Abstract
Preimplantation genetic diagnosis technique has been changed in the past few years from a cytogenetic technique that analyzes a limited number of chromosomes targeting only one or two points to a molecular diagnostic technique that permits to analyze a complete comprehensive chromosome screening targeting thousands of points in each chromosome. That, together with a huge increase in culture conditions on the laboratory (low pressure oxygen incubators, single culture medium, vitrification protocols, and time-lapse), has change the rules of the game. In this chapter, we analyze new issues to face: what to do with mosaicism, fresh or frozen embryo transfer, segmental aneuploidies, etc.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Preimplantation genetic screening
- Trophectoderm biopsy
- Segmental aneuploidies
- Embryo mosaicism
- Embryo aneuploidy in assisted reproduction
1 Background
The prevalence of primary and secondary infertility has been estimated in 1.9% and 10.5%, respectively, in women of 20–44 years of age from 190 countries according published data in 2010 [1]. Aneuploidy is the main genetic factor that influences human reproductive success [2]. As it has been published, aneuploid embryos account for at least 10% of human pregnancies, and the incidence could exceed 50% for women over 35 years of age [3, 4]. Most aneuploidies compromise the implantation of the conceptuses that perish in utero, and those that implant may result in an early miscarriage or cause congenital birth defects.
Medically assisted reproduction (MAR) allows for the treatment of most infertile couples with the aim of securing a healthy birth. Therefore, in vitro fertilization (IVF) laboratories are challenged to reduce the risk associated with multiple pregnancy. For that, most of the IVF clinics have moved to the strategy of a single embryo transfer, diagnosed as chromosomally normal, since selecting just the morphologically normal ones to transfer is not enough to guarantee its success. Morphology of an embryo is weakly correlated with its viability and, hence, with its chromosome constitution. All type of uniform aneuploidies can survive to the blastocyst stage [3, 5,6,7,8,9,10,11,12,13]. Moreover, 40–50% blastocysts with optimal morphology can be chromosomally abnormal [14, 15], and euploid embryos do not always demonstrate better morphology than chaotic mosaics [16]. On the other hand, there is a correlation between aneuploidy and maternal age due to an increase of premature sister chromatids separation and meiotic nondisjunction of homologous chromosomes [17]. As an example, aneuploidy increases from 40% in fertile egg donors to 80% in patients of 41–42 years old [18]. However, Harton et al. in 2013 [19] demonstrated that if a chromosomally normal embryo is transferred to the uterus, the chance to implant is independent of maternal age. The transfer of abnormal embryos in an IVF cycle is related to higher rates of implantation failure and miscarriage. Although there is a direct correlation between embryo aneuploidy and maternal age, there is also positive correlation with other factors such as sperm chromosome abnormalities, altered male meiosis, or nongenetic male factor [20, 21].
