Abstract
Toxicology tests on earthworms were conducted in artificial soil, which is composed of 10% Kaolin clay (Fisher Scientific), 20% of coir pith and 70% of sand (grade 70 particle size 0.1–0.3 mm). Melanoidins were applied to artificial soil in different concentrations ranging from 0 (control), 250, 500, 750 and 1000 µg per kg. The toxicological analysis of earthworms treated with synthetic melanoidins and distillery effluent includes antioxidant enzyme analysis (superoxide dismutase, catalase, glutathione peroxidase and glutathione S-transferase), comet assay and histopathological analysis. The investigation facilitates us to conclude that melanoidin stimulates adverse effects on earthworms such as oxidative stress and DNA damage which may be the important mechanisms of melanoidin toxicity to earthworms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Molecular markers or biological markers are referred as the indicators of biological effects of contaminants on organisms which may perhaps be used as diagnostic and extrapolative tests to identify and levy the pollution effects, predominantly to find the effects of low concentrations of composite mixtures of contaminants on the quality of the environment (Livingstone 1993). Biological responses are classified in molecular, subcellular and cellular level. In ecotoxicology, the biomarkers have a major rationale as the conventional tactics have certain limitations about the quantity of chemicals that can cause adverse effects on plants and animals which are considered as the endpoints.
The use of living organisms as biomarkers is a fundamental contemplation which provides the unsurpassed consideration of authentic circumstances of ecosystems. The trials are possible by means of either structural or functional, which are based on the ecosystems. In general, oligochaetes are vastly considered to be used as bioindicators (Peijnenburg et al. 2009). Their significance is copious as they are the ecologically chief invertebrate group. Other reasons to use oligochaetes for ecotoxicological studies are that they are easy to handle and could be cultured under laboratory conditions.
Earthworms prevail in various types of soils ranging from temperate to tropical regions. They are considered as an imperative number in food chains as earthworms are considered as a prime food source for many organisms including birds and mammals which have turned out as the major incentive for the ecologists and ecotoxicologists to study about earthworms (Lokke and Gestel 1998). Taking this into account, earthworm is habitually used as experimental organisms to resolve the deposition of chemicals in the soil (Oste et al. 2001).
As the behavior and morphology of earthworms are created accordingly to cope up with the aqueous and solid phases of the soil, it may be concluded that the earthworms can be used to study about the ill effects caused by both the organic (Vijver et al. 2003) and inorganic (Jager et al. 2003) contaminants added to the soil.
Roughly, the majority of the earthworm species do not react to low concentrations of chemicals, as the chemical constitution of their body takes time for the proper understanding about the chemical or pollutant and the mechanism of its toxicity on their body (Sturzenbaum et al. 1998). As portrayed earlier in the literatures, the cellular organization of the oligochaetes is not convolute, and it also constitutes distinguished organs.
The other purpose to use earthworms as bioindicators is that they have the ability to ensure metals to a great extent. Chlorogocytes play an important role in accumulating metals in the earthworm’s body in different patterns resulting in tissue accumulation for different types of metals (Morgan and Morgan 1990).
Hitherto, in the literatures, various effects of biomarkers have been explained. The effects include:
-
1.
Alternations in DNA induced by toxic contaminants which have genotoxic properties.
-
2.
Immunological responses.
-
3.
Stimulation of metal-binding proteins.
-
4.
Hindrance of enzymes.
-
5.
Cohesion of lysosomal membrane.
1.1 Alternations in DNA Induced by Toxic Contaminants Which Have Genotoxic Properties
The conventional reactions include binding of the contaminant or its secondary metabolites to the DNA, DNA strand breakage, exchange of bases, etc.
