Abstract
Regulatory subsets of B cells (Bregs) modulate immune responses in autoimmunity, cancer, and other inflammation diseases. In the present study, we investigated the numbers of circulating Breg cells in patients with rheumatoid arthritis (RA). We evaluated 59 RA patients and 25 healthy controls. CD19+CD5+CD1dhi B cells and granzyme B-secreting B cells (CD19+CD5+GzmB+) were analyzed by flow cytometry in peripheral blood mononuclear cells. We detected serum interleukin 10 (IL-10), interleukin 21 (IL-21), granzyme B (GzmB) using enzyme-linked immunosorbent assay (ELISA). Compared to control subjects, we found a decreased proportion of CD19+CD5+CD1dhi B cells in RA patients. The number of CD19+CD5+CD1dhi B cells negatively correlated with DAS28 (P < 0.05). Moreover, serum IL-10 and IL-21 concentrations were significantly lower in RA patients compared to healthy controls (P < 0.05). Conversely, the number of CD19+CD5+GzmB+ B cells was significantly higher in RA patients (P < 0.05), and the number of CD19+CD5+GzmB+ B cells did not correlate with DAS28, IL-21, or GzmB (P > 0.05, all). Interestingly, IL-21 and GzmB levels positively correlated in RA patients (P < 0.05). Our data indicate that CD19+CD5+CD1dhi B cells influence RA disease activity. CD19+CD5+GzmB+ B cells may be involved in RA development and progression. Our data strongly suggest a role for Bregs in RA, and Bregs may be a viable therapeutic strategy for RA disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that is characterized by persistent inflammation in the synovium, which eventually leads to joint destruction and deformity [1, 2]. RA has a prevalence of approximately 0.5–1 % in the adult population [3]. Previous studies have shown that immune dysfunction is considered as the main pathogenesis in RA and involves various immune cells and inflammatory cytokines. The function of regulatory B cells (Bregs) is abnormal in inflammatory diseases. In this study, we explored the roles of Bregs in RA disease.
The term ‘regulatory B cells’, which defines B cell subsets with regulatory properties, was first introduced by Mizoguchi and Bhan in 2006 [4]. Similar to regulatory T cells (Treg), the regulatory function of B cells is exerted by the production of regulatory cytokines, such as IL-10 [5, 6], Fas ligand (FasL), transforming grown factor-β (TGF-β) [7], Foxp3 [8], and the ability to express inhibitory molecules that suppress pathogenic T cells and autoreactive B cells in a cell-to-cell contact-dependent manner [9]. Recent studies on autoimmune diseases have identified several new subsets of human B cells, including CD19+CD5+CD1dhi B cells, which have the potential ability to produce IL-10 and CD19+CD5+GzmB+ B cells, a new cell phenotype that secretes granzyme B (GzmB).
In collagen-immunized arthritis (CIA) mice, the cell-surface phenotype of murine IL-10-producing regulatory B cells is reported to be CD1dhiCD5+. There are significantly decreased numbers of CD19+CD5+CD1dhi B cells but increased IL-17-producing CD4+T (Th17) cells. However, after adoptive transfer of in vitro expanded CD1dhiCD5+ B cells, CIA mice show a marked delay of arthritis onset with reduced severity in both clinical symptoms and joint damage, accompanied by a substantial reduction in the number of proinflammatory Th17 cells [10]. It is therefore apparent that CD19+CD5+CD1dhi B cells are important for disease suppression in RA. CD19+CD5+CD1dhi B cell subsets have recently been identified in human [5]. Therefore, we aimed to elucidate the probable relation between the number of CD19+CD5+CD1dhi B cells and RA disease activity.