Preimplantation genetic testing (PGT) has been used since the 1990s to diagnose genetically abnormal embryos for selecting, with some certainty, those genetically normal embryo(s) to be transferred to the uterus with the maximum guarantees to implant and to reach term. PGT has been incorporated into IVF laboratories to improve the efficiency of ART, increasing implantation rates while lowering pregnancy loss rate [22,23,24,25,26,27,28,29]. The success of PGT for aneuploidy screening (PGT-AS) is not limited to the technique itself but depends on different factors: (1) the optimization of the PGT-AS technique; (2) the proper selection of patients for PGT-AS; (3) the number of analyzed chromosomes (limited or comprehensive chromosome screening, CCS); and (4) the protocols of ovarian stimulation, in vitro embryo culture, and embryo(s) transfer. Focusing on PGT-AS technique, over the past years, different methodologies have been optimized to overcome many of the technical limitations intrinsic to the analysis of a single cell or a few number of them. Fluorescence in situ hybridization (FISH) on fixed nuclei from biopsied blastomeres was the technique of choice over the past two decades. However, the classic FISH technique analysis was limited to a restricted number of chromosomes [30] restricting the improvement of IVF outcomes with PGT-AS, as reported by several authors [31,32,33,34,35,36,37,38] and advised by the ESHRE PGD Consortium [39]. Therefore, the natural evolution of the PGT-AS has driven to the development, clinical validation, and application of the new emerging CCSs methodologies. Currently, the available CCS techniques developed and clinical validated for PGT-AS are array comparative genomic hybridization (aCGH) [5, 11, 15, 40], 24-chromosome FISH (FISH-24) [41], single nucleotide polymorphism (SNP) microarray [42], quantitative real-time polymerase chain reaction (qPCR)-based CCS [43], and more recently next-generation sequencing (NGS) [44,45,46,47,48,49,50]. The application of CCS techniques also produces a change in the protocol of biopsy, moving from day 3 to day 5 of embryo development in order to have more quantity and quality of DNA for amplification and to overcome the high rate of mosaicism detected on cell stage embryos that can lead to misdiagnosis. Among these technologies, NGS seems to detect with higher accuracy for segmental imbalances [51, 52] and chromosomal mosaicism [51, 53,54,55]. Recently after three randomized control trials (RCT) testing day 5 blastocyst biopsies in good prognosis patients, there appear to be significant improvements in ongoing pregnancy rates [26, 27, 56], encouraging physicians to recommend PGT-AS on trophectoderm samples.
2 Is There an Optimal PGT-AS and Embryo Transfer Program?
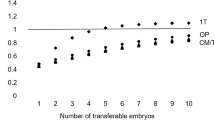
One of the more recent discussions about PGT-AS using the new CCS platforms is which is the most efficient operating way in terms of maximizing pregnancy rates. When PGT-AS by FISH was established, most centers did the biopsy on day 3, and euploid embryos were transferred in day 5 in a fresh cycle, but pregnancy rates were not as good as expected. In the last few years, there is published evidence showing that transferring cryopreserved embryos in a non-stimulated cycle increases clinical implantation rates [57,58,59,60,61,62,63] and decreases low birth weight and preterm delivery rate [64, 65]. Coates et al. in 2017 [66] published a RCT comparing both approaches: to perform day 5 biopsy and vitrify all embryos while waiting for the PGT-AS results and to carry out the euploid embryo transfer in a non-stimulated cycle versus biopsing embryos at day 5 and transferring the euploid embryos on day 6 in a fresh cycle. Embryos showing slow development were biopsied on day 6 and kept frozen for a future non-stimulated transfer, in case of failed outcomes. The study was performed in a US institute with a long standing experience in embryo vitrification, embryo culture, and biopsy procedures, and the results showed, in terms of ongoing pregnancy rates and live birth rates, a trend in favor of a freeze all strategy and transference of the euploid embryos in non-stimulated cycles. Another RCT published by Rubio et al. [67] compared the effectiveness of clinical outcome with and without PGT-AS in women with advanced maternal age (from 38 to 41 years old) after embryo analysis by aCGH. They published a higher delivery rate per transfer after the first transfer attempt (52.9% vs 24.2%) and higher delivery rate per patient (36.0% vs. 21.9%) in the group that performed PGD aneuploidy screening compared to the group that did not perform PGD.
The main issue when applying the freeze all strategy is that the laboratory must optimize its culture conditions to achieve the highest rates of blastocyst formation. Moreover, vitrification and thawing protocols must be optimized in order to achieve the highest post-warming survival and cleavage development rate. Unfortunately, not all IVF laboratories around the world have standardized protocols, and, even among those following the highest quality standards, results may drastically differ from one center to another. This suggests that although publications are in favor of a specific strategy, each center should analyze its own laboratory efficiency and which strategy is the best for them. For one laboratory that does not have a good established blastocyst vitrification protocol and presents a high incidence of lysed cells and low development rate post-warming, the best approach would be to perform day 5 biopsy and transfer in a fresh cycle and only keep vitrified the D6 biopsied blastocyst for a second transfer. Another scenario may be a laboratory that presents a poor embryo culture conditions. In that way, the best approach should be to biopsy on day 3 and transfer in day 3/4 in a fresh cycle to avoid losing embryo potential.
