
5.3.8 Osteoporosis
Osteoporosis represents one of the most common bone loss conditions, leading to higher fragility and bone fractures often related to advanced age and post-menopausal conditions [137, 138]. Osteoporosis is often a neglected disease with more than 200 million affected individuals worldwide, thus also referred as a “silent killer” [139, 140]. In the bone marrow, B cells are a major source of the osteoclastogenesis inhibitor osteoprotegerin (OPG), in the presence of activated T cells signaled by CD40L-CD40 interaction on B cells. Moreover, a CD40L-CD40-deficient mice showed reduced bone mass compared to the control mice. B cells also express RANKL along with OPG, which in the long run affects bone physiology. Furthermore, mice with B cell deficiency show suppressed OPG production and high prevalence of osteoporosis [141]. Bregs suppress various proinflammatory cytokines such as IL-1 and TNF-α, which are osteoclastogenic in nature, therefore leading to enhanced bone loss. The ratio of Th1/Th2 is an important parameter defining bone strength [142], including the rate of bone resorption and the resulting bone loss. Moreover, several subtypes of Bregs have now been reported with the suppression of Th1-, Th2-, or Th17-mediated autoimmune responses with a subsequent increase in Foxp3+ Treg cells along with conversion of effector T cells into Tr1 cells (CD4+ Foxp3+IL-10+ Treg 1 cells). Bregs have also been observed to suppress the expression of Th17 cells [59, 109], which are responsible for enhanced osteoclastogenesis and bone loss [142]. Recent observations (unpublished) from our lab clearly demonstrate the role of CD19hiCD1dhiCD5hiIL-10hi Bregs in modulating bone health. Thus, further research is needed to establish the precise role of Bregs in regulating bone health.
5.4 Therapeutic Potential of Bregs: From Bench to Bedside
The present global scenario arising from various studies using experimental models and human disorders validate the vital role of Bregs in several diseases. Together, these studies indicate that Bregs have the potential to modulate a number of immune pathologies. Tedder et al. demonstrated that Bregs are involved in autoimmune responses and also provide protection to host tissues during the immunopathogenesis of infectious diseases [143]. More importantly, understanding the basic principle underlying the induction of Bregs will help in tweaking cellular tolerance and amend the influence of disease. As a small number of Bregs are inefficient in inhibiting inflammation, mechanisms that can enhance both the number and effector functions of Bregs can result in enhanced immune-suppressive functions. In the context of immunological conditions such as autoimmunity and transplantation, long-term usage of immunosuppressive drugs increases the likelihood of life-threatening infections. In certain conditions such as during graft transplantation, autoimmune diseases, and so on, expansion of the immunosuppressive Bregs population is needed. Thus, strategies that can be exploited by therapeutically targeting Bregs can open new avenues in treating various immune-mediated diseases such as the following: (a) ex vivo expansion of Bregs: stimulation of B cells in patient-derived PBMCs, leading to expansion of Bregs, followed by adoptive transfer of Bregs sorted by FACS may suppress the inflammation and re-induce tolerance. (b) in vivo modulation of Bregs for expansion: stimuli that can shift the differentiation of B cells toward immunosuppressive regulatory B cells. Some evidence suggest that pro-inflammatory cytokines such as B cell–activating factor (BAFF), IL-1β, IL-6, IL-21, IFN-α, and IFN- γ [23, 68] are the key cytokines that expand the Bregs population upon exposure. Interestingly, in arthritic mice, the gut microbiota has the potential to induce the expression of IL-1β and IL-6, which further promote Bregs differentiation and production of IL-10 cytokine [23]. (c) Depletion of Bregs: B cell depletion therapies (viz. rituximab), usage of targeted B cell therapies, that can target a specific subtype of B cells is more advantageous than total B cell depletion. Thus, further in-depth studies are required to develop Breg-dependent immunotherapies and to enhance their applications in treating various immune disorders and pathologies.
