Abstract
Quinones serve as redox-active cofactors in photosynthetic reaction centers. To understand the energetics of electron transfer along the electron transfer pathways in protein environments, the redox potentials (Em) of the cofactors in water versus normal hydrogen electrode (NHE) are required. However, ubiquinone, menaquinone (phylloquinone), and plastoquinone, which are found in photosynthetic reaction centers, have insoluble hydrophobic isoprene side chains, and thus far only Em in dimethylformamide (DMF) versus saturated calomel electrode (SCE) had been reported. Recently, Em in water versus NHE was reported for the quinone species of photosynthetic reaction centers. These results confirmed that Em(Q/Q●−) in water versus NHE was more relevant to Em(Q/Q●−) in protein environments than Em(Q/Q●−) in DMF versus SCE. It has also been demonstrated that Em for one-electron reduction can also be calculated based on the lowest unoccupied molecular orbital (LUMO) level of the quinone molecules.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bacterial photosynthetic reaction centers
- Blastochloris viridis
- Cytochrome b6 f
- Cytochrome bc1
- Photosystem II
- Rhodobacter sphaeroides
1 Introduction
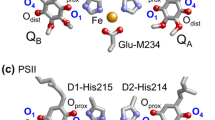
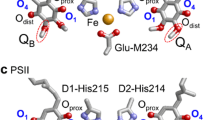
Quinones are redox-active cofactors in many photosynthetic reaction centers. Ubiquinone serves as an electron acceptor at the QA and QB binding sites in photosynthetic reaction centers of purple bacteria (PbRC) from Rhodobacter sphaeroides and as an electron donor in cytochrome bc1. Menaquinone (vitamin K2) is the acceptor at the QA site in PbRC from Blastochloris viridis, whereas phylloquinone (vitamin K1) is the active center at the A1A and A1B sites in photosystem I (PSI). Plastoquinone serves as an electron acceptor at the QA and QB sites in photosystem II (PSII) (Fig. 1) (Robinson and Crofts 1984; Rutherford et al. 1984; Okamura et al. 2000; Brettel and Leibl 2001; Wraight 2004) and as an electron donor in cytochrome b6f.
Prince et al. measured the redox potential for one-electron reduction, Em(Q/Q●−), of 1,4-quinones, including ubiquinone, menaquinone (phylloquinone), and plastoquinone, in dimethylformamide (DMF) versus saturated calomel electrode (SCE) (Prince et al. 1983). Swallow also measured Em(Q/Q●−) for small 1,4-quinones in water versus normal hydrogen electrode (NHE) (Swallow 1982). As mentioned (Kishi et al. 2017), experimentally measured Em(Q/Q●−) in DMF versus SCE can be practically converted to Em(Q/Q●−) in water versus NHE by adding 480 mV.
2 Em for Quinones in Water and in Protein Environments
Kishi et al. reported the Em(Q/Q●−) values in water versus NHE as −163 mV for ubiquinone, −260 mV for menaquinone (phylloquinone), and −154 mV for plastoquinone (Table 1) by quantum chemical calculation of the free energy difference between the neutral state Q and the reduced state Q●− in the aqueous phase (Kishi et al. 2017). Before that study, it was a matter of debate whether Em(Q/Q●−) for quinone in DMF could be relevant to calculate the Em values in photosynthetic reaction centers when using theoretical approaches, namely, electrostatic calculations. Notably, in electrostatic calculations, only the difference between the Em of quinone in bulk water and quinone in the protein environment can be computed. Thus, to obtain, for example, Em(A1) in PSI, it is necessary to determine the Em(Q/Q●−) for phylloquinone in a reference system (preferentially in water) and add the calculated Em difference.
Previously, to calculate Em(A1) in PSI, Ptushenko et al. used Em(Q/Q●−) = −800 mV for phylloquinone in DMF versus NHE, by assuming a liquid junction potential between SCE in DMF and NHE in water (Ptushenko et al. 2008). However, it should be noted that Em(Q/Q●−) for quinones in the two systems differs by 600 mV even in the absence of the liquid junction potential, i.e., the discrepancy between the Em(Q/Q●−) values in the two systems cannot be explained by the liquid junction potential, as previously demonstrated (Kishi et al. 2017).