GzmB can induce target cell apoptosis, and several in vitro studies suggest that GzmB is involved in chondrocyte apoptosis and has important extracellular functions. Recent studies of chronic lymphocytic leukemia and systemic lupus erythematosus (SLE) have demonstrated CD5+ B cell subsets can express GzmB, and IL-21 can directly induce GzmB expression and secretion by CD5+ B cells in vitro [11, 12]. Furthermore, recent results in various solid tumors indicate that CD19+CD5+GzmB+ B cells can suppress CD4+ T cell proliferation [13]. These results suggest GzmB is a predominant regulatory molecule and an important novel marker of human Bregs. Moreover, CD19+CD5+GzmB+ cells may contribute to the suppression of anti-tumor immune responses and have important roles in autoimmune diseases. In RA patients, there are increased GzmB levels in serum and synovium. GzmB may be a useful prognostic marker in early RA and may provide important clues to the pathogenesis of the disease [14]. However, whether CD19+CD5+GzmB+ B cell numbers in RA patients are altered and related to disease activity, and whether the number of CD19+CD5+GzmB+ cells is related to IL-21, remain to be determined.
However, few studies have aimed to understand the biology of Bregs, particularly the role of GzmB-secreting B cells. In the present study, we aimed to determine the numbers of circulating CD19+CD5+CD1dhi B cells and CD19+CD5+GzmB+ B cells in RA patients with different disease activity. We also analyzed the relationship between Breg cells and regulatory cytokines.
Materials and methods
Patients and control subjects
The Ethics Committee of the Taizhou Hospital of Zhejiang Province approved this study. Written informed consent was obtained from all participants. From June 2013 to April 2014, 59 patients diagnosed with RA based on the 1987 American College of Rheumatology criteria [15] and 25 healthy controls were recruited for this study. The disease severity of each patient was evaluated based on the 28-joint disease activity score (DAS28) [16]. Patients were divided into three groups: the low activity (LA) group (DAS <3.2), the moderate activity (MA) group (3.2 <DAS <5.1), and the high activity (HA) group (DAS >5.1). The clinical laboratory markers of patients, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and rheumatoid factor (RF), were recorded. Patients with liver diseases, renal diseases, or other inflammatory or autoimmune diseases were excluded. The clinical features of all patients and healthy volunteers are provided in Table 1.
Clinical characteristic are presented as the mean ± standard error (SE), median (range), or number. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RF, rheumatoid factor; DAS28, 28-joint count disease activity score;
Blood samples and laboratory tests
Venous blood samples (5 mL) were collected into vacutainer tubes containing sodium lithium. Serum was obtained after centrifugation and stored at −80 °C. Whole blood differential counts and ESR were determined by routine blood examination (Alifax TEST I, Alifax S.p.A, Italy). The levels of high-sensitive C-reactive protein (hs-CRP) and RF were determined using a blood chemistry analyzer (IMMAGE800, Beckman Coulter).
Cell preparation and flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were immediately separated from freshly anticoagulated blood by Ficoll density gradient centrifugation (MD Pacific, China). After washing twice with phosphate-buffered saline (PBS) and adding 500 µL AIM-V media (MD Pacific), the absolute value of lymphocytes was determined using blood routine examinations and adjusting the concentration to have at least 1 × 106cells/mL. The Cell mixture (200 µL) and 100 µL pre-made brefeldin A (BFA; final concentration 1 µg/mL)/monensin solution (Multisciences, China) were cultured and incubated for at least 4 h in 96-well plates. Cells were maintained at 37 °C in 5 % CO2. Unstimulated PBMCs (106cells/tube) were stained in the dark for 20 min at room temperature with anti-CD19-Percp-CyTM5.5, anti-CD5-fluorescein isothiocyanate (FITC), and anti-CD1d-phycoerythrin (PE). Isotype IgG was added as a negative control. For GzmB analyses, stimulated PBMCs were incubated at room temperature for 20 min with Percp/FITC-labeled anti-human CD19/CD5. After surface staining, cells were stained with PE-labeled anti-human GrB (eBioscience) for Breg detection. Fixation and permeabilization were performed according to the manufacturer’s instructions (eBioscience). Isotype IgG-PE was used to show correct compensation and confirm antibody specificity. After washing with PBS, 500 µL 1 % paraformaldehyde solution was added to the tubes, and CD1d and GzmB expression were analyzed by flow cytometry. At least 30,000 cells were analyzed using Cell Quest software, version 3.3 (BD Bioscience).