Abbreviations
- Bregs:
-
B regulatory cells
- BCR:
-
B cell receptor
- TLR:
-
Toll-like receptor
- PAMPs:
-
pathogen-associated molecular patterns
- EAE:
-
experimental autoimmune encephalomyelitis
- IL:
-
interleukin
- LPS:
-
lipopolysaccharide
- TGF-β:
-
transforming growth factor Beta
- Tregs:
-
T regulatory cells
- MHC:
-
major histocompatibility complex
- AIA:
-
antigen-induced arthritis
- Th:
-
T helper cells
- STAT:
-
Signal Transducer and Activator of Transcription
- IFN-γ:
-
interferon gamma
- TNF-α:
-
tumor necrosis factor alpha
- mAbs:
-
monoclonal antibodies
- T2-MZP:
-
transitional 2 marginal-zone precursor
- TIM-1:
-
T-cell Ig mucin domain-1
- CTLA-4:
-
cytotoxic T lymphocyte-associated protein 4
- iBreg:
-
induced B regulatory cells
- IDO:
-
indoleamine 2,3-dioxygenase
- MS:
-
multiple sclerosis
- SLE:
-
systemic lupus erythematosus
- RA:
-
rheumatoid arthritis
- NOD:
-
non-obese diabetic
- RANKL:
-
receptor activator of nuclear factor-κB ligand
- OPG:
-
osteoprotegerin
- T1D:
-
Type 1 diabetes
- Tr1:
-
T regulatory type 1
References
Katz S, Parker D, Turk J (1974) B-cell suppression of delayed hypersensitivity reactions. Nature 251:550. https://doi.org/10.1038/1550a0
Wolf SD, Dittel BN, Hardardottir F, Janeway CA (1996) Experimental autoimmune encephalomyelitis induction in genetically B cell–deficient mice. J Exp Med 184:2271–2278. https://doi.org/10.1084/jem.184.6.2271
Fillatreau S, Sweenie CH, Mcgeachy MJ, Gray D, Anderton SM (2002) B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3:944. https://doi.org/10.1038/ni833
Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK (2002) Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16:219–230. https://doi.org/10.1016/S1074-7613(02)00274-1
Mauri C, Gray D, Mushtaq N, Londei M (2003) Prevention of arthritis by interleukin 10–producing B cells. J Exp Med 197:489–501. https://doi.org/10.1084/jem.20021293
Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC (2003) B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-β1. J Immunol 170:5897–5911. https://doi.org/10.4049/jimmunol.170.12.5897
Mizoguchi A, Bhan AK (2006) A case for regulatory B cells. J Immunol 176:705–710. https://doi.org/10.4049/jimmunol.176.2.705
Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF (2008) A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28:639–650
Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN (2007) B cell regulation of CD4+ CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol 178:3447–3456. https://doi.org/10.4049/jimmunol.178.6.3447
Ray A, Basu S, Williams CB, Salzman NH, Dittel BN (2012) A novel IL-10–independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol 1103354. https://doi.org/10.4049/jimmunol.1103354
Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG (2010) Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol 125:1114–1124.e8. https://doi.org/10.1016/j.jaci.2010.01.018
Carter NA, Vasconcellos R, Rosser EC, Tulone C, Muñoz-Suano A, Kamanaka M et al (2011) Mice lacking endogenous IL-10–producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol 1100284. https://doi.org/10.4049/jimmunol.1100284
Lundy SK (2009) Killer B lymphocytes: the evidence and the potential. Inflamm Res 58:345. https://doi.org/10.1007/s00011-009-0014-x
Lundy SK, Boros DL (2008) Fas ligand-expressing B-1a lymphocytes mediate CD4(+)-T-cell apoptosis during schistosomal infection: induction by interleukin 4 (IL-4) and IL-10. Infect Immun 70:812
Kaku H, Cheng KF, Al-Abed Y, Rothstein TL (2014) A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J Immunol 193:5904
Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG (2015) PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun 6:5997
Oleinika K, Rosser EC, Matei DE, Nistala K, Bosma A, Drozdov I, Mauri C (2018) CD1d-dependent immune suppression mediated by regulatory B cells through modulations of iNKT cells. Nat Commun 9(1):684
Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF (2007) Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis 13:1365–1368
Dass S, Vital EM, Emery P (2007) Development of psoriasis after B cell depletion wit rituximab. Arthritis Rheum 56:2715–2718
Blair PA et al (2010) CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus Erythematosus patients. Immunity 32(1):129–140
Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA et al (2009) Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol 182:3492–3502. https://doi.org/10.4049/jimmunol.0803052
Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM et al (2011) Characterization of a rare IL-10–competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117:530–541. https://doi.org/10.1182/blood-2010-07-294249
Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA et al (2014) Regulatory B cells are induced by gut microbiota–driven interleukin-1β and interleukin-6 production. Nat Med 20:1334. https://doi.org/10.1038/nm.3680
Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL (2001) Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol 167:1081–1089. https://doi.org/10.4049/jimmunol.167.2.1081
Yoshizaki A, Miyagaki T, Dilillo DJ, Matsushita T, Horikawa M, Kountikov EI et al (2012) Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491:264. https://doi.org/10.1038/nature11501
Bankoti R, Gupta K, Levchenko A, Stäger S (2012) Marginal zone B cells regulate antigen-specific T cell responses during infection. J Immunol 188(8):3961–3971. https://doi.org/10.4049/jimmunol.1102880
Yanaba K, Bouaziz J-D, Matsushita T, Tsubata T, Tedder TF (2009) The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol 182:7459–7472. https://doi.org/10.4049/jimmunol.0900270
Fagraeus A (1948) The plasma cellular reaction and its relation to the formation of antibodies in vitro. J Immunol 58:1–13
Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD et al (2004) Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med 200:967–977
Lino AC, Lampropoulou V, Welle A, Joedicke J, Pohar J, Simon Q, Thalmensi J, Baures A, Fluhler V, Sakwa I, Stervbo U (2018) LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity 49(1):120–133
Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY (2007) IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 37(11):3021–3029. https://doi.org/10.1002/eji.200737810
Yu CR, Choi JK, Uche AN, Egwuagu CE (2018) Production of IL-35 by Bregs is mediated through binding of BATF-IRF-4-IRF-8 complex to il12a and ebi3 promoter elements. J Leukoc Biol 104(6):1147–1157
Corneth OB, Verstappen GM, Paulissen SM, de Bruijn MJ, Rip J, Lukkes M, Hendriks RW (2017) Enhanced Bruton’s tyrosine kinase activity in peripheral blood B lymphocytes from patients with autoimmune disease. Arthritis Rheum 69(6):1313–1324
Hardy RR (2006) B-1 B cell development. J Immunol 177:2749–2754. https://doi.org/10.4049/jimmunol.177.5.2749
Kantor A (1991) A new nomenclature for B cells. Immunol Today 12:388. https://doi.org/10.1016/0167-5699(91)90135-G
Stall AM, Adams S, Herzenberg LA, Kantor AB (1992) Characteristics and development of the murine B-lb (Ly-1 B sister) cell population. Ann N Y Acad Sci 651:33–43. https://doi.org/10.1111/j.1749-6632.1992.tb24591.x
Kawahara T, Ohdan H, Zhao G, Yang Y-G, Sykes M (2003) Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol 171:5406–5414. https://doi.org/10.4049/jimmunol.171.10.5406
Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N (2012) B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol 42:120–129. https://doi.org/10.1002/eji.201141890
Bos NA, Kimura H, Meeuwsen CG, Visser HD, Hazenberg MP, Wostmann BS et al (1989) Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol 19:2335–2339. https://doi.org/10.1002/eji.1830191223
Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A (1997) The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol 27:1557–1563. https://doi.org/10.1002/eji.1830270635
Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA (1999) Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci 96:2250–2255. https://doi.org/10.1073/pnas.96.5.2250
Kantor AB, Merrill CE, Herzenberg LA, Hillson JL (1997) An unbiased analysis of V (H)-DJ (H) sequences from B-1a, B-1b, and conventional B cells. J Immunol 158:1175–1186
Notkins AL (2004) Polyreactivity of antibody molecules. Trends Immunol 25:174–179. https://doi.org/10.1016/j.it.2004.02.004
Stewart J (1992) Immunoglobulins did not arise in evolution to fight infection. Immunol Today 13:396–399. https://doi.org/10.1016/0167-5699(92)90088-O