3 Mosaicism
Transferring high morphological quality euploid embryos has increased pregnancy rates, but we are still faced with the challenge that some euploid embryos with a good morphology fail to implant. In this scenario, many programs have started to utilize time-lapse PGD-AS studies to correlate morphokinetics parameters and the type of aneuploidy in an attempt to identify which embryos have better competence to implant, but that said, efficiency is still not 100%. This can mainly be due to two factors: mosaicism and technical limitations.
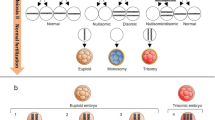
Embryo mosaicism is one of the main sources of error when performing PGT-AS [68,69,70,71,72,73,74,75,76]. To establish the rate of mosaicism in preimplantation embryos is a complex task since it varies according to the embryo stage, the technology used for the diagnosis, and the skills of the genetic laboratory for the interpretation. In cleavage-stage embryos, the estimated levels of mosaicism vary from 15 to 75% while in blastocyst have been estimated in 3–24%, according to a published review [77]. The great variability on reported data can also be influenced by different factors other than PGT-AS procedure itself, including the etiology of infertility, female’s age, or even in vitro culture and environmental conditions. All these elements can also impact the abnormal chromosome segregation leading to embryonic mosaicism. However, it appears that there is a general agreement for the observation that a gradual decrease in aneuploidy takes place during embryo development most probably due to self-correction mechanisms and preferential development of euploid cells.
Mosaicism occurs during mitotic division of the embryo, giving rise to chromosomally different cell lines. When analyzing one cell from the embryo, it is assumed that the result is representative of the whole embryo. In order to avoid mosaicism misdiagnosis when performing PGT-AS, two different strategies have been proposed. The first is to perform polar body 1 and 2 analysis. Using this strategy, only chromosomal abnormalities of maternal meiotic origin are analyzed, while paternal meiotic abnormalities and abnormal chromosomal mitotic segregations are not evaluated. The second is to perform trophectoderm biopsy at blastocyst stage, analyzing more than one embryonic cell in in a developmental stage with a lower rate of mosaicism compared to day 3 embryos. This strategy can be used only if a good system for day 5 embryo culture is available and if a high number of embryos is achieved. However, although mosaicism rate is lower, it can be present so there is still a risk of misdiagnosis. At the blastocyst stage, different types of mosaics have been described [78]: mosaicism that affect both trophectoderm (TFE) and inner cell mass (ICM), when the abnormal cells are confined to the TFE or ICM exclusively or when the ICM is normal TFE is abnormal (or vice versa). Depending on the type of mosaicism we are facing and the TFE cells we are biopsing by chance will condition PGT-AS misdiagnosis rate on blastocyst stage. Some studies have tried to estimate this correlation between ICM and TFE cell lines by biopsing two or three different groups of cells of the same embryo. They observed a diagnosis correlation of 95–100%, and the discordance between ICM and TFE cell lines was estimated to be around 3–4% [79, 80].
Another strategy to avoid misdiagnosis due to mosaicism on the PGT-AS results has been to perform two cells biopsy on day 3. However, this strategy has been demonstrated to be detrimental for embryo developmental competence and has not been recommended any longer.
