Abstract
A novel dissimilatory iron-reducing bacteria, Klebsiella sp. IR21, was isolated from the anode biofilm of an MFC reactor. Klebsiella sp. IR21 reduced 27.8 % of ferric iron to ferrous iron demonstrating that Klebsiella sp. IR21 has electron transfer ability. Additionally, Klebsiella sp. IR21 generated electricity forming a biofilm on the anode surface. When a pure culture of Klebsiella sp. IR21 was supplied into a single chamber, air–cathode MFC fed with a mixture of glucose and acetate (500 mg L−1 COD), 40–60 mV of voltage (17–26 mA m−2 of current density) was produced. Klebsiella sp. IR21 was also utilized as a biocatalyst to improve the electrical performance of a conventional MFC reactor. A single chamber, air–cathode MFC was fed with reject wastewater (10,000 mg L−1 COD) from a H2 fermentation reactor. The average voltage, current density, and power density were 142.9 ± 25.74 mV, 60.5 ± 11.61 mA m−2, and 8.9 ± 3.65 mW m−2, respectively, in the MFC without inoculation of Klebsiella sp. IR21. However, these electrical performances of the MFC were significantly increased to 204.7 ± 40.24 mV, 87.5 ± 17.20 mA m−2, and 18.6 ± 7.23 mW m−2, respectively, with inoculation of Klebsiella sp. IR21. The results indicate that Klebsiella sp. IR21 can be utilized as a biocatalyst for enhancement of electrical performance in MFC systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cells (MFCs) are devices that can directly convert chemical energy to electrical energy due to the degradation of organic compounds by microorganisms as biocatalysts [1–3]. Microorganisms in MFCs oxidize organic compounds producing electrons and transferring electrons to the anode electrode [1, 3]. MFCs are able to use various organic matter ranging from simple carbon sources such as glucose, acetate, and lactose to complex carbon compounds such as organic waste, food industry based wastewater, agricultural wastes and renewable biomass [2, 4]. MFCs are recognized as environmentally friendly systems because MFCs are able to treat organic waste and do not generate additional pollutants such as sludge [2]. MFCs can be applied in various environments such as water treatment, bioremediation, and biosensor [5–8]. MFCs convert the chemical energy in wastewater into electrical energy [5, 6], and it can be used for nutrient removal such as nitrogen and phosphorus in wastewater [7]. In addition, MFCs are also widely used as biosensor for monitoring DO and BOD in environment water because of its high sensitivity [8].
The electrochemical performances of MFCs are mainly based on three factors: the physico-chemical, operating, and biological components of systems [2]. Among these factors, biological components, especially biocatalysts are the most important factor which is controlling overall MFC performance [2]. Among the microorganisms used in MFCs, Geobacter and Shewanella were the most widely used as biocatalysts in the early stages of this type of research [3]. However, they produce electricity by oxidizing simple molecular carbon sources such as acetate or lactate. Thus, it is difficult to decompose complex carbon compounds using them [3, 9]. MFCs have been applied for organic wastewater treatment as well as electricity production [9]. Therefore, microorganisms, which can utilize only simple molecular carbon, are not suitable for wastewater because it contains various complex organic compounds [3]. Since 2004, fermentative facultative anaerobes have been used as biocatalysts to oxidize complex organic carbon [3, 9]. Zhang et al. utilized a fermentative facultative anaerobe, Klebsiella pneumonia, as a biocatalyst in a two chamber MFC reactor [3]. Their research demonstrated that the K. pneumoniae strain has electrocatalytic activity in oxidizing complex starch molecules and produced a maximum power density of 218.51 mW m−2 [3]. Clostridium is also a fermentative facultative anaerobe that can be used as a biocatalyst for converting organic compounds into electric energy [10]. Clostridium has the capability to oxidize various ranges of substrates from simple molecules like lactate to complex molecules including starch or sucrose [10]. In 2004, Niessen et al. investigated electricity production using C. butyricum [10]. They inoculated C. butyricum in a two chamber MFC and utilized starch and molasses as substrates [10]. C. butyricum quickly started fermenting starch and molasses upon inoculation and generated a power density of 1.1–1.3 mA cm−2 in a fed batch system [10]. In addition, Clostridium can reduce CO2 to organic compounds using electrons at the cathode in MFC [11]. In another study, a fermentative bacterium, Enterobacter aerogenes, was used as a biocatalyst in a single-chamber MFC without additional mediators [12]. E. aerogenes can also grow using various substrates ranging from low molecular compounds such as lactate to complex carbohydrates such as starch and cellulose [12]. Zhuang et al. demonstrated that E. aerogenes possesses electron transfer ability through cyclic voltammetry analysis and it produced a maximum power density of 1.79–2.51 W m−3 from glucose [12]. Several microorganisms such as P. aeruginosa and Desulfovibrio vulgaris secrete self-mediators such as pyocyanin and pyoverdine and these endogenous mediators [2]. Therefore, it is important to find new microorganisms that can be utilized as biocatalysts in MFC systems without artificial mediators.