Baumgarth N, Tung JW, Herzenberg LA (2005) Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol 26:347–362
O’Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M (1992) Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol 22:711–717
Velupillai P, Sypek J, Harn DA (1996) Interleukin-12 and -10 and gamma interferon regulate polyclonal and ligand-specific expansion of murine B-1 cells. Infect Immun 64:4557–4560
Al-Qaoud KM, Fleischer B, Hoerauf A (1998) The Xid defect imparts susceptibility to experimental murine filariosis – association with a lack of antibody and IL-10 production by B cells in response to phosphorylcholine. Int Immunol 10(1):17–25
Zhang X, Deriaud E, Jiao X, Braun D, Leclerc C, Lo-Man R (2007) Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J Exp Med 204:1107
Shimomura Y, Mizoguchi E, Sugimoto K et al (2008) Regulatory role of B-1 B cells in chronic colitis. Int Immunol 20:729
Lee KM, Stott RT, Zhao G, Soohoo J, Xiong W, Lian MM et al (2014) TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol 44:1728–1736. https://doi.org/10.1002/eji.201344062
Matsushita T, Horikawa M, Iwata Y, Tedder TF (2010) Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol 1001307. https://doi.org/10.4049/jimmunol.1001307
Maseda D, Candando KM, Smith SH, Kalampokis I, Weaver CT, Plevy SE et al (2013) Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-γ+ CD4+ T cell numbers during colitis development in mice. J Immunol 1300649. https://doi.org/10.4049/jimmunol.1300649
Maseda D, Smith SH, Dilillo DJ, Bryant JM, Candando KM, Weaver CT et al (2012) Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol 188:1036–1048. https://doi.org/10.4049/jimmunol.1102500
Kuchroo VK, Dardalhon V, Xiao S, Anderson AC (2008) New roles for TIM family members in immune regulation. Nat Rev Immunol 8:577
Xiao S, Brooks CR, Sobel RA, Kuchroo VK (2015) Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J Immunol 194:1602
Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H et al (2011) Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest 121. https://doi.org/10.1172/JCI46274
Matsushita T, Yanaba K, Bouaziz J-D, Fujimoto M, Tedder TF (2008) Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 118:3420–3430. https://doi.org/10.1172/JCI36030
Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H et al (2010) Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol 184(9):4789–4800. https://doi.org/10.4049/jimmunol.0902391
Yanaba K, Yoshizaki A, Asano Y, Kadono T, Tedder TF, Sato S (2011) IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model. Am J Pathol 178:735–743
Le Huu D, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K, Tedder TF, Fujimoto M (2013) Donor-derived regulatory B cells are important for suppression of murine sclerodermatous chronic graft-versus-host disease. Blood 121:3274–3283
Mauri C, Mars LT, Londei M (2000) Therapeutic activity of agonistic monoclonal antibodies against CD40 in a chronic autoimmune inflammatory process. Nat Med 6:673. https://doi.org/10.1038/76251
Horikawa M, Weimer ET, Dilillo DJ, Venturi GM, Spolski R, Leonard WJ et al (2013) Regulatory B cell (B10 cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol 190:1158–1168. https://doi.org/10.4049/jimmunol.1201427
Dilillo DJ, Griffiths R, Seshan SV, Magro CM, Ruiz P, Coffman TM et al (2011) B lymphocytes differentially influence acute and chronic allograft rejection in mice. J Immunol 1002983. https://doi.org/10.4049/jimmunol.1002983
Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR et al (2007) Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 178:7868–7878. https://doi.org/10.4049/jimmunol.178.12.7868
Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S et al (2011) B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc Natl Acad Sci 201100994. https://doi.org/10.1073/pnas.1100994108
Bosma A, Abdel-Gadir A, Isenberg DA, Jury EC, Mauri C (2012) Lipid-antigen presentation by CD1d+ B cells is essential for the maintenance of invariant natural killer T cells. Immunity 36:477–490. https://doi.org/10.1016/j.immuni.2012.02.008
Menon M, Blair PA, Isenberg DA, Mauri C (2016) A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 44:683–697. https://doi.org/10.1016/j.immuni.2016.02.012
Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A et al (2010) IL-10 produced by activated human B cells regulates CD4+ T-cell activation in vitro. Eur J Immunol 40:2686–2691. https://doi.org/10.1016/j.immuni.2012.02.008
Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA et al (2013) CD19+ CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 5:173ra23–173ra23. https://doi.org/10.1126/scitranslmed.3005407
Tarique M, Naz H, Kurra SV, Saini C, Naqvi RA, Rai R et al (2018) Interleukin-10 producing regulatory B cells transformed CD4+ CD25− into Tregs and enhanced regulatory T cells function in human leprosy. Front Immunol 9. https://doi.org/10.3389/fimmu.2018.01636
Van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG et al (2013) IgG4 production is confined to human IL-10–producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol 131(4):1204–1212. https://doi.org/10.1016/j.jaci.2013.01.014
Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, Kallies A, Nutt SL, Sakaguchi S, Takeda K, Kurosaki T (2014) Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 41(6):1040–1051. https://doi.org/10.1016/j.immuni.2014.10.016
Aravena O, Ferrier A, Menon M, Mauri C, Aguillón JC, Soto L, Catalán D (2017) TIM-1 defines a human regulatory B cell population that is altered in frequency and function in systemic sclerosis patients. Arthritis Res Ther 19(1):8
Wang T, Li Z, Li X, Chen L, Zhao H, Jiang C, Song L (2017) Expression of CD19+ CD24highCD38high B cells, IL-10 and IL-10R in peripheral blood from patients with systemic lupus erythematosus. Mol Med Rep 16(5):6326–6333. https://doi.org/10.3892/mmr.2017.7381
Cherukuri A, Rothstein DM, Clark B, Carter CR, Davison A, Hernandez-Fuentes M, Baker RJ (2014) Immunologic human renal allograft injury associates with an altered IL-10/TNF-α expression ratio in regulatory B cells. J Am Soc Nephrol 25(7):1575–1585. https://doi.org/10.1681/ASN.2013080837
Kristensen B, Hegedus L, Lundy SK, Brimnes MK, Smith TJ, Nielsen CH (2015) Characterization of regulatory B cells in Graves’ disease and Hashimoto’s thyroiditis. PLoS One 10(5):e0127949
Bankó Z, Pozsgay J, Szili D, Tóth M, Gáti T, Nagy G et al (2017) Induction and differentiation of IL-10–producing regulatory B cells from healthy blood donors and rheumatoid arthritis patients. J Immunol 198(4):1512–1520. https://doi.org/10.4049/jimmunol.1600218
Amel Kashipaz MR, Huggins ML, Lanyon P, Robins A, Powell RJ, Todd I (2003) Assessment of Be1 and Be2 cells in systemic lupus erythematosus indicates elevated interleukin-10 producing CD5+ B cells. Lupus 12:356–363
Llorente L, Richaud-Patin Y, Fior R, Alcocer-Varela J, Wijdenes J, Fourrier BM, Galanaud P, Emilie D (1994) In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjogren’s syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum 37:1647–1655
Liu HJ, Guo XF, Hu FL, Yan CP, Cui XJ, Yan XL et al (2018) Increased receptor activator of nuclear factor kappa B ligand expressed on B10 cells in rheumatoid arthritis. Beijing da xuexue bao. Yi xue ban. J Peking Univ Health Sci 50(6):968–974. PMID: 30562766
Sperling S, Fiedler P, Lechner M, Pollithy A, Ehrenberg S, Schiefer AI et al (2019) Chronic CD30 signaling in B cells results in lymphomagenesis by driving the expansion of plasmablasts and B1 cells. Blood 133(24):2597–2609. https://doi.org/10.1182/blood.2018880138
Hartung HP, Kieseier BC (2010) Atacicept: targeting B cells in multiple sclerosis. Ther Adv Neurol Disord 3:205–216
Lin W, Zhang P, Chen H, Chen Y, Yang H, Zheng W et al (2017) Circulating plasmablasts/plasma cells: a potential biomarker for IgG4-related disease. Arthritis Res Ther 19(1):25. https://doi.org/10.1186/s13075-017-1231-2
Kubo S, Nakayamada S, Zhao J, Yoshikawa M, Miyazaki Y, Nawata A et al (2017) Correlation of T follicular helper cells and plasmablasts with the development of organ involvement in patients with IgG4-related disease. Rheumatology 57(3):514–524. https://doi.org/10.1093/rheumatology/kex455
Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V (2001) Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol 167:2361–2369
Arbore G, Henley T, Biggins L, Andrews S, Vigorito E, Turner M, Leyland R (2019) MicroRNA-155 is essential for the optimal proliferation and survival of plasmablast B cells. Life Sci Alliance 2(3):e201800244
Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth T et al (2013) Interleukin-21-induced granzyme B-expressing B lymphocytes regulate T cells and infiltrate tumors (P1088). Cancer Res. https://doi.org/10.1158/0008-5472.CAN-12-3450
Hagn M, Ebel V, Sontheimer K, Schwesinger E, Lunov O, Beyer T, Fabricius D, Barth TF, Viardot A, Stilgenbauer S, Hepp J, Scharffetter-Kochanek K, Simmet T, Jahrsdörfer B (2010) CD5+ B cells from individuals with systemic lupus erythematosus express granzyme B. Eur J Immunol 40(7):2060–2069
Hagn M, Schwesinger E, Ebel V, Sontheimer K, Maier J, Beyer T, Syrovets T, Laumonnier Y, Fabricius D, Simmet T, Jahrsdörfer B (2009) Human B cells secrete Granzyme B when recognizing viral antigens in the context of the acute phase cytokine IL-21. J Immunol 183(3):1838–1845
Xu L, Liu X, Liu H, Zhu L, Zhu H, Zhang J et al (2017) Impairment of granzyme B-producing regulatory B cells correlates with exacerbated rheumatoid arthritis. Front Immunol 8(768). https://doi.org/10.3389/fimmu.2017.00768
Nouel A, Pochard P, Simon Q, Segalen I, Le Meur Y, Pers J et al (2015) B-cells induce regulatory T cells through TGF-β/IDO production in a CTLA-4 dependent manner. J Autoimmun 59:53–60. https://doi.org/10.1016/j.jaut.2015.02.004
Fehres CM, van Uden NO, Yeremenko NG, Fernandez L, Franco Salinas G, Van Duivenvoorde LM et al (2019) APRIL induces a novel subset of IgA+ regulatory B cells that suppress inflammation via expression of IL-10 and PD-L1. Front Immunol 10:1368
Liu R, Lu Z, Gu J, Liu J, Huang E, Liu X et al (2018) MicroRNAs 15A and 16–1 activate signaling pathways that mediate chemotaxis of immune regulatory B cells to colorectal tumors. Gastroenterology 154(3):637–651. https://doi.org/10.1053/j.gastro.2017.09.045
Porakishvili N, Mageed R, Jamin C, Pers JO, Kulikova N, Renaudineau Y et al (2001) Recent progress in the understanding of B-cell functions in autoimmunity. Scand J Immunol 54:30–38
Isenberg DA (2006) B cell targeted therapies in autoimmune diseases. J Rheumatol Suppl 33:24–28. PMID: 16652442
Correale J, Farez M, Razzitte G (2008) Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol 64:187–199. https://doi.org/10.1002/ana.21438
Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW et al (2011) Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naive/memory Breg ratio during a relapse but not in remission. J Neuroimmunol 239:80–86. https://doi.org/10.1016/j.jneuroim.2011.08.019
Grutzke B, Hucke S, Gross CC, Herold MV, Posevitz-Fejfar A, Wildemann BT et al (2015) Fingolimod treatment promotes regulatory phenotype and function of B cells. Ann Clin Transl Neurol 2:119–130. https://doi.org/10.1002/acn3.155
Thompson SA, Jones JL, Cox AL, Compston DA, Coles AJ (2010) B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol 30:99–105. https://doi.org/10.1007/s10875-009-9327-3
Bouaziz J-D, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC et al (2007) Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci 104:20878–20883. https://doi.org/10.1073/pnas.0709205105
Lund FE (2008) Cytokine-producing B lymphocytes key regulators of immunity. Curr Opin Immunol 20:332–338. https://doi.org/10.1016/j.coi.2008.03.003
Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF et al (2006) Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol 168:812–821. https://doi.org/10.2353/ajpath.2006.050923
Talley NJ, Abreu MT, Achkar J-P, Bernstein CN, Dubinsky MC, Hanauer SB et al (2011) An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol 106:S2. https://doi.org/10.1038/ajg.2011.58
Molodecky NA, Soon S, Rabi DM, Ghali WA, Ferris M, Chernoff G et al (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142:46–54.e42. https://doi.org/10.1053/j.gastro.2011.10.001
Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK (1997) Suppressive role of B cells in chronic colitis of T cell receptor α mutant mice. J Exp Med 186:1749–1756. https://doi.org/10.1084/jem.186.10.1749
Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M et al (2015) Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci 102:2010–2015. https://doi.org/10.1073/pnas.0409449102