1.2 Immunological Responses
The immune system crashes in acute conditions which may lead even to mortality. The changes in the immune system namely sublethal are considered as the first level of toxicity induced by the contaminants. The immunological system is compliant and pliable, wherein earthworms, it was observed that the alteration on the immune system restores immediately after the confiscation of earthworms from the contaminant source. Some studies have proved the influence of chemicals in the immune system of the earthworms. Nevertheless, the dosage and the retaliation of the immune system to the contaminants are yet to be unearthed.
1.3 Stimulation of Metal-Binding Proteins
The heavy metals in different concentrations to which the earthworm is exposed may be detoxified by metal-binding proteins such as metallothionein (Huang et al. 1987; Klaassen et al. 1999). The roles of these proteins are not fully understood, but it is thought that these proteins may involve in the regulation of the metal levels in tissues. The use of these proteins as bio indicators may be proficient to quantify the exposure and metal toxicity.
1.4 Hindrance of Enzymes
The inhibition or the hindrance of the enzymes due to the exposure of the contaminants is considered as the conventional biomarker. For example, inhibition of cholinesterases has been studied to understand the exposure of pesticides such as carbamate and organophosphorous using earthworms. The enzymes were responsible for the transmission of nerve signals, and it is reported that the pesticides can trigger a decline of the production and the activity of the specific enzyme (Dikshith and Gupta 1981).
1.5 Cohesion of Lysosomal Membrane
Lysosomes are membrane-bound cell organelles which are structurally and chemically spherical vessels which are capable of breaking down almost all biomolecules including protein and DNA. Any alteration in the membrane of this organelle is considered to be a measure of stress in the organism which is considered to be bioindicator of contaminants in the soil.
2 Materials and Methods
2.1 Earthworm Exposures
Earthworms used in this assay (Eudrilus eugeniae) were purchased from an earthworm culturing farm at Periyar Maniyammai University in Thanjavur. Toxicology tests on earthworms were conducted in OECD artificial soil (OECD 2004), which is composed of 10% Kaolin clay (Fisher Scientific), 20% of coir pith and 70% of sand (grade 70 particle size 0.1–0.3 mm). Melanoidins were applied to artificial soil in concentration of 0 (control), 250, 500, 750 and 1000 µg per kg. For each concentration, 20 earthworms were added.
2.2 Enzyme Extraction
Earthworms were killed by introducing into the formaldehyde solution for a minute and were placed in a dissection tray. They were dissected vertically, and its gut was cleared and then placed into a prechilled mortar and pestle and was crushed under ice-cold condition with 0.5 M Phosphate buffer (pH 7.0). The homogenate was centrifuged at 8000 rpm at 4 °C for 30 min. The supernatant was used for the assay enzyme activity and for protein determination (Song et al. 2009).
2.3 Protein Estimation by Bradford Assay
Accurate determination of protein concentration was done by Bradford method (1976). It is widely used and is found to be more reliable for detecting 20–400 µg of protein.
-
Procedure
To the clean test tubes, about 2.50 ml of Bradford reagent was added. To the reagent, 10–20 µl of earthworm extract is added which produces a blue color by reaction. Its absorbance is measured at 595 nm.
-
Formula
2.4 Enzyme Assays
2.4.1 Superoxide Dismutase Assay
Superoxide dismutase is an enzyme that catalyzes the dismutation of superoxide (O2−) into oxygen and hydrogen peroxide. Thus, they are an important antioxidant defense in nearly all the cells exposed to oxygen.
-
Procedure
100 µl of earthworm extract was taken and mixed with 250 µl of absolute alcohol. Then, 150 µl of chloroform was added, and 1 ml of distilled water was added to the mixture. The reaction mixture was centrifuged at 2500 rpm for 15 min. The whole supernatant was taken and to that 2 ml of Tris buffer was added. OD was measured with time interval of 0, 1, 2 and 3 min (Marklund and Marklund 1974) following the addition of 500 µl of pyrogallol.