In this study, a novel fermentative facultative anaerobic bacterium, Klebsiella sp. IR21, was isolated as a biocatalyst in MFC reactors used to treat organic wastewater in the absence of artificial mediators. The direct electron transfer and electricity generation of Klebsiella sp. IR21 were evaluated in a MFC system inoculated with only its pure culture. Additionally, the performance enhancement of a conventional MFC reactor by the inoculation of Klebsiella sp. IR21 was investigated.
Methodology
Isolation and identification of strain IR21
A new fermentative facultative anaerobe was isolated from an air–cathode MFC (AC-MFC) inoculated with anaerobic sludge (3565 ± 91.9 mg L−1 MLVSS) from a sewage treatment plant in Seoul, South Korea. After operating the AC-MFC reactor with 3.5 g of glucose (20 mM) for 80 days, a 0.5 × 0.5 cm2 anode biofilm from the AC-MFC was sampled. The biomass detached from the anode biofilm was inoculated 120 mL of serum. A mineral salt solution was supplemented with 20 mM glucose as a carbon source and electron donor, and 50 mM iron pyrophosphate [Fe4(P2O7)3] as an electron acceptor [14]. The mineral salt solution contained 0.31 g L−1 NH4Cl, 0.13 g L−1KCl, 21.84 g L−1 Na2HPO4·12H2O, and 6.08 g L−1 NaH2PO4·2H2O (pH 7). The bottle was purged with N2 gas and then sealed with a rubber stopper to maintain anaerobic conditions. The bottle was incubated at 35 °C under shaking (200 rpm) for 4 days. The culture broth was serially diluted and then spread onto a glucose medium with 1.5 % agar. The plates were cultivated at 35 °C for 4 days in an anaerobic chamber while being purged with 99 % N2 gas. Among the colonies on the plates, the ivory colony, which dominated the plates, was isolated and named strain IR21. Strain IR21 was identified by 16S rRNA gene sequencing analysis. To identify the strain IR21, PCR was performed using a bacterial universal primer set consisting of 341F (5′-CCTACGGGAGGCAGCAG-3′) and 907R (5′-CCCCGTCAATTCATTTGAGTTT-3′). The PCR mixture included 5 μL of 10× PCR buffer (Genenmed Inc., Seoul, Korea), 4 μL of 2.5 mM dNTPs, 1 μL of each forward/reverse primer, 1 U of ACE TaqMan polymerase (Genenmed Inc., Seoul, Korea), and 2 μL of DNA template in a total reaction volume of 50 μL. PCR was conducted by using a 2700® PCR system (Applied Biosystems, Foster City, USA). Initial denaturation was performed at 95 °C for 4 min followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 30 s, and final extension at 72 °C for 5 min. The DNA sequences of the PCR products were analyzed by Macrogen Incorporation (Seoul, Korea) and compared with the National Center for Biotechnology Information (NCBI) GenBank database nucleotide BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The strain IR21 was assigned under the accession number, KR351311 under NCBI GenBank database, and deposited in the Korean Collection for Type Cultures (KCTC) under accession number KCTC12571BP.
Reduction of ferric iron by strain IR21
Strain IR21 was cultivated in 120 mL serum bottles to investigate the electron transfer ability. First, to obtain cells of strain IR21, a colony of strain IR21 was cultivated in 120 mL serum bottles with a mineral salt solution containing 20 mM glucose and 50 mM iron pyrophosphate [Fe4(P2O7)3] [13, 14]. After 4 days, the culture medium became turbid and the cells were centrifuged at 10,000 rpm for 10 min to collect the cells. The collected cells were washed once with a mineral salt solution. After that, 10 mL of the cells in the mineral salt medium was mixed with 90 mL of a mineral salt solution with an identical composition containing 20 mM glucose as a carbon source and 50 mM iron pyrophosphate as an electron acceptor in 500 mL serum bottles. A serum bottle containing the medium without inoculation of IR21 was prepared as a control. All serum bottles were purged with 99 % N2 gas and sealed with a rubber septum to maintain anaerobic conditions. The bottles were incubated in a shaking incubator at 35 °C and 200 rpm. All experiments were conducted in triplicate.
Reduced ferric iron was measured by a ferrozine method [15, 16]. First, 0.5 mL of sample was mixed with 0.5 mL of 0.5 N HCl. Then, 0.1 mL of the mixture was removed and mixed with 4.9 mL of a 0.1 % (wt/wt) ferrozine solution in 50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer (pH 7.0 with NaOH) for 15 s. The solution was filtered using a filter (0.45-μm pore diameter) and the absorbance was measured at 562 nm.
