Abstract
Aluminium-based water treatment sludge (WTS) was investigated for its potential to remove chromium(VI) from simulated wastewater. Batch sorption tests and column tests were carried out using poly aluminium chloride sludge. Results of the batch study showed that chromium sorption decreased with increase in pH for the pH range studied (2.0–12.0). Long-duration column test with an empty bed contact time of 3 h indicated good Cr(VI) removal capacity of WTS as no chromium was present in the effluent for 115 bed volumes, and a sorption capacity of 1.62 mg/g of dry sludge was obtained which was lower than the sorption capacity reported in the literature. In the presence of other metals such as Co, Cu, Hg and Pb, both percentage Cr(VI) removal and sorption capacity was negatively affected in column tests, and Cr(VI) sorption capacity was only 0.96 mg/g of dry sludge.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In recent years, the problems caused due to accumulation of heavy metals in the environment have been receiving increased attention. Heavy metals are still being used in large quantities due to their technological importance in different industries and this has made heavy metals as unavoidable pollutants. Due to low removal efficiency, heavy metals at its lower concentration and higher costs in conventional techniques paved the way for the identification of adsorbents with high sorption capacity for heavy metals, and several adsorbents obtained from low-cost and waste materials have been identified.
In water treatment facilities all around the world, large quantities of water treatment sludge or residual (WTS) are generated during the coagulation and flocculation process which results from the removal of suspended impurities in the raw surface water. At present, disposal methods for these WTS include (a) discharge into municipal wastewater (b) disposal into inland water bodies and (c) use for soil improvement, with the assumption that these wastes do not cause any harm to the receiving environment [1, 2]. In recent years, with the realization of negative impacts of WTS on the environment, stringent regulations have been implemented in many countries. This resulted in an interest in the reuse of these residuals. Studies have been reported in the literature on the possible reuse of the water treatment sludge in different ways which include its use for soil improvement, as a construction material and in water and wastewater treatment [3, 4].
Coagulation is a common treatment process in water treatment industry which uses iron or aluminium ions, and the resulting sludge namely Fe-WTS and Al-WTS contain iron and aluminium hydroxides, respectively. These sludges also contain humic substances and suspended solids originally present in the raw water [5]. Further, due to their amorphous nature, WTS will add more sorption sites on sorption surface [6]. Thus, this highly reactive nature makes water treatment sludge suitable for several potential applications as sorbents [7]. Two different approaches have been employed in the reuse of WTS for wastewater treatment [3]. In the first approach, aluminium or iron ions are first recovered from the waste material and the recovered chemical is used generally as a coagulant in the treatment of water/wastewater. The raw residual in dry/wet form itself can also be used for the removal of different pollutants present in wastewater. The second approach where wet/dry sludge is utilized is more popular compared to the first approach and has been employed to treat types of wastewaters containing a variety of pollutants such as heavy metals, nutrients and colour [3, 4, 6, 8]. Several studies have been reported in the literature assessing the potential of WTS for removing various heavy metals [7, 9,10,11,12,13,14]. As heavy metal itself is a major environmental problem in consideration, the reuse of these residuals in the removal and recovery of metals can be considered as an environmentally friendly option for both sludge and metals.
The present study investigated the suitability of water treatment residuals obtained from a treatment plant which uses poly aluminium chloride as a coagulant for removing heavy metals from simulated wastewater. The performance of water treatment sludge was evaluated in batch and long-duration column tests using single metal and multi-metal systems. Heavy metals tested included chromium, copper, mercury, cobalt and lead.
2 Materials and Methods
2.1 Collection and Characterisation of Water Treatment Sludge
In the present study, WTS was collected from the coagulation–flocculation unit of the water treatment plant in Katargram, Surat, India. The plant treats 240 million litres of River Tapi water per day using poly aluminium chloride (PACl) as the coagulant. The collected residual was transported to the laboratory and was dried at 105 °C for 2 days. No pretreatment was given to the sludge before use. The sludge was crushed after drying and passed through 150-micrometre sieve and the retained portion on the sieve was employed in all the tests. A single batch of sludge collected on a particular day was used in all the tests reported in this study.