ELISA detection of serum IL-10, IL-21, granzyme B
Serum cytokine IL-10, IL-21, GzmB concentrations in 59 RA patients and 25 healthy controls were determined using commercially available human ELISA kit (IL-10 and IL-21 ELISA kits from Multisciences; human GzmB Platinum ELISA kits from eBioscience). The detection limits for IL-10, IL-21, and GzmB were 0.59, 11.99, 0.2 pg/mL, respectively. Assays were preformed strictly following the manufacturer’s instructions. All samples were measured in duplicate.
Statistical analysis
Statistical analyses were performed using SPSS software (version 17.0, SPSS). Data are described as the mean ± standard error (SE). Statistical comparisons were performed by using Mann–Whitney U-tests. Correlation analyses between two parameters were performed using Spearman’s correlation coefficient. A two-sided P value <0.05 was considered statistically significant.
Results
Serum IL-10, IL-21, and GzmB concentrations in RA patients and healthy controls
To assess the cytokine microenvironment in the peripheral blood from patients with RA, we examined serum IL-10, IL-21, and GzmB levels in RA patients and healthy controls. Compared with healthy controls, the serum concentrations of IL-10 and IL-21 in RA patients were decreased (IL-10: 4.632 ± 0.380 vs. 3.563 ± 0.205 pg/mL, P = 0.0094; IL-21: 398.5 ± 25.28 vs. 320.9 ± 14.61 pg/mL, P = 0.0076) (Fig. 1a, b). However, GzmB levels in RA patients did not differ compared to controls (P = 0.4772) (Fig. 1c). Data are shown in Table 2.
Decreased numbers of CD19+CD5+CD1dhi B cells in RA patients and inversely correlation with RA disease activity
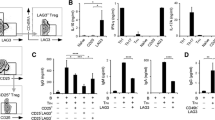
We observed that the percentage of CD19+CD5+CD1dhi B cells decreased in HA group (2.742 ± 0.432 %) and MA group (3.306 ± 0.418 %) compared with the healthy control (HC) group (4.208 ± 0.468 %) (P HA = 0.0038, P MA = 0.0409). However, there were no obvious differences between the three RA groups (P > 0.05) (Fig. 2B). Additionally, we analyzed the association between CD19+CD5+CD1dhi B cell numbers and clinical features in RA patients. Remarkably, the number of CD19+CD5+CD1dhi B cells negatively correlated with the DAS28 (r = −0.396, P = 0.005) (Fig. 4e). In addition, there were no statistical correlations between CD19+CD5+CD1dhi B cells and serum IL-10, ESR, CRP, RF levels (P > 0.05).
A Flow cytometry analyses of the percentage of circulating CD19+CD5+CD1dhi B cells in peripheral blood (PB). At least 30,000 events were analyzed for each sample. Data shown are representative charts. a Lymphocyte subsets were gated from isolated PBMCs by FCM. b CD19+ B subsets were gated by FCM. c Plots in the top right box represent CD19+CD5+ B cell subsets. d and e Representative pictures of CD1d expression in CD19+CD5+ B cell subsets from RA patients (2.8 %) and healthy controls (4.3 %), respectively. B: Frequency of circulating CD19+CD5+CD1dhi B cells is significantly higher in healthy controls (n = 25) compared to RA patients (n = 59). *P < 0.05; **P < 0.01
Increased numbers of CD19+CD5+GzmB+ cells in RA patients
We detected the expression of GzmB in CD19+CD5+ B cell subset in RA patients and healthy controls. In contrast to CD19+CD5+CD1dhi B cells, we observed increased percentages of CD19+CD5+GzmB+ B cells in RA patients compared to healthy controls (LA: 2.467 ± 0.418 %, P = 0.0328; MA: 2.200 ± 0.232 %, P = 0.0352; HA: 2.114 ± 0.212 %, P = 0.0446; HC:1.632 ± 0.191 %) (Fig. 3d). However, no statistically significant differences were observed between RA groups (P > 0.05).