New CCS platforms for PGT-AS such as NGS can detect low levels of diploid/aneuploid mosaicism with high accuracy (lower than 20%). Mosaic or potentially mosaic embryos have become a new category to classify embryos [81]. According PGDIS recommendations [82], embryos with a mosaicism rate lower than 20% can be considered as euploid (and then transferable), while embryos with more than 80% of abnormal cells are classified as aneuploid. The remaining ones (20–80%) can be classified as mosaics. However, to establish the thresholds between which the embryos can be considered transferable or not is a controversial issue. Simon et al. recently suggested [81] that one consider above 50% of mosaicism embryo to be classified as aneuploid and nontransferable. According to a worldwide survey from 32 countries, <10% of the analyzed embryos are classified as mosaics [81]. These embryos have a theoretically decreased implantation rate and increased risk of miscarriage, pregnancy complications, and clinically affected life births [81, 82]. Transferring embryos categorized as mosaic, although can raise some ethical considerations, is generally accepted when the couple does not have any euploid embryos [82, 83]. Different factors should be taken into consideration such as the methodology used for testing, the involved chromosomes, or the reproductive medical history of the couple [81,82,83]. Regarding this matter, PGDIS consortium published a suggested guideline to prioritize mosaic embryos for transfer. Patients may consider transferring a mosaic embryo only after a proper genetic counselling about the risks of miscarriage and adverse outcomes they can face.
4 Conclusion
One of the most important challenges for the embryologist is to discern which is the most competent embryo to transfer. Many efforts to find the magical wand have been made in studying the cytoplasmic and nuclear competence, the morphology, and morphokinetics during embryo development or in developing the most paramount technique to detect all chromosome aneuploidies. Yet still, just when we thought that we had the most comprehensive technology that permits us to screen all chromosomes, some new question arises and makes us go back in time and question all we know. Is embryo mosaicism an indicator of euploidy? Do we have to discard mosaic embryos?
References
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356.
Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nature Rev Genet. 2001;2:280–91.
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT. Aneuploidy across individual chromosomes at the embryonic level in trophectoderm biopsies: changes with patient age and chromosome structure. J Assist Reprod Genet. 2014 Nov;31(11):1501–9.
Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504.
Fragouli E, Alfarawati S, Daphnis DD, Goodall N, Mania A, Griffiths T, Wells D. Cytogenetic analysis of human blastocysts with the use of FISH, CGH and aCGH: scientific data and technical evaluation. Hum Reprod. 2011;26(2):480–90.
Evsikov S, Verlinsky Y. Mosaicism in the inner cell mass of human blastocysts. Hum Reprod. 1998;13(11):3151–5.
Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson O. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod. 2000;15(8):1781–6.
Sandalinas M, Sadowy S, Alikani M, Calderon G, Cohen J, Munné S. Developmental ability of chromosomally abnormal human embryos to develop to the blastocyst stage. Hum Reprod. 2001;16(9):1954–8.
Bielanska M, Tan SL, Ao A. High rate of mixoploidy among human blastocysts cultured in vitro. Fertil Steril. 2002;78(6):1248–53.
Coonen E, Derhaag JG, Dumoulin JC, et al. Anaphase lagging mainly explains chromosomal mosaicism in human preimplantation embryos. Hum Reprod. 2004;19(2):316–24.
Fragouli E, Katz-Jaffe M, Schoolcraft WB, Ross R, Munne S, Wells D. Comprehensive cytogenetic analysis of the human blastocyst stage. Fertil Steril. 2008;90(11):S36.
Santos MA, Teklenburg G, Macklon NS, Van Opstal D, Schuring-Blom GH, Krijtenburg PJ, de Vreeden-Elbertse J, Fauser BC, Baart EB. The fate of the mosaic embryo: chromosomal constitution and development of day 4, 5 and 8 human embryos. Hum Reprod. 2010;25(8):1916–26.
Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20(2):117–26.
Wells D, Alfarawati S, Fragouli E. Use of comprehensive chromosomal screening for embryo assessment: microarrays and CGH. Mol Hum Reprod. 2008;14(12):703–10.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, Ubaldi FM. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81.
Johnson DS, Cinnioglu C, Ross R, Filby A, Gemelos G, Hill M, Ryan A, Smotrich D, Rabinowitz M, Murray MJ. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol Hum Reprod. 2010;16(12):944–9.