Kalampokis I, Yoshizaki A, Tedder TF (2013) IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 15:S1. https://doi.org/10.1186/ar3907
Oka A, Ishihara S, Mishima Y, Tada Y, Kusunoki R, Fukuba N et al (2014) Role of regulatory B cells in chronic intestinal inflammation: association with pathogenesis of Crohn’s disease. Inflamm Bowel Dis 20:315–328. https://doi.org/10.1097/01.MIB.0000437983.14544.d5
Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J (2001) Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294:1540–1543. https://doi.org/10.1126/science.1064890
Carroll M (2001) Innate immunity in the etiopathology of autoimmunity. Nat Immunol 2:1089. https://doi.org/10.1038/ni1201-1089
Miyagaki T, Fujimoto M, Sato S (2015) Regulatory B cells in human inflammatory and autoimmune diseases: from mouse models to clinical research. Int Immunol 27:495–504. https://doi.org/10.1093/intimm/dxv026
Mauri C, Bosma A (2012) Immune regulatory function of B cells. Annu Rev Immunol 30:221–241. https://doi.org/10.1146/annurev-immunol-020711-074934
Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA et al (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum 58:26–35. https://doi.org/10.1002/art.23176
Feldmann M, Brennan FM, Maini RN (1996) Rheumatoid arthritis. Cell 85:1277–1289. https://doi.org/10.1016/s0092-8674(00)81109-5
Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH et al (2006) Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest 116:961–973. https://doi.org/10.1172/JCI25422
Daien CI, Gailhac S, Mura T, Audo R, Combe B, Hahne M et al (2014) Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheum 66:2037–2046. https://doi.org/10.1002/art.38666
Cui D, Zhang L, Chen J, Zhu M, Hou L, Chen B et al (2015) Changes in regulatory B cells and their relationship with rheumatoid arthritis disease activity. Clin Exp Med 15:285–292. https://doi.org/10.1007/s10238-014-0310-9
Anderson MS, Bluestone JA (2005) The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 23:447–485. https://doi.org/10.1146/annurev.immunol.23.021704.115643
Silveira PA, Grey ST (2006) B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends Endocrinol Metab 17:128–135. https://doi.org/10.1016/j.tem.2006.03.006
Fox CJ, Danska JS (1998) Independent genetic regulation of T-cell and antigen-presenting cell participation in autoimmune islet inflammation. Diabetes 47:331–338. https://doi.org/10.2337/diabetes.47.3.331
Xiu Y, Wong CP, Bouaziz J-D, Hamaguchi Y, Wang Y, Pop SM et al (2008) B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in FcγR effector functions. J Immunol 180:2863–2875. https://doi.org/10.4049/jimmunol.180.5.2863
Mariño E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST (2009) CD4+ CD25+ T cells control autoimmunity in the absence of B cells. Diabetes. https://doi.org/10.2337/db08-1504
Smith SH, Tedder TF (2009) Targeting B-cells mitigates autoimmune diabetes in NOD mice: what is plan b? Diabetes 58:1479–1481. https://doi.org/10.2337/db09-0497
Hussain S, Delovitch TL (2007) Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol 179:7225–7232. https://doi.org/10.4049/jimmunol.1201427
Kleffel S, Vergani A, Tezza S, Ben Nasr M, Niewczas MA, Wong S et al (2015) Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens. Diabetes 64:158–171. https://doi.org/10.2337/db13-1639
Van Rensburg IC, Kleynhans L, Keyser A, Walzl G, Loxton AG (2017a) B-cells with a FasL expressing regulatory phenotype are induced following successful anti-tuberculosis treatment. Immun Inflamm Dis 5(1):57–67. https://doi.org/10.1002/iid3.140
Van Rensburg IC, Wagman C, Stanley K, Beltran C, Ronacher K, Walzl G, Loxton AG (2017b) Successful TB treatment induces B-cells expressing FASL and IL5RA mRNA. Oncotarget 8(2):2037. https://doi.org/10.18632/oncotarget.12184
Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Kennedy PT (2012) IL-10–producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol 189(8):3925–3935. https://doi.org/10.4049/jimmunol.1103139
Siewe B, Stapleton JT, Martinson J, Keshavarzian A, Kazmi N, Demarais PM, Landay A (2013) Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8+ T cell function in vitro. J Leukoc Biol 93(5):811–818. https://doi.org/10.1189/jlb.0912436