C Flow cytometry analyses of the percentage of circulating CD19+CD5+GzmB+ B cells in peripheral blood (PB). At least 30,000 events were analyzed for each sample. Data shown are representative charts. a Green plots represent CD19+ B subsets that were gated by FCM. b Blue plots represent GzmB expressed in CD19− cell subsets (an internal control). c and d Representative pictures of GzmB expression in CD19+CD5+ B cell subsets from RA patients (2.7 %) and healthy controls (1.3 %), respectively. D: The frequency of circulating CD19+CD5+GzmB+ B cells was higher in RA patients (n = 47) compared to healthy controls (n = 22) *P < 0.05 (color figure online)
In addition, serum IL-21 and GzmB positively correlated in the RA group (r = 0.4316, P = 0.0048), (Fig. 4f). However, there was no statistical correlation between CD19+CD5+GzmB+ cells and DAS28 scores or serum IL-21 levels in RA patients (P > 0.05) (data not shown).
Discussion
Traditionally, B cells are considered to contribute to the development and progress of autoimmune disease due to their capability to produce antibodies. B cells can also facilitate CD4+T cells activation through antigen presentation and contribute to cytokines secretion and expression of co-stimulatory molecules [17]. However, certain B cells have recently been shown to be capable of negatively regulating the immune response by producing regulatory cytokines and directly interacting with pathogenic T cells via cell-to-cell contact [18]. Many different Breg phenotypes have been demonstrated in mouse models of autoimmunity as well as in humans. To our knowledge, IL-10-producing human B cells (B10) are not restricted to one subset. Several B cell subsets, including CD19+CD5+CD1dhi cells and CD27+CD24hi cells, are suggested to be precursors of B10 cells. Various stimuli increase the number of B10 cells in vitro, such as toll-like receptor (TLR) signaling, BCR signaling, and CD40 signaling [19].
In the present study, we found that the frequency of CD19+CD5+CD1dhi cells was significantly decreased in RA patients. Additionally, we found that the frequency of CD19+CD5+CD1dhi cells negatively correlated with the DAS28. Our results are consistent with the previous results from Ma et al. [20]. They also found that RA patients had significantly fewer CD19+CD5+CD1dhi cells; after drug therapy, however, the number of CD19+CD5+CD1dhi cells gradually increased. These results indicate that CD19+CD5+CD1dhi cells are negative regulators of the RA process and may contribute to RA pathogenesis. In addition, Ma et al. detected CD19+CD5+CD1dhi B cells after phorbol myristate acetate (PMA) and ionomycine stimulation of PBMCs from RA patients (the mean number of cells was approximately 250 per mL). Based on our initial counts of isolated PBMCs per milliliter of blood from RA patients, we found that the absolute numbers of CD19+CD5+CD1dhi B cells ranged from 40 to 850 per mL (298 ± 205 per mL). In addition, Daien et al. [21] recently reported that the number of IL-10 CD19+ cells were higher after stimulation with CpG + PIB, but were not specific to the CD5+CD1dhi phenotype. Thus, it is critical to note the stimulation method, and different stimulation protocols require further study.
CD5+CD1dhi B cells that are capable of producing IL-10 are the predominant B cell source of IL-10. IL-10 promotes the proliferation of antigen-specific B cell [22, 23]. IL-10 release by B10 cells is pivotal for Foxp3+Tregs resulting in immune regulation at the site of inflammation, such as the joint and synovium [19]. Consistent with previous data [24], we found that the concentration of serum IL-10 in RA patients was less than in healthy controls. We also found that CD19+CD5+CD1dhi B cells were not related to serum IL-10 in normal controls or RA patients. To the best of our knowledge, IL-10 is a multifunctional cytokine that is secreted by a variety of cells. IL-10 exerts its biological functions through various pathways, and others signaling members might also adjust IL-10 production. In addition, most in vitro experiments only found an interaction between B10 cells and IL-10, which may be influenced by other factors in vivo. Therefore, these results still need to be clarified.