-
Formula
x = 0 min EDTA OD − 1 min OD
y = 0 min OD − 2 min OD/2
z = 0 min OD − 3 min OD/3
a = Control
b = (x + y + z)/3
c = b/a * 100
2.4.2 Catalase Assay
Catalase enzyme is a common enzyme present nearly in all living organisms exposed to oxygen. It catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting the cells from oxidative damage by reactive oxygen species. Likewise, catalase has one of the highest turnover numbers of all enzymes; one catalase molecule can convert millions of molecule of hydrogen peroxide to water and oxygen/second.
-
Procedure
Accurately, 1.5 ml of phosphate buffer was taken, and 0.5 ml of hydrogen peroxide was added to the buffer. To the mixture, 100 µl of earthworm extract is added. At this stage, to the reaction mixture, 250 µl of potassium dichromate was added which when added arrests the reaction at different time intervals. Then, the whole set is kept in boiling water bath for 10 min. After 10 min, the reaction develops a green color for which the absorbance is measured (Sinha 1972).
-
Formula
2.4.3 Glutathione Peroxidase
Glutathione peroxidase is the general name of an enzyme family with peroxidase activity whose main biological role is to protect the organism from oxidative damage. The biochemical function of glutathione peroxidase is to reduce lipid hydro peroxides to their corresponding alcohols and to reduce free hydrogen peroxide to water.
-
Procedure
200 µl of EDTA was added to the centrifuge tubes. To that, 100 µl of sodium azide was added, and along with that 100 µl of hydrogen peroxide was added. To the reaction mixture, reduced glutathione was mixed. 400 µl of phosphate buffer is added simultaneously. To that, earthworm extract is added followed by incubation of 37 °C for 10 min. Then, the reaction is arrested by adding 500 µl of 10% TCA. Then, the whole mixture is centrifuged at 3000 rpm for 3 min. The supernatant is transferred to a test tube, and 3 ml of disodium hydrogen phosphate is added. One ml of DTNB was further added which develops a yellow color. Absorbance is measured for the reaction mixture at 412 nm (Rotruck et al. 1973).
-
Formula
2.4.4 Glutathione S-Transferase
Glutathione S-transferases (GSTs), previously known as ligandins, comprise a family of eukaryotic and prokaryotic phase II metabolic isozymes best known for their ability to catalyze the conjugation of the reduced form of glutathione (GSH) to xenobiotic substrates for the purpose of detoxification.
-
Procedure
To a clean centrifuge tube, 1 ml of phosphate buffer, 100 µl of CDNB and 100 µl of enzyme extract are added. Then, it was made up to the volume of 3 ml and was incubated at 37 °C for 5 min. The reaction gets started by adding 100 µl of reduced glutathione. The absorbance is measured at 340 nm with an interval of 30 s for 5 min. Reaction mixture without enzyme source is used as blank (Mozer et al. 1983).
-
Formula
2.5 Comet Assay
After exposure of the earthworms to the synthetic melanoidins, their coelomocytes were obtained using the non-invasive extrusion method described by Eyambe et al. (1991). Individual earthworms were rinsed in the extrusion medium composed of 5% ethanol, 95% saline, 2.5 mg/ml EDTA and 10 mg/ml guaiacol glyceryl ether (pH 7.3). Coelomocytes were spontaneously secreted in the medium and washed with phosphate-buffered saline (PBS) prior to the comet assay. The cells were collected by centrifugation (3000 µg, 10 min) and placed on ice prior to the comet assay. The comet assay was performed according to Singh et al. (1988), with slight modifications. The cell suspension was mixed with 100 ml of 0.7% low melting agar (LMA) in PBS at 37 °C and pipetted onto fully frosted slides precoated with a layer of 100 ml 0.8% normal melting agar (NMA). After solidification on ice, another layer of 85 ml LMA was added, and the slides were immersed into a lysis solution (2.5 M NaCl, 10 mM Tris, 100 mM Na2EDTA (pH 10.0), 1% Na-sarcosinate, 10% dimethyl sulfoxide (DMSO) and 1% Triton X-100). Slides were then incubated in an electrophoresis tank containing 300 mM NaOH with 1 mM Na2EDTA for 20 min prior to electrophoresis for 15 min at 25 V (300 mA). The slides were then neutralized (0.4 M Tris, pH 7.5) thrice at 5 min intervals and stained with 40 ml ethidium bromide (13 mg/ml) for fluorescence microscopy analysis (a fluorescence microscope) using a digital imaging system. The images of the SCGE were analyzed using CASP (Konca et al. 2003). One hundred cell cores on each slide were counted. The parameter used to quantify the extent of DNA damage was the olive tail moment (OTM). OTM is the product of the distance between the centers of gravity of the head and the center of gravity of the tail and percent tail DNA.