Van Steirteghem A, Liu J, Nagy Z, Joris H, Tournaye H, Liebaers I, Devroey P. Use of assisted fertilization. Hum Reprod. 1993 Nov;8(11):1784–5.
Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, Munné S. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod Biomed Online. 2012 Jun;24(6):614–20.
Harton GL, Munné S, Surrey M, Grifo J, Kaplan B, McCulloh DH, Griffin DK, Wells D, PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013 Dec;100(6):1695–703.
Sánchez-Castro M, Jiménez-Macedo AR, Sandalinas M, Blanco J. Prognostic value of sperm fluorescence in situ hybridization analysis over PGD. Hum Reprod. 2009;24(6):1516–21.
Sarrate Z, Vidal F, Blanco J. Role of sperm fluorescent in situ hybridization studies in infertile patients: indications, study approach, and clinical relevance. Fertil Steril. 2010;93(6):1892–902.
Gianaroli L, Magli MC, Ferraretti AP, Munné S. Preimplantation diagnosis for aneuploidies in patients undergoing in vitro fertilization with a poor prognosis: identification of the categories for which it should be proposed. Fertil Steril. 1999;72(5):837–44.
Munné S, Magli C, Cohen J, Morton P, Sadowy S, Gianaroli L, Tucker M, Márquez C, Sable D, Ferraretti AP, Massey JB, Scott R. Positive outcome after preimplantation diagnosis of aneuploidy in human embryos. Hum Reprod. 1999;14:2191–9.
Munné S, Sandalinas M, Escudero T, Velilla E, Walmsley R, Sadowy S, Cohen J, Sable D. Improved implantation after preimplantation genetic diagnosis of aneuploidy. Reprod Biomed Online. 2003;7(1):91–7.
Munné S, Chen S, Fischer J, Colls P, Zheng X, Stevens J, Escudero T, Oter M, Schoolcraft B, Simpson JL, Cohen J. Preimplantation genetic diagnosis reduces pregnancy loss in women aged 35 years and older with a history of recurrent miscarriages. Fertil Steril. 2005;84(2):331–5.
Scott RT Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, Tao X, Treff NR. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703.
Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, Peck AC, Sills ES, Salem RD. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24.
Rubio C, Bellver J, Rodrigo L, Bosch E, Mercader A, Vidal C, De los Santos MJ, Giles J, Labarta E, Domingo J, Crespo J, Remohí J, Pellicer A, Simón C. Preimplantation genetic screening using fluorescence in situ hybridization in patients with repetitive implantation failure and advanced maternal age: two randomized trials. Fertil Steril. 2013;99(5):1400–7.
Forman EJ, Hong KH, Franasiak JM, Scott RT. Obstetrical and neonatal outcomes from the BEST trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol. 2014;210(2):157.e1–6.
Munne S, Marquez C, Magli C, Morton P, Morrison L. Scoring criteria for preimplantation genetic diagnosis of numerical abnormalities for chromosomes X, Y, 13, 16, 18 and 21. Mol Hum Reprod. 1998;4:863–70.
Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, Devroey P, Liebaers I, Van Steirteghem A. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19(12):2849–58.
Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357(1):9–17.
Fritz MA. Perspectives on the efficacy and indications for preimplantation genetic screening: where are we now? Hum Reprod. 2008;23(12):2617–21.
Hardarson T, Hanson C, Lundin K, Hillensjö T, Nilsson L, Stevic J, Reismer E, Borg K, Wikland M, Bergh C. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod. 2008;23(12):2806–12.
Staessen C, Verpoest W, Donoso P, Haentjens P, Van der Elst J, Liebaers I, Devroey P. Preimplantation genetic screening does not improve delivery rate in women under the age of 36 following single-embryo transfer. Hum Reprod. 2008;23(12):2818–25.