Electricity generation by strain IR21 and its dynamics in a MFC reactor
MFC reactor structure and operation
Single chamber, air–cathode MFCs were constructed from acrylic with an inner volume of 7 L × 15 H × 10 W cm3. 13 × 9 cm2 anode carbon felt (CaraMaterials, Port Jervis, USA) was pretreated at 450 °C for 30 min and it was connected with an air diffusion cathode (ADE75, MEET, Ilsan, Korea) using titanium wire at an external resistance of 100 Ω. 1 L of the MFC reactor was filled with 500 mL of strain IR21 in a culture broth and 500 mL of the mineral salt medium containing 20 mM glucose and 50 mM Fe4O21P6. A control MFC reactor was filled with 1 L of the mineral salt medium with the same amount of glucose and Fe4O21P6. The reactors were operated in a fed-batch system for 34 days. 5 mL of concentrated glucose was added to all reactors (final concentration of 20 mM) when the glucose concentration decreased below 5 mM. The glucose concentration was analyzed using a glucose monitor (Inforpia Co., Ltd., Anyang, Korea). All materials and medium were sterilized before use.
Quantification of strain IR21 through quantitative real-time PCR (qRT-PCR)
To quantify strain IR21 in the biofilm and suspended cells in the pure culture AC-MFC reactor, quantitative real-time PCR (qRT-PCR) was conducted. 0.5 × 0.5 cm2 of anode carbon felt was removed using a sterilized cutter in triplicate to quantify strain IR21 on the anode biofilm. 2 mL of the medium was sampled in triplicate in an Eppendorf tube to quantify strain IR21 in the suspended cells. The samples were centrifuged at 11,000 rpm for 2 min and then the supernatant was eliminated. DNA was extracted by a NucleoSpin® Soil Kit (Macherey–Nagel GmbH, Düren, Germany) and a BeadBeater-8 system (Biospec, Bartlesville, USA). DNA samples were eluted in 50 μL elution buffer and quantified by ASP-2680 (ACTGene Inc., Piscataway, USA). Extracted DNA samples were kept at −20 °C before use.
To detect strain IR21 in the biofilm and suspended cells, two sets of specific forward/reverse primers were designed based on the DNA sequence of strain IR21. The specific primer sets were Kleb_F1 (5′-GGCAGGCTGGAGTCTTGTAG-3′) and Kleb_R1 (5′-GCCACTCCTCAAGGGAACAA-3′), and Kleb_F2 (5′-GGCAGGCTGGAGTCTTGTAG-3′) and Kleb_R2 (5′-AAGCCACTCCTCAAGGGAAC-3′). PCR was conducted using a 7300 Real-time PCR system (Applied Biosystems, Foster City, USA). The PCR mixture contained 2.5 μL of 10× PCR buffer (Genenmed Inc., Seoul, Korea), 2 μL of 2.5 mM dNTPs (Genenmed Inc., Seoul, Korea), 0.5 μL of forward primer, 1 μL of reverse primer, 0.2 μL SYBR (50×), 0.5 μL ROX dye (50×), 1 U of ACE Taq polymerase (Genenmed Inc., Seoul, Korea), and 2 μL of DNA template in a 25 μL reaction volume. Initial denaturation was performed at 95 °C for 4 min followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 57 °C for 30 s, extension at 72 °C for 30 s, and finally, the reading step was performed at 82 °C for 30 s.
To evaluate whether strain IR21 was successfully attached on the anode biofilm for power production, the relative amounts of strain IR21 in the biofilm and suspended cells were investigated. The amount of strain IR21 in the biofilm was estimated using the total DNA elution buffer volume, anode surface area, and organic contents. The amount of strain IR21 in the suspended cells was estimated considering the DNA elution buffer volume, internal medium volume of the anode chamber, and organic contents.
Inoculation effect of strain IR21 in a conventional MFC reactor
Electricity production and COD removal
To evaluate the inoculation effects of strain IR21 in a MFC reactor, a single chamber, air–cathode MFC reactor was used, as described above. Anaerobic sludge (3.565 ± 91.9 mg L−1 MLVSS) from a sewage treatment plant (Seoul, Korea) was used as the initial inoculum. 450 mL of anaerobic sludge and 450 mL of a mineral salt solution (pH 7) were mixed for the initial inoculation. 5 mL of the concentrated solution of glucose:acetate (1:1, w/w, 1 g L−1) was used as the substrate and electron donor, and the final concentration was 500 mg L−1 COD. After the substrate addition, the voltage production was observed. When the voltage decreased below 10 mV, substrate was supplied into the reactor. This operation was repeated ten times to obtain a stable electrical capacity of the MFC reactor. Then, the internal medium was replaced with reject wastewater (10,000 mg L−1 COD) from a hydrogen reactor fed molasses wastewater. The reactor was operated for 71 days in continuous mode with a hydraulic retention time (HRT) of 2 days and the electrochemical performance data were collected at steady state.