2.6 Histology of Earthworm
The histology of gut of earthworm was studied adopting the routine paraffin method (Humason 1979). Gut of earthworm, dissected out from the control and experimental animals, was blotted free of mucus, washed thoroughly in physiological saline, cut into pieces of desired size and fixed in Bouin’s fluid fixative immediately after autopsy. Fixation was carried out at room temperature for 24 h, after which the tissues were transferred to 70% alcohol. Several changes of 70% alcohol were given until the yellow color disappeared from the tissues. The tissues were then dehydrated by passing through ascending grades of alcohol, cleared in xylene, infiltrated with molten paraffin and finally embedded in paraffin wax (58 °C MP). Tissue section of 5-μm thick transverse and longitudinal sections were obtained using a rotary microtome (Leica, Germany). The sections, thus obtained, were stained in Harris hematoxylene and eosin, dehydrated using alcohol, cleared in xylene and mounted using dihydroxy phthalate xylol (DPX). The stained slides were observed using a Research Microscope (NikonE400).
3 Results and Discussion
The experimental set up used for the study conducted to understand the effect of differnt concentrations of melanoidin and distillery effluent along with the control is as represented in Plate 1.
3.1 Mortality of Earthworms
Table 1 denotes the mortality of earthworms during the period of the study. The mortality of the earthworms increased significantly as the concentration of the synthetic melanoidins increased. This signifies that the melanoidins induce mortality in the earthworms.
Initially, 20 earthworms were inoculated in each of the treatment groups.
3.2 Estimation of Protein Concentration of Earthworm Sample Treated with Varying Concentrations of Synthetic Melanoidin
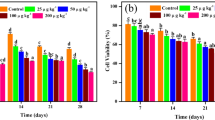
In the present study, the total protein content got subsequently increased in the second week of the study period for melanoidins treated group compared to control animals. The total protein concentration in all the four concentrations melanoidins (250, 500, 750, 1000 µg kg−1) and the distillery effluent increased during 14 days and then decreased after 21 days compared to the controls (Table 2 and Fig. 1). Upon all the concentrations, 1000 µg kg−1 and distillery effluent showed drastic reduction (0.0512 and 0.0263 mg/ml) in the protein concentration.
The total protein content subsequently increased in the second week of the study period for melanoidins treated group compared to control animals. This could be attributed to the fact that melanoidins greatly increase the gene transcription and enhance expression of mRNA (Lescoat et al. 2000).
3.3 Estimation of Superoxide Dismutase Concentration of Earthworm Sample Treated with Varying Concentrations of Synthetic Melanoidin
As shown in Table 3 and Fig. 2, the SOD activity in all the four concentrations of melanoidins (250, 500, 750, 1000 µg kg−1) and the distillery effluent increased at the initial stage and then decreased throughout the study period compared to the controls. The SOD activity of the groups treated with distillery effluent was significantly higher on the 7th day (23.492 Unit/mg protein/min) when compared to the other treated groups and control.
The decrease in SOD activity signifies an imbalance between the pre-oxidant and antioxidant states in the body leading to an imbalance in systemic redox status (Chakraborty et al. 2007) caused by the melanoidins.