Scanning electron micrographs of the powdered sludge shown in Fig. 1 indicate that the sludge is of various sizes and shapes and is of highly porous nature. The SEM images do not show classical well-crystalline appearance on WTS surface. Hence, the aluminium present in the WTS may be in the amorphous form.
2.2 Batch Sorption Tests
The batch tests to determine the adsorption capacity of different metal ions was performed at ambient temperature. Five metals, namely lead, cobalt, mercury, copper and chromium were used. Tests were conducted in duplicate at a constant ionic strength using 0.01 M sodium nitrate solution. The desired concentration of metals was prepared by using lead nitrate Pb(NO3)2, cobalt nitrate Co(NO3)2, mercuric nitrate Hg(NO3)2, copper nitrate Cu(NO3)2 and sodium chromate Na2CrO4. A 50 mL of solution containing equal volume of 0.01 M NaNO3 solution and individual metal solution was taken in a bottle and the calculated quantity of WTS was added to a particular solution. This solution was shaken for the desired period in a shaker at 150 rpm until equilibrium was reached. After the equilibrium period, centrifugation was done (2000 × g) for 10 min and was analyzed for the metal content using ICP-AES/spectrophotometer (ARCOS Spectro, Germany). For the batch adsorption experiments, different variables were considered including contact time, initial concentration of metal, pH and WTS dosage and the optimum conditions for metal adsorption were determined. Effect of each parameter on the extent of adsorption was analyzed by maintaining other variables constant.
2.3 Column Study
Two columns were set up, one for the removal of chromium alone and the other for multi-metal (chromium, copper, mercury, lead and cobalt) removal. Both these columns were made of a cylindrical glass tube of inner diameter 2.4 cm. Sludge was packed between two supporting layers of glass wool for 15 cm height and the upper portion of the bed was covered with pebbles. The synthetic metal solution of 10 mg/L concentration was prepared to feed the column. Column operation was done in upflow mode using a peristaltic pump with a flowthrough time maintained at 1 h for chromium and multi-metal removal studies. The effluent was collected and metal contents were determined. Leaching of aluminium and organic matter from sludge were checked by measuring effluent aluminium and COD, respectively.
3 Results and Discussion
3.1 Characteristics of Water Treatment Residual
Table 1 presents the physical and chemical properties of the sludge employed. The solid content of the sludge was high at 67% while TVS/TS ratio showed a low value of 0.45. The low TVS/TS ratio shows a larger proportion of inorganic materials in the sludge. The elemental spectra analysis using EDS showed that the important contents in the sludge were Al, Si, Ca, and Fe. Aluminium and iron contents were 88.7 and 48.2 mg/g dry sludge, respectively. Scanning electron micrographs indicated that residuals consist of particles with irregular shape and sizes. High silica content in sludge (165.1 mg/g dry sludge) indicated the predominated silica surface sites on the surface of the water treatment sludge that can involve in surface complex reactions of metals with silica oxide.
3.2 Batch Tests
In order to determine the suitable contact time for the sorption of metal onto water treatment sludge, the adsorption rate of metal ions was determined as a function of time (Fig. 2). It was observed that equilibrium had occurred at a contact time of 8 h. Adsorption did not seem to increase beyond a contact time of 8 h, and hence, further tests were conducted with a contact time of 8.0 h.
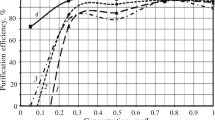
In order to study the effect of pH on heavy metal removal, the solution pH was varied in the range of 2–12 for the metals considered, and the results are shown in Fig. 3. As evident from Fig. 3, for Cr(VI), sorption decreased with increase in initial pH. For Cu(II) removal, increased pH till pH 10 showed a decreased value at pH 12. Co(II) and Hg(II) showed increasing removal with increasing pH, while Pb(II) was unaffected by pH and was completely removed in the pH range studied (2–12). pH is the key parameter that governs surface charge of WTS. The extent of sorption on adsorbent surface depends on the zero point charge (pHzpc) of the sorbent material. In this study, pHzpc of sludge was observed as 6.9. It is known that above pHzpc, the sorbent would be negatively charged and cationic metal adsorption would be favoured. Further, it is reported that increase in pH would result in increased generation of cation hydroxide phase [15] and as a result, higher metal cation removal was observed at higher pH values.