After operation with reject wastewater from the hydrogen reactor, strain IR21 was inoculated into the identical AC-MFC reactor to evaluate the effects of strain IR21. 20 mL of the culture broth of strain IR21 (6 g cell dry weight L−1) in the mineral salt solution containing 20 mM glucose and 50 mM iron pyrophosphate was inoculated in the AC-MFC reactor fed with reject wastewater (10,000 mg L−1 COD) from a hydrogen reactor fed molasses wastewater. The AC-MFC reactor was operated for 130 days in continuous mode with a HRT of 2 days. 20 mL of the culture broth of strain IR21 in the mineral salt solution was supplied into the AC-MFC reactor at an interval of 2 days. All performance data were collected at steady state.
The voltage was monitored by a digital multimeter GL220 (Graphtec Co., Yokohama, Japan) at an interval of 1 h. The current and power were calculated according to:
where I (A) is the current, V (V) is the voltage, R (Ω) is the external resistance, and P (W) is the power. The obtained current and power were divided by the anodic surface area (m2) to obtain the current density and power density, respectively. Polarization and power density curves were used to characterize the MFC systems as a function of voltage. The voltage values were obtained by changing the external resistances at 10, 100, 500, 1000, 2000, and 10,000 Ω. The current and power densities in the curves were calculated as described above.
The chemical oxygen demand (COD) was measured using a COD Test Kit (Hach Co., Loveland, USA) and a thermostat reactor (DRB200, Hach Co., Loveland, USA). 2 mL of the sample was added into the COD test kit and heated at 150 °C for 2 h using the DRB 200 thermostat reactor. The COD concentration was evaluated using a COD colorimeter (Thermo Fisher Scientific, Inc., Waltham, USA). The COD removal rate was calculated based on the difference between the influent and effluent COD concentrations as follows:
where I COD is the influent COD and O COD is the effluent COD.
Comparison of Klebsiella sp. abundance by qRT-PCR
To demonstrate whether inoculated strain IR21 formed a biofilm on the anode surface of the conventional MFC reactor, the abundance of strain IR21 was analyzed by quantitative real-time PCR (qRT-PCR) using ribosomal RNA. 0.5 × 0.5 cm2 of the anodic biofilm was sampled at 67 days after operation of the AC-MFC without strain IR21 and at 89 days after operation with strain IR21. The anode biofilm was sampled using a sterilized cutter. RNA extraction and cDNA synthesis were conducted, as previously described [17]. qRT-PCR was conducted using specific primer sets, the Kleb_primer set 1 and Kleb_primer set 2 (described above). The amount of strain IR21 in the biofilm was estimated from the total DNA elution buffer volume, anode surface area, and organic contents.
Results
Identification of strain IR21
The DNA sequence of the strain IR21 was compared with the National Center for biotechnology Information (NCBI) GenBank database nucleotide BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The strain IR21 had 99 % of similarity with Klebsiella michiganensis strain AG-57 16S ribosomal RNA gene, partial sequence (accession number, KF817669.1) under NCBI GenBank database. Phylogenetic analysis of strain IR21 sequence based on the GenBank database showed that strain IR21 belongs to genus Klebsiella with K. michiganensis (KF817789.1, bootstrap value of 92.4 %), K. oxytoca (KF254665.1, bootstrap value of 70.2 %), and K. planticola (NR119279.1, bootstrap value of 70.2 %) (Fig. 1).
Klebsiella michiganensis was isolated from a tooth brush holder in 2013 [18]. It is a glucose fermenter and can also utilize citrate as a carbon source under facultative anaerobic conditions [18]. The electron transfer ability and applicability in MFC systems of K. michiganensis have not been reported yet. K. oxytoca, which is known as citrate fermenting bacteria, was isolated from sediments under an iron mat [19, 20]. K. oxytoca produces exopolysaccharide (EPS) which binds with Fe(III) under anaerobic environments [20]. The binding Fe(III)-EPS precipitates and thus, K. oxytoca can tolerate a high iron concentration [20]. During citrate fermentation, K. oxytoca reduced Fe(III), which did not bind with EPS, to Fe(II) [19, 20]. K. planticola was isolated from salt marsh sediments [21]. K. planticola is a cadmium-resistant bacteria and it is tolerant of other toxic metals such as Cr(VI), As(V), Co(II), Zn(II), and Pb(II) [21].
Ferric iron reduction of strain IR21
First, 50 mM ferric iron was added into a serum bottle to evaluate the ferric iron reduction ability of strain IR21. A day after cultivation, Klebsiella sp. IR21 started to reduce Fe(III) at 4.9 ± 0.40 mg L−1 and the Fe(II) concentration increased up to 11.6 ± 0.47 mg L−1after 9 days (Fig. 2). Klebsiella sp. IR21 reduced approximately 27.8 % of Fe(III) to Fe(II), demonstrating that Klebsiella sp. IR21 has the capacity for electron transfer. There was no abiotic Fe(III) reduction in the control group.