Although not clearly stated by Ptushenko et al. (2008), it seems likely that in their computational model, the electrostatic interaction between the PSI protein environment and the A1 phylloquinone molecule was originally underestimated and that they needed the unusually low Em value of −800 mV for phylloquinone as a reference, mainly to reproduce the reported low Em(A1) in PSI (e.g., −810 mV (Vos and van Gorkom 1990), −754 mV (Iwaki and Itoh 1994), and −700 mV (Brettel and Leibl 2001)). Using the unusually low Em value of −800 mV for phylloquinone in DMF versus NHE allowed them to conveniently match their calculated value to the reported low Em(A1) value. However, using the unusually low Em value would simultaneously cause a problem in reproducing the Em(QA) of −150 mV for the same quinone species (menaquinone) in PbRC (Brettel and Leibl 2001). That is, they must explain how the PbRC protein environment is able to increase Em(Q/Q●−) = −800 mV in DMF versus NHE for phylloquinone to −150 mV at the QA site in the PbRC protein environment. Obviously, this is impossible in the PbRC protein electrostatic environment, as demonstrated in numerous electrostatic calculations (Rabenstein et al. 1998; Ishikita and Knapp 2004; Zhu and Gunner 2005). It seems plausible that the Em values measured in water versus NHE (−260 mV for menaquinone (phylloquinone)) (Kishi et al. 2017) are more relevant to the Em values in proteins than the Em values measured in DMF versus SCE (unless the proteins are solvated in DMF).
This fact would be more understandable when considering Em of QB near the protein bulk surface in PbRC and PSII. Em(Q/Q●−) is −154 mV for plastoquinone in water versus NHE, which is more consistent with Em(QB) = +90 mV versus NHE in PSII determined using spectroelectrochemistry (Kato et al. 2016) than Em(QB) ≈ −750 mV in DMF versus NHE (Kishi et al. 2017). Again, these results confirm that Em(Q/Q●−) in water versus NHE is more relevant to Em(Q/Q●−) in protein environments than Em(Q/Q●−) in DMF versus SCE.
3 Alternative Approach for Calculating Em of Quinones and Other Cofactors
There are other approaches for calculating Em of redox-active groups isolated in a solvent, including quinone molecules. As the basis of quantum chemistry, the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels are associated with Em for one-electron oxidation and for one-electron reduction, respectively (Watanabe and Kobayashi 1991). Indeed, the experimentally measured Em for ten 1,4-quinones in dimethylformamide (DMF) versus SCE (Prince et al. 1983) is strongly correlated with the LUMO level of quinone in the neutral state in the aqueous phase (coefficient of determination R2 = 0.97, Fig. 2).
Correlation between experimentally measured Em for ten 1,4-quinones in dimethylformamide (DMF) versus saturated calomel electrode (SCE) (Prince et al. 1983) and calculated LUMO energy level (ELUMO) for neutral quinones (coefficient of determination R2 = 0.97). The 1,4-quinones shown are 1,4-benzoquinone, methyl-1,4-benzoquinone, 2,3-dimethyl-1,4-benzoquinone, 2,5-dimethyl-1,4-benzoquinone, 2,6-dimethyl-1,4-benzoquinone, trimethyl-1,4-benzoquinone, tetramethyl-1,4-benzoquinone, 1,4-naphthoquinone, 2-methyl-1,4-naphthoquinone, and 2,3-dimethyl-1,4-naphthoquinone. ELUMO can be calculated using a quantum chemical approach. We employed the restricted density functional theory (DFT) method with the B3LYP functional and 6-31g++∗∗ basis sets for Q (S = 0), using the Gaussian (Frisch et al. 2004) program code with the PCM method for DMF. Solvent molecules were considered implicitly, using the SCRF = water option and the SCRF = dimethylformamide option with dielectric constants of 78.355 for water and 37.219 for DMF (i.e., default values). (For further details, including the atomic coordinates, see ref. Kishi et al. 2017)
The MO-based approach presented herein requires quantum chemical calculation of the neutral state only, whereas the previous approach reported by Kishi et al. (2017) requires quantum chemical calculation of both the neutral and reduced states. The strong correlation between the calculated Em values and the LUMO energy levels indicates that the Em values are in fact determined by the molecular structures in the neutral states (prior to reduction of the quinones) and that structural changes that may be induced in response to reduction of the quinones are negligibly small in terms of Em. This approach can also be applied to other redox-active cofactors, e.g., chlorophylls (Watanabe and Kobayashi 1991).
References
Brettel, K., & Leibl, W. (2001). Electron transfer in photosystem I. Biochimica et Biophysica Acta, 1507, 100–114.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Montgomery, J., A, J., Vreven, T., Kudin, K. N., Burant, J. C., Millam, J. M., Iyengar, S. S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G. A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J. E., Hratchian, H. P., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Ayala, P. Y., Morokuma, K., Voth, G. A., Salvador, P., Dannenberg, J. J., Zakrzewski, V. G., Dapprich, S., Daniels, A. D., Strain, M. C., Farkas, O., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Ortiz, J. V., Cui, Q., Baboul, A. G., Clifford, S., Cioslowski, J., Stefanov, B. B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Gonzalez, C., & Pople, J. A. (2004). Gaussian 03. Wallingford: Gaussian, Inc.