3.3 Column Tests
Two column tests were conducted, and in the first test, initial chromium content was maintained around 10 mg/L while the second column test was conducted using a solution containing all the five metals. Bed flowthrough time of 1.0 h was used in the column tests. Both the tests were performed with an initial solution pH of 6.38. Breakthrough time for each of the metals and the corresponding breakthrough concentration were found out.
Breakthrough curve for the first column test is presented in Fig. 4. The Cr(VI) concentration was below detectable limit till 116th bed volume and breakthrough occurred suddenly after that. The column run was continued till 308th bed volume where the removal reached <7.0%. Generally, the breakthrough is said to have occurred when Ce/C0 value approaches 0.05, and adsorption bed is said to be exhausted when the outlet value reaches 95% of the initial value [16]. Maximum equilibrium capacity of the sludge was given by area behind the breakthrough curve and it was obtained as 1.62 mg/g of sludge. It is much higher than that obtained from batch adsorption study (0.24 mg/g of sludge).
Figure 5 presents the results of the column study with all the five metals. Though the targeted initial concentrations for all the metals were 10.0 mg/L, it resulted in precipitation of some metals. Thus the column was clogged after 1 day operation. It was decided to filter the prepared metal solution which resulted in reduced metal concentration in the influent to the column. The average metal concentrations were Cu(II): 0.732 mg/L, Pb(II): 0.381 mg/L, Co(II): 9.019 mg/L, Cr(VI): 9.5 mg/L and Hg(II): 0.433 mg/L.
Figure 5 clearly shows that the presence of other metals affected Cr(VI) removal. For Cu(II), Pb(II) and Hg(II) breakthrough occurred at 220th, 228th and 200th bed volumes, respectively. For Co(II), no breakthrough occurred even after passage of 228th bed volume in spite of a high influent concentration of 9.019 mg/L. Column effluents were monitored for COD and aluminium also, and the results presented in Table 2 show no evidence for leaching of organic matter or Al from the sludge with effluent showing very low values for these two parameters. Influent and effluent pH were also measured and the values are given in Fig. 6. An increase in pH was observed, and it was expected due to removal of metals from the solution.
Maximum equilibrium sorption potential of the sludge for chromium in the presence of other metals is 0.96 mg/g of sludge. It is less than that from column study done for Cr(VI) alone (1.62 mg/g of sludge). Thus, it is evident that the presence of cationic metals inhibits the removal of anionic metals like Cr(VI).
From these studies, it can be concluded that the sludge used in the study has a stronger affinity toward cationic metals compared to anionic metals. In the presence of cationic metals, chromium, which is anionic, shows lower removal efficiency. It might also be due to the higher pH values of solution. Higher pH range favours the removal of cationic metals and at the same time, reduces anionic metal removal [12].
4 Conclusions
The potential of water treatment sludge to treat chromium(VI) containing wastewater was assessed in the present study. Batch adsorption experiments and column tests conducted showed a significant effect of pH on chromium removal with decreased sorption at higher pH values. In long-duration column test, good Cr(VI) removal was obtained and the medium had a Cr(VI) sorption capacity of 1.62 mg/g of dry sludge. Presence of other heavy metals Co, Cu, Hg and Pb reduced both percentage chromium removal and sorption capacity, and the maximum chromium sorption capacity was only 0.96 mg/g of dry sludge. No significant leaching of organic content or aluminium from the sorbent occurred with the column effluent showing very low values for these two parameters.
