Abstract
This project explores the use of grinding aids to control the resulting particle-size distribution of calcined clay and limestone. It was observed that after grinding, calcined clays exhibit a strongly bimodal particle-size distribution, where the clay minerals concentrate mainly in the finer particle population. A significant increase in compressive strength at early age was observed in systems incorporating alkanolamines. The effect appears to be restricted to the aluminate reaction, as the silicate peak remains virtually unmodified. Particle classification (air separation) techniques were applied to remove the impurities (mainly quartz and iron oxides) and, therefore, increase the amount of kaolinite in the resulting material. An increase in the kaolinite content from 29 to 45% by mass was achieved in one step and without pre-dispersion of the particles.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Calcined clays are a promising opportunity to lower clinker levels in cements because of their widespread availability and their excellent reactivity in blended cements. The combination of metakaolin and limestone in OPC-based systems produces a synergy that enables the production of high-performance cement with a significantly lower clinker factor. Clays are mixtures of clay minerals (such as kaolinite, illite and montmorillonite) and other impurities, such as quartz, iron oxide and other rock forming minerals. Due to this inherent heterogeneity of the material, the grinding of clays results in a characteristic bimodal particle-size distribution. This characteristic distribution is observed when grinding both raw and calcined clay and also at industrial-scale grinding setups. This opens the possibility of applying particle classification processes to increase the kaolinite content in low-grade clays.
Grinding aids (GAs) are incorporated during comminution of clinker to reduce electrostatic forces and minimize agglomeration of clinker and SCM grains [1]. Such additions are commonly used to increase cement fineness and compressive strength for given specific energy consumption (Ec) of the grinding mill [1,2,3]. After the grinding process, GAs may not preserve their original molecule structures. However, they do remain adsorbed onto the cement particles to entail variations of cement properties whether in the fresh or hardened state [1]. Thus, grinding aids may have beneficial effects on rheology and hydration of limestone calcined clay cements (LC3).
2 Materials and Methods
The effect of grinding aids on the agglomeration of calcined clay during grinding was studied using unground calcined clay passing #8 sieve, and a lab-scale rotary jar mill. The clay had a calcined kaolinite content of 62%. The grinding was performed in controlled conditions of time and load of the mill, incorporating different dosages of commercial grinding aids based on polycarboxylate ether (PCE), glycol or amines. As the clay was calcined before grinding, the workability of LC3 cast using these clays was assessed using the mini-cone slump test, while hydration kinetics was studied using isothermal calorimetry.
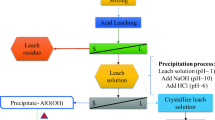
To assess the potential of particle classification as a mean to increase the kaolinite content of ground clays, a natural clay with 30% kaolinite content was selected. Two different particle classification techniques, gravimetric precipitation and air separation, were applied over the same material. The first one is a classic and accurate method normally used by geologists on soil science, while the second one has the biggest potential for industrial scalability. The procedure was applied to a previously grounded and dried batch of raw clay. A Hermle Z 206 lab centrifuge was used with 50 mL tubes. The clay dispersion was composed of raw ground clay as solute and sodium metaphosphate (0.1%) aqueous solution in water-to-solid ratio of 6. At the end of the centrifugation, the liquid solution was placed in plastic container. The containers were placed in a 60 °C oven to speed-up the evaporation and to allow the retrieval of the dry samples. The gravimetric separation was performed to obtain 5 batches with their own target separation size (the limit size between particles sedimented and in suspension): 0.8, 3, 5, 11 and 50 microns. The time required to achieve the desired separation limit was established using the Stoke’s law adapted for centrifugation [4].
Cement plants normally perform grinding in close circuit configuration, combining a mill with an air classifier that controls the particle size of the output. In order to demonstrate the potential for clay concentration using this technology, a lab-scale air separator was used. The speed of the classifier was initially adjusted using a limestone calibration curve. Afterwards, a clay curve was constructed based on experimental results. The reactivity of the fine and rejected (coarse) fractions of clay was analysed by means of the R3 test [5], while their physical (particle-size distribution by laser diffraction and specific surface area by nitrogen adsorption) and chemical (kaolinite content by TGA) properties were also assessed.
3 Results and Discussion
3.1 Effect of the Incorporation of Grinding Aids in the Agglomeration of Clay During Grinding
Calcined clay was ground in the conditions described above for 60 min in order to observe the effects of grinding aids incorporation on the strong agglomeration observed in clays without the addition of these molecules. The three commercial grinding aids were included in the dosages recommended by the manufacturer. As observed in Fig. 1, all of the products used showed a strong effect on reducing the clay covering layer of the mill walls and grinding media, which impacts the efficiency of the process. Particle-size distribution of samples collected every 15 min during the grinding process allowed to observe that fine clay is obtained faster with the use of grinding aids. However, the ultimate fineness is similar as this is mainly controlled by the mill geometry and the grinding media load.
Particle-size distribution and specific surface area measurements of the samples were collected every 15 min and compared. It was observed that the increase in the surface area as a function of particle size is almost negligible (2% increase of surface area, for a DV50 reduction from 50 to 10 m), in contrast to what is normally observed in cement and other SCMs. This is explained as the main source of surface area from clays is their internal porosity, which is independent of the particle size over the range explored in this study.
3.2 Effect of the Incorporation of Grinding Aids on Workability and Hydration
The effect of the grinding aids addition on the workability of LC3 is illustrated on Fig. 2. Results are presented as slump versus addition of a commercial PCE superplasticizer, and compared against a reference OPC w/c 0.5 mixture. As observed, the incorporation of some grinding aids can improve the flowability of LC3 to levels approaching the behaviour of OPC. The most effective grinding aid is the PCE based. For this reason, another batch of clay was ground with an increased dosage of this product to see if the effects could be further increased. Thus, the addition of PCE both in the solids or during mixing as a regular superplasticizer is effective to increase the slump of LC3-based mixtures.