Ishikita, H., & Knapp, E.-W. (2004). Variation of Ser-L223 hydrogen bonding with the QB redox state in reaction centers from Rhodobacter sphaeroides. Journal of the American Chemical Society, 126, 8059–8064.
Iwaki, M., & Itoh, S. (1994). Reaction of reconstituted acceptor quinone and dynamic equilibration of electron transfer in the photosystem I reaction center. Plant & Cell Physiology, 35, 983–993.
Kato, Y., Nagao, R., & Noguchi, T. (2016). Redox potential of the terminal quinone electron acceptor QB in photosystem II reveals the mechanism of electron transfer regulation. Proceedings of the National Academy of Sciences of the United States of America, 113, 620–625.
Kishi, S., Saito, K., Kato, Y., & Ishikita, H. (2017). Redox potentials of ubiquinone, menaquinone, phylloquinone, and plastoquinone in aqueous solution. Photosynthesis Research, 134, 193–200.
Okamura, M. Y., Paddock, M. L., Graige, M. S., & Feher, G. (2000). Proton and electron transfer in bacterial reaction centers. Biochimica et Biophysica Acta, 1458, 148–163.
Prince, R. C., Dutton, P. L., & Bruce, J. M. (1983). Electrochemistry of ubiquinones: Menaquinones and plastoquinones in aprotic solvents. FEBS Letters, 160, 273–276.
Ptushenko, V. V., Cherepanov, D. A., Krishtalik, L. I., & Semenov, A. Y. (2008). Semi-continuum electrostatic calculations of redox potentials in photosystem I. Photosynthesis Research, 97, 55–74.
Rabenstein, B., Ullmann, G. M., & Knapp, E.-W. (1998). Energetics of electron-transfer and protonation reactions of the quinones in the photosynthetic reaction center of Rhodopseudomonas viridis. Biochemistry, 37, 2488–2495.
Robinson, H. H., & Crofts, A. R. (1984). Kinetics of proton uptake and the oxidation-reduction reactions of the quinone acceptor complex of photosystem II from pea chloroplasts. In C. Sybesma (Ed.), Advances in photosynthesis research (Vol. 1, pp. 477–480). The Hague: Martinus Nijhoff/Dr. W. Junk Publishers.
Rutherford, A. W., Renger, G., Koike, H., & Inoue, Y. (1984). Thermoluminescence as a probe of photosystem II. The redox and protonation states of the secondary acceptor quinone and the O2-evolving enzyme. Biochimica et Biophysica Acta, 767, 548–556.
Swallow, A. J. (1982). Physical chemistry of semiquinones. In B. L. Trumpower (Ed.), Function of quinones in energy conserving systems (pp. 59–72). New York: Academic Press.
Vos, M. H., & Van Gorkom, H. J. (1990). Thermodynamical and structural information on photosynthetic systems obtained from electroluminescence kinetics. Biophysical Journal, 58, 1547–1555.
Watanabe, T., & Kobayashi, M. (1991). Electrochemistry of chlorophylls. In H. Scheer (Ed.), Chlorophylls (pp. 287–303). Boca Raton: CRC Press.
Wraight, C. A. (2004). Proton and electron transfer in the acceptor quinone complex of photosynthetic reaction centers from Rhodobacter sphaeroides. Frontiers in Bioscience, 9, 309–337.
Zhu, Z., & Gunner, M. R. (2005). Energetics of quinone-dependent electron and proton transfers in Rhodobacter sphaeroides photosynthetic reaction centers. Biochemistry, 44, 82–96.
Acknowledgments
This research was supported by JST CREST (JPMJCR1656), JSPS KAKENHI (18H01186 to K.S., JP16H06560 to K.S. and H.I., and JP26105012 to H.I.), Japan Agency for Medical Research and Development (AMED), Materials Integration for engineering polymers of Cross-ministerial Strategic Innovation Promotion Program (SIP), and Interdisciplinary Computational Science Program in CCS, University of Tsukuba.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ishikita, H., Saito, K. (2020). Redox Potentials of Quinones in Aqueous Solution: Relevance to Redox Potentials in Protein Environments. In: Wang, Q. (eds) Microbial Photosynthesis. Springer, Singapore. https://doi.org/10.1007/978-981-15-3110-1_5
Download citation
DOI: https://doi.org/10.1007/978-981-15-3110-1_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-3109-5
Online ISBN: 978-981-15-3110-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)