Electrical performance of strain IR21 in a MFC reactor
The voltage generated by Klebsiella strain IR21 in an AC-MFC reactor is shown in Fig. 3. The AC-MFC with Klebsiella sp. IR21 was monitored for 34 days and 20 mM glucose was added when the glucose concentration decreased below 100 mg L−1 (data not shown). The AC-MFC with the inoculation of Klebsiella sp. IR21 started to generate approximately 35 mV of voltage (current density of 15 mA m−2) within 3 days; 30–35 mV of voltage (current density of 13–15 mA m−2) was maintained and a maximum voltage of 50–55 mV (current density of 21–24 mA m−2) was generated during operation. Voltage production did not occur in the control reactor without the inoculation of Klebsiella sp. IR21. The voltage production results indicate that Klebsiella sp. IR21 participated in electron transfer in the MFC reactor.
To quantify Klebsiella sp. IR21 in the anode biofilm and suspended cells in the AC-MFC reactor, qRT-PCR was conducted using two primer sets targeting Klebsiella sp. genes. 3.8 × 106 ± 6.9 × 104 gene copy number g biomass−1 was detected in the anode biofilm while 2.3 × 105 ± 7.2 × 103 gene copy number g biomass−1 was quantified in the suspended cells when primer set 1 was used (p < 0.05, Table 1). Additionally, 3.8 × 106 ± 5.5 × 104 gene copy number g biomass−1was measured in the anode biofilm while 2.2 × 105 ± 6.9 × 103 gene copy number g biomass−1 was quantified in the suspended cells when using primer set 2 (p < 0.05, Table 1). The results indicate that Klebsiella sp. IR21 successfully formed a biofilm on the anode surface and thus, the voltage production shown in Fig. 3 was generated by Klebsiella sp. IR21 on the anode surface and not by the suspended cells.
Enhancement of electrochemical performance by inoculation with strain IR21
Figure 4 shows the COD removal and voltage production of an AC-MFC without and with the inoculation of Klebsiella sp. IR21. When reject wastewater (10,000 mg L−1 COD) from a H2-producing reactor was fed into the AC-MFC reactor without the inoculation of Klebsiella sp. IR21, the effluent concentration was 1300–2500 mg L−1 and the voltage production increased gradually from 60 up to 120 mV within 30 days. After 30 days of operation with reject wastewater, the effluent COD concentration decreased to 700–1800 mg L−1 and the voltage production increased to 120–250 mV. On the 70th day, Klebsiella sp. IR21 was inoculated into the same AC-MFC fed with identical reject wastewater (10,000 mg L−1 COD). After inoculation of Klebsiella sp. IR21, the effluent COD concentration was maintained in the range of 700–2000 mg L−1 and the voltage production increased to 130–300 mV.
The electrochemical characteristics of the AC-MFC are shown in Table 2. The electrical performance including the average voltage, current density, and power density were measured at an external resistance of 100 Ω with a HRT of 2 days. Each performance value was analyzed when the AC-MFC reactor became stable. The average voltage, current density, and power density were 142.9 ± 25.74 mV, 60.5 ± 11.61 mA m−2, and 8.9 ± 3.65 mW m−2, respectively, in the AC-MFC without the inoculation of Klebsiella sp. IR21. However, the electrical performance efficiencies increased after inoculation of Klebsiella sp. IR21 (p < 0.05). The average voltage, current density, and power density increased up to 204.7 ± 40.24 mV, 87.5 ± 17.20 mA m−2, and 18.6 ± 7.23 mW m−2, respectively, in the AC-MFC with the inoculation of Klebsiella sp. IR21. However, there was not a significant difference of the COD removal rate between the AC-MFC without and with the inoculation of Klebsiella sp. IR21 (p > 0.05).
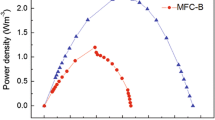
The COD removal rates were 86.7 ± 5.91 and 86.1 ± 4.70 % in the AC-MFC without and with the inoculation of Klebsiella sp. IR21, respectively. To characterize the cell voltage and power density as a function of current in the AC-MFC with the inoculation of Klebsiella sp. IR21, the polarization and power density curves are shown in Fig. 5. To obtain the polarization curve, the voltage was measured at a series of different resistances, as shown in Fig. 5a. The maximum cell voltage was 444 mV at an external resistance of 10,000 Ω (Fig. 5a) and the voltage in the AC-MFC with inoculation of Klebsiella sp. IR21 was maintained as a function of the current density production (Fig. 5b). The maximum power density was 15.04 mW m−2 at an external resistance of 100 Ω (Fig. 5b).