Lundy SK, Berlin AA, Martens TF, Lukacs NW (2005) Deficiency of regulatory B cells increases allergic airway inflammation. Inflamm Res 54(12):514–521. https://doi.org/10.1007/s00011-005-1387-0
Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG (2004) Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol 173(10):6346–6356. https://doi.org/10.4049/jimmunol.173.10.6346
Lee JH, Noh J, Noh G, Choi WS, Cho S, Lee SS (2011) Allergen-specific transforming growth factor-β-producing CD19 (+) CD5 (+) regulatory B-cell (Br3) responses in human late eczematous allergic reactions to cow’s milk. J Interf Cytokine Res 31(5):441–449. https://doi.org/10.1089/jir.2010.0020
Kamekura R, Shigehara K, Miyajima S, Jitsukawa S, Kawata K, Yamashita K et al (2015) Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol 158(2):204–211. https://doi.org/10.1016/j.clim.2015.02.016
Mauri C, Menon M (2017) Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest 127(3):772–779. https://doi.org/10.1172/JCI85113
Wiest M, Upchurch K, Hasan MM, Cardenas J, Lanier B, Millard M, Joo H (2019) Phenotypic and functional alterations of regulatory B cell subsets in adult allergic asthma patients. Clin Exp Allergy. https://doi.org/10.1111/cea.13439
Dar HY, Pal S, Shukla P, Mishra PK, Tomar GB, Chattopadhyay N et al (2018) Bacillus clausii inhibits bone loss by skewing Treg-Th17 cell equilibrium in postmenopausal osteoporotic mice model. Nutrition 54:118–128. https://doi.org/10.1016/j.nut.2018.02.013
Dar HY, Shukla P, Mishra PK, Anupam R, Mondal RK, Tomar GB et al (2018) Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep 8:46–56. https://doi.org/10.1016/j.bonr.2018.02.001
Dar HY, Azam Z, Anupam R, Mondal RK, Srivastava RK (2018) Osteoimmunology: the Nexus between bone and immune system. Front Biosci 23:464–492. https://doi.org/10.2741/4600
Dar HY, Singh A, Shukla P, Anupam R, Mondal RK, Mishra PK et al (2018) High dietary salt intake correlates with modulated Th17-Treg cell balance resulting in enhanced bone loss and impaired bone-microarchitecture in male mice. Sci Rep 8:2503. https://doi.org/10.1038/s41598-018-20896-y
Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN (2007) B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 109(9):3839–3848. https://doi.org/10.1182/blood-2006-07-037994
Srivastava RK, Dar HY, Mishra PK (2018) Immunoporosis: immunology of osteoporosis-role of T cells. Front Immunol 9:657. https://doi.org/10.3389/fimmu.2018.00657
Dilillo DJ, Matsushita T, Tedder TF (2010) B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci 1183:38–57. https://doi.org/10.1111/j.1749-6632.2009.05137.x
Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE (2005) Identification and characterization of circulating human transitional B cells. Blood 105(11):4390–4398. https://doi.org/10.1182/blood-2004-11-4284
Acknowledgment
This work was financially supported by projects: DST-SERB (EMR/2016/007158), Govt. of India and intramural project from All India Institute of Medical Sciences (AIIMS), New Delhi-India sanctioned to RKS; National Academy of Sciences (NASI), Allahabad-India sanctioned to GCM. HYD, LS, ZA, AB and RKS acknowledge the Department of Biotechnology, AIIMS, New Delhi-India for providing infrastructural facilities. ZA thanks Dr. Harisingh Gour Central University, Sagar (MP). LR and GCM acknowledge National Centre for Cell Science (NCCS), Pune-India for providing infrastructural facilities. HYD thanks ICMR for research fellowship. LR thanks NASI for research fellowship. LS and ZA thank the UGC and CSIR for their respective research fellowships.
Author Contributions
RKS and GCM suggested the focus and outline of the review and wrote the review. HYD, LR, LS and ZA participated in writing of the review. RKS suggested and HYD and ZA created the illustrations.
Conflicts of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dar, H.Y. et al. (2020). A Chronological Journey of Breg Subsets: Implications in Health and Disease. In: Singh, S. (eds) Systems and Synthetic Immunology . Springer, Singapore. https://doi.org/10.1007/978-981-15-3350-1_5
Download citation
DOI: https://doi.org/10.1007/978-981-15-3350-1_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3349-5
Online ISBN: 978-981-15-3350-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)