Twisk M, Mastenbroek S, Hoek A, Heineman MJ, van der Veen F, Bossuyt PM, Repping S, Korevaar JC. No beneficial effect of preimplantation genètic screening in women of advanced maternal age with a high risk for embryonic aneuploidy. Hum Reprod. 2008;23(12):2813–7.
Schoolcraft WB, Katz-Jaffe MG, Stevens J, Rawlins M, Munne S. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil Steril. 2009;92(1):157–62.
Harper JC, Harton G. The use of arrays in preimplantation genetic diagnosis and screening. Fertil Steril. 2010;94(4):1173–7.
Harper J, Coonen E, De Rycke M, Fiorentino F, Geraedts J, Goossens V, et al. What next for preimplantation genetic screening (PGS)? A position statement from the ESHRE PGD consortium steering committee. Hum Reprod. 2010;25(4):821–3.
Gutiérrez-Mateo C, Colls P, Sánchez-García J, Escudero T, Prates R, Ketterson K, Wells D, Munné S. Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertil Steril. 2011;95(3):953–8.
Fernández S, Toro E, Colomar A, López-Teijón M, Velilla E. A 24-chromosome FISH technique in preimplantation genetic diagnosis: validation of the method. Syst Biol Reprod Med. 2015;61(3):171–7. https://doi.org/10.3109/19396368.2014.1002869.
Treff NR, Su J, Tao X, Levy B, Scott RT. Accurate single cell 24 chromosome aneuploidy screening using whole genome amplification and single nucleotide polymorphism microarrays. Fertil Steril. 2010;94(6):2017–21.
Treff NR, Tao X, Ferry KM, Su J, Taylor D, Scott RT. Development and validation of an accurate quantitative real-time polymerase chain reaction-based assay for human blastocyst comprehensive chromosomal aneuploidy screening. Fertil Steril. 2012;97(4):819–24.
Wells D, Kaur K, Grifo J, Glassner M, Taylor JC, Fragouli E, Munne S. Clinical utilisation of a rapid low-pass whole genome sequencing technique for the diagnosis of aneuploidy in human embryos prior to implantation. J Med Genet. 2014;51(8):553–62.
Fiorentino F, Biricik A, Bono S, Spizzichino L, Cotroneo E, Cottone G, Michel CE. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertil Steril. 2014;101(5):1375–82.
Fiorentino F, Bono S, Biricik A, Nuccitelli A, Cotroneo E, Cottone G, Greco E. Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum Reprod. 2014;29(12):2802–13.
Tan Y, Yin X, Zhang S, Jiang H, Tan K, Li J, Xiong B, Gong F, Zhang C, Pan X, Chen F, Chen S, Gong C, Lu C, Luo K, Gu Y, Zhang X, Wang W, Xu X, Vajta G, Bolund L, Yang H, Lu G, Du Y, Lin G. Clinical outcome of preimplantation genetic diagnosis and screening using next generation sequencing. Gigascience. 2014;3(1):30.
Zheng H, Jin H, Liu L, Liu J, Wang WH. Application of next-generation sequencing for 24-chromosome aneuploidy screening of human preimplantation embryos. Mol Cytogenet. 2015;8:38.
Yang Z, Lin J, Zhang J, Fong WI, Li P, Zhao R, Liu X, Podevin W, Kuang Y, Liu J. Randomized comparison of next-generation sequencing and array comparative genomic hybridization for preimplantation genetic screening: a pilot study. BMC Med Genet. 2015;8:30.
Kung A, Munné S, Bankowski B, Coates A, Wells D. Validation of next-generation sequencing for comprehensive chromosome screening of embryos. Reprod Biomed Online. 2014;31(6):760–9.
Lai H-H, Chuang T-H, Wong L-K, Lee M-J, Hsieh C-L, Wang H-L, Chen S-U. Identification of mosaic and segmental aneuploidies by next-generation sequencing in preimplantation genetic screening can improve clinical outcomes compared to array-comparative genomic hybridization. Mol Cytogenet. 2017;10:14. https://doi.org/10.1186/s13039-017-0315-7.