Regarding the effects of grinding aids on LC3 hydration, it was observed that alkanolamines (TEA, TIPA and DEIPA) have a strong effect on the intensity of the aluminate (second) peak of hydration. The effect seems to be more intense in the system containing DEIPA, followed by TIPA and finally TEA. Previous research has suggested that this heat increase is linked to the complexation of iron contained in calcined clay, preventing the formation of a solid ferrous hydroxide phase that prevents further dissolution of clay. In order to confirm this hypothesis, model systems with synthetic iron-free clay (prepared by mixing quartz and pure metakaolin to match the grade of the natural clay used) and also incorporating white OPC instead of grey cement were tested. Each system was intended to test the effect of alkanolamines on the aluminate peak in LC3 mixtures with iron-free clays and iron-free clays and cement. Results show that in the iron-free clay system, the effects of TEA, TIPA and DEIPA are comparable as the one observed in the natural (iron rich) clay LC3. On the other hand, the complete iron-free system (synthetic clay and white OPC) showed a very minor effect of the molecules studied. This confirms that the interaction is indeed related to the presence of iron, but not sourced from calcined clay. In contrast, the effect appears to be linked to the iron contained in cement, in particular, C4AF.
3.3 Particle Classification of Ground Clay to Increase the Kaolinite Content
The measurements of kaolinite content on the fine fraction collected after gravimetric separation showed that it is concentrated in the fine fraction of the particle-size distribution, corresponding typical first bump observed between about 0.1 and 1 m. This is linked to the different grindability of kaolinite and the impurities normally found on clays (quartz and rock forming minerals). In this regard, clay minerals become finer quicker generating the characteristic bimodal distribution, while the impurities remain concentrated in the coarser fraction. Thus, particle classification techniques based on size difference could provide a technically suitable way to concentrate kaolinite in low-grade natural clays.
A consolidated plot containing the results of characterization of the fine fraction obtained by air separation is shown on Fig. 3. It can be observed that, as gravimetric separation, air separation is effecting in removing the impurities (mainly quartz) from the raw materials and concentrating the kaolinite in the remaining one. The amount of kaolinite was increased from 30% in the raw clay to about 45% under the most restrictive conditions. However, the reactivity as measured by the R3 test shows an increase on the heat release at 24 h from 70 to 130 J/g of solids. This is explained due to the simultaneous increase of kaolinite content and specific surface area of the final material as compared to the initial clay.
4 Conclusions
Based on the presented results, the following conclusions can be drawn:
-
The incorporation of grinding aids effectively reduces the agglomeration of clays upon grinding. This leads to a reduction of the time required to reach a fine material suitable for LC3 manufacture under the same grinding energy conditions.
-
The incorporation of grinding aids in calcined clay can lead in some cases to significant improvements in the flowability of fresh LC3.
-
Alkanolamines have a strong impact on the aluminate reaction during LC3 hydration. This effect is linked to the interaction between alkanolamines and iron-bearing phases of OPC.
-
Kaolinite is concentrated in the finer portion of the particle-size distribution of ground clays. Particle classification techniques can be used to remove part of the (coarser) impurities in order to increase the kaolinite content of the final material.
References
Assaad, J.J., Issa, C.A.: Effect of clinker grinding aids on flow of cement-based materials. Cem. Concr. Res. 63, 1–11 (2014). https://doi.org/10.1016/j.cemconres.2014.04.006
Katsioti, M., Tsakiridis, P.E., Giannatos, P., Tsibouki, Z., Marinos, J.: Characterization of various cement grinding aids and their impact on grindability and cement performance. Constr. Build. Mater. 23, 1954–1959 (2009). https://doi.org/10.1016/j.conbuildmat.2008.09.003
Sohoni, S., Sridhar, R., Mandal, G.: The effect of grinding aids on the fine grinding of limestone, quartz and Portland cement clinker. Powder Technol. 67, 277–286 (1991). https://doi.org/10.1016/0032-5910(91)80109-V
Hathaway, J.C.: Procedure for clay mineral analyses used in the sedimentary petrology laboratory of the USGS. US. Geol. Surv. Methods, 8–13 (1955)
Avet, F., Snellings, R., Alujas Diaz, A., Ben Haha, M., Scrivener, K.: Development of a new rapid, relevant and reliable (R3) test method to evaluate the pozzolanic reactivity of calcined kaolinitic clays. Cem. Concr. Res. 85, 1–11 (2016). https://doi.org/10.1016/j.cemconres.2016.02.015
Acknowledgements
The authors would like to acknowledge financial support by the Swiss Agency of Development and Cooperation (SDC) grant 81026665. The Swiss federal commission for scholarships for foreign students (FCS) is acknowledged for supporting Franco Zunino’s studies through scholarship 2016.0719.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 RILEM
About this paper
Cite this paper
Zunino, F., Scrivener, K.L. (2020). Improving the Behaviour of Calcined Clay as Supplementary Cementitious Materials by a Combination of Controlled Grinding and Particle Selection. In: Bishnoi, S. (eds) Calcined Clays for Sustainable Concrete. RILEM Bookseries, vol 25. Springer, Singapore. https://doi.org/10.1007/978-981-15-2806-4_18
Download citation
DOI: https://doi.org/10.1007/978-981-15-2806-4_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2805-7
Online ISBN: 978-981-15-2806-4
eBook Packages: EngineeringEngineering (R0)