At present, the mechanism of activation of GzmB-expressing B cells remains undefined, with few studies related to CD19+CD5+GzmB+ cells involved in autoimmune diseases. To our knowledge, this is the first study to describe CD19+CD5+GzmB+ cells in PBMCs from RA patients and to analyze the relationships between CD19+CD5+GzmB+ cell numbers and serum IL-21 and GzmB levels. It is difficult to directly detect CD19+CD5+GzmB+ cells in PBMCs; however, when we stimulated PBMCs with BFA/monensin, the percentage of CD19+CD5+GzmB+ cells in PBMCs significantly increased. Our data show that GzmB-expressing B cells are present in the peripheral blood of healthy controls and their frequency is increased in RA patients. We also found that CD19+CD5+GzmB+ cells have a tendency to decline with increased RA disease activity; however, no significant correlation between CD19+CD5+GzmB+ cells and DAS28 score were observed. The reason for this discrepancy remains unknown but could reflect the complicated functions of GzmB-expressing B cells. Recent studies have shown that GzmB-expressing B cells can induce target cells death and suppress CD4+T cell proliferation [11–13]. Upon activation, B cells can differentiate into various subpopulations, bearing specific surface markers and exhibiting either regulatory or inflammatory properties. We suggest that CD19+CD5+GzmB+ cells contribute to either the suppression or amplification of inflammatory signals that depend on the inflammatory context. In the initial stage of RA disease, many original B cells are activated and differentiated into GzmB-expressing B cells. As the disease progresses, B cells function is impaired and their numbers decline. In addition, differentiation of naive B cells into GzmB-expressing B cells is promoted by CD4+T cells via a mechanism that is strictly dependent on IL-21 [25]. Because serum IL-21 is decreased in RA patients in our study, we cannot explain this discrepancy. However, studies in synovial fluid could help confirm the role of CD19+CD5+GzmB+ cells in the pathogenesis of RA disease. Synovial fluid is difficult to obtain considering the clinical characteristics of our patients. Taken together, our results indicate that GzmB represents a predominant regulatory molecule and a novel marker of human Bregs. CD19+CD5+GzmB+ cells are significant for the initiation of RA.
IL-21 has a pleiotropic role in immunity by controlling B cell functions. In certain cases, IL-21 can induce B cell proliferation, survival, differentiation into plasma cells, or isotype switching [26]. In other situations, B cells rather undergo apoptosis and cell cycle arrest after IL-21 stimulation [27]. In this study, we attempted to explore the relationship between IL-21 and Bregs. Compared with healthy individuals, RA patients’ serum IL-21 levels were obviously decreased, but the correlation between serum IL-21 and Bregs was not significant. However, we found that IL-21 and IL-10 in serum showed a weak positive correlation (r = 0.262, P = 0.049), due to limitation of sample size, which remain to be further studied. It is interesting that one recent mouse study proved that stimulation with IL-21 purified B cells induced a threefold increase in IL-10 + B cells within the spleen CD5+CD1dhi B cell subset and 4.4- to 5.3-fold more IL-10 secretion [28]. Therefore, it is noteworthy how IL-21 controls Bregs expansion and cytokines secretion in vivo.
GzmB is an enzymatic component of cytotoxic granules produced by CTL and NK cells that mediates caspase cleavage and initiates apoptosis in virus-infected cells [29]. There were markedly increased levels of soluble GzmB in plasma and synovial fluid from RA patients. As an independent variable, GzmB could help correctly predict 84 % of RA patients [30]. In our study, we found that serum GzmB levels did not significantly increase; in contrast, serum GzmB levels were reduced in low activity RA patients. Moreover, we found that serum IL-21 and GzmB were positively correlated, which is consistent with the results in SLE [11].
In summary, different stimulation methods can be used to improve the expression of Bregs and there is no unified standard. In this study, we measured the number of CD19+CD5+CD1dhi B cells without stimulation, as well as the number of CD19+CD5+GzmB+ cells with BFA/monensin stimulation. Bregs may provide a novel and effective treatment option for autoimmune diseases. In particular, IL-10 and IL-21 may modulate immune responses through complicated mechanisms that remain to be determined. Our data indicate that CD19+CD5+CD1dhi B cells influence RA disease activity. CD19+CD5+GzmB+ B cells may be involved in the development and progression of RA and may be viable therapeutic strategy for RA disease.