3.4 Estimation of Catalase Concentration of Earthworm Sample Treated with Varying Concentrations of Synthetic Melanoidin
Compared to the control, the CAT activity of the earthworm exposed to distillery effluent was markedly higher (52.5 µg mol/mg protein/min) on the 7th day and started to decrease in the following weeks of the study. The CAT activity at the other four concentrations of melanoidins was lower than that of the controls on the 7th day, and statistically, significant reduction was found on the all other days.
The catalase activity may be induced or hindered in the cells that were exposed to Melanoidins. Induction and deterioration of catalase activity are regulated at the mRNA level (Mutoh and Hayashi 1988). The catalase activity tended to increase in the tissues of the earthworm at the beginning treated with effluent and then tend to decrease which shows that the melanoidins affect the catalytic mechanism of the earthworm tissues (Table 4 and Fig. 3).
3.5 Estimation of Glutathione Peroxidase Concentration of Earthworm Sample Treated with Varying Concentrations of Synthetic Melanoidin
There was a significant reduction in the GPx activity of earthworms exposed to four concentrations melanoidins (250, 500, 750, 1000 µg kg−1) and the distillery effluent when compared to control except on the 7th day of the study period (Table 5 and Fig. 4).
The GPx activity decreased from the 14th day throughout the period in all the test groups suggesting that antioxidant defense is overwhelmed by ROS. Mostly, in a system, there will be a balance between the GPx production and lipid peroxide level. Glutathione has ability to control, the levels of lipid peroxides in body. In the animal, the system lost its critical balance of redox states compared to control animals (Song et al. 2009).
3.6 Estimation of Glutathione S-Transferase Concentration of Earthworm Sample Treated with Varying Concentrations of Synthetic Melanoidin
The experimental results obtained from plants suggest that glutathione S-transferase may be closely linked with stress response. Glutathione S-transferase activity seemed to increase at seven days’ exposure and then significantly decrease from 14th day. The trend observed after seven days was more convicting as the activity in the control was particularly low at this time. This could explain the lack of a significant effect of melanoidins on GST activity (Table 6 and Fig. 5) (Saint-Denis et al. 2001).
3.7 DNA Damage Evaluation by Comet Assay in Earthworm Coelomocytes
The alkaline comet assay was conducted in earthworm coelomocytes of negative and positive controls exposed to different doses of melanoidins and distillery effluent. The data shown in Table 7 represent the DNA damage. As expected, comparison between positive and negative controls showed that methyl methanesulfonate (MMS) induced a significant increase in DNA migration. In control group, the cells with intact DNA and few comets with very short tail length were seen. The extent of DNA damage along with number of comets was increased with increased doses of melanoidins (250, 500, 750, 1000 µg kg−1) and distillery effluent. As compared to positive control, there was a significant decrease in those of 250, 500 and 750 µg/kg melanoidins test groups, whereas no appreciable difference between those of 1000 µg/kg melanoidins, distillery effluent and positive control. The % DNA in comet head decreased in treated groups compared to negative control.
But only, the group of 1000 µg/kg melanoidins and distillery effluent showed significant decrease in % DNA than in positive control. Sharp and significant increase was observed in % DNA in comet tail and tail moment in treated groups compared to negative control. Regarding positive control, there was a notable difference between low dose and positive control, but the other two doses had no significant difference in % DNA in comet tail and tail moment (Table 7, Fig. 6). Correspondingly, Collins and Harrington 2002 have reported the determining of steady-state damage levels following induction of oxidative stress.
3.8 Histopathological Analysis of Earthworms When Treated with Varying Concentrations of Synthetic Melanoidin
There are no visible alterations in the vertical histology of the body wall of E. eugeniae in the control (Plate 2). The earthworms exposed to different concentrations of melanoidins and distillery effluent developed varied histological changes (Plate 2). The most prominent changes included degeneration of the circular and longitudinal muscles.