References
Babatunde, A.O., Zhao, Y.Q.: Constructive approaches toward water treatment works sludge management: an international review of beneficial re-uses. Crit. Rev. Environ. Sci. Technol. 37, 129–164 (2007)
Makris, K.C., O’Connor, G.A.: Beneficial utilization of drinking-water treatment residuals as contaminant-mitigating agents. In: Sarkar, D., Datta, R., Hannigan, R. (eds.) Developments in Environmental Science, Concepts and Applications in Environmental Geochemistry, vol. 5, pp. 607–636. Elsevier Science, Amsterdam (2007)
Nair, A.T., Ahammed, M.M.: Coagulant recovery from water treatment plant sludge and reuse in post-treatment of UASB reactor effluent treating municipal wastewater. Environ. Sci. Pollut. Res. 21, 10407–10418 (2014)
Ahmad, T., Ahmad, K., Alam, M.: Sustainable management of water treatment sludge through 3’R’ concept. J. Cleaner Prod. 124, 1–13 (2016)
Makris, K.C., Sarkar, D., Parsons, J.G., Datta, R.: X-ray absorption spectroscopy as a tool investigating arsenic(III) and arsenic(V) sorption by an aluminium-based drinking-water treatment residual. J. Hazard. Mater. 171, 980–986 (2009)
Makris, K.C., Harris, W.G., O’Connor, G.A., Obreza, T.A.: Phosphorus immobilization in micropores of drinking water treatment residuals: implications for long-term stability. Environ. Sci. Technol. 38, 6590–6596 (2004)
Ippolito, J.A., Barbarick, K.A., Elliott, H.A.: Drinking water treatment residuals: a review of recent uses. J. Environ. Qual. 40, 1–12 (2011)
Gadekar, M.R., Ahammed, M.M.: Coagulation/flocculation process for dye removal using water treatment residuals: modelling through artificial neural networks. Desalin. Water Treat. 57, 26392–26400 (2016)
Caporale, A.G., Punamiya, P., Pigna, M., Violante, A., Sarkar, D.: Effect of particle size of drinking-water treatment residuals on the sorption of arsenic in the presence of competing ions. J. Hazard. Mater. 260, 644–651 (2013)
Castaldi, P., Silvetti, M., Garau, G., Demurtas, D., Deiana, S.: Copper(II) and lead(II) removal from aqueous solution by water treatment residues. J. Hazard. Mater. 283, 140–147 (2015)
Putra, R.S., Tanaka, S.: Aluminum drinking water treatment residuals (Al-WTRs) as an entrapping zone for lead in soil by electrokinetic remediation. Sep. Purif. Technol. 79, 208–215 (2011)
Zhou, Y.F., Haynes, R.J.: Removal of Pb(II), Cr(III) and Cr(VI) from aqueous solutions using alum-derived water treatment sludge. Water Air Soil Pollut. 215, 631–643 (2015)
Hovsepyan, A., Bonzongo, J.J.: Aluminum drinking water treatment residuals (Al-WTRs) as sorbent for mercury: implications for soil remediation. J. Hazard. Mater. 164, 73–80 (2009)
Ghorpade, A., Ahammed, M.M.: Water treatment sludge for removal of heavy metals from electroplating wastewater. Environ. Eng. Res. 23, 92–98 (2017)
Elzinga, E.J., Sparks, D.L.: Reaction condition effects on nickel sorption mechanisms in illite-water suspensions. Soil Sci. Soc. Am. J. 65, 94–101 (2009)
Metcalf and Eddy: Waste Water Engineering, 4th edn. Tata McGraw-Hill Education Private Limited, New Delhi, India (2003)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Thomas, T.N., Ahammed, M.M. (2020). Removal of Chromium Using Water Treatment Sludge. In: Kalamdhad, A. (eds) Recent Developments in Waste Management . Lecture Notes in Civil Engineering, vol 57. Springer, Singapore. https://doi.org/10.1007/978-981-15-0990-2_23
Download citation
DOI: https://doi.org/10.1007/978-981-15-0990-2_23
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0989-6
Online ISBN: 978-981-15-0990-2
eBook Packages: EngineeringEngineering (R0)