To demonstrate that the inoculation of Klebsiella sp. IR21 improved the reactor performance, relative quantification of Klebsiella sp. IR21 was accessed using qRT-PCR (Table 3). In the AC-MFC reactor without the inoculation of Klebsiella sp. IR21, 3.6 × 106 ± 1.9 × 105 gene copy number g biomass−1 was detected when primer set 1 was used. However, after inoculation of Klebsiella sp. IR21 into the identical reactor, the gene copy number increased to 7.7 × 106 ± 7.8 × 105 gene copy number g biomass−1 (p < 0.05). When primer set 2 was used, the amount of Klebsiella sp. IR21 increased from 4.2 × 106 ± 5.7 × 105 gene copy number g biomass−1 to 9.3 × 106 ± 7.4 × 104 gene copy number g biomass−1 (p < 0.05). The results demonstrate that Klebsiella sp. IR21 was successfully attached on the anode surface forming a biofilm and it acted as a biocatalyst to enhance the electrochemical performances in the AC-MFC system.
Discussion
Dissimilatory iron-reducing bacteria (DIRB) are microorganisms that reduce ferric iron to ferrous iron by transferring electrons from organic compounds under anoxic or anaerobic conditions [22, 23]. In the past, DIRB have been utilized for the bioremediation of soils contaminated with metals, radionuclides, and organics because Fe(III) reduction can occur with catabolism of organic contaminants [22]. Recently, many DIRB have demonstrated that they have the ability to generate electrical energy in microbial fuel cell (MFC) systems through their capacity for electron transfer [24]. Among Klebsiella species, K. pneumoniae is a representative fermentative DIRB [25, 26]. Li et al. demonstrated that K. pneumoniae reduced various Fe(III) oxides such as HFO, α-FeOOH, γ-FeOOH, and α-Fe2O3 using a fermentable substrate [25]. 0.07–0.50 mM Fe(II) was produced by K. pneumonia from 50 mM Fe(III) oxides [25]. The Fe(II) concentration increased significantly up to 0.45–4.80 mM when an artificial mediator, anthraquinone-2,6-disulfonate (AQDS), was added [25]. In other research, K. pneumoniae produced 0.426 mM Fe(II) without additional mediators from 30 mM α-FeOOH and a 4.5-fold higher concentration of Fe(II) with artificial mediators [26]. In previous research, K. pneumoniae reduced <2 % of Fe(III) and the efficiency of iron reduction did not exceed 6–10 % although artificial mediators were added [25, 26]. In this study, a novel DIRB, Klebsiella sp. IR21, was isolated from the anode biofilm of a MFC reactor. Klebsiella sp. IR21 reduced about 27 % of Fe(III) to Fe(II) without any artificial mediators (Fig. 2). When compared to values obtained in other studies which used K. pneumonia, the new fermentative DIRB, Klebsiella sp. IR21, demonstrated a higher efficiency of iron reduction than other Klebsiella species. This result indicates that Klebsiella sp. IR21 can be effectively utilized as an electron shuttle.
Electron transfer between microorganisms and the anode electrode occurs through three different mechanisms: oxidation–reduction mediators, redox enzyme on the cell membrane, and direct transfer by pili [27]. K. pneumoniae, which is the most well-known fermentative DIRB, transfers electrons through pili like G. sulfurreducens and S. oneidensis [3, 27]. They establish a biofilm by pili on the anode surface and it acts as an electron shuttle from the substrate to electrode where they are attached [3]. Zhang et al. demonstrated that K. pneumoniae established a biofilm on the anode surface and it is a key factor for electron transfer [3]. The anode surface which K. pneumoniae covered with pili was observed by a scanning electron microscope (SEM) [3]. In other research, it was reported that K. pneumoniae secreted 2,6-di-tert-butyl-p-benzoquinone (2,6-DTBBQ) which functioned as an redox mediator [28]. Among Klebsiella species, mechanisms of electron transport have only been investigated for K. pneumoniae while other Klebsiella species have not been evaluated to determine whether they have electrochemical capabilities. Therefore, this study accessed whether Klebsiella species other than K. pneumoniae have electron transfer and electricity production capabilities.
It is difficult to directly compare electrical efficiencies of MFCs because the performances of MFCs are different as various factors such as system designs, operation condition, and biological components [2]. In general, the power densities of MFCs have various range from tens to thousands as conditions [29]. The power densities obtained in this study were relatively lower than other researches [29, 30]. The most likely reason for the low power density was due to the large inner volume of the MFCs. In this study, the MFCs had comparatively larger anode inner volume of 1 L than other systems. Generally, a large inner volume of the MFC causes high internal resistance, thus it reduces average power density [31].