The longitudinal muscle has shown the signs of crakes in major parts of the regions. Also, there was generalized cellular cytolysis, tissue vacuolization and necrosis. Degenerative zones in the longitudinal muscles were noticeable as well as tissue erosion. There were also noticeable cytoplasmic and nuclear alterations in the epidermal cells and those of both circular and longitudinal muscles. Similarly, Gobi and Paramasamy (2010) studied the effect of butachlor herbicide on earthworm Eisenia fetida where they have investigated about the histological changes of the earthworm.
In short, the midgut region of distillery effluent exposed earthworms is severely affected as the tissue necrosis is severe. The 750 and 500 µg/kg melanoidins show tissue vacuolization and cellular cytolysis. This shows evidently that the melanoidins and the distillery effluent affect the tissue organization of the earthworms.
4 Conclusion
The effect of raw distillery effluent and different concentrations of melanoidin was understood by estimating the antioxidative enzymes. The impact on DNA was made clear following the Comet assay. Further the histological studies makes us understand that the increase in melanoidin increases the damage caused to the earthworms. This shows that melanoidins induce an adverse effect on earthworms which trigger mutilation in the antioxidant enzyme mechanism, DNA damage and cytological damages. And to add a note, this is alleged to be the first investigation to find out the toxicity of melanoidins on earthworms.
References
Bradford, M. M. (1976). Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry, 72, 248–254.
Chakraborty, I., Mondal, S., Rout, D., Chandra, K., & Islam, S. S. (2007). Structural investigation of a heteroglycan isolated from the fruit bodies of an ectomycorrhizal fungus Astraeus hygrometricus. Carbohydrate Research, 342(7), 982–987.
Collins, A., & Harrington, V. (2002). Repair of oxidative DNA damage: Assessing its contribution to cancer prevention. Mutagenesis, 17, 489–493.
Dikshith, T. S. S., & Gupta, S. K. (1981). Carbaryl induced biochemical changes in earthworm (Pheretima posthuma). Indian Journal of Biochemistry & Biophysics, 18, 154–169.
Eyambe, S. G., Gaven, A. J., Fitzpatrick, L. C., Venables, B. J., & Cooper, E. L. (1991). A non-invasive technique or sequential collection of earthworm leukocytes during sub chronic immune toxicity study. Laboratory Animals, 25, 61–70.
Gobi, M., & Paramasamy, G. (2010). Effect of butachlor herbicide on earthworm Eisenia fetida—Its histological perspicuity. Applied and Environmental Soil Science, 758–850.
Huang, P. C., Morris, S., Dinman, J., Pine, R., & Smith, B. (1987). Role of metallothionein in detoxification and tolerance to transition metals. Experientia Suppl, 52, 439–446.
Humason, G. L. (1979). Animal tissue techniques (4th ed.). San Francisco: W. H. Freeman and Co.
Jager, T., Fleuren, R. H. L. J., Hogendoorn, E. A., & De Korte, G. (2003). Elucidating the routes of exposurefor organic chemicals in the earthworm, Eisenia andrei (Oligochaeta). Environmental Science and Technology, 37, 3399–3404.
Klaassen, C. D., Liu, J., & Choudhuri, S. (1999). Metallothionein: an intracellular protein to protect against cadmium toxicity. Annual Review of Pharmacology and Toxicology, 39, 267–294.
Konca, K., Lankoff, A., Banasik, A., Lisowska, H., Kuszewski, T., Gozdz, S., et al. (2003). A cross-platform public domain PC image-analysis program for the comet assay. Mutation Research, 534, 15–20.
Lescoat, P., Ali-Haimoud-Lekhal, D., & Bayourthe, C. (2000). Effets de Saccharomyces cerevisiae et Aspergillus oryzae sur la digestion et le fonctionnement ruminal: Rencontres autour des Recherches sur les Ruminants, Paris, France.