Bauckman K, Ribustello L, Escudero T, Armenti E, Capaldi R, Munne S. Validation of next generation sequencing (NGS) for PGD of structural chromosome abnormalities (ESHRE Abstract). Hum Reprod. 2016;31:i410.
Fragouli E, Alfarawati S, Spath K, Tarozzi N, Borini A, Wells D. The developmental potential of mosaic embryos (ASRM Abstract). Fertil Steril. 2015;104:e96.
Grifo J, Colls P, Ribustello L, Escudero T, Liu E, Munne S. Why do array-CGH(ACGH) euploid embryos miscarry? Reanalysis by NGS reveals undetected abnormalities which would have prevented 56% of the miscarriages. Fertil Steril. 2015:104e14.
Liu E, Escudero T, Ribustello L, Bauckman K, Colls P, Munne S. Chromosome abnormalities detected by array comparative genomic hybridization and next-generacion sequencing: results on >38000 embryos (ESHRE abstracts). Hua Reprod. 2016;31:i388.
Forman EJ, Li X, Ferry KM, Scott K, Treff NR, Scott RT Jr. Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: a novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril. 2012 Sep;98(3):644–9.
Chattacharya S. Maternal and perinatal outcomes after fresh versus frozen embryo transfer- what is the risk-benefit ratio? Fertil Steril. 2016;106:241–3.
Ozgur K, Berkkanoglu M, Bulut H, Humaidan P, Coetzee K. Perinatal outcomes after fresh versus vitrified–warmed blastocyst transfer: retrospective analysis. Fertil Steril. 2015;104:899–907.
Shapiro BS, Daneshmand ST, Garner FC, Aguieer M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102:3–9.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen–thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344–8.
Roque M, Valle M, Guimaraes F, Sampaio M, Geber S. Freeze-all policy: fresh versus frozen–thawed embryo transfer. Fertil Steril. 2015;103:1190–3.
Goma H, Baydoum R, Sachak S, Lapana I, Soliman S. Elective single embryo transfer: Is frozen better than fresh. JBRA Assist Reprod. 2016;20:3–7.
Shi Y, Wei D, Liang X, Sun Y, Liu J, Cao Y, et al. Live birth after fresh embryo transfer in women with polycistic ovary syndrome undergoing IVF /FreFro-PCOS: study protocol for a multicenter, prospective, randomized controlled trial. Trials. 2014;15:154.
Wennerholm UB, Henningsen AK, Romundstad LB, Bergh C, Pinborg A, Skajaerven E, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from CoNARTaS group. Hum Reprod. 2013;28:2545–53.
Maheshwari A, Battachanya S. Ellective frozen replacement cycles for all: ready for prime time? Hum Reprod. 2013;28:6–9.
Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, Ata B, Cohen J, Munné S. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril. 2017 Mar;107(3):723–30.
Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, Giles J, Ferrando M, Cabanillas S, Remohí J, Pellicer A, Simón C. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017 May;107(5):1122–9.
Los FJ, van Opstal D, van den Berg C, Braat AP, Verhoef S, Wesby-van Swaay E, van den Ouweland AM, Halley DJ. Uniparental disomy with and without confined placental mosaicism: a model for trisomic zygote rescue. Prenat Diagn. 1998 Jul;18(7):659–68.
Los FJ, Van Opstal D, van den Berg C. The development of cytogenetically normal, abnormal and mosaic embryos: a theoretical model. Hum Reprod Update. 2004 Jan–Feb;10(1):79–94.
Baart EB, Van Opstal D, Los FJ, Fauser BC, Martini E. Fluorescence in situ hybridization analysis of two blastomeres from day 3 frozen-thawed embryos followed by analysis of the remaining embryo on day 5. Hum Reprod. 2004 Mar;19(3):685–93.