During the past decade, interest in MFC systems has increased because it is considered a sustainable technique for simultaneous energy production and organic waste treatment [2]. Since the 1990s, MFC systems have been reported and interest in MFC systems has rapidly increased recently [2]. In accordance with these interests, various trials have been conducted to improve the efficiency of MFCs [2, 32]. Most strategies to improve the performance of MFC systems have focused on changing the structure, materials, or operating conditions [2, 32]. On the other hand, the effects of biocatalyst inoculation in MFC systems have rarely been reported although the biocatalyst is considered the most important component controlling overall MFC performance [2]. However, investigations in which artificial catalysts are supplied into MFC systems to enhance reactor performance are readily available [33]. Rahimnejad et al. demonstrated that the artificial catalyst of methylene blue enhanced the power generation of a MFC reactor by a factor of two [33]. In other research, the voltage production increased by a factor of four after artificial resazurin was supplied into a MFC reactor [34]. In research using Klebsiella species in a MFC system, it was demonstrated that K. pneumoniae produced endogenous catalyst 2,6-DTBBQ, which acted as an electron shuttle between K. pneumoniae and the electrode [28]. Although the exact mechanism for electron transfer for isolated strain IR21 has not been investigated, it may be possible to secrete an endogenous mediator as with K. pneumoniae [28]. Thus, it may act as a performance enhancer for MFC systems. In our study, when comparing the electrochemical performance before and after inoculation of Klebsiella sp. IR21, the COD removals were not significantly different, but the electrical performances significantly increased after inoculation. That is, before inoculation of Klebsiella sp. IR21, the microbial anode biofilm community could utilize wastewater as a substrate, but it had a low efficiency of electron transfer to the electrode. However, the increasing electrical performance demonstrates improved efficiency of electron transfer to the electrode, indicating that inoculated Klebsiella sp. IR21 may be used as a catalyst for electron transfer.
Conclusions
A novel facultative fermentative DIRB, Klebsiella sp. IR21, was isolated from an anode biofilm of a MFC reactor. Klebsiella sp. IR21 could successfully form a biofilm on the anode and generate voltage from a single-chamber MFC reactor without any additional artificial electron shuttles. In addition, Klebsiella sp. IR21 could improve the electrical performance of a MFC reactor treating reject wastewater. When Klebsiella sp. IR21 was added into the MFC reactor, the efficiency of electron transfer to the electrode increased. The combined results indicated that Klebsiella sp. IR21 has potential as a biocatalyst for application in MFC systems.
References
Logan BE, Hamelers B, Rozendal R, Schroder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells, methodology and technology. Environ Sci Technol 40:5191–5192
Mohan SV, Velvizhi G, Modestra JA, Srikanth S (2014) Microbial fuel cell: critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew Sust Energy Rev 40:779–797
Zhang L, Zhou S, Zhuang L, Li W, Zhang J, Lu N, Deng L (2008) Microbial fuel cell based on Klebsiella pneumoniae biofilm. Electrochem Comm 10:1641–1643
ElMekawy A, Srikanth S, Bajracharya S, Hegab HM, Nigam PS, Singh A, Mohan SV, Pant D (2015) Food and agricultural wastes as substrates for bioelectrochemical system (BES): the synchronized recovery of sustainable energy and waste treatment. Food Res Int 73:213–225
Schröder U, Harnisch F, Angenent LT (2015) Microbial electrochemistry and technology: terminology and classification. Energy Environ Sci 8:513–519
Zhang Y, Olias LG, Kongjan P, Angelidaki I (2011) Submersible microbial fuel cell for electricity production from sewage sludge. Wat Sci Tech 64:50–55
Kelly PT, He Z (2014) Nutrients removal and recovery in bioelectrochemical systems: a review. Bioresour Technol 153:351–360
Zhang Y, Angelidaki I (2012) A simple and rapid method for monitoring dissolved oxygen in water with a submersible microbial fuel cell (SBMFC). Bios Bioelec 38:189–194
Wang H, Ren ZJ (2013) A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol Adv 31:1796–1807
Niessen J, Schröder U, Scholz F (2004) Exploting complex carbohydrates for microbial electricity generation—a bacterial fuel cell operating on starch. Electrochem Commun 6:955–958