References
Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10.
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61.
Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108.
Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–10.
Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10 competent B cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–41.
Flores-Borja F, Bosma A, Mauri C, et al. CD19 + CD24hiCD38hi B cells Maintain Regulatory T Cells While Limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;V5N173:173ra23.
Lee JH, Noh J, Noh G, et al. Allergen-specific transforming growth factor-β-producing CD19(+)CD5(+)regulatory B cell(Br 3) responses in human late eczematous allergic reactions to cow’s milk. J Interferon Cytokine Res. 2011;31:441–9.
Noh J, Choi WS, Noh S, et al. Presence of Foxp3-expressing CD19(+)CD5(+) B cells in human peripheral blood mononuclear cells: human CD19(+)CD5(+)Foxp3(+) regulatory B cell (Breg). Immune Netw. 2010;10:247–9.
Lundy SK. Killer B lymphocytes: the evidence and the potential. Inflamm Res. 2009;58(7):345–57.
Yang M, Den J, Liu J, et al. IL-10-producing regulatory B10 cells sameliorate collagen induced arthritis via suppressing Th17 cell generation. Am J Pathol. 2012;180(6):2375–85.
Hagn M, Ebel V, Sontheimer K, et al. CD5 + B cells from individuals with systemic lupus erythematosus express granzyme B. Eur J Immunol. 2010;40(7):2060–9.
Jahrsdörfer B, Blackwell SE, Wooldridge JE, et al. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 2006;108(8):2712–9.
Lindner S, Dahlke K, Sontheimer K, et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 2013;73(8):2468–9.
Goldbach-Mansky R, Suson S, et al. Raised granzyme B levels are associated with erosions in patients with early rheumatoid factor positive rheumatoid arthritis. Ann Rheum Dis. 2005;64(5):715–21.
Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
Prevoo ML, Van’t Hof MA, Kuper HH, et al. Modified disease activity scores that includes twenty-eight joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8.
Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells(B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15(Suppl 1):S1.
Yang M, Rui K, Wang S, et al. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10(2):122–32.
Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–41.
Ma L, Liu B, Jiang Z, et al. Reduced numbers of regulatory B cells are negatively correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Rheumatol. 2014;33(2):187–95.
Daien CI, Gailhac S, et al. (2014) Regulatory B10 cels are decreased in patients with rheumatoid arthritis and inversely correlated with disease activity.Arthritis Rheumatol. [Epup ahead of print].
DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57.
Tian G, Li JL, Wang DG, et al. (2014) Targeting IL-10 in Auto-immune Diseases. Cell Biochem Biophys s12013-14-9903-x.
Chen R, Tao Y, Qiu K, et al. Association of circulating Treg cells with disease activity in patients with rheumatoid arthritis. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32(6):886–9.
Hagn M, Sontheimer K, Dahlke K, et al. Human B cells differentiate into granzyme B-secreting cytotoxic B lymphocytes upon incomplete T-cell help. Immunol Cell Biol. 2012;90(4):457–67.
Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–4.
Konforte D, Simard N, Paige CJ. IL-21: an executor of B cell fate. J Immunol. 2009;182:1781–7.
Yoshizaki A, Miyagaki T, Tedder TF. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–8.
Trapani JA, Sutton VR. Granzyme B: pro-apoptotic, antiviral and antitumor functions. Curr Opin Immunol. 2003;15(5):533–43.
Tak PP, Spaeny-Dekking, et al. The levels of soluble granzyme A and B are elevated in plasma and synovial fluid of patients with rheumatoid arthritis (RA). Clin Exp Immunol 1999; 116(2): 366–70.
Acknowledgments
This study was supported by Grants from the Zhejiang Province Natural Science Fund (LY12H10002). We thank Prof. Jahrsdörfer, Thamara Beyer for helpful advice.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, D., Zhang, L., Chen, J. et al. Changes in regulatory B cells and their relationship with rheumatoid arthritis disease activity. Clin Exp Med 15, 285–292 (2015). https://doi.org/10.1007/s10238-014-0310-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-014-0310-9