Baart EB, Martini E, van den Berg I, Macklon NS, Galjaard RJ, Fauser BC, Van Opstal D. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum Reprod. 2006 Jan;21(1):223–33.
Bielanska M, Jin S, Bernier M, Tan SL, Ao A. Diploid-aneuploid mosaicism in human embryos cultured to the blastocyst stage. Fertil Steril. 2005 Aug;84(2):336–42.
Barbash-Hazan S, Frumkin T, Malcov M, Yaron Y, Cohen T, Azem F, Amit A, Ben-Yosef D. Preimplantation aneuploid embryos undergo self-correction in correlation with their developmental potential. Fertil Steril. 2009 Sep;92(3):890–6.
Daphnis DD, Fragouli E, Economou K, Jerkovic S, Craft IL, Delhanty JD, Harper JC. Analysis of the evolution of chromosome abnormalities in human embryos from Day 3 to 5 using CGH and FISH. Mol Hum Reprod. 2008 Feb;14(2):117–25.
Frumkin T, Malcov M, Yaron Y, Ben-Yosef D. Elucidating the origin of chromosomal aberrations in IVF embryos by preimplantation genetic analysis. Mol Cell Endocrinol. 2008 Jan 30;282(1–2):112–9.
Munné S, Sandalinas M, Escudero T, Márquez C, Cohen J. Chromosome mosaicism in cleavage-stage human embryos: evidence of a maternal age effect. Reprod Biomed Online. 2002 May–Jun;4(3):223–32.
Harton GL, Cinnioglu C, Fiorentino F. Current experience concerning mosaic embryos diagnosed during preimplantation genetic screening. Fertil Steril. 2017 May;107(5):1113–9.
Liu J, Wang W, Sun X, Liu L, Jin H, Li M, Witz C, Williams D, Griffith J, Skorupski J, Haddad G, Gill J. DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploid and mosaic. Biol Reprod. 2012 Dec 27;87(6):148.
Johnson DS, Cinnioglu C, Ross R, Filby A, Gemelos G, Hill M, Ryan A, Smotrich D, Rabinowitz M, Murray MJ. Comprehensive analysis of karyotypic mosaicism between trophectoderm and inner cell mass. Mol Hum Reprod. 2010 Dec;16(12):944–9.
Capalbo A, Wright G, Elliott T, Ubaldi FM, Rienzi L, Nagy ZP. FISH reanalysis of inner cell mass and trophectoderm samples of previously array-CGH screened blastocysts shows high accuracy of diagnosis and no major diagnostic impact of mosaicism at the blastocyst stage. Hum Reprod. 2013 Aug;28(8):2298–307.
Simon C. Introduction: to transfer or not transfer…a mosaic embryo, that is the question. Fertil Steril. 2017 May;107(5):1083–4.
PGDIS Newsletter. PGDIS position statement on chromosome mosaicism and preimplantation aneuploidy testing at the blastocyst stage. Chicago: PGDIS Newsletter; 2016.
Sachdev NM, Maxwell SM, Besser AG, Grifo JA. Diagnosis and clinical management of embryonic mosaicism diagnosis and clinical management of embryonic mosaicism. Fertil Steril. 2017 Jan;107(1):6–11. https://doi.org/10.1016/j.fertnstert.2016.10.006.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Velilla, E., Morales, C. (2020). Relevance of Embryo Aneuploidy in Medically Assisted Reproduction. In: Allahbadia, G.N., Ata, B., Lindheim, S.R., Woodward, B.J., Bhagavath, B. (eds) Textbook of Assisted Reproduction. Springer, Singapore. https://doi.org/10.1007/978-981-15-2377-9_71
Download citation
DOI: https://doi.org/10.1007/978-981-15-2377-9_71
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2376-2
Online ISBN: 978-981-15-2377-9
eBook Packages: MedicineMedicine (R0)