Livingstone, D. M. (1993). Temporal structure in the deep-water temperature of four Swiss lakes: A short-term climatic change indicator. Verhandlungen des Internationalen Verein Limnologie, 25, 75–81.
Lokke, H., & Gestel, C. A. M. (1998). Handbook of soil invertebrate toxicity tests. Chichester: Wiley.
Marklund, S., & Markulnd, G. (1974). Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 469–474.
Morgan, J. E., & Morgan, A. J. (1990). The distribution of cadmium, copper, lead, zinc and calcium in the tissues of the earthworm Lumbricus rubellus sampled from one uncontaminated and four polluted soils. Oecologia, 84, 559–566.
Mozer, T. J., Tiemeier, D. C., & Jaworski, E. G. (1983). Purification and characterization of corn glutathione S-transferase. Biochemistry, 22, 1068–1072.
Mutoh, N., & Hayashi, Y. (1988). Isolation of mutants of Schizosaccharomyces pombe unable to synthesize cadystin, small cadmiumbinding peptides. Biochemical and Biophysical Research Communications, 151, 32–39.
OECD. (2004). Guidelines for Testing of Chemicals No. 222: Earthworm Reproduction Tests (Eisenia foetida/Eisenia andrei). Organisation for Economic Co-operation and Development (OECD), Paris.
Oste, L. A., Dolfing, J. K., Ma, W. C., & Lexmond, T. M. (2001). Cadmium uptake by earthworms as related to the availability in the soil and the intestine. Environmental Toxicology and Chemistry, 20, 1785–1791.
Peijnenburg, Vijiver, M. G., Martina, G. V. (2009). Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicology and Environmental Safety, 67, 163–179.
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., & Hoekstra, W. G. (1973). Selenium: Biological role as a component of glutathione peroxidase. Science, 179, 588–590.
Saint-Denis, M., Narbonne, J. F., Arnaud, C., & Ribera, D. (2001). Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: Effects of lead acetate. Soil Biology & Biochemistry, 33, 395–404.
Singh, N. P., McCoy, M. T., Tice, R. R., & Schneider, E. L. (1988). A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research, 175, 184–191.
Sinha, K. A. (1972). Colorimetric assay of catalase. Analytical Biochemistry, 47, 389–394.
Song, Y., Zhu, L. S., Wang, J., Wang, J. H., Liu, W., & Xie, H. (2009). DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biology & Biochemistry, 41, 905–909.
Sturzenbaum, S. R., Kille, P., & Morgan, A. J. (1998). Heavy metal-induced molecular responses in the earthworm, Lumbricus rubellus genetic fingerprinting by directed differential display. Applied Soil Ecology, 9, 495–500.
Vijver, M. G., Vink, J. P. M., Miermans, C. J. H., & Van Gestel, C. A. M. (2003). Oral sealing using glue: A new method to distinguish between intestinal and dermal uptake of metals in earthworms. Soil Biology & Biochemistry, 35, 125–132.
Acknowledgements
The authors are grateful and would like to thank Department of Science and Technology (DST), MoEF & CC and UGC, Government of India, New Delhi, for the financial support and encouragement. They also acknowledge the DST-FIST, DST-PURSE, UGC-Non-SAP, UGC-SAP and MHRD-RUSA for providing the instrumentation facilities in the department.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Vivekanandam, S., Muniraj, S., Muthunarayanan, V. (2020). Melanoidin Induced DNA Damage and Effects on Antioxidative Enzymes in Earthworm (Eudrilus eugeniae). In: Ghosh, S., Sen, R., Chanakya, H., Pariatamby, A. (eds) Bioresource Utilization and Bioprocess. Springer, Singapore. https://doi.org/10.1007/978-981-15-1607-8_22
Download citation
DOI: https://doi.org/10.1007/978-981-15-1607-8_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1606-1
Online ISBN: 978-981-15-1607-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)