Bajracharya S, Heijne A, Benetton XD, Vanbroekhoven K, Buisman CJN, Strik DPBTB, Pant D (2015) Carbon dioxide reduction by mixed and pure cultures in microbial eletrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Biores Technol 195:14–24
Zhuang L, Zhou S, Yuan Y, Liu T, Wu Z, Cheng J (2011) Development of Enterobacter aerogenes fuel cells: from in situ biohydrogen oxidization to direct electroactive biofilm. Bioresour Technol 102:284–289
Lee Y-Y, Kim TG, Cho K-S (2015) Novel Klebsiella sp. or Candida sp. and microbial fuel cells comprising the same. PCT/KR2015/004055
Caccavo F, Lonergan DJ, Lovley DR, Davis M, Stolz JF, McInerney MJ (1994) Geobacter sulfurreducens sp. nov., a hydrogen- and acetate- oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol 60:3752–3759
Lovley DR, Phillips EJP (1986) Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol 51:683–689
Sørensen J (1981) Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol 43:319–324
Kim TG, Moon K-E, Yun J, Cho K-S (2013) Comparison of RNA- and DNA-based bacterial communities in a lab-scale methane-degrading biocover. Appl Microbiol Biotechnol 97:3171–9181
Saha R, Farrance CE, Verghese B, Hong S, Donofrio RS (2013) Klebsiella michiganensis sp. nov., a new bacterium isolated from a tooth brush holder. Curr Microbiol 66:72–78
Baldi F, Minacci A, Pepi M, Scozzafava A (2001) Gel sequenstration of heavy metals by Klebsiella oxytoca isolated from iron mat. FEMS Microbiol Ecol 36:169–174
Baldi F, Marchetto D, Battistel D, Daniele S, Faleri C, De Castro C, Lanzetta R (2009) Iron-binding characterization and polysaccharide production by Klebsiella oxytoca strain isolated from mind acid drainage. J Appl Microbiol 107:1241–1250
Sharma PK, Balkwill DL, Frenkel A, Vairavamurthy MA (2000) A new Klebsiella planticola strain (Cd-1) grows anaerobically at high cadmium concentrations and precipitates cadmium sulfide. Appl Environ Microbiol 66:3083–3087
Fredrickson JK, Gorby YA (1996) Environmental processes mediated by iron-reducing bacteria. Curr Opin Biotechnol 7:287–294
Liu T-X, Li X-M, Li F-B, Zhang W, Chen M-J, Zhou S-G (2011) Reduction of iron oxides by Klebsiella pneumoniae L17: kinetics and surface properties. Colloids Surf A Physicochem Eng Asp 379:143–150
Zuo Y, Xing D, Regan JM, Logan BE (2008) Isolation of the exoelectrogenic bacterium Ochrobactrum anthropi YZ-1 by using a u-tube microbial fuel cell. Appl Environ Microbiol 74:3130–3137
Li XM, Zhou SG, Li FB, Wu CY, Zhuang L, Xu W, Liu L (2009) Fe(III) oxide reduction and carbon tetrachloride dechlorination by a newly isolated Klebsiella pneumoniae strain L17. J Appl Microbiol 106:130–139
Li X, Liu L, Liu T, Yuan T, Zhang W, Li F, Zhou S, Lo Y (2013) Electron transfer capacity dependence of quinone-mediated Fe(III) reduction and current generation by Klebsiella pneumoniae L17. Chemosphere 92:218–224
Zeng LZ, Zhao SF, Wang YQ, Li H, Li WS (2012) Ni/β-Mo2C as novel-metal-free anodic electrocatalyst of microbial fuel cell based on Klebsiella pneumoniae. Int J Hydrogen Energy 37:4590–4596
Deng LF, Li FB, Zhou SG, Huang DY, Ni JR (2010) A study of electron-shuttle mechanism in Klebsiella pneumoniae based-microbial fuel cells. Chin Sci Bull 55:99–104
Sharma M, Bajracharya S, Gildemyn S, Patil SA, Alvarez-Gallego Y, Pant D, Rabaey K, Dominguez-Benetton X (2014) A critical revisit of the key parameters used to describe microbial electrochemical systems. Electrochim Acta 140:191–208
Pasupuleti SB, Srikanth S, Mohan SV, Pant D (2015) Continuous mode operation of microbial fuel cell (MFC) stack with dual gas diffusion cathode design for the treatment of dark fermentation effluent. Int J Hydrogen Energy 40:12424–12435
Lee Y-Y, Kim TG, Cho K-S (2015) Effects of proton exchange membrane on the performance and microbial community composition of air–cathode microbial fuel cells. J Biotechnol 211:130–137
Oliveira VB, Simões M, Melo LF, Pinto AMFR (2013) Overview on the developments of microbial fuel cells. Biochem Eng J 73:53–64
Rahimnejad M, Najafpour GD, Ghoreyshi AA, Shakeri M, Zare H (2011) Methylene blue as electron promoters in microbial fuel cell. Int J Hydrogen Energy 36:3335–3341
Sund CJ, McMasters S, Crittenden SR, Harrell LE, Sumner JJ (2007) Effect of electron mediators on current generation and fermentation in a microbial fuel cell. Appl Microbiol Biotechnol 76:561–568
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF-2012R1A2A2A0346724).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, YY., Kim, T.G. & Cho, Ks. Enhancement of electricity production in a mediatorless air–cathode microbial fuel cell using Klebsiella sp. IR21. Bioprocess Biosyst Eng 39, 1005–1014 (2016). https://doi.org/10.1007/s00449-016-1